Abstract
Objective To investigate whether PALSA PLUS, an on-site educational outreach programme of non-didactic, case based, iterative clinical education of staff, led by a trainer, can increase access to and comprehensiveness of care for patients with HIV/AIDS.
Design Cluster randomised trial.
Setting Public primary care clinics offering HIV/AIDS care, antiretroviral treatment (ART), tuberculosis care, and ambulatory primary care in Free State province, South Africa.
Participants Fifteen clinics all implementing decentralisation and task shifting were randomised. The clinics cared for 400 000 general primary care patients and 10 136 patients in an HIV/AIDS/ART programme. There were 150 nurses.
Intervention On-site outreach education in eight clinics; no such education in seven (control).
Main outcome measures Provision of co-trimoxazole prophylaxis among patients referred to the HIV/AIDS/ART programme, and detection of cases of tuberculosis among those in the programme. Proportion of patients in the programme enrolled through general primary care consultations.
Results Patients referred to the HIV/AIDS programme through general primary care at intervention clinics were more likely than those at control clinics to receive co-trimoxazole prophylaxis (41%, (2253/5523) v 32% (1340/4210); odds ratio 1.95, 95% confidence interval 1.11 to 3.40), and tuberculosis was more likely to be diagnosed among patients with HIV/AIDS/ART (7% (417/5793) v 6% (245/4343); 1.25, 1.01 to 1.55). Enrolment in the HIV/AIDS and ART programme through HIV testing in general primary care was not significantly increased (53% v 50%; 1.19, 0.51 to 2.77). Secondary outcomes were similar, except for weight gain, which was higher in the intervention group (2.3 kg v 1.9 kg, P<0.001).
Conclusion Though outreach education is an effective and feasible strategy for improving comprehensiveness of care and wellbeing of patients with HIV/AIDS, there is no evidence that it increases access to the ART programme. It is now being widely implemented in South Africa.
Trial registration Current Controlled Trials ISRCTN 24820584.
Introduction
As the price and funding of antiretroviral drugs become less of a barrier to provision of antiretroviral treatment (ART) in low and middle income countries1 attention has turned to the challenges that remain2 3: shifting tasks from doctors to nurses to mitigate the worst shortages in human resources,4 decentralisation from hospitals to peripheral clinics to broaden geographical access to ART and equity,5 and integration of HIV/AIDS and ART care with tuberculosis care and other primary care to strengthen quality and efficiency of care delivery.6 Task shifting and decentralisation are being implemented in pilot7 and national8 HIV/AIDS/ART programmes funded by African countries and by the US Presidential Emergency Program for Aids Relief (PEPFAR)9 and the Global Fund to Fight AIDS, Tuberculosis, and Malaria,10 showing large improvements in coverage and in increased volume of patients served.
There is less experience with integration as few health systems in low or middle income countries have tried to integrate HIV/AIDS/ART care. Instead a “vertically” organised delivery system is established, separately managed, staffed, and supplied from other primary care services in the same facilities. These vertical programmes contradict global recommendations favouring integration of HIV/AIDS and ART care with tuberculosis11 12 and primary care.13 Vertical approaches are thought to result in poor quality of care14 because clinicians not trained in HIV care might manage patients for their presenting problem, missing an underlying HIV infection or complication, while clinicians trained in HIV/AIDS care might miss or mistreat tuberculosis15 among their patients. Vertical organisation of service delivery requires that patients make separate visits for problems other than HIV, which is inefficient for the healthcare system and difficult for patients, potentially raising costs and reducing the quality and cohesiveness of their care as well as their long term adherence and follow-up. Vertical organisation could weaken health systems16 as scarce professionals might move to better paid jobs in externally supported vertical HIV care programmes, which do not share the new resources for HIV/AIDS care with other parts of the health system. Concerns have been raised regarding the quality and sustainability of vertical ART programmes.17 Integration is presumed to improve18 19 HIV/AIDS/ART outcomes, but this is supported by only two case studies, which did not compare outcomes or processes of integrated care with usual care or an alternative.20 21
