Wounding downregulates PTEN and activates the PI3 kinase/Akt pathway. Pharmacologic inhibition of PTEN stimulates the motility of corneal epithelial cells and corneal wound healing. These results imply that the inhibition of PTEN may be a plausible approach for corneal wounds.
Abstract
Purpose.
The PI3K/Akt pathway is required for cell polarization and migration, whereas the phosphatase and tensin homologue deleted on chromosome 10 (PTEN) has inhibitory effects on the PI3K/Akt pathway. The authors therefore hypothesized that wounding would downregulate PTEN and that this downregulation would enhance wound healing.
Methods.
In human corneal epithelial (HCE) cell monolayer and rat cornea scratch wound models, the authors investigated PTEN and Akt expression using Western blot and immunofluorescence analyses. The effects of PTEN and PI3K inhibitors dipotassium bisperoxo (picolinato) oxovanadate (bpv(pic)) and LY294002 on cell migration and wound closure were investigated using time-lapse imaging. Finally, the authors investigated the effect of PTEN inhibition on wound healing in whole rat eyes.
Results.
In HCE cell monolayer and rat cornea, PTEN was downregulated at the wound edges within 30 minutes of wounding. The downregulation of PTEN was causal in a simultaneous increase in Akt activation, which was responsible for a significant increase in individual cell migration rate from 8.8 μm/h to 17.3 μm/h. An increased migration rate was maintained for 20 hours. PTEN inhibition significantly enhanced the wound healing rate in the HCE cell monolayer from 10 minutes onward after treatment and reduced the healing time in eye organ culture from 30 to 20 hours.
Conclusions.
Injury to the corneal epithelium downregulates the expression of PTEN at wound edges, allowing increased PI3K/Akt signaling, thereby contributing to a significant enhancement of cell migration and wound healing. These results suggest that PTEN inhibition may be an effective treatment for corneal injury.
A major factor in the outcome of all keratorefractive surgical procedures is biological diversity in the corneal wound healing response,1 which is orchestrated by a variety of cytokines, growth factors (GFs), and chemokines.2,3 Epithelial wound healing comprises a complex cascade of events that ultimately culminate in wound closure and reestablishment of normal epithelial function. To close a wound cells must survive, proliferate, and migrate directionally from the edges to the center of the wound. Epithelial migration is initiated by the polarized rearrangement of the actin cytoskeleton and the formation of lamellipodia and filopodia until the wound has completely closed.4–7 Cellular migration into the center of the wound depends on the synthesis of cytoskeletal proteins such as vinculin, actin, talin, and integrins and on cell surface receptors such as the hyaluronan (HA) receptor CD44. However, the events that initiate and control wound healing are not yet fully understood.8
Injury to the epithelium results in the release of GFs and adenosine triphosphate (ATP), which initiate the phosphoinositide 3-kinase (PI3K) and the serine/threonine protein kinase B (Akt) signaling pathway. Indeed, this signaling pathway has been shown to be essential for the directional migration of corneal and skin epithelial cells in response to injury and in response to physiological electrical signals at wound edges.9–11 It has therefore been proposed that effective and specific modulation of this pathway may provide novel clinical therapies, leading to improved wound healing.12
The phosphatase and tensin homologue deleted on chromosome 10 (PTEN) was identified in 1997.13 Located on human chromosome 10q23, it was found as a candidate tumor suppressor gene in a variety of human cancers.13–16 PTEN is a dual-specificity phosphatase demonstrating phosphatase activity against protein13 and lipid substrates, most notably the 3-phosphorylated phosphoinositides (PI) PtdIns(3)P, PtdIns(3,4)P2 (PIP2), and PtdIns(3,4,5)P3 (PIP3).17 PI lipids are generated by PI3K in response to extracellular stimuli such as GFs that activate receptor tyrosine kinases (RKTs) and G-protein–coupled receptors. In dephosphorylating PIP3, PTEN directly antagonizes the PI3K pathways.18 PIP3 leads to the activation of Akt, a serine/threonine kinase that is involved in numerous cellular processes including transcription, proliferation, and migration.19
PTEN reconstitution or overexpression inhibits cell migration20,21 through several different mechanisms, one of which is its dephosphorylation of PIP3 and the resultant downregulation of the actin cytoskeleton regulators ras-related C3 botulinum toxin substrate (Rac) and cell division cycle 42 (Cdc42).21 PTEN has also been shown to inactivate focal adhesion kinase,22 which is required for the correct turnover of focal adhesions, necessary for effective cell movement and actin rearrangement. Inhibition of PTEN has been found to significantly increase the number of cells migrating to scraped areas and the rate of wound closure in vitro.23 Therefore, there is an important question to be raised: does the downregulation of PTEN play a key role in the mechanisms of wound healing in vivo?
