The authors demonstrate that the post-illumination pupil response is reduced in glaucoma patients compared with age-matched controls with healthy eyes.
Abstract
Purpose.
The post-illumination pupil response (PIPR), which is driven by the intrinsic response of melanopsin-containing, intrinsically photosensitive retinal ganglion cells, has previously been characterized in healthy eyes. The present study examined whether the PIPR is affected in patients with glaucoma compared with healthy subjects.
Methods.
Sixteen glaucoma patients (mean age, 63.7 years) were tested by presenting a 60°, 10-second light stimulus (13 log quanta/cm2/s retinal irradiance) of either 470 nm (blue) or 623 nm (red) to one eye after dilation. The consensual pupil response of the fellow undilated eye was recorded by infrared pupillometry for 50 seconds after light offset. These pupillary responses were compared with those of 19 age-matched controls (mean age, 59 years).
Results.
The glaucoma patients displayed a net PIPR (blue PIPR minus red PIPR) that was significantly (t-test, P < 0.001) smaller (0.6 mm, SEM 0.12; P < 0.05) than in age-matched controls (1.3 mm, SEM 0.16; P < 0.001). For the patient population, the magnitude of the net PIPR was inversely correlated with the measured visual field loss (mean deviation) of the tested eye.
Conclusions.
This study demonstrates that there is a significant decrease in the ipRGC-mediated PIPR in glaucomatous patients when compared to age-matched controls. As the severity of the glaucomatous neuropathy increases, there is a correlated decrease in the PIPR. Therefore, this test has the potential for use as a clinical tool in evaluating patients with glaucoma.
The pupillary light reflex was, until recently, thought to be primarily driven by rods and cones.1,2 In 2000, a novel class of retinal ganglion cells—the melanopsin containing, intrinsically photosensitive retinal ganglion cells (ipRGCs)—were discovered that, in addition to receiving classical photoreceptor input, are intrinsically photosensitive.3–8 Recent studies have shown that ipRGCs drive pupillary responses and entrain circadian rhythms.8–12 A study in macaques and a limited sample of humans showed that, after light offset, the ipRGCs are responsible for a sustained pupilloconstriction, termed the post-illumination pupil response (PIPR).9 Using a newly developed, wide-field optical system, we have recently demonstrated that all subjects with healthy eyes display a post-illumination pupil response.13
Glaucoma is a group of diseases that have in common a characteristic optic neuropathy associated with visual field loss. There are multiple factors contributing to glaucoma; increased intraocular pressure (IOP) is the most significant risk factor.14–19 Serial visual field measurements is one of the standard methods used to follow progression of the disease in patients in the United States. Reduction in the peripheral visual field is the usual initial functional finding in glaucomatous optic neuropathy (GON) followed by progressive loss of the field, which is correlated with retinal ganglion cell (RGC) loss. 20,21 Currently, it is unclear whether all classes of RGCs are equally damaged in glaucoma.22–28 To investigate the effect of GON on one specific class of RGCs, the ipRGCs, we tested the PIPR (a specific measure of the light-evoked intrinsic activity of ipRGCs) in patients with GON and compared it with that in age-matched normal population, extracted from our previous study.13 We also correlated the PIPR of the patients with their mean deviation (MD) values (a measure of central visual field loss) in the tested eye.