Promising free diagnosis, monitoring, and ART for those with AIDS, the 2003 South African National Operational Plan allocated funding for 240 ART treatment centres across all nine provinces.22 This was to be the world’s largest free ART programme23—a response to an epidemic killing 300 000 South Africans each year.24 Eight provinces chose to begin by providing ART through hospital based physicians, delaying task shifting and decentralisation, but one, the Free State province, chose to begin by decentralising all ART care after the initial prescription of ART to existing community based primary care clinics, and to deliver that ART care largely through task shifting to nurses.25 As full structural integration of HIV/AIDS and ART care with other healthcare delivery might require substantial reorganisation of clinic processes and staff, entailing risk to all primary care delivery, this province also simultaneously tested a low risk strategy for promoting integration of HIV/AIDS/ART care with general primary care and tuberculosis care, using outreach education to integrate these teams functionally, rather than structurally.26
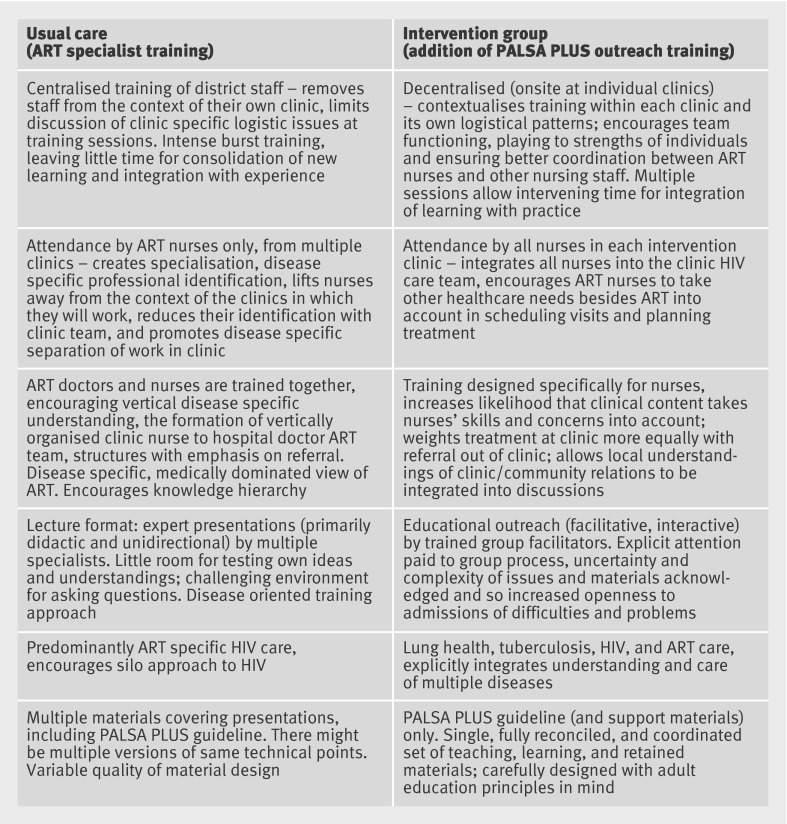
We evaluated the incremental impact of educational outreach on healthcare quality and health status outcomes among patients with HIV/AIDS receiving ART. Educational outreach was delivered as a clinic based, on-site adult education programme of non-didactic, case based, iterative clinical education of all staff together, including staff providing general primary care, tuberculosis care, and HIV/AIDS/ART care, led by an outreach trainer (fig 1). This aimed to achieve sharing of knowledge and integration of care among staff in primary care clinics. We evaluated this added intervention in a cluster randomised trial, in which clinics and their staff in both arms also received the usual approach to task shifting and decentralisation.
Fig 1 Comparison of ART specialist training and PALSA PLUS outreach approach
Methods
Study design
We conducted a pragmatic27 cluster randomised trial28 of the effects of adding outreach education to a roll-out of decentralised nurse run HIV/AIDS/ART services at primary care clinics.
Randomisation
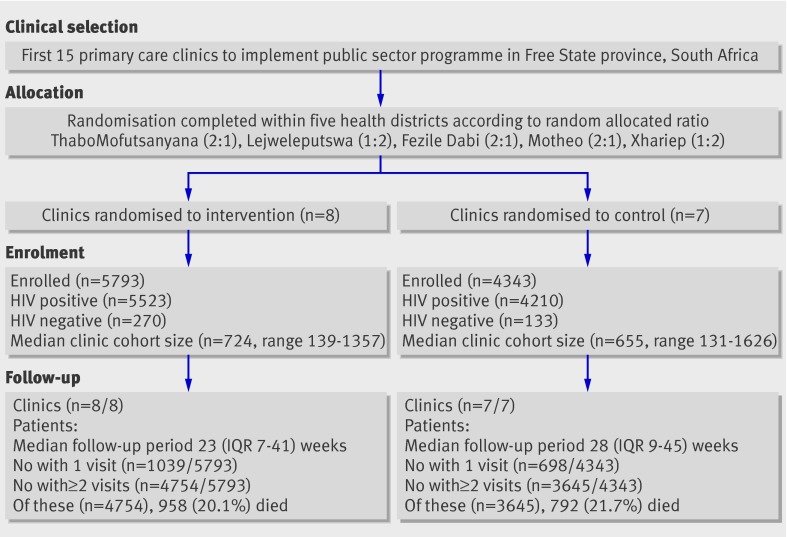
The unit of randomisation was the clinic, stratified by health district. Urn randomisation (chosen for simplicity, given the small number of units) was done with folded slips of paper marked with clinic names, selected blindly from a container by the trial statistician before the intervention or recruitment of patients.29 Outcome data were collected on individual patients. Allocation was conducted centrally at the same time as randomisation and thus concealed. No blinding was possible because of the obvious nature of the intervention. Each stratum (district) contained three ART clinics and a single hospital, to which those clinics referred patients for physician care (fig 2). Three randomly selected districts were randomised at a ratio of two intervention clinics to one control clinic, leaving two districts with a ratio of one intervention to two control clinics (total eight intervention and seven control clinics).
Fig 2 Details of clinic selection, allocation, enrolment, and follow-up in trial (IQR=interquartile range)
Setting and programme
Usual care
The Free State HIV/AIDS/ART care programme (other than initial ART prescription and care of complications, which were to be provided by physicians) was being decentralised to nurse clinicians at each of the 15 community based general primary care (including tuberculosis care) clinics accredited as having the facilities, such as private consultation space, for ART care.