We found that wounding the corneal epithelium induced a downregulation of PTEN, which significantly increased the activation of Akt. Inhibition experiments showed that cellular migration in wound healing was dependent on Akt activation; PTEN inhibition significantly enhanced migration, and simultaneous Akt inhibition was found to counteract this response. PTEN inhibition also increased wound healing in whole rat eyes. These results suggest that PTEN is an important regulator of the molecular events associated with the mechanisms of corneal wound healing in vivo and that inhibition of PTEN may be a pharmacologic target for clinical treatment of injured epithelia.
Materials and Methods
Materials and Cell Culture
HCE cells were kindly provided by Kaoru Araki-Sasaki (Osaka University, School of Medicine, Osaka, Japan). HCE cells were cultured in Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12; Gibco, Grand Island, NY), supplemented with 15% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 5 μg/mL insulin (Sigma-Aldrich, Paisley, UK), 0.1 μg/mL cholera toxin, 10 ng/mL human epidermal growth factor (Sigma-Aldrich USA), and 40 μg/mL gentamicin (Invitrogen-Gibco, Paisley, UK) at 37°C with 5% CO2. Once they were 70% to 80% confluent, the cells were subcultured onto a 100-mm tissue culture dish (Corning, Corning, NY). DMEM/F-12 was changed every 2 to 3 days. A PTEN inhibitor, dipotassium bisperoxo (picolinato) oxovanadate (bpv(pic)), was purchased from Calbiochem (Nottingham, UK), and an inhibitor of phosphorylated AKT (pAKT; LY294002) was purchased from Sigma-Aldrich UK. Anti-PTEN (1:200), anti-Akt (1:200), anti-phospho PTEN (1:200), and anti-phospho Akt (1:200) were obtained from Cell Signaling (Herts, UK). Anti–mouse and anti–rabbit secondary antibodies (1:5000) were obtained from Sigma-Aldrich UK.
Western Blot Analysis
Proteins were isolated using RIPA cell lysis buffer (Cell Signaling, Beverly, MA). Thirty micrograms of protein was electrophoresed through a 4%–12% bis-tris gel (Invitrogen). Sample proteins were transferred to nitrocellulose membrane (Invitrogen), and the membrane was blocked in 1× phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) and 5% skimmed milk. The membrane was washed with PBS-T, and then primary antibody was diluted in PBS-T containing 3% BSA and added to the membrane, which had been incubated overnight at 4°C. Bound antibodies were detected by horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich UK), followed by enhanced chemiluminescence detection (Amersham, Buckinghamshire, UK).
Immunofluorescence
Preparations for tissue sections and fluorescence staining of rat cornea were obtained from four rats (Sprague-Dawley, male or female) euthanatized for other purposes. Preparations were placed in M199 medium (Gibco). One hour after wounding, the eyes were fixed in paraformaldehyde for 15 minutes. The preparations were frozen at −80°C, and sections were obtained by slicing (Sliding Arc CO2 Freezing Microtome; E. Leitz Inc., Rockleigh, NJ) and were collected in PBS (Invitrogen) with 0.02% sodium azide (NaN3; Sigma-Aldrich USA). Sections were floated onto gelatin-coated microscope slides and allowed to dry. For the staining of PTEN in the HCE monolayer and cornea, samples were blocked for 30 minutes in blocking solution (10% normal serum from the same species as the secondary antibody, 1% BSA, and 0.02% NaN3 in PBS. A monoclonal antibody against PTEN (1:2000; Cell Signaling) was used to label the protein (1 hour at room temperature). After washing, the samples were incubated with Texas Red conjugated secondary antibody (1:2000; Jackson ImmunoResearch Laboratories, Bar Harbor, ME) and phalloidin-FITC (1:1000; Sigma-Aldrich) for 1 hour at room temperature. Images were obtained with an inverted fluorescence microscope (Axiovert 100; Carl Zeiss, Oberkochen, Germany) using controlled Axion software.
Cell Migration and Wound Healing Assays
Single-cell migration (low density of cells seeded) was recorded with a time-lapse recording system (Axiovert 100 microscope; Carl Zeiss) with 10-minute intervals. The images were analyzed with research imaging software (MetaMorph; Universal Imaging Corporation, Downingtown, PA). Trajectories of individual cells were tracked and quantified with the research imaging software (MetaMorph; Universal Imaging Corporation). The total length of the migration trajectory of a cell (Tt) was defined as the migration distance. The trajectory length (Tt) divided by the given period (T) yields the trajectory speed (Tt/T) or the migration rate. The details have been described previously.24
HCE cells were cultured on 24-well plates or 35-mm cell culture dishes (Corning) to form an intact monolayer. Once confluent, two types of wounds were made to analyze wound healing rates. The first type of wounds was a hole of approximately 200 μm in diameter made in the center of each well with a 200-μL tip. Holes of too large and too small diameters were excluded from further experiments.25 The second type was scratch wounds made using a 200-μL pipette tip to form an approximately 200-μm wide “wound” as previously described.26 After 1-hour recovery in growth media, DMEM/F-12 growth media were then changed to the media with or without bpv(pic) (0.1, 1.0, and 10.0 μM, as indicated; Calbiochem) or 30 μM LY294002 (Sigma-Aldrich) before experimentation. A time-lapse recording system (Axiovert 100 microscope; Carl Zeiss) was used to record the images at 10-minute intervals or 1-hour intervals. The images were analyzed using research imaging software (MetaMorph; Universal Imaging Corporation).