Materials and Methods
Participant Selection
Patients with glaucoma were recruited as part of ongoing studies within the Glaucoma Service of the Eye Foundation Hospital (EFH) at University of Alabama at Birmingham. Data from age-matched controls were selected from a group of subjects with healthy eyes collected for a previous study in our laboratory who underwent a similar testing protocol.13 The study adhered to the Declaration of Helsinki and was approved by the Institutional Review Board. Patients with open angle glaucoma were enrolled upon written consent of each subject. These patients were identified from previous studies performed at the Glaucoma Service of the EFH. All subjects underwent complete eye examination, including fundus examination, gonioscopy, IOP measurement, and visual fields. Visual fields (Humphrey Field Analyzer 750; Carl Zeiss Meditec, Dublin, CA) measured with the SITA (Swedish Interactive Threshold Algorithm) standard 24–2 program were defined as normal when MD and pattern standard deviation (PSD) were within 95% confidence limits, with fewer than three non edge contiguous points identified as significant (P < 0.05) on the same side of the horizontal meridian in the PSD plot and within the normal limits of the glaucoma hemifield test (Carl Zeiss).29 A visual field was considered reliable when the fixation losses were <20% and false-negative and false positive rates were <25%.29 Patients were required to have repeatable and reliable abnormal visual fields reviewed by a fellowship-trained ophthalmologist (CAG) in a masked fashion. Only patients with open angles who were not taking any medications that affected the pupil or who had not undergone surgery that altered the shape of the pupil and who were judged to have GON on fundus examination and on masked evaluation of fundus stereophotographs by a fellowship-trained glaucoma specialist (CAG) were included in the study. For the subjects who had cataracts removed and had intraocular lenses in place, there was no statistically significant difference in pupil size compared with those who did not and with the age-matched control group (P < 0.05). Given that the purpose of this initial study was to determine whether the PIPR is diminished by the glaucomatous process, we avoided enrolling subjects with early glaucoma.
Testing Apparatus
A novel optical system that has previously been described was used for this study.13 The viewing eye of the subject was dilated with 1% tropicamide (Mydral; Bausch & Lomb, Rochester, NY) and 2.5% phenylephrine (Neofrin; Bausch & Lomb), and the light stimulus was presented to this eye while the consensual pupil response in the undilated eye was recorded by infrared camera (Digivue EC-PC-Cam; Elyssa Corporation, Briarcliff Manor, NY) and computer.
Stimulus Presentation
The experiment was run under data acquisition and analysis software (LabView; National Instruments, Austin, TX). Light stimuli were adjusted to present a retinal irradiance of 13 log quanta/cm2/s assuming normal prereceptoral filtering.9 Each test was run as 2 epochs (either a blue or a red stimulus), each of 80 seconds' duration, and was separated by up to 5 minutes. During each epoch, after a 20-second fixation period, the stimuli were presented to the dilated eye for a period of 10 seconds. The red stimulus primarily served as a control for nonspecific influences such as fatigue on the post-illumination pupil response. Three or four tests were conducted, and the duration of the entire session was approximately 45 minutes.
Statistical Analysis
Data from all tests were stored and analyzed off-line. Traces showing pupillary diameter and eye position were displayed for analysis, and regions of the data were selected for further analysis by data analysis software (Excel [Microsoft, Redmond, WA], SAS [SAS Institute, Chicago, IL], and MatLab [MathWorks, Natick, MA]), and data plots were generated (SigmaPlot; SyStat Software, San Jose, CA).
As described previously,13 we defined the following measures for data analysis. Baseline pupil diameter was the average pupil diameter, over a 7-second period, before light stimulus. Sustained pupil diameter was the average pupil diameter over a period of 30 seconds, starting 10 seconds after light offset. The variables used for subsequent analyses were:
 |
Descriptive statistics (mean and standard errors) were calculated for baseline, sustained response, and PIPR. Test for normalcy was performed using the Shapiro-Wilk test. Statistical analyses were performed using linear regression models, and statistical significance was obtained with a nominal P ≤ 0.05. Blue and red light responses were compared using the Student's t-test and the slopes for regression were tested with the F-test. Relationships between baseline pupil sizes and the PIPR parameters were investigated by means of Pearson's correlation coefficients and analysis of covariance within patient and control groups.
Results
Sixteen persons with GON were recruited for the study (10 women and 1 man of European ancestry; 2 women and 3 men of African ancestry). Demographic characteristics of the patients and controls are shown in Table 1. The patients' average age was 63.7 years (range, 42–88 years). There were 19 control subjects (3 women and 6 men of European ancestry; 3 women and 7 men of African ancestry) with an average age of 59 years (range, 49–80 years), whose data were collected in our previous study.13 There was no significant difference in the mean age between the two groups (t-test, P > 0.05). The number of subjects in the race and sex categories was not sufficient to test for differences between the two groups.
Table 1.