One or two experienced, fully qualified nurse clinicians from each of the 15 clinics were selected to receive a five day lecture based training course on ART, delivered by health department experts, mainly physicians, at a central venue. All ART specialist nurses also received a copy of the current guideline for management of HIV, including physician initiated, nurse maintained ART, and adult respiratory diseases including tuberculosis. The guideline was evidence based, and adhered to national HIV/AIDS clinical recommendations and to provincial prescribing rules and referral criteria applying to nurses. Once trained, these newly appointed ART nurse specialists were to see HIV/AIDS/ART patients, referring HIV patients to hospital based physicians for initiation of ART when the CD4 count fell below 200 cells/μl. They were also to provide ongoing guideline based care for patients in whom ART was initiated, issuing monthly repeat prescriptions, and conducting laboratory and clinical monitoring for side effects and complications, with referral back to hospital physicians as needed. Every six months nurses were also responsible for taking blood samples for measurement of CD4 counts and viral loads the month before the patient returned to the doctor for their scheduled six monthly repeat prescription, so that the results were available for the doctor to review at the time of renewing the prescription. They were also expected to support the general primary care team (who provide HIV counselling and testing to general patients).
The specialist nurse run ART programme was established in the same small building as the tuberculosis and general primary care clinics but in different consultation rooms. The general primary care clinic is the planned entry point for new patients to the HIV/AIDS programme, and the planned site of prescription of co-trimoxazole for patients with a new diagnosis of HIV before their referral to the specialist run HIV/AIDS/ART programme. Thus the planned model of care (the usual care provided by the control group clinics in the trial reported here) could be defined as decentralisation of ART with task shifting, but not integration, as tasks for HIV/AIDS/ART care and tuberculosis care were to remain divided between specialists and generalists in the same clinic building, the former focusing on clinical care of people already identified as HIV positive and people with AIDS defining conditions or CD4 counts already receiving ART, including the tuberculosis care of such patients. General patients, patients with tuberculosis, and patients seeking or needing HIV screening were looked after by general or tuberculosis staff until an HIV diagnosis and then referred on to the specialist nurses in the same building.
Intervention
The intervention was additive to the usual care described above and delivered only in the eight intervention clinics. It was based on educational outreach30: a well proved and modestly but variably effective strategy for change in professional practice, consisting of short, face to face, interactive education of clinicians at the workplace by a trusted non-commercial educator. The intervention aimed to produce cognitive, rather than physical or organisational, integration—that is, to enable specialist ART nurses to think beyond HIV aspects of the care of their patients, and tuberculosis and general nurses to think of caring for HIV aspects of their patients’ care, irrespective of presenting complaints. In consultation with their managers and the individuals themselves, the research team selected 10 nurse managers, already employed by the Free State, as outreach educators for the intervention clinics. They received no payment from the research team, gained no extra benefits from their employer, and were drawn equally from the provincial HIV and tuberculosis programmes. All attended a five day workshop led by members of the research team on educational outreach techniques and on the clinical content of the guidelines.31 32 To maintain the competence of the outreach educators the research team also provided three one day follow-up workshops—not at primary care clinics—over a six month period.
Over this period the outreach educators were asked to deliver at least eight outreach sessions of one to two hours at each intervention clinic to a group of all nurses (including the ART specialists), lay counsellors, community health workers, and administrative staff available at each clinic on outreach visit days. All professional staff at the intervention clinics received a copy of the integrated syndromic guideline. Visits were repeated until the educators were satisfied that all staff were familiar with the diagnosis, treatment, and complications of HIV/AIDS, tuberculosis, and other conditions covered in the guideline described above, at a level appropriate to their responsibilities in the clinic. Four outreach educators supported one intervention clinic each; six worked in pairs, each pair supporting one or two clinics.
Clinics
We enrolled all three clinic based ART sites in each of the five administrative districts of the Free State Province. These 15 were the first wave of ambulatory clinics to provide decentralised HIV/AIDS/ART care in the province. No sampling was used. Clinics were staffed by a median of 10 nurses, one to three of whom were newly trained to run the separately organised ART service in the same building (table 1).
Table 1.
Characteristics of clinics and patients according to additional outreach intervention or usual care in decentralising HIV/AIDS/ART programme. Figures are numbers (percentages) unless stated otherwise
| Outreach group | Control group | |
|---|---|---|
| Clinics | ||
| No of clinics | 8 | 7 |
| Median quarterly adult attendances | 8929 | 7236 |
| Median No of nurses per clinic | 11 | 9 |
| Median distance from referral site (km) | 25 | 25 |
| Patients with HIV | ||
| No of patients | 5523 | 4210 |
| Sex (women) | 3576/5521 (65) | 2764 (66) |
| Mean age (years) | 35.4 | 35.6 |
| Any previous antiretroviral treatment | 59 (1) | 39 (1) |
| CD4 count at enrolment (cells/μl): | ||
| Median (IQR) | 166 (71-319) | 185 (84-339) |
| <25 | 388 (7) | 278 (7) |
| 25-49 | 344 (6) | 219 (5) |
| 50-99 | 615 (11) | 427 (10) |
| 100-199 | 1064 (19) | 758 (18) |
| 200-349 | 798 (14) | 725 (17) |
| ≥350 | 892 (16) | 749 (18) |
| Unknown | 1422 (26) | 1054 (25) |
| Mean weight at enrolment (kg) | 56.7 | 57.0 |
| WHO stage at doctor assessment: | ||
| Stage 1/2 | 761/1815 (42) | 435/1359 (32) |
| Stage 3/4 | 1057/1815 (58) | 924/1359 (68) |
| Employed | 127/1014 (13) | 102/1044 (10) |
| Median (IQR) household size | 4 (3-6) | 4 (3-6) |
| Household receiving social welfare | 445/1006 (44) | 500/1027 (49) |
IQR=interquartile range.