Organ Culture
The eyes of adult rats (Sprague-Dawley, male and female, n = 20; age range, 12–16 weeks; weight range, 180–210 g) were enucleated immediately after euthanatization and placed in 50-mL tubes (Becton Dickinson, Franklin Lakes, NJ) containing cold M199 medium. The corneal epithelium was removed according to the published method.27,28 Briefly, a circle 2 mm in diameter was cut in the center of the cornea with a biopsy cutter (Miltex, York, PA) under a dissecting microscope with a cold light source. A cotton swab saturated with n-Heptanol was applied with moderate pressure to the cornea for approximately 30 seconds. A disposable scalpel (BD Biosciences, Franklin Lakes, NJ) was used to scrape and remove the entire or superficial layers of epithelial layer within the demarcated area in a standardized fashion avoiding damage to the basement membrane. The wounded eyes were placed in six-well plates, 6 mL serum-free M199 with antibiotic solution (with or without inhibitor) was added to the wells, and the medium was refreshed every 10 hours because of the high stability of bpv(pic) in the medium.29 The plates were incubated at 37°C with 5% CO2. The reepithelialization process was monitored by imaging every 10 hours using a dissecting microscope (SteREO LumarV12; Carl Zeiss) for up to 30 hours. Fluorescein dye (100 μL of 0.5%; Sigma-Aldrich USA) was applied to stain the wound just before every imaging time point. Excess fluorescein dye was rinsed away with 5 mL PBS. The corneal wound area was measured on the images with research imaging software (MetaMorph; Universal Imaging Corporation). The “healing area” is the difference in area between the original wound area and the wound area at a later time point. The healing rate was presented as: percentage: healing area/original wound area × 100%.
Statistical Analysis
Values are expressed as the mean ± SEM. The Student's t-test was used for comparisons of biochemical data between the control and one drug-treated sample. The percentages of wound closure and the speed of cell migration after treatment with multiple drugs or concentrations were compared by one-way ANOVA and Tukey's multiple comparison tests using PHStat software (Prentice Hall, Upper Saddle River, NJ). Statistical significance for differences between groups of data was accepted with P < 0.05.
Results
Downregulation of PTEN in Wounded HCE Monolayer and Rat Cornea
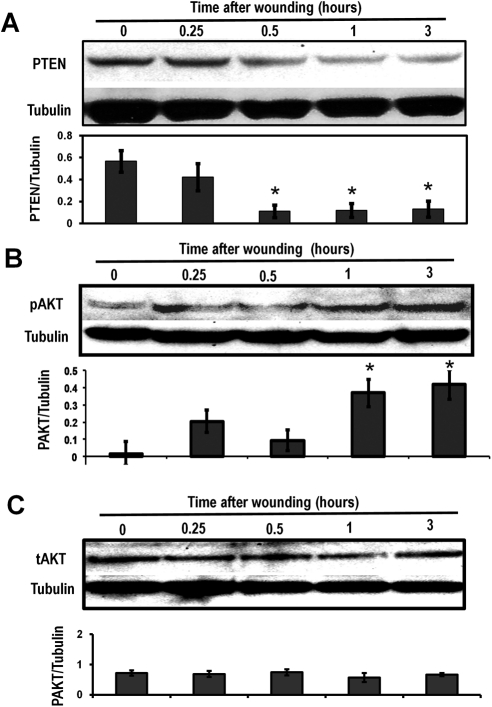
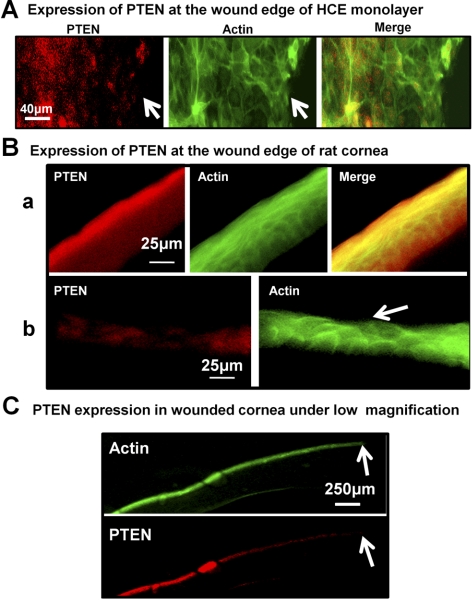
The expressions of PTEN and Akt in the HCE monolayer were assessed at 0.25, 0.5, 1, and 3 hours after wounding in the HCE monolayer. Western blot analysis revealed the downregulation of PTEN at 0.5 hour after wounding, with a further decline through 1 and 3 hours (Fig. 1A). Simultaneously, Akt was activated after wounding in the HCE monolayer (Fig. 1B). Immunofluorescence staining showed a downregulation of PTEN expression around wound edges in both the HCE monolayer (Fig. 2A) and the rat corneal epithelium removed wound (Figs. 2B, 2C).
