Abstract
Besides the genetic framework, there are two critical requirements for the development of tissue-specific autoimmune diseases. First, autoreactive T cells need to escape thymic negative selection. Second, they need to find suitable conditions for autoantigen presentation and activation in the target tissue. We show here that these two conditions are fulfilled in diabetic NOD mice. A set of autoreactive CD4+ T cells specific for an insulin peptide, with the noteworthy feature of not recognizing the insulin protein when processed by the antigen presenting cells (APC) escape thymic control, participate in diabetes and can cause disease. We also find that APCs situated in close contact with the beta cells in the islets of Langerhans bear vesicles with the antigenic insulin peptides and activate the peptide-specific T cells. These findings may be relevant for other cases of endocrine autoimmunity.
Although autoimmune diabetic NOD mice exhibit a wide range of autoreactivities1, the one directed against the insulin molecule is prominent2. T cells reactive to insulin were identified in NOD mice and shown to transfer diabetes into non-diabetic mice3–7. T cells from T cell receptor (TCR) transgenic mice specific for insulin were also diabetogenic8. Additional findings point to insulin as an important primary autoantigen for disease initiation. The amplitude of insulin expression in the thymus was linked to diabetes incidence9–12, and high expression of insulin in APCs using a transgene ablated diabetes development13,14. Moreover, mice expressing a mutant insulin gene product not recognized by T cells did not develop diabetes7. It is noteworthy that the T cell response to the insulin molecule is highly focused on a segment of the β-chain, encompassing residues 9-23 (B:9-23)15–18. This peptide binds poorly (μM affinity and has a high dissociation rate) to the class II major histocompatibility complex (MHC) molecule I-Ag7 (ref18,19). How a small protein that yields a very weak binding peptide and circulates at ηM concentrations can function as a significant autoantigen is surprising, and raises a number of important considerations regarding the molecular and cellular basis of autoreactivity. The current view is that weak binding MHC epitopes, such as those from the insulin B:9-23 peptide18 or the insulin C-peptide20, may favor the development of autoreactivity because they may escape thymic selection21,22.
Two sets of CD4+ T cells have been identified by studying CD4 T cell responses to hen-egg white lysozyme (HEL)23,24. One set, the conventional T cells, termed ‘type A’, respond to the protein and to the peptide presented by APC. The second set, termed ‘type B’, have the unique features of responding only to peptides offered exogenously to the APC, but not to the identical peptide derived from the processing of the protein. The peptides were identical, but had different conformations when bound to the class II molecules. It was suggested that ‘type B’ T cells directed to autologous proteins participate in autoreactivity induction 23–27. Here we show that ‘type B’ T cells specific for insulin appear spontaneously in the diabetic NOD mice, along with weakly reactive ‘type A’ T cells. The ‘type B’ T cells react with dendritic cells (DC) containing the B:9-23 peptides and induce diabetes.