Patients
All patients aged 16 or older enrolling in the ART programme through any channel (including voluntary counselling and testing) at any of the 15 clinics during one year were included in the trial and monitored until death or completion of the trial. In every clinic in each district (stratum), the study began the first month after the specialised HIV/AIDS/ART nurses had been trained and deployed in intervention and control clinics and the local outreach educators had completed their training and were ready to provide outreach support to the intervention clinics. Districts began data collection for the study between 1 July 2004 and 30 November 2005, and patients were recruited and followed up in all facilities in each district for 12 months after data collection began.
Data collection
Each time a patient with HIV visited the clinic (or hospital), a nurse (or doctor) recorded clinical information on paper forms. Trained data capturers then entered the data on the province’s computerised HIV record system by trained data capturers and uploaded them to a central database. Entries were checked with automated quality control routines, with records with missing or contradictory information returned for correction. Deaths were identified by electronically linking patients’ data with the national death register by using national identity numbers.33 Diagnoses of tuberculosis, made according to national guidelines,34 were obtained from the HIV records or through linkage with the tuberculosis treatment register.
Outcome measures
Because the intervention is complex, it could have independent effects on several different aspects of care, and as such the effect of the intervention needs to be quantified by a “family” of three separate primary outcome measures: firstly, integration of HIV/AIDS and ART care with tuberculosis care; secondly, overall quality of pre-ART care provided at the clinic for patients who are HIV positive; and, thirdly, integration of general primary care provided by nurses with HIV screening. The first primary outcome, detection of tuberculosis among patients with HIV, reflects the quality of awareness of tuberculosis and care among the HIV/AIDS/ART programme specialists; the second, co-trimoxazole prophylaxis among patients with a new diagnosis of HIV, reflects the quality of pre-physician clinic care for HIV positive patients, which is provided mainly by general primary care nurses immediately after they diagnose HIV in their general clinics, and also by specialist nurses after the general nurse refers the patients with a new diagnosis into the HIV/AIDS and ART programme. The third outcome, the proportion of patients enrolled in the ART programme through newly conducted HIV testing, reflects the index of suspicion for HIV among general nurses treating a full range of presenting complaints in primary care patients.
Other outcomes were precautionary as we had no reason to expect that ART or its outcomes (viral load suppression, mortality, weight gain) would differ between arms; ART was initiated by physicians and monitored in both arms by similar specialist ART nurses. We also included a further marker of the quality of pre-ART care (CD4 follow-up in those not eligible for ART at first presentation) and counted clinic visits to track use of healthcare.
Sample size and statistical power
As we included all eligible patients in all proposed ART clinics there was no sampling. We carried out a power calculation, rather than an estimation of necessary sample size. Decision makers expected that the 15 clinics would have a mean of 500 patients in the HIV/AIDS/ART programme and were looking for an improvement of 20% or more in any one of the three primary outcomes. Outcomes of our previous study of outreach education in the province had intracluster correlation coefficients between 0.019 and 0.007,25 meaning that, with an α of 0.05, our proposed trial had over 80% power to detect the desired improvement in the primary outcomes.35
Statistical analysis
Descriptive statistics were pooled over the five districts, unweighted. We use four regression models to estimate effects, with district as the covariate for stratification: logistic regression for binary outcomes (such as co-trimoxazole prescribing), linear regression for continuous outcomes (such as weight gain), Cox proportional hazards for time-to-event outcome (such as mortality), and Poisson regression for count outcomes (such as clinic attendances). The model parameters were estimated by the generalised estimating equations approach, which takes into account clustering of outcomes within clinics. We estimated treatment effects (odds ratios, difference in means, hazard ratio, and incidence rate ratio) with 95% confidence intervals. All outcomes were analysed on an intention to treat basis, except for mortality, for which we excluded from the analysis patients with only one recorded visit.
Consent
Enrolment of clinics in the trial was at the behest of the Free State Department of Health. Individual patients were not approached for consent as all 15 clinics had the same access to ART medications, guidelines, physicians and designated, trained ART nurses. All data were obtained from computerised routine clinical records or other existing administrative and demographic databases.
Results
Characteristics of clinics and patients—Characteristics of patients and clinics were comparable at baseline (table 1). All clinics completed the trial (fig 2). The eight intervention clinics enrolled a median of 724 patients per clinic, with a median follow-up of 23 weeks (interquartile range 7-41 weeks) to the end of the study or loss to follow-up; the seven control clinics enrolled 655 patients per clinic, with a median follow-up of 28 weeks (9-45 weeks). Intervention clinics had more patients and more staff (table 1). The median CD4 count at enrolment was slightly lower in the intervention group, though control patients were slightly more likely to have advanced clinical infection.
Intervention—Trainers delivered a median of 14.5 (range 6-20) educational outreach sessions to each intervention clinic. Clinic nurses attended a median of five (range 0-14) sessions each.
Outcomes—The intervention was effective for two of the three prespecified primary outcomes (table 2). Co-trimoxazole prophylaxis was more likely to be prescribed (1.95, 95% confidence interval 1.11 to 3.40) and tuberculosis was more likely to be diagnosed (1.25, 1.01 to 1.55) among patients attending intervention group clinics, but there was no evidence that the intervention was effective in increasing recruitment into the HIV/AIDS/ART programme from primary care (1.19, 0.51 to 2.77).