Demographic Characteristics of Patients and Controls
| Patients (n = 16) | Age-Matched Controls (n = 19) | P | |
|---|---|---|---|
| Age, y | 63.7 | 59 | >0.05 |
| Sex* | |||
| Female | 12 | 6 | |
| Male | 4 | 13 | |
| Race* | |||
| European ancestry | 11 | 9 | |
| African ancestry | 5 | 10 | |
| Baseline pupil diameter, mm | 4.5 | 4.8 | >0.05 |
| Mean deviation, dB | −12.44 | WNL |
Values are rounded to 1 decimal point. WNL, within normal limits.
Sample size was too small to test for differences between the groups.
Average pupil diameters of the patients were plotted against time for both control and test stimuli (623 and 470 nm, respectively; Fig. 1A). For comparison, pupil traces for the same stimuli in the control subjects are presented in Figure 1C. PIPR values to both red and blue stimuli were plotted against the baseline pupil diameters (Fig. 1B; blue light, R2 = 0.335, P < 0.05; red light, R2 = 0.034, P > 0.05). PIPR values for the controls subjects were also plotted as a function of baseline pupil diameter (Fig. 1D; blue light, R2 = 0.303, P < 0.05; red light, R2 = 0.045, P > 0.05).
Figure 1.
Time trace plots of the pupillary response to the control (red) and test (blue) LEDs. Bar indicates light stimulus duration. (A) Average pupil diameter (mm) plotted against time for all patients (n = 16). The red and blue traces depict pupil diameter for red and blue lights respectively. (B) PIPR values (mm) plotted against baseline measurements (mm) for the patient population (n = 16) with linear regression lines (solid line) and 95% confidence intervals (dashed lines) for both red (red diamonds, R2 = 0.034) and blue (blue diamonds, R2 = 0.335) light stimuli. (C) Pupil time course for age-matched controls (n = 19). (D) PIPR values (mm) plotted against baseline measurements (mm) for the age-matched controls (n = 19) with linear regression lines (solid line) and 95% confidence intervals (dashed lines) for both red (red circles, R2 = 0.0448) and blue (blue circles, R2 = 0.303) light stimuli.
Values for the baseline pupil diameter, sustained pupil diameter, and PIPR measures for red (control) and blue (test) stimuli, respectively, of the patients and age-matched controls are shown in Table 2. For the patient population, the mean response to the blue stimulus was 0.7 mm (SEM 0.14, P < 0.05), with a net PIPR of 0.6 mm (SEM 0.12, P < 0.05). For the control subjects, the mean response to the blue stimulus was 1.5 mm (SEM 0.15, P < 0.05),) with a net PIPR of 1.3 mm (SEM 0.16, P < 0.001).
Table 2.
Pupil Measurements in Patients and Age-Matched Controls
| Patients (n = 16) |
Age-Matched Controls (n = 19) |
|||||
|---|---|---|---|---|---|---|
| R | B | B-R | R | B | B-R | |
| Baseline | 4.6 | 4.5 | 0.1 | 4.9 | 4.8 | 0.1 |
| Sustained | 4.5 | 3.8 | 0.7 | 4.7 | 3.3 | 1.4 |
| PIPR | 0.1 (0.05) | 0.7* (0.14) | 0.6* (0.12) | 0.2 (0.05) | 1.5* (0.15) | 1.3† (0.16) |
Baseline measure is the average pupil diameter over a 7-second period before light onset. Sustained measure is the average pupil diameter over a period of 30 seconds, starting 10 seconds after light offset. The difference between the two measures provides the PIPR value. SEM is indicated in parentheses. Values are rounded to 1 decimal point. R, red; B, blue.
P < 0.05;
P < 0.001.
For subsequent analyses, we used the net PIPR values. Figures 2A and 2B show the relationship of the net PIPR (Fig. 2A; R2 = 0.433, P < 0.05) and the net PIPR change (%) (Fig. 2B; R2 = 0.239, P = 0.055) as a function of the baseline pupil diameter for the patient population. Figures 2C and 2D show the relationship of the net PIPR (Fig. 2C; R2 = 0.237, P < 0.05) and the net PIPR change (%) (Fig. 2D; R2 = 0.004, P > 0.05) as a function of the baseline pupil diameter for the control population. It should be noted in Figures 2B and 2D that there is no significant relationship between baseline pupil diameter and net PIPR change (%), showing that this metric effectively controls for baseline pupil diameter in both the patients and the age-matched controls.