RESULTS
Insulin-reactive CD4+ ‘Type B’ T cells
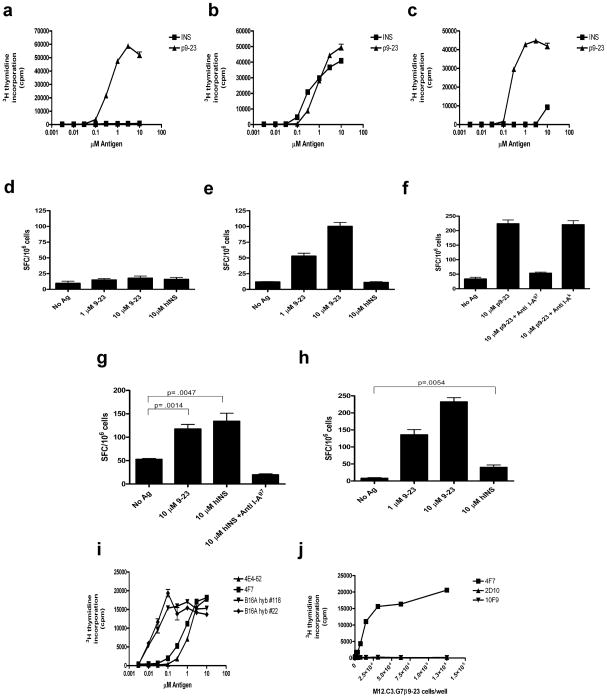
CD4+ T cells reactive against insulin were identified in islets and the peri-pancreatic lymph nodes of pre-diabetic/early diabetic mice and T cell hybridomas were generated. Forty-two T cell hybridomas (7% of total) from 6 different fusions, were reactive to insulin and/or to the B:9-23 peptide (617 hybridomas were screened of which 32 were further characterized). A number of T cell hybridomas, the conventional ‘type A’, responded to insulin as well as to the B:9-23 peptide. Several T cells hybridomas, the ‘typeB’, only recognized the peptide B:9-23 but not the insulin molecule (Fig. 1a). The distribution of ‘type A’ and ‘type B’ T cells was equal: among the 32 insulin-reactive CD4+ T cells which were characterized, 16 responded to peptide but not to insulin (‘type B’), and an equal number, 16, responded to both (‘type A’). The number is too small to derive definitive conclusions on their relative incidence. However, most of ‘type A’ T cells reacted weakly (in the low μM range) with either insulin or the peptide. About half of the ‘type A’ T cells had a 10–100-fold lower affinity to insulin than to the B:9-23 peptide, while the remaining reacted equally to both (Fig. 1b,c). Both ‘type A’ and ‘type B’ T cell subsets were found in the peri-pancreatic node as well as within the infiltrated islets. These results imply that ‘type B’ T cells were specifically recruited to the islets together with weakly reactive ‘type A’ T cells, suggesting the involvement of both ‘type B’ and low affinity ‘type A’ T cells in the early disease process.
Figure 1.
Insulin reactive T cells in NOD mice. (a–c) IL-2 production in response to insulin or B:9-23 peptide stimulation by three representative CD4+ T cell hybridomas using a standard CTLL proliferation bioassay. (a) ‘type B’ T cell (2D10), no response to insulin.(b) ‘type A’ T cell (4F7), equal response to insulin and peptide. (c) ‘type A’ T cell (IIT-9) very poor response to insulin. (d) ELISPOT of IL-2 production in NOD mice immunized with insulin and re-stimulated with insulin or the B:9-23 peptide. Results represent number of IL-2 secreting cells per 106 draining lymph node cells. SFC, spot forming cells. (e) ELISPOT of IL-2 secretion in NOD mice immunized with B:9-23 peptide and restimulated with peptide or insulin. (f) ELISPOT of IL-2 secretion in NOD mice immunized with B:9-23 peptide and restimulated with peptide in the presence of specific (anti-I-Ag7) blocking antibody. (g) ELISPOT of IL-2 secretion in B16:A-dKO mice immunized with insulin and re-stimulated as indicated. (h) ELISPOT of IL-2 secretion in B16:A-dKO mice immunized with B:9-23 peptide and re-stimulated as indicated. (i) IL-2 production in two T cell hybridomas derived from the B16:A-dKO mice(#118 & # 22) and two hybridomas derived from NOD mice (4F7 & 4E4-62) in response to insulin presented by the C3.G7 cell line. (j) IL-2 production in ‘type A’ (4F7) and ‘type B’ (2D10 &10F9) hybridomas incubated with the C3.G7 cell line bearing a B:9-23 peptide-I-Ag7 covalent complex. P values in experiments were determined using one-tailed unpaired Student’s t test. Error bars, s.e.m.