Table 2.
Trial outcomes in intervention clinics (with added PALSA PLUS outreach education) and control clinics (with specialist ART nurse training only)
| No (%, SD, or IQR) | OR, HR, MD, or IRR* (95% CI) | P value | Intracluster correlation coefficient | ||
|---|---|---|---|---|---|
| Intervention clinics | Control clinics | ||||
| Primary outcomes | |||||
| Enrolment in programme through new HIV testing (1 year)† | 3048/5793 (53%) | 2187/4343 (50%) | OR 1.19 (0.51 to 2.77) | 0.695 | 0.108 |
| Provision of co-trimoxazole prophylaxis over 12 months | 2253/5523 (41%) | 1340/4210 (32%) | OR 1.95 (1.11 to 3.40) | 0.020 | 0.034 |
| Tuberculosis case detection† | 417/5793 (7%) | 245/4343 (6%) | OR 1.25 (1.01 to 1.55) | 0.038 | 0.027 |
| Preplanned secondary outcomes | |||||
| Viral load suppressed after 6 months’ antiretroviral treatment | 332/389 (85%) | 332/389 (88%) | OR 0.96 (0.74 to 1.26) | 0.779 | 0.088 |
| Mortality | 958/4754 (20%) | 792/3645 (22%) | HR 0.90 (0.67 to 1.19) | 0.466 | NA |
| CD4 follow-up among patients with initial CD4 200-500 cells/μl | 1127/1276 (88%) | 954/1141 (84%) | OR 1.39 (0.65 to 2.96) | 0.40 | 0.022 |
| Weight gain (kg) | 2.3 (SD 5.8) | 1.9 (SD 6.1) | MD 0.55 (0.30 to 0.79) | <0.001 | 0.10 |
| Clinic visits | 6 (IQR 3-15) | 8 (IQR 4-19) | IRR 0.95 (0.77 to 1.18) | 0.658 | 0.026 |
| Post hoc exploratory analyses of primary outcomes | |||||
| Enrolment in programme through new HIV testing (1 month) * | 357/653 (55%) | 214/499 (43%) | OR 1.58 (1.01 to 2.48) | 0.054 | 0.113 |
| Provision of co-trimoxazole prophylaxis to patients with ≤200 CD4 cells/μl, AIDS, or tuberculosis | 1762/2419 (73%) | 1025/1572 (65%) | OR 1.88 (1.07 to 3.31) | 0.029 | 0.035 |
IQR=interquartile range.
*OR=odds ratio; HR=hazard ratio; MD=mean difference; IRR=incidence rate ratio.
†Denominator includes all patients enrolled in treatment programme, as well as patients who were tested through programme with negative results.
Other outcomes—Viral load, mortality, use of healthcare, and monitoring of CD4 counts were similar in both arms, while weight gain favoured the intervention group (mean difference 0.55 kg, 0.30 to 0.79 kg; P<0.001; table 2).
Discussion
Educational outreach to promote shared knowledge and awareness of all staff on HIV/AIDS/ART, tuberculosis, and general primary care substantially increased prescription of co-trimoxazole and diagnosis of tuberculosis and initiation of tuberculosis care among patients with HIV/AIDS. There was no evidence that it increased the probability of patients in general primary care being screened and referred for HIV/AIDS/ART care. The increase in diagnosis of tuberculosis is appropriate as all were microbiologically confirmed or, if a sputum smear yielded negative results, diagnosed by a physician. The increased co-trimoxazole prescribing, which concentrated on patients with a CD4 count <200/ cells/μl, also adheres to guidelines (see table 2). Weight gain also improved slightly, a clinical sign of improved care. These successes are important as they increase survival36 and reduce the transmission of tuberculosis.37
Policy implications
PALSA PLUS combined outreach education, simplification of treatment,38 39 and supportive supervision,40 resulting in important improvements in care under realistic conditions, within local constraints on human resources and organisations. It is an effective addition to decentralisation and task shifting, which is worth implementing in nurse led services in South Africa, even in the absence of structural integration of HIV/AIDS/ART and general primary and tuberculosis care (and possibly as an initial step towards it).
Even though it increased the probability that general primary care and tuberculosis clinicians would prescribe co-trimoxazole prophylaxis, the intervention did not increase the likelihood that these same clinicians would screen for HIV and refer. We explored this in a post hoc analysis (see table 2), which showed a large and significant increase (intervention relative to control group) in the proportion of the general clinic attendees who the general nurses screened for HIV and referred to the HIV/AIDS/ART programme in the first month of the trial, but this initial intervention advantage equalised between arms over the year of follow-up. Our qualitative study alongside this randomised controlled trial suggests that this lack of sustained success could be because of the intensive counselling that non-HIV specialist staff must provide to patients to encourage their consent for screening. Staff find this counselling emotionally draining, and it requires skills that were not provided by the intervention training.41 We have since adapted the training to provide more of this kind of skill and support.