Figure 1.
PTEN was downregulated and Akt was activated after wounding of the HCE cell monolayer, as determined by Western blot analysis. (A) PTEN was increasingly downregulated in a time-dependent manner after wounding. Time 0 = unwounded. (B) pAkt levels were upregulated in a time-dependent manner. The results of Western blot analysis showed that pAkt increased over time. (C) Total Akt levels showed no variation in nonwounded or wounded HCE monolayers. Proteins were isolated using RIPA buffer at 0.25, 0.5, 1, and 3 hours after wounding/without wound. Tubulin was used as a protein loading control. Scanning densitometry of Western blot analysis under each western image was from three different experiments. Bars show mean ± SD. *P < 0.05 compared with unwounded control (T0).
Figure 2.
PTEN was downregulated around wound edges in the HCE cell monolayer and rat cornea. (A) PTEN expression was reduced near the wound edges in the HCE cell monolayer at 1 hour after wounding. (B) In the normal cornea, (a) PTEN expression in the epithelium was higher on the surface than on the lower epithelium of the cornea. After wounding of the cornea, (b) PTEN expression was reduced near the wounded epithelium at 1 hour after wounding, when partial scraping of corneal epithelium, as indicated by the arrow. (C) Under low magnification, PTEN was downregulated distally from the wound edge in the cornea with the full thickness of the epithelium wound. All samples were collected at 1 hour after wounding. Red: PTEN expression was determined by immunofluorescence; green: actin expression; arrow: wound edge.
Inhibition of PTEN-Activated Akt
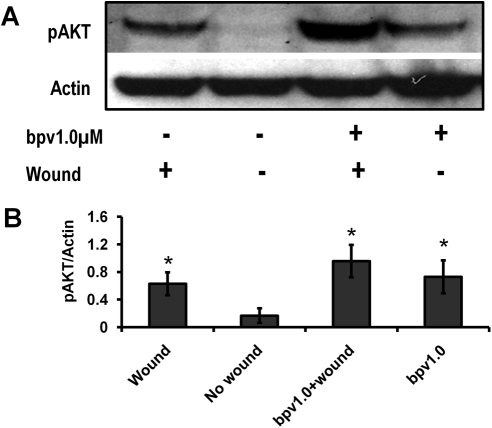
Bpv(pic) is a tyrosine phosphatase with a high specificity for PTEN. To investigate whether PTEN downregulation was directly responsible for the simultaneous activation of Akt as previously described, we administered 1.0 μM bpv(pic) to wounded and nonwounded HCE monolayers for 1 hour before protein isolation. Western blot analysis confirmed that the inhibition of PTEN resulted in a significant activation of Akt in wounded and nonwounded HCE monolayer samples (Fig. 3).
Figure 3.
Inhibition of PTEN-enhanced activation of Akt. HCE monolayer was treated with PTEN inhibitor (bpv(pic)) 1 hour after wounding for Western blot analysis. pAkt levels increased after 30 minutes' treatment with bpv and increased even more so after wounding in the presence of bpv(pic). Actin was used as a protein loading control. Scanning densitometry of Western blot analysis under each Western blot image was from three separate experiments. *P < 0.05 compared with unwounded control (T0).
Inhibition of PTEN Increased the Migration of Individual HCE Cells by the Activation of Akt
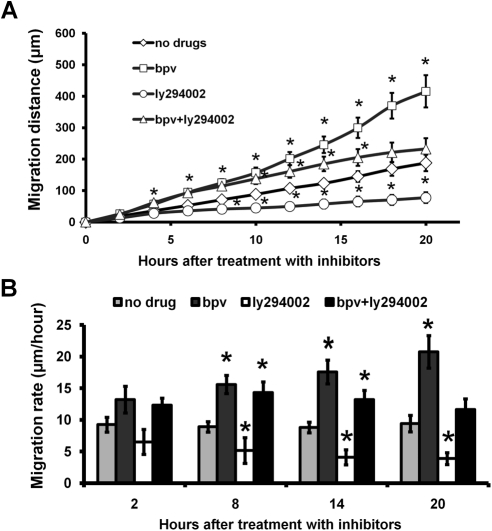
The migration of individual HCE cells treated with PTEN and Akt inhibitors was analyzed by time-lapse imaging. The PTEN inhibitor bpv(pic) (1 μM) significantly increased the rate of single-cell migration from 8.8 to 17.3 μm/h, and this increase was maintained for 20 hours (Figs. 4A, 4B). This suggested that bpv(pic) is very stable in the cell culture medium. We then treated HCE cells with both bpv(pic) (1 μM) and the Akt inhibitor LY294002 (30 μM) and found that the inhibition of Akt partially abrogated the increased migration induced by PTEN inhibition (Fig. 4), strongly suggesting that the increased migration seen after PTEN inhibition resulted from the phosphorylation of Akt.