Mice respond to immunization with B:9-23 peptide
To examine in detail the immune response to insulin or to the B:9-23 peptide, mice were immunized with one or the other and the incidence of ‘type A’ and ‘type B’ insulin reactive CD4+ T cells were examined. When NOD mice were immunized with insulin, few, if any, insulin- specific T cells were detected in the draining lymph nodes (Fig. 1d). Previous studies detected a weak insulin T cell proliferative response14. In contrast, a prominent CD4+ T cell response was found upon immunization with the B:9-23 peptide. The response was entirely blocked by an anti-I-Ag7 antibody (Fig. 1e,f). These T cells were peptide specific, because they responded only to B:9-23 peptide, but not to the insulin protein. Of 351 T cell hybridomas generated from the draining lymph nodes of the immunized mice, the majority (74%) were peptide specific, while only a small number (1.7%) recognized both peptide and the insulin molecule (Fig. 1a–c and Supplementary Table 1). The remaining hybridomas were either non-responsive (17.7%) or autoreactive (6.6%). The ‘type B’ T cells also recognized denatured insulin, indicating that they were not recognizing an artificial determinant arising from the synthetic peptide (Supplementary Fig. 1a).
We next compared the insulin-reactive T cells from NOD wild-type and NOD mice with genetic deletions of both insulin genes but expressing a transgene encoding a mutant proinsulin7. The mutant transgene encodes an insulin molecule containing a single tyrosine to alanine amino acid substitution at residue16 of the insulin beta-chain, referred to as B16:A-dKO. In the absence of native insulin genes, the transgenic mutant insulin restored biological activity, however, the substitution abrogated recognition by insulin-specific T cells7. Unlike NOD mice, B16:A-dKO mice immunized with insulin generated a T cell response specific to insulin protein (Fig. 1g and d). Immunization of B16:A-dKO mice with the B:9-23 peptide generated a peptide response and a weak response to insulin (Fig. 1h). In addition, ‘type A’ hybridomas generated from B16:A-dKO mice immunized with insulin displayed a much greater sensitivity for insulin compared to hybridomas derived from NOD mice (Fig. 1i). This indicates that in the NOD mouse the high affinity T cells to insulin are normally deleted. T cells found in peripheral tissue are less reactive, reflecting that they escaped negative selection and bear the stamp of tolerance.
Differential recognition of covalent Peptide-MHC complexes
The explanation for the different recognition of the B:9-23 peptide-I-Ag7 complex between the two sets of T cells is presently unclear. Initial studies indicate that in contrast to the HEL system, H2-DM did not affect the development of ‘type A’ or ‘type B’ peptide-MHC (pMHC) complexes (data not shown). Moreover, H2-DM favored the assembly of B:9-23 to I-Ag7. We have searched extensively for changes in the peptide or for post-translational modifications that may account for recognition by one or the other set of T cells and have failed to identify any (data not shown). Both ‘type A’ and ‘type B’ CD4+ T cells reacted to purified I-Ag7 molecules incubated with the identical B:9-23 peptide (Supplementary Fig. 1b). The differential recognition of the B:9-23 peptide by the two T cells types most likely reflects different conformational states of the pMHC complex. In support of this interpretation, ‘type A’ and ‘type B’ T cells recognized differently a covalently-linked pMHC complex. M12.C3 cells engineered to express I-Ag7 with the B:9-23 peptide covalently tethered15 activated the ‘type A’ T cells, including those that reacted weakly with insulin, but not the ‘type B’ T cells (Fig. 1j and Supplementary Table 1). Thus, the covalently-linked complex was presented in a state or a conformation that allowed the TCR of ‘type A’ T cells to recognize it, but not the TCR of ‘type B’ T cells. It is probable that the intracellular pathway by which insulin reaches the class II molecule and the site within the APC are the two critical factors in generating a unique complex that T cells recognize.