Figure 2.
Net PIPR plotted against baseline pupil diameters, with linear regression lines (solid line) and 95% confidence intervals (dashed lines) comparing the patients (n = 16) (A, B) and the age-matched controls (n = 19) (C, D). (A) Baseline diameter of patients plotted against absolute net PIPR values (R2 = 0.433; P < 0.05). (B) Net PIPR change (%) of patients plotted against baseline diameter (R2 = 0.239; P = 0.055). (C) Baseline diameter of the age-matched controls plotted net PIPR (mm) (R2 = 0.237, P < 0.05). (D) Net PIPR change (%) of age-matched controls plotted against baseline diameter (R2 = 0.004; P > 0.05). The offset for all the parameters was not significantly different from zero (P > 0.05) for patients.
When we compared the results from our glaucoma patients with those of the age-matched controls, we found that all PIPR measures were significantly lower (P < 0.001) in the patient population than in the control group (Table 3). When we compared the net PIPR change (%) among the patients and the controls (Fig. 3), we noted that most patients had a PIPR change of <20% (n = 16; mean, 13%), whereas most control subjects had a PIPR change of >20% (n = 19; mean, 27%).
Table 3.
Comparison of PIPR Values between Glaucomatous Patients and Age-Matched Controls
| Patients (n = 16) | Age-Matched Controls (n = 19) | Difference | P | |
|---|---|---|---|---|
| Baseline pupil, mm | 4.5 | 4.8 | 0.3 | >0.05 |
| Net PIPR, mm | 0.6 | 1.3 | 0.7 | <0.001 |
| Net PIPR change, % | 13.0 | 27.3 | 14.3 | <0.001 |
Baseline pupil diameter was similar in both populations, but all net PIPR measures were significantly lower in the patient population. Values are rounded to 1 decimal point.
Figure 3.
Histogram of the net PIPR change (%) values among the patients and the controls plotted against the number of subjects. Almost all patients had a PIPR change of < 20% (n = 16, mean 13%) and most of the controls had a PIPR change > 20% (n = 19, mean 27%).
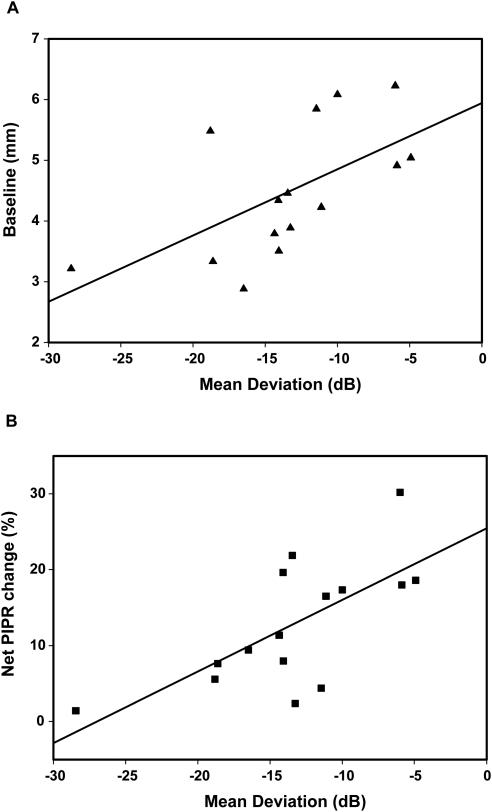
We then examined whether the magnitude of central visual field loss, as measured by the MD (in dB), affected the net PIPR change (%) (Fig. 4). For this analysis we used data from all the patients whose MD values were outside the normal limits (n = 15). We observed a tendency for baseline pupil diameter to become smaller as the visual field loss became worse (Fig. 4A; R2 = 0.363, P < 0.05). However, multiple regression analysis of visual field loss as a function of baseline pupil diameter and net PIPR change (%) indicated that visual field loss was correlated with the net PIPR change (%) (P < 0.05) but not with the baseline pupil diameter (P > 0.05). This latter observation is consistent with the observation that the mean and variance of the baseline pupil diameter in the age-matched controls (4.85 mm; SD, 0.99 mm) are very similar to those of the patient population (4.54 mm; SD, 1.07 mm). Given the results of the multiple regression analysis, we investigated the relationship between MD and the net PIPR change (%) with single linear regression. These data show that as visual field loss increases in severity, there is a reduction in the net PIPR change (%) (Fig. 4B; R2 = 0.466, P < 0.05). Importantly, the intercept of the regression line with the 0 dB value was at a net PIPR change (%) of 25.5%, which was similar to the value of 27.3% for the net PIPR change (%) in the age-matched controls, who had visual fields within normal limits.