Figure 4.
PTEN inhibition accelerated the migration rate of HCE cells. (A) Immediately after media were changed with different inhibitors, time-lapse images were recorded for 20 hours. Migration distance of single cells was analyzed and presented. There are significant different between bpv(pic) treated and without treatment (no drug) group (P < 0.05) at each time point. (B) Immediately after media were changed with different inhibitors, time-lapse images were recorded for more than 20 hours. Migration rates were presented. PTEN inhibition with 1 μM bpv(pic) enhanced the migration rate, and this was partially counteracted by the addition of the PI3K inhibitor LY294002 (30 μM). Research imaging software was used to analyze cell migration. Values are presented as mean ± SE. *P < 0.05 compared with vehicle treatment by one-way ANOVA and individual comparisons by Tukey's procedure (n = 30–32 per group).
Inhibition of PTEN Increased the Rate of Wound Closure Within 10 Minutes after bpv(pic) Treatment
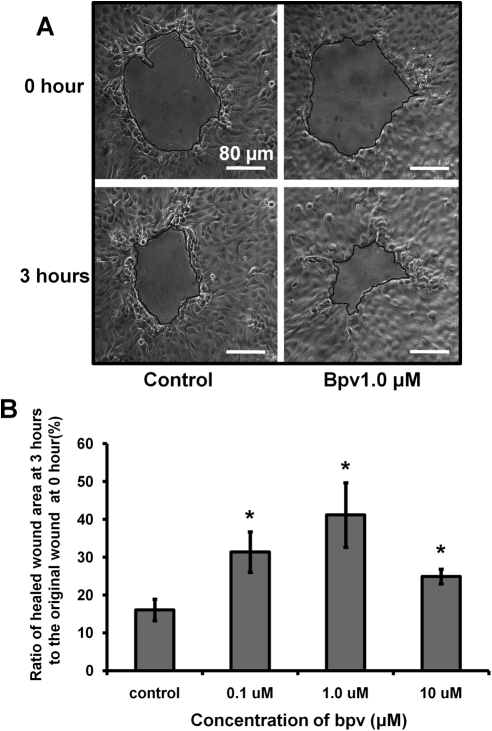
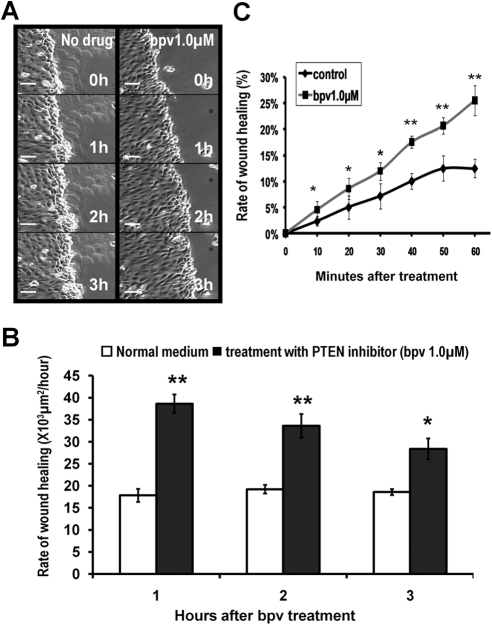
To determine the effect of PTEN inhibition on the wound healing rate, we used two recording methods. First, a small wound was made in the HCE monolayer and treated with bpv(pic) (0.1, 1, and 10 μM). Images were taken every hour, and results showed that PTEN inhibition significantly promoted wound healing at 0.1 and 1 μM bpv(pic). At 10 μM bpv(pic), the wound healing rate was slightly reduced but still significantly enhanced, possibly because of the nonspecific inhibition of additional proteins (Fig. 5). Second, time-lapse imaging was used to analyze the wound healing rate in HCE monolayers treated with 1 μM bpv(pic) (Fig. 6), and results showed that PTEN inhibition significantly enhanced the wound healing rate in HCE monolayer 10 minutes after bpv(pic) treatment (Fig. 6C).
Figure 5.
PTEN inhibition increased the wound healing rate in the HCE monolayer. (A) HCE monolayer was scratch wounded. Time-lapse images were taken every hour during the 3-hour experiment. (B) Percentage changes in wound size over 3 hours are presented. The values are presented as the mean ± SD taken from three independent experiments. *P < 0.05 compared with vehicle treatment by one-way ANOVA and individual comparisons by Tukey's procedure (n = 9 per group). Scale bars, 80 μm.