Strengths and weaknesses of the study
Study arms were balanced at baseline, and there was no loss of clusters to follow-up. Cluster randomisation permitted uncontaminated estimation of the effects of a complex intervention, while use of three primary outcomes showed the different direction of the effects of the intervention on separate aspects of care, justifying our choice not to adjust for multiple comparisons. Randomisation and implementation of the intervention proceeded smoothly. The inclusion of all ART clinics and patients in one of South Africa’s poorer provinces, rather than the common reliance on atypically resourced demonstration sites, supports wider applicability of these results to other constrained healthcare systems.
Differences in outcomes between arms might have been reduced by the distribution and widespread use of our evidence based syndromic guideline in control sites. This element of the intervention is obviously less useful without the shared outreach training to use it correctly but could still have had some benefit; as a new programme, there are no comparable before and after data on HIV screening with which to separately estimate the effect of the guideline. Our reliance on sources of routinely collected clinical data, with some missing values, could also slightly blur contrast between the groups, though our large sample size counteracts this imprecision. The fact that ART initiation remained a physician and hospital activity in both arms might place a ceiling on the impact that could be obtained by the intervention.42 These limitations suggest our study might lean towards underestimation of the potential impact of this approach.
Mortality was high in both arms, in keeping with previously reported observational cohort data for these patients, probably because of the large number of late stage patients in the start-up ART programme.43
There was a moderately large intracluster correlation coefficient for most outcomes, suggesting variability in the effectiveness of the intervention between clusters. The intervention was permitted to vary in response to local conditions, through, for example, adding training sessions as needed.43 This could have contributed to variability in outcomes between clusters, though we believe responsiveness to local need probably had the opposite effect. The overall outcomes of the study, however, were broadly positive, supporting a recent review suggesting that adaptation to local conditions is associated with successful scale-up.44
Applicability
There were no signs of disruption in ART care, use of healthcare remained unchanged, and, despite the added responsibilities, staff morale improved.40 We chose outreach educators from among existing nurse supervisors and incorporated the new roles into their existing responsibilities (no extra positions were created nor were any replacements hired). There was no extra financial reward, facilitating sustainability and widespread implementation. With this reassuring news for managers, the provincial Department of Health has incorporated the educational outreach intervention into the control clinics as a province-wide roll-out. Each outreach educator now supports four clinics, with one clinic visit every second week in the first four months, and one visit per quarter thereafter, which is a sustainable ratio in South Africa.
Further research
We cannot tell whether the upper limit of tuberculosis detection has been reached as the prevalence in clinic populations has not been studied against a standard diagnostic, but there clearly remains room for further improvement in co-trimoxazole prophylaxis. We believe this will come about through increasing the role of nurses in the initiation of ART rather than through further increases in efforts by general and tuberculosis staff, and this element will be evaluated in our ongoing trial of an expanded intervention that shifts responsibility for most ART initiation and follow-up to nurses.45 PALSA PLUS has recently been implemented as the training and support model for accelerated implementation of HIV/AIDS/ART care throughout South Africa, but even though it has been designed for widespread implementation and applicability, the actual effectiveness of the intervention as it is adapted or applied elsewhere should be further monitored. Where the context is different, a full pragmatic trial is recommended; one is now underway of an adapted version of PALSA PLUS in Malawi.46
This pragmatic randomised trial provided timely, trustworthy, real world estimates of effects of a novel locally developed intervention for strengthening and integration of health services. In the Free State province our rigorous evaluation, unobtrusively integrated alongside early stages of programme implementation, provided formative information for improving the programme and supported evidence based decision making on system-wide implementation. This is a rare example of synergy between research and policy for strengthening health systems, for which there is a growing call.47
What is already known on this topic
In low and middle income countries, especially in Africa, there are not enough physicians and limited accessibility to hospital based care for HIV/AIDS and antiretroviral treatment (ART)
Decentralisation and task shifting are being widely implemented and integration of HIV/AIDS care and antiretroviral treatment with tuberculosis care and general primary care is recommended
What this study adds
Educational outreach—on-site, in-service education of all clinicians from separate clinical specialty teams, together—was effective in improving the comprehensiveness of care of patients with HIV/AIDS/ART and might be an alternative to organisational integration or a low risk starting point
While co-trimoxazole prophylaxis in patients awaiting ART was improved (higher coverage)—and tuberculosis was more often detected (both appropriately)—educational outreach did not increase the detection of HIV in patients in general and tuberculosis clinics, probably because of insufficient training and support for general clinic staff in counselling for HIV screening
We thank P Shai-Mhatu, formerly senior HIV programme manager in the Free State Department of Health, for support and help with design and implementation of the intervention; the PALSA PLUS trainers from the Free State Department of Health (Leona Smith, Ruth Marumo, Sandra Korkie, Khasiane Tumahole, Seipati Mothlanke, Mojola Mokokoane, Jacqui Sishuba, Sinah Samusho, Kuki Mokoena, and Elizabeth Bolofo) for support and guidance; Yolisa Tsibolane from the Free State Department of Health’s tuberculosis programme and Lindiwe Mvusi from the National Department of Health’s tuberculosis programme; Sonja Botha for supporting the initial training of PALSA PLUS trainers and for training the Free State’s nurses in completion of structured clinical records and data capturers in their electronic capturing; Glenn Campbell and Val Myburgh for guideline layout and illustration of training materials; Chris Seebregts, Clive Seebregts, and Gillian Staniland, from the Biomedical Informatics Research Division, Medical Research Council of South Africa, for data collection support, database design, collation, and initial extraction of data for analysis; Gloria Rembe and Thulani Mazibuko for data collection, checking, and cleaning; Debbie Bradshaw, from the Burden of Disease Unit, Medical Research Council, for facilitating linkage with the national mortality register; Venessa Timmerman, from the University of Cape Town Lung Institute, for assistance with extraction of data for analysis and Jani Brett Driskell, from the University of Cape Town Lung Institute, for administrative assistance throughout; and Ronald Chapman, formerly of the Free State Department of Health, for crucial early support and guidance.