Type B T cells cause diabetes
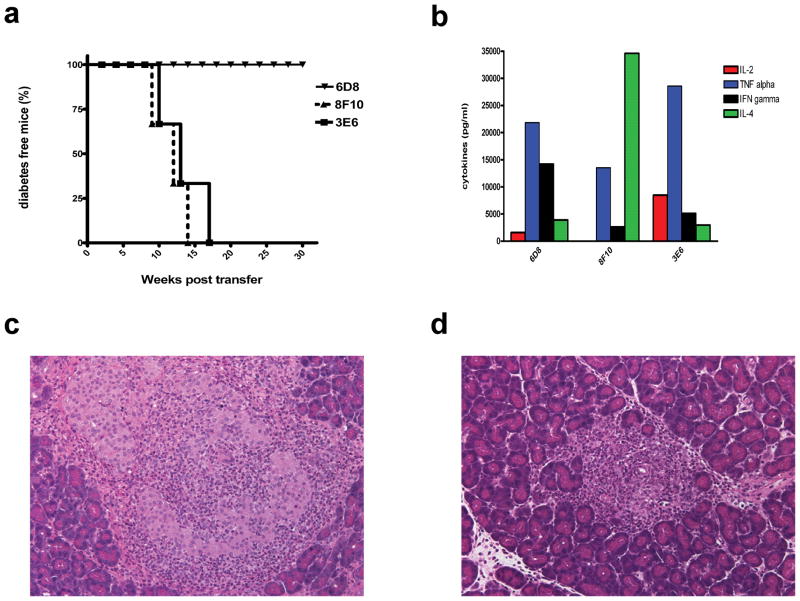
To determine if ‘type B’ insulin T cells are diabetogenic, eleven primary T cell lines were generated from the draining lymph nodes of B:9-23 immunized NOD mice, expanded and adoptively transferred into NOD.SCID recipients. All T cell lines were ‘type B’, meaning they recognized the B:9-23 peptide, but not the insulin molecule (Supplementary Fig. 2). None of them recognized the covalently linked pMHC complex on M12.C3.G7β9-23 cells (data not shown). All T cell lines induced extensive insulitis, with 7 of 11 lines also inducing diabetes (Fig. 2a,c,d and Supplementary Fig. 3a). A number of these lines also accelerated diabetes in young NOD mice (Supplementary Fig. 3a–d). The extent of disease transfer varied among the clones and was irrespective of their cytokine profiles (Fig. 2b and Supplementary Fig. 3a). Thus, the peptide reactive, ‘type B’ T cells could damage the islets and induce the same histopathology as other diabetogenic T cells, suggesting that they participate in the autoimmune process.
Figure 2.
‘Type B’ T cells are diabetogenic. (a) Diabetes incidence of three representative ‘type B’ T cell lines, when transferred into NOD.SCID recipients ( n=3 for each); mice were considered diabetic after two consecutive blood glucose readings of ≥ 250 mg/dl. (b) Cytokine profile of representative CD4+ T cell lines 48 h post stimulation with plate bound anti-CD3/CD28 antibodies. Haematoxylin and eosin stained pancreatic section of a NOD.SCID recipient that received 6D8 T cells and developed insulitis but not diabetes (c) and a NOD.SCID recipient that received 3E6 T cells and developed overt diabetes (d).