Figure 6.
PTEN inhibition increased the wound healing rate in the HCE monolayer within 10 minutes after bpv(pic) treatment. (A) HCE monolayer was scratch wounded. Time-lapse images were taken every 10 minutes for 3 hours. (B) Image analysis with research imaging software demonstrated that the rate of wound healing increased significantly when PTEN was inhibited. (C) The rate of wound healing was analyzed within the first hour (10, 20, 30, 40, 50, and 60 minutes) after bpv treatment. The wound healing rate increased as quickly as 10 minutes after bpv treatment. Values are presented as the mean ± SD taken from three independent experiments. *P < 0.05. **P < 0.01. Scale bars, 60 μm.
Inhibition of PTEN Promoted Wound Healing in Whole Rat Eyes
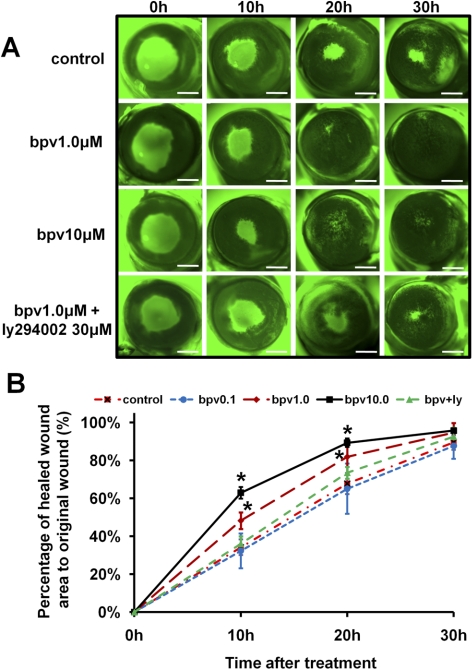
We also investigated the effect of PTEN inhibition on corneal wound healing in whole rat eyes cultured in vitro. PTEN inhibition significantly promoted wound healing in whole rat eyes. The average time of wound healing was reduced significantly from 30 to 20 hours at 1 μM bpv(pic), further confirming the effectiveness of PTEN inhibition in the promotion of wound healing. Similar to healing rates in the HCE monolayer, at 10 μM bpv(pic), healing rates were increased slightly compared with those at 1 μM bpv(pic) (Fig. 7). In addition, there is weak fluorescein staining at 30 hours in the 10-μM bpv(pic) treatment group. It may indicate that minimal cellular toxicity appeared at a high concentration of bpv(pic).
Figure 7.
PTEN inhibition increased the corneal wound healing rate in whole rat eyes. (A) Each eye was scraped to form a wound and was then cultured in serum-free M199 medium with or without bpv(pic) at 37°C and 5% CO2. Wounds were stained with fluorescein, and images were taken under a fluorescence dissection microscope every 10 hours. (B) The wound healing area was measured and calculated using research imaging software. Values are presented as the mean ± SD taken from three independent experiments. *P < 0.05 for comparison of drug treatments with vehicle treatment (control) by one-way ANOVA and individual comparisons by Tukey's procedure (n = 6–8 per group). Scale bars, 1 mm.
Discussion
Enhanced signaling through the PI3K/Akt pathway is a significant contributor to cell migration and wound healing10,30–32 as well as other cellular behaviors in normal development and in many malignant neoplasms,33 and PTEN is a negative regulator of this pathway.34 PTEN indirectly inactivates Akt, the downstream effector of PI3K, by dephosphorylating PIP3 at the D3 position.35
The present investigation showed that PTEN expression decreases after wounding in the HCE cell monolayer and whole rat cornea, the inhibition of PTEN is causal in the activation of Akt, and the inhibition of PTEN can promote wound healing in the HCE monolayer and rat cornea. PTEN expression was reduced as quickly as 30 minutes after wounding, and Akt was concurrently activated (Figs. 1A, 1B). Immunofluorescence imaging also showed that PTEN expression was significantly reduced at the wound edges of the HCE monolayer and the rat cornea (Fig. 2). This suggests that in addition to the ensuing stimuli, such as GFs and ATP initiating PI3K/Akt signaling after wounding,9,10 another mechanism to enhance this signaling was the downregulation of PTEN. Hence, we consider PTEN downregulation to be an important physiological mechanism associated with the healing of injured epithelia.