Contributors: MZ and LRF led the design of the trial protocol and application for funding with help from CL and EB. LRF led the data collection, CL led the analysis, and MZ led the interpretation and write-up, with help from SL, MOB, and EB. LRF led the design and implementation of the intervention with help from PM, AB, RGE, MZ, MOB, and EB. All authors had full access to the data on request and approved the final version. MZ is guarantor.
Funding: The study was funded by the International Development Research Centre (IDRC) of Canada, who had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the article, or in the decision to submit it for publication. The researchers were completely independent of IDRC.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study was approved by the human research ethics committee at the University of Cape Town.
Data sharing: No additional data available.
Cite this as: BMJ 2011;342:d2022
References
- 1.Steinbrook R. Closing the affordability gap for drugs in low-income countries. N Engl J Med 2007;357:1996-9. [DOI] [PubMed] [Google Scholar]
- 2.Kurowski C, Wyss K, Abdulla S, Mills A. Scaling up priority health interventions in Tanzania: the human resources challenge. Health Policy Plan 2007;22:113-27. [DOI] [PubMed] [Google Scholar]
- 3.Kober K, Van Damme W. Scaling up access to antiretroviral treatment in southern Africa: who will do the job? Lancet 2004;364:103-7. [DOI] [PubMed] [Google Scholar]
- 4.Samb B, Celletti F, Holloway J, Van Damme W, De Cock KM, Dybul M. Rapid expansion of the health workforce in response to the HIV epidemic. N Engl J Med 2007;357:2510-4. [DOI] [PubMed] [Google Scholar]
- 5.Bedelu M, Ford N, Hilderbrand K, Reuter H. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. J Infect Dis 2007;196(suppl 3):S464-8. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme W, Kober K, Kegels G. Scaling-up antiretroviral treatment in Southern African countries with human resource shortage: how will health systems adapt? Soc Sci Med 2008;66:2108-21. [DOI] [PubMed] [Google Scholar]
- 7.Shumbusho F, van Griensven J, Lowrance D, Turate I, Weaver MA, Price J, et al. Task shifting for scale-up of HIV care: evaluation of nurse-centered antiretroviral treatment at rural health centers in Rwanda. PLoS Med 2009;6:e1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assefa Y, Jerene D, Lulseged S, Ooms G, Van Damme W. Rapid scale-up of antiretroviral treatment in Ethiopia: successes and system-wide effects. PLoS Med 2009;6:e1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendavid E, Bhattacharya J. The president’s emergency plan for AIDS relief in Africa: an evaluation of outcomes. Ann Intern Med 2009;19:150,688-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global fund grants—progress report. http://web.theglobalfund.org/TGFWebReports3/ReportOutput.jsp?chartId=SummaryReport.
- 11.Wood R. The case for integrating tuberculosis and HIV treatment services in South Africa. J Infect Dis 2007;196(suppl):S497-9. [DOI] [PubMed] [Google Scholar]
- 12.Dong K, Thabethe Z, Hurtado R, Sibaya T, Dlwati H, Walker B, et al. Challenges to the success of HIV and tuberculosis care and treatment in the public health sector in South Africa. J Infect Dis 2007;1(suppl):S491. [DOI] [PubMed] [Google Scholar]
- 13.UNAIDS. Assessment report on scaling up towards universal access to HIV prevention, treatment, care and support. UNAIDS, 2006.
- 14.World Health Organization. Health systems and services. Taking stock: health worker shortages and the response to AIDS. WHO, 2006.
- 15.Coetzee D, Hilderbrand K, Goemaere E, Matthys F, Boelaert M. Integrating tuberculosis and HIV care in the primary care setting in South Africa. Trop Med Int Health 2004;9:A11-5. [DOI] [PubMed] [Google Scholar]
- 16.Frenk J. The global health system: strengthening national health systems as the next step for global progress. PLoS Med 2010;7:e1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philips M, Zachariah R, Venis S. Task shifting for antiretroviral treatment delivery in sub-Saharan Africa: not a panacea. Lancet 2008;371:682-4. [DOI] [PubMed] [Google Scholar]
- 18.McCoy D, Chopra M, Loewenson R, Aitken JM, Ngulube T, Muula A, et al. Expanding access to antiretroviral therapy in sub-Saharan Africa: avoiding the pitfalls and dangers, capitalizing on the opportunities. Am J Public Health 2005;95:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Task shifting: rational redistribution of tasks among health workforce teams. Global recommendations and guidelines, I. WHO, 2008.
- 20.Pfeiffer J, Montoya P, Baptista AJ, Karagianis M, Pugas M, Micek M, et al. Integration of HIV/AIDS services into African primary health care: lessons learned for health system strengthening in Mozambique—a case study. J Int AIDS Soc 2010;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topp SM, Chipukuma JM, Giganti M, Mwango LK, Chiko LM, Tambatamba-Chapula B, et al. Strengthening health systems at facility-level: feasibility of integrating antiretroviral therapy into primary health care services in Lusaka, Zambia. PLoS One 2010;5:e11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.South African Government Information. Operational plan for comprehensive HIV and AIDS care, management and treatment for SA. 2010. www.info.gov.za/otherdocs/2003/aidsplan/index.html.