Presentation of B:9-23 by islet DC
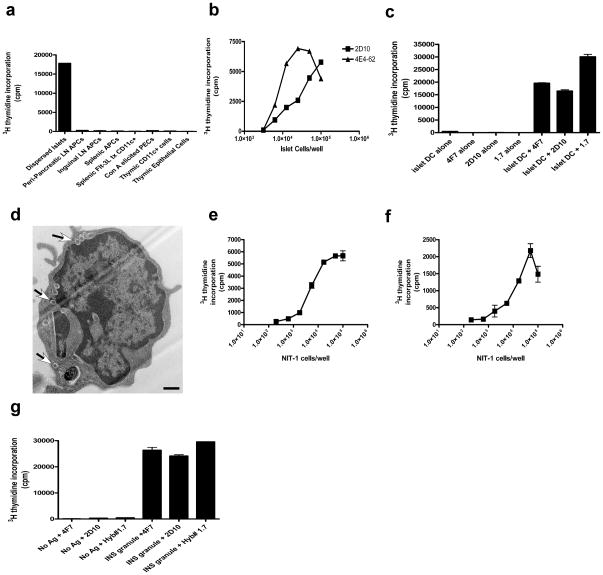
In order to address where insulin peptide presentation leading to ‘type B’ T cells activation occurs, antigen presenting cells (APC) from various tissues were examined. We could not detect antigen presentation to T cells by APCs from spleen, inguinal or peri-pancreatic lymph nodes from pre-diabetic mice, suggesting that circulating insulin was not the source of peptide. In addition, neither dendritic cells (DC) nor epithelial cells isolated from thymi activated ‘type A’ hybridomas, indicating that presentation in the thymus is quite low. Of all tissues examined the only APCs that presented insulin peptides to either ‘type A’ or ‘type B’ T cells were the intra-islet DC (Fig. 3a). In all mouse strains, the islets of Langerhans harbor an average of about 10 DC per islet, most of which are constitutively expressing pMHC complexes derived from beta cell proteins, irrespective of the diabetic status, islet inflammation or beta cell death28. Whole islets cell fractions containing DC, or purified intra-islet DC from NOD.Rag1−/− mice constitutively presented antigen to both ‘type A’ and ‘type B’ T cells in the absence of inflammation or overt cellular death (Fig. 3b,c). Furthermore, when Nit-1 insulinoma cells, which do not express MHC class II molecules, were incubated with purified splenic DC, uptake of insulin granules by DC was detected by electron microscopy (Fig. 3d). These DC were capable of activating both the ‘type A’ and ‘type B’ insulin-reactive T cells (Fig. 3e,f), showing that both peptide-MHC conformers are presented by APC within close contact to beta cells.
Figure 3.
Intra-islet DC pulsed with secretory granules, present insulin peptides. (a) B:9-23 peptide presentation by a panel of cells from various tissues to a ‘type B’ hybridoma (Hyb #1.7). (b) IL-2 production from ‘type A’ (4E4-62) and ‘type B’ (2D10) hybridomas stimulated with dispersed islets from NOD.Rag1−/− mice. (c) IL-2 secretion from ‘type A’ (4E4-62) and ‘type B’ (2D10) hybridomas stimulated with CD11c+ cells isolated from the islets of 8 week old NOD males. (d) Electron microscopy analysis of Flt-3L CD11c+ cell coincubated with NIT-1 insulinoma cells for 24 h (arrows highlight granules in DC). Scale bar 500 ηm. (e,f) IL-2 production from ‘type B’ (2D10) (e) and ‘type A’ (4F7) (f) hybridomas stimulated with Nit-1 cells co-incubated with purified splenic CD11c+ DCs. (g) IL-2 production in ‘type A’ (4F7) and ‘type B’ (2D10 and Hyb #1.7) hybridomas stimulated with splenic CD11c+ cells co-incubated with purified insulin granules from primary dispersed islet cells. Error bars, s.e.m.
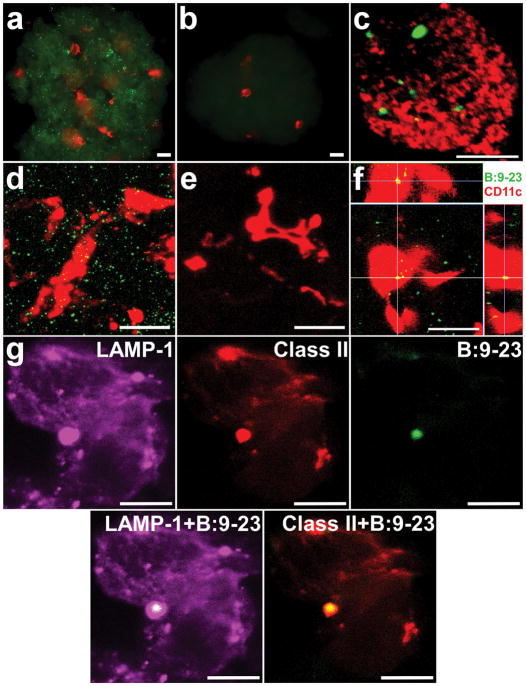
The secretory granules of beta cells can constitute a source of the B:9-23 peptide, due to the fact each granule is comprised of about 106 insulin molecules, as well as catabolic products from its processing29. Purified secretory granules from primary beta cells incubated with splenic DC led to robust activation of both ‘type A’ and ‘type B’ hybridomas (Fig. 3g). To assess whether these granules contain insulin peptides, we generated a monoclonal antibody that specifically binds to the B:9-23 peptide but not to insulin or proinsulin molecules (Supplementary Fig. 4). Specific granule staining was observed in whole islets or purified beta cells from NOD or NOD.Rag1.−/− mice (Fig. 4a,d). The reaction was blocked by free peptide (Fig. 4b,e), but not by an excess of insulin or proinsulin (Supplementary Fig. 4). Cells containing B:9-23 positive granules represented a subset of total beta cells. The majority of granules inside beta cells were positive for insulin but negative for B:9-23 and did not contain LAMP-1 (Fig. 4c and Supplementary Fig. 5). In contrast, all vesicles containing the B:9-23 peptide in beta cells were positive for LAMP-1, and only very few of these vesicles (less that 10%), were also positive for insulin (Supplementary Fig. 5).