The bisperoxovanadium compounds with polar side chains, including bpv(phen) and bpv(pic), have a strong affinity for PTEN (IC50, 20–40 nM) with minimal cellular toxicity, but they are also inhibitors of several other phosphatases,29 such as insulin receptor kinase36 and glucose 6-phosphatase.37 However, a recent study reported a distinct IC50 for PTEN approximately 10- to 100-fold lower than other tyrosine phosphatases that stimulated Akt phosphorylation.29 Further experiments may further differentiate the effects of glucose 6-phosphatase and PTEN. Using pharmacologic agents targeting PTEN (bpv(phen), bpv(pic)), it has been shown that PTEN inhibition significantly activates Akt, resulting in significant acceleration of wound healing in the rabbit cornea and monolayer in human lung and airway epithelia in vitro.23,38 Similarly, our data demonstrated that bpv(pic) (1 μM) significantly raised levels of pAkt in the wounded and nonwounded HCE monolayer (Fig. 3). The enhanced wound healing rate observed in the HCE monolayer treated with bpv(pic) was dose dependent. Bpv(pic) concentrations of 0.1 and 1 μM produced a greater enhancement of the wound healing rate than did 10 μM bpv(pic) (Fig. 5). However, the inhibition of PTEN (with 1 or 10 μM bpv(pic)) promoted wound healing in the rat cornea (stratified epithelium), reducing healing time by one third (Fig. 7).
The downregulation of PTEN has also been implicated in the enhanced motility of cancer cells. PKC-β has been shown to mediate IGF-β–induced proliferation and motility in pancreatic cells by downregulating PTEN expression.39 In addition, the proto-oncogenic transcription factor Jun suppresses PTEN expression by binding to a variant AP-1 site on the PTEN promoter.40,41 Hence, when we collated the results from these individual studies, it was clear that enhanced cell motility, in various cell types, under different conditions (e.g., wounding and metastasis), is dependent on increased Akt activation, mediated by the downregulation of PTEN.
We previously discovered that applied electric fields activate PI3 kinase signaling pathway and significantly increase corneal epithelial wound healing in vitro10 and inferred that it could be a promising pharmacologic target to enhance epithelial wound healing,12 but it was not known whether the downregulation of PTEN was a physiological response to wounding. We reported that keratinocytes from PI3Kγ null mice (p110γ−/−) showed the activation of Akt after electrical stimulation, whereas keratinocytes from conditional knockouts of PTEN (pten−/−) showed increased Akt activation after electrical stimulation.10 From this we concluded that the deletion of PTEN enhanced the response in keratinocytes.10 In this study we showed that reduced PTEN expression is a physiological response to wounding, with levels dropping as quickly as 30 minutes after wounding (Figs. 1, 2). We have already discovered that PTEN inhibition enhances the response in keratinocytes. When the corneal epithelium is wounded, the transepithelial potential instantaneously drops to 0 mV at the wound edge but is maintained distally, creating lateral electric fields of approximately 42 mV/mm in the first 0.25 mm from the wound edge.12 Therefore, it may be that PTEN is being downregulated by the electric current arising at the wound edge. Further investigation into PTEN expression at wound edges under electrical stimulation may shed some light on this hypothesis.
Although PTEN expression appears to be a good therapeutic target to enhance wound healing, its functions are complex and, as yet, largely unknown. It is a known tumor suppressor; therefore, tumor promotion through its inhibition must be taken into consideration and means must be found to prevent such possible eventualities. However, the discovery of new compounds with higher potency and specificity may add to the bank of PTEN inhibitors19 and reduce the likelihood of any unwanted outcomes.
Acknowledgments
The authors thank Laura Chalmers for English correction.
Footnotes
Supported by National Institutes of Health Grant 1R01EY019101, in part by Wellcome Trust Grant 068012, Research to Prevent Blindness, Inc., NSFC Grant 30628026, UC Davis Dermatology Developmental Fund (MZ), California Institute of Regenerative Medicine Grant RB1-01417 (MZ), and NSF Grant MCB-0951199 (MZ).