Figure 4.
Linear regression plots of mean deviation (MD) values in decibels (dB), as determined by visual field charts, plotted against baseline (A), and net PIPR change percent (B) values for the patients who had MD outside normal limits (n = 15). These panels show a weak relationship between MD (dB loss increased) and baseline pupil diameter (R2 = 0.363; P < 0.05) and a stronger relationship between MD and net PIPR percent change (%) (R2 = 0.466; P < 0.05). The offsets for all the plots were significantly different from zero (P < 0.05).
Discussion
The results of the present study demonstrate a significantly reduced PIPR in glaucoma patients compared with those in age-matched controls without ocular disease. To our knowledge, this is the only study to show such a significant reduction in the ipRGC-mediated PIPR in human subjects with GON. Indeed, there is an ongoing debate about the classes of RGC that are damaged in glaucoma. For example, it has been suggested that RGC with larger soma are preferentially affected in GON.22–25 Other studies in rodents suggest that both ipRGC and RGC death is topological and not related to cell size or class.27,28 Li et al.10 reported that rodent ipRGCs, which have generally large cell bodies with extensive dendritic arbors,10 are preferentially spared in glaucoma.26 In contrast to this latter observation, the present study clearly demonstrates a loss of PIPR in patients with GON, indicating that ipRGCs are significantly affected in glaucomatous injury. However, it is unclear from the present study whether the ipRGCs are lost preferentially or the PIPR reduction reflects a generalized RGC loss.
It is important to note that the PIPR response is not significantly reduced in amplitude as part of the normal aging process. We have previously shown in healthy subjects that as age increased, there was a decrease in baseline pupil diameter accompanied by an insignificant tendency for the net PIPR amplitude to decrease.13 We also showed that there was no affect of age on the net PIPR change (%) because this metric takes into account any age-related differences in baseline pupil diameter.
The loss of PIPR in the patients was correlated with their MD values as determined by central visual field measurements. As the MD values increased, we noticed a decline in PIPR. This correlation was present for both the net PIPR and the net PIPR change (%) (slope for all regression lines, P < 0.05) thus demonstrating that MD was a determinant of PIPR independently of baseline pupil diameters.
Compared with the control subjects in our previous study,13 our age-matched sample had smaller baseline pupil diameters. This smaller baseline pupil is almost certainly attributed to the older age of this group compared with the control group in our previous study because it is well known that baseline pupil size decreases as age progresses.30–33
In conclusion, this study has demonstrated a significant reduction in the PIPR in patients with GON and has shown that reduced PIPR is correlated with the severity of visual field loss. It is clear from our results that measuring PIPR may provide an additional objective method to detect and monitor glaucoma. Furthermore, given that the PIPR is significantly associated with visual field severity, monitoring this response over time may provide an objective measurement of progressive GON. However, to be of significant use, the PIPR will have to be evaluated in larger populations of healthy subjects and in disease categories other than glaucoma. In addition, studies will be required to better define intratest variability in patients with disease and healthy subjects, the relationship of the PIPR across disease severity, and the prospective performance of the PIPR test.
In summary, a novel wide-field optical system has the potential to be used as a clinical screening tool, along with other clinical parameters, in patients with retinal disorders such as glaucoma.
Acknowledgments
The authors thank all the subjects for participating in the study; Bobbie Hill, Cassandra Page, and the staff of Glaucoma Service of the Eye Foundation Hospital for their invaluable help in recruitment; and David McDougal, Jerry Millican, and Mark Bolding for their help in the development of the testing apparatus.
Footnotes
Supported by National Institutes of Health Grants EY09380 (PDG) and P30 EY03039, The EyeSight Foundation of Alabama, and a Research to Prevent Blindness Award (CAG).