Figure 4.
Secretory granules contain proteolytic fragments of the insulin β-chain in NOD.Rag1 −/− islets. (a) Immunofluorescence analysis at low magnification (20X) of an islet stained for CD11c+ (red) and intracellular B:9-23 (green). (b) Immunofluorescence analysis of an islet stained for CD11c+ (red) and B:9-23 (green) in the presence of competing exogenous B:9-23 peptide. Scale bars, 20μm. (c) Confocal microscopy of an isolated beta cell, stained for B:9-23 (green) and insulin (red). The reconstruction is from a single stack (1.5μm thickness). Scale bar, 15μm. (d, e) Reconstruction from a stack of 60 compiled optical sections acquired in 0.5-μm increments stained for CD11c+ (red) and intracellular B:9-23 (green) in the absence (d) or presence of competing B:9-23 free peptide (e). Scale bars, 20μm.. (f) Three-dimensional reconstruction of an islet by confocal microscopy stained for CD11c+ (red) and intracellular B:9-23 (green). The bottom left image is a projection along the z-axis (top view) from a stack of 30 optical sections acquired in 0.5μm increments. The top and right-sided images are zx- and zy-reconstructions (side view) of the same image stack (indicated as white lines). B:9-23 staining in the islet DC (yellow merge). Scale bar, 20μm. (g) Reconstruction of an isolated islet DC co-stained for B:9-23, class II MHC and for LAMP-1. Images represent a single stack with thickness of 1.5μm. Scale bars, 10μm.
Confocal microscopy showed that the majority of DC in the examined islets had taken up B:9-23 positive and/or insulin granules (Fig. 4f). Screening of purified islet DC showed that about 80% of them contained insulin positive granules with an average of 7 granules per DC. Many of these DC stained positive for both insulin containing vesicles and B:9-23 containing vesicles. Granules within islet DC co-localized with LAMP-1 and I-Ag7 (Fig. 4g), consistent with trafficking to cellular compartments involved in antigen processing and presentation. These observations suggest that the B:9-23 peptides presented by I-Ag7 could be derived from beta cell vesicles containing peptides of the insulin β-chain and/or from the processing of insulin granules within DC. Evidence for granules within intra-islet DC under physiological conditions was presented previously by electron microscopy26. In addition, granules isolated from Nit-1 cells contained 8.8 ηmoles of anti-insulin-reactive material and 12 ρmoles of anti-9-23 reactive material. Initial mass spectrometry studies on purified secretory granules showed they contained C-peptide (data not shown), but also peptides from the B:9-23 segment, which included peptides centered on the 12-21 residues (VEALYLVGGE) of the insulin β-chain (Supplementary Fig. 6). These observations suggest that peptides immunogenic for ‘type B’ T cells are produced during the physiological processing of mature insulin molecules, and are not dependent on any prior inflammation.