Disclosure: L. Cao, None; E.O. Graue-Hernandez, None; V. Tran, None; B. Reid, None; J. Pu, None; M.J. Mannis, None; M. Zhao, None
References
- 1. Netto MV, Mohan RR, Ambrosio R, Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522 [DOI] [PubMed] [Google Scholar]
- 2. Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293–309 [DOI] [PubMed] [Google Scholar]
- 3. Imanishi J. Expression of cytokines in bacterial and viral infections and their biochemical aspects. J Biochem. 2000;127:525–530 [DOI] [PubMed] [Google Scholar]
- 4. Pfister RR. The healing of corneal epithelial abrasions in the rabbit: a scanning electron microscope study. Invest Ophthalmol. 1975;14:648–661 [PubMed] [Google Scholar]
- 5. Kuwabara T, Perkins DG, Cogan DG. Sliding of the epithelium in experimental corneal wounds. Invest Ophthalmol. 1976;15:4–14 [PubMed] [Google Scholar]
- 6. Matsuda M, Ubels JL, Edelhauser HF. A larger corneal epithelial wound closes at a faster rate. Invest Ophthalmol Vis Sci. 1985;26:897–900 [PubMed] [Google Scholar]
- 7. Crosson CE, Klyce SD, Beuerman RW. Epithelial wound closure in the rabbit cornea: a biphasic process. Invest Ophthalmol Vis Sci. 1986;27:464–473 [PubMed] [Google Scholar]
- 8. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med. 2001;226:653–664 [DOI] [PubMed] [Google Scholar]
- 9. Vanhaesebroeck B. Charging the batteries to heal wounds through PI3K. Nat Chem Biol. 2006;2:453–455 [DOI] [PubMed] [Google Scholar]
- 10. Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460 [DOI] [PubMed] [Google Scholar]
- 11. Huttenlocher A, Horwitz AR. Wound healing with electric potential. N Engl J Med. 2007;356:303–304 [DOI] [PubMed] [Google Scholar]
- 12. Zhao M. PTEN: a promising pharmacological target to enhance epithelial wound healing. Br J Pharmacol. 2007;152:1141–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947 [DOI] [PubMed] [Google Scholar]
- 14. Bostrom J, Cobbers JM, Wolter M, et al. Mutation of the PTEN (MMAC1) tumor suppressor gene in a subset of glioblastomas but not in meningiomas with loss of chromosome arm 10q. Cancer Res. 1998;58:29–33 [PubMed] [Google Scholar]
- 15. Li DM, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci U S A. 1998;95:15406–15411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waite KA, Eng C. Protean PTEN: form and function. Am J Hum Genet. 2002;70:829–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378 [DOI] [PubMed] [Google Scholar]
- 18. Leslie NR, Downes CP. PTEN: the down side of PI 3-kinase signalling. Cell Signal. 2002;14:285–295 [DOI] [PubMed] [Google Scholar]
- 19. Rosivatz E. Inhibiting PTEN. Biochem Soc Trans. 2007;35:257–259 [DOI] [PubMed] [Google Scholar]
- 20. Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617 [DOI] [PubMed] [Google Scholar]
- 21. Liliental J, Moon SY, Lesche R, et al. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol. 2000;10:401–404 [DOI] [PubMed] [Google Scholar]
- 22. Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703 [DOI] [PubMed] [Google Scholar]
- 23. Lai JP, Dalton JT, Knoell DL. Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair. Br J Pharmacol. 2007;152:1172–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao M, Jin T, McCaig CD, Forrester JV, Devreotes PN. Genetic analysis of the role of G protein-coupled receptor signaling in electrotaxis. J Cell Biol. 2002;157:921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shanley LJ, McCaig CD, Forrester JV, Zhao M. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pu J, Zhao M. Golgi polarization in a strong electric field. J Cell Sci. 2005;118:1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lambiase A, Manni L, Bonini S, Rama P, Micera A, Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41:1063–1069 [PubMed] [Google Scholar]
- 28. Song B, Zhao M, Forrester J, McCaig C. Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J Cell Sci. 2004;117:4681–4690 [DOI] [PubMed] [Google Scholar]
- 29. Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38 [DOI] [PubMed] [Google Scholar]
- 30. Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–2271 [DOI] [PubMed] [Google Scholar]
- 31. Nakao T, Shiota M, Tatemoto Y, Izumi Y, Iwao H. Pravastatin induces rat aortic endothelial cell proliferation and migration via activation of PI3K/Akt/mTOR/p70 S6 kinase signaling. J Pharmacol Sci. 2007;105:334–341 [DOI] [PubMed] [Google Scholar]
- 32. Stahle M, Veit C, Bachfischer U, et al. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci. 2003;116:3835–3846 [DOI] [PubMed] [Google Scholar]
- 33. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501 [DOI] [PubMed] [Google Scholar]
- 34. Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–279 [DOI] [PubMed] [Google Scholar]
- 35. Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192 [DOI] [PubMed] [Google Scholar]
- 36. Band CJ, Posner BI, Dumas V, Contreres JO. Early signaling events triggered by peroxovanadium [bpV(phen)] are insulin receptor kinase (IRK)-dependent: specificity of inhibition of IRK-associated protein tyrosine phosphatase(s) by bpV(phen). Mol Endocrinol. 1997;11:1899–1910 [DOI] [PubMed] [Google Scholar]
- 37. Van Schaftigen E. Glucosamine-sensitive and -insensitive detritiation of [2–3H]glucose in isolated rat hepatocytes: a study of the contributions of glucokinase and glucose-6-phosphatase. Biochem J. 1995;308(pt 1):23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kakazu A, Sharma G, Bazan HE. Association of protein tyrosine phosphatases (PTPs)-1B with c-Met receptor and modulation of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2008;49:2927–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chow JY, Dong H, Quach KT, Van Nguyen PN, Chen K, Carethers JM. TGF-beta mediates PTEN suppression and cell motility through calcium-dependent PKC-alpha activation in pancreatic cancer cells. Am J Physiol. 2008;294:G899–G905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hettinger K, Vikhanskaya F, Poh MK, et al. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007;14:218–229 [DOI] [PubMed] [Google Scholar]
- 41. Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007;120:4071–4079 [DOI] [PubMed] [Google Scholar]