- 23.World Health Organization. Progress on global AIDS access to antiretroviral therapy: an update on “3 by 5”. 2010. www.who.int/3by5/fullreportJune2005.pdf.
- 24.Groenewald P, Nannan N, Bourne D, Laubscher R, Bradshaw D. Identifying deaths from AIDS in South Africa. AIDS 2005;19:193-201. [DOI] [PubMed] [Google Scholar]
- 25.Chapman R. Proposed plan for implementation of ARVs in the Free State Province. Free State Department of Health (South Africa), 2003.
- 26.Fairall LR, Zwarenstein M, Bateman ED, Bachmann M, Lombard C, Majara BP, et al. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. BMJ 2005;331:750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutic trials. J Chronic Dis 1967;20:637-48. [DOI] [PubMed] [Google Scholar]
- 28.Campbell MK, Elbourne DR, Altman DG: CONSORT group. CONSORT statement: extension to cluster randomised trials. BMJ 2004;20:702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei LJ, Lachin JM. Properties of urn randomization in clinical trials. Control Clin Trials 1988;9:345-64. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2007;4:CD000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bheekie A, Buskens I, Allen S, English R, Mayers P, Fairall L, et al. The Practical Approach to Lung Health in South Africa (PALSA) intervention: respiratory guideline implementation for nurse trainers. Int Nurs Rev 2006;53:261-8. [DOI] [PubMed] [Google Scholar]
- 32.University of Cape Town Lung Institute, 2007. www.knowledgetranslation.co.za/content/palsa_movie.html.
- 33.Statistics South Africa. Mortality and causes of death in South Africa, 1997-2003. Findings from death notification. Statistical release P0309.3. Statistics South Africa, 2005.
- 34.Department of Health. Tuberculosis and HIV and AIDS. Clinical guidelines: 2000. 2010. www.doh.gov.za/aids/docs/tuberculosis.html.
- 35.Campbell M. Cluster sample size software guide. 1999. www.abdn.ac.uk/hsru/uploads/files/calculationmanual.pdf.
- 36.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, Walensky RP, et al. Cost-effectiveness of HIV treatment in resource-poor settings—the case of Côte d’Ivoire. N Engl J Med 2006;355:1141-53. [DOI] [PubMed] [Google Scholar]
- 37.Chaisson RE, Martinson NA. Tuberculosis in Africa—combating an HIV-driven crisis. N Engl J Med 2008;358:1089-92. [DOI] [PubMed] [Google Scholar]
- 38.Calmy A, Klement E, Teck R, Berman D, Pecoul B, Ferradini L. Simplifying and adapting antiretroviral treatment in resource-poor settings: a necessary step to scaling-up. AIDS 2004;18:2353-60. [PubMed] [Google Scholar]
- 39.Harries AD, Makombe SD, Schouten EJ, Ben-Smith A, Jahn A. Different delivery models for antiretroviral therapy in sub-Saharan Africa in the context of universal access. Trans R Soc Trop Med Hyg 2008;102:310-1. [DOI] [PubMed] [Google Scholar]
- 40.Rowe AK, de Savigny D, Lanata CF, Victora CG. How can we achieve and maintain high-quality performance of health workers in low-resource settings? Lancet 2005;366:1026-35. [DOI] [PubMed] [Google Scholar]
- 41.Stein J, Lewin S, Fairall L, Mayers P, English R, Bheekie A, et al. Building capacity for antiretroviral delivery in South Africa: a qualitative evaluation of the PALSA PLUS nurse training programme. BMC Health Serv Res 2008;18:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colvin CJ, Fairall L, Lewin S, Georgeu D, Zwarenstein M, Bachmann MO, et al. Expanding access to ART in South Africa: the role of nurse-initiated treatment. S Afr Med J 2010;100:210-2. [DOI] [PubMed] [Google Scholar]
- 43.Fairall LR, Bachmann MO, Louwagie GMC, van Vuuren C, Chikobvu P, Steyn D, et al. Effectiveness of antiretroviral treatment in the South African public-sector programme: cohort study. Arch Intern Med 2008;168:86-93. [DOI] [PubMed] [Google Scholar]
- 44.Ovretveit J, Siadat B, Peters DH, Thota T, El-Saharty S. Review of strategies to strengthen health services. In: Peters DH, El-Saharty S, Siadat B, Janovsky K, Vujicic M, eds. Improving health service delivery in developing countries: from evidence to action. World Bank, 2009.
- 45.Fairall LR, Bachmann MO, Zwarenstein MF, Lombard CJ, Uebel K, van Vuuren C, et al. Streamlining tasks and roles to expand treatment and care for HIV: randomised controlled trial protocol. Trials 2008;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schull M. Strengthening human resources for health through simplified clinical tools and educational outreach: a cluster-randomised trial. 2010. http://isrctn.org/ISRCTN47805230.
- 47.Oxman AD, Bjørndal A, Becerra-Posada F, Gibson M, Block MA, Haines A, et al. A framework for mandatory impact evaluation to ensure well informed public policy decisions. Lancet 2010;375:427-31. [DOI] [PubMed] [Google Scholar]