DISCUSSION
Three sets of insulin-reactive T cells were distinguished in this study. One set had the unique feature of being specific to exogenous insulin peptide, but unreactive to insulin. The peptide that activated these ‘type B’ T cells was normally derived from the insulin secretory granule and was loaded on MHC class II in the intra-islet DC. These T cells were capable of transferring diabetes and were found spontaneously in infiltrated islets, indicating their participation in the autoimmune process. These T cells are compatible with the ‘type B’ T cells described before23–26.
The two other sets of T cells were insulin and peptide-reactive T cells, but differed in their sensitivity to insulin. We refer to them as ‘type A’ T cells, identified in the NOD or in the B16:A-dKO mice, respectively. In the B16:A-dKO mice ‘type A’ T cells were readily found after insulin immunization and showed a higher response to the insulin molecule or the B:9-23 peptide compared to ‘type A’ T cells from the NOD mouse. The strong insulin reactivity found in B16:A-dKO mice supports the conclusion reached by others9–12 that native insulin expression in the thymus influences the selection of insulin-reactive T cells, and that partial tolerance or unresponsiveness to the insulin molecule exists in NOD mice. Thus, the few insulin reactive T cells found in NOD mice had an imprint of tolerance, with weak reactivity to insulin, which might explain why they escaped negative selection.
The frequency of ‘type B’ T cells is markedly greater than ‘type A’ T cells in the precursor population of NOD mice. However, because both ‘type A’ and ‘type B’ T cells were isolated from the islets, we cannot conclude which set is the major participant, or if they act synergistically in disease development. More than one factor can influence the diabetogenicity of transferred T cell cells, such as time in culture and expression of adhesion molecules30. Due to this variability, strict comparisons between the diabetogenicity of ‘type A’ and ‘type B’ T cells were not done systematically. Our results and previous reports5,8 indicate that both ‘type A’ and ‘type B’ insulin-specific T cells are diabetogenic.
The B:9-23 peptide could be generated following DC handling of insulin upon capture of the insulin granules or from uptake of the granules that already contained the peptide. The finding that islet DC contain insulin granules and B:9-23 peptide, and the observation that feeding DC with purified secretory granules results in strong presentation explains another conundrum concerning insulin presentation. There is a relatively poor yield of peptide selected by MHC molecules after protein processing31. Furthermore, due to the low binding affinity of the B:9-23 peptide for I-Ag7 and the rapid dissociation of the peptide from I-Ag7 (ref 18), a high concentration of insulin within the APC is required for effective presentation to T cells. The concentration of insulin in blood and tissue is in the low ηM level. In antigen presentation assays, the concentration of insulin or B:9-23 peptide required for presentation is in the low μM level which is unphysiological in the whole mouse. If all these aspects are taken into consideration, it is most likely that the islet DC have two essential roles: to migrate into the pancreatic draining lymph node to initiate the sensitization process32; and to present antigen to sensitized T cells in the effector stage of diabetogenesis.
Collectively, these results suggest that self-reactive ‘type B’ T cells and low affinity ‘type A’ T cells escape negative selection and give rise to pathogenic T cells in autoimmune diabetes. A situation akin to insulin autoimmunity may also be found in other endocrine autoimmune diseases such as in autoimmune thyroiditis in which DC are found surrounding the follicles, some of which have class II-positive epithelial cells33,34.
Supplementary Material
Acknowledgments
The authors thank P. Bittner and K. Frederick for technical assistance, R. Belizaire and M. Colonna for comments on the manuscript, M. Gross and H. Rohrs for mass spectrometry and K. Green for electron microscopy. We thank G.S. Eisenbarth and N. Abiru for the M12.C3.G7β9-23 cell line. This research was supported by National Institutes of Health grants AI024742, DK020579 and P60DK20579, by the Juvenile Diabetes Research Foundation grant JDRF 1-2007-731 and by grants from the Kilo Diabetes and Vascular Research Foundation.