Disclosure: L. Kankipati, None; C.A. Girkin,, None; P.D. Gamlin, None
References
- 1. Alpern M, Campbell FW. The spectral sensitivity of the consensual light reflex. J Physiol. 1962;164:479–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loewenfeld IE. The Pupil Anatomy, Physiology, and Clinical Applications. Vol. 1 Ames, IA: Iowa State University Press; 1993 [Google Scholar]
- 3. Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626 [DOI] [PubMed] [Google Scholar]
- 5. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073 [DOI] [PubMed] [Google Scholar]
- 7. Gooley JJ, Lu J, Fisher D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal color and irradiance and project to the LGN. Nature. 2005;433:749–754 [DOI] [PubMed] [Google Scholar]
- 9. Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vis Res. 2007;47:946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007;27:195–204 [DOI] [PubMed] [Google Scholar]
- 11. Zaidi FH, Hull JT, Peirson SN, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDougal DH, Gamlin PD. The influence of intrinsically photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010;50:72–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kankipati L, Girkin CA, Gamlin P. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010;51:2764–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leske MC. The epidemiology of open-angle glaucoma: a review. Am J Epidemiol. 1983;118:166–191 [DOI] [PubMed] [Google Scholar]
- 15. Van Buskirk EM, Cioffi GA. Glaucomatous optic neuropathy. Am J Opthalmol. 1992;113:447–452 [DOI] [PubMed] [Google Scholar]
- 16. Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–1106 [DOI] [PubMed] [Google Scholar]
- 17. Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57 [DOI] [PubMed] [Google Scholar]
- 18. Friedman DS, Wilson MR, Liebmann JM, Fechtner RD, Weinreb RN. An evidence-based assessment of risk factors or ocular hypertension and glaucoma. Am J Opthalmol. 2004;38:S19–S31 [DOI] [PubMed] [Google Scholar]
- 19. Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007;16:406–418 [DOI] [PubMed] [Google Scholar]
- 20. Harwerth RS, Carter-Dawson L, Smith EL, 3rd, Barnes G, Holt WF, Crawford ML. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45:3152–3160 [DOI] [PubMed] [Google Scholar]
- 21. Harwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Opthalmol. 2006;124:853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95:357–363 [DOI] [PubMed] [Google Scholar]
- 23. Quigley HA, Sanchez RM, Dunkelberger GR, et al. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987;28:913–920 [PubMed] [Google Scholar]
- 24. Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991;32:484–491 [PubMed] [Google Scholar]
- 25. Glovinsky Y, Quigley HA, Pease ME. Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1993;34:395–400 [PubMed] [Google Scholar]
- 26. Li RS, Chen BY, Tay DK, Chan HH, Pu ML, So KF. Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Invest Opthalmol Vis Sci. 2006;47:2951–2958 [DOI] [PubMed] [Google Scholar]
- 27. Wang HZ, Lu QJ, Wang NL, Liu H, Zhang L, Zhan GL. Loss of melanopsin-containing retinal ganglion cells in a rat glaucoma model. Chin Med J (Engl). 2008;121:1015–1019 [PubMed] [Google Scholar]
- 28. Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ojima T, Tanabe T, Hangai M, Yu S, Morishita S, Yoshimura N. Measurement of retinal nerve fiber layer thickness and macular volume for glaucoma detection using optical coherence tomography. Jpn J Ophthalmol. 2007;51:197–203 [DOI] [PubMed] [Google Scholar]
- 30. Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Optics. 1987;26:1437–1440 [DOI] [PubMed] [Google Scholar]
- 31. Winn B, Whitaker D, Elliott DB, Phillips NJ. Factors affecting light-adapted pupil size in normal human subjects. Invest Opthalmol Vis Sci. 1994;35:1132–1137 [PubMed] [Google Scholar]
- 32. Newsome DA. After image and pupillary activity following strong light exposure. Vision Res. 1971;11:275–288 [DOI] [PubMed] [Google Scholar]
- 33. Fotiou DF, Brozou CG, Tsiptsios CJ, et al. Effect of age on pupillary light reflex: evaluation of pupil mobility for clinical practice and research. Electromyogr Clin Neurophysiol. 2007;47:11–22 [PubMed] [Google Scholar]