Footnotes
Author Contributions. J.F.M., M.G.L. and E.R.U designed and evaluated the experimental work. J.F.M., M.G.L. and J.W.H. carried out most of the cellular experiments. Immunofluorescence and confocal microscopy was done by B.C. S.J.P. isolated insulin granules for mass spectrometry and quantified insulin content within the granules. J.F.M. and E.R.U. wrote the manuscript
Supplementary Information is linked to the online version of the paper at www.nature.com/nature
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune disregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 4.Wegmann DR, Gill RG, Norbury-Glaser M, Schloot N, Daniel D. Analysis of the spontaneous T cell response to insulin in NOD mice. J Autoimmun. 1994;7:833–843. doi: 10.1006/jaut.1994.1066. [DOI] [PubMed] [Google Scholar]
- 5.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 6.Moriyama H, et al. Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc Natl Acad Sci U S A. 2003;100:10376–10381. doi: 10.1073/pnas.1834450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasinski JM, et al. Transgenic insulin (B:9-23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes. 2006;55:1978–1984. doi: 10.2337/db06-0058. [DOI] [PubMed] [Google Scholar]
- 9.Chentoufi AA, Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes. 2002;51:1383–1390. doi: 10.2337/diabetes.51.5.1383. [DOI] [PubMed] [Google Scholar]
- 10.Garcia CA, et al. Dendritic cells in human thymus and periphery display a proinsulin epitope in a transcription-dependent, capture-independent fashion. J Immunol. 2005;175:2111–2122. doi: 10.4049/jimmunol.175.4.2111. [DOI] [PubMed] [Google Scholar]
- 11.Pugliese A, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 12.Pugliese A, et al. Self-antigen-presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. J Clin Invest. 2001;107:555–564. doi: 10.1172/JCI10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French MB, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1997;46:34–39. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 14.Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nature Immunol. 2004;5:1028–1035. doi: 10.1038/ni1120. [DOI] [PubMed] [Google Scholar]
- 15.Abiru N, et al. Dual overlapping peptides recognized by insulin peptide B:9-23 T cell receptor AV13S3 T cell clones of the NOD mouse. J Autoimmun. 2000;14:231–237. doi: 10.1006/jaut.2000.0369. [DOI] [PubMed] [Google Scholar]
- 16.Abiru N, et al. Peptide and major histocompatibility complex-specific breaking of humoral tolerance to native insulin with the B9-23 peptide in diabetes-prone and normal mice. Diabetes. 2001;50:1274–1281. doi: 10.2337/diabetes.50.6.1274. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama M, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117:1835–1843. doi: 10.1172/JCI31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol. 2007;178:6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- 19.Hausmann DH, Yu B, Hausmann S, Wucherpfennig KW. pH-dependent peptide binding properties of the type I diabetes-associated I-Ag7 molecule: rapid release of CLIP at an endosomal pH. J Exp Med. 1999;189:1723–1734. doi: 10.1084/jem.189.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levisetti MG, Lewis DM, Suri A, Unanue ER. Weak proinsulin peptide-MHC complexes are targeted in autoimmune diabetes in mice. Diabetes. 2008;57:1852–1860. doi: 10.2337/db08-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu GY, et al. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 22.Fairchild PJ, Wildgoose R, Atherton E, Webb S, Wraith DC. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int Immunol. 1993;5:1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- 23.Pu Z, Lovitch SB, Bikoff EK, Unanue ER. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity. 2004;20:467–476. doi: 10.1016/s1074-7613(04)00073-1. [DOI] [PubMed] [Google Scholar]
- 24.Pu Z, Carrero JA, Unanue ER. Distinct recognition by two subsets of T cells of an MHC class II-peptide complex. Proc Natl Acad Sci U S A. 2002;99:8844–8849. doi: 10.1073/pnas.092260499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 26.Lovitch SB, Walters JJ, Gross ML, Unanue ER. APCs present A beta(k)-derived peptides that are autoantigenic to type B T cells. J Immunol. 2003;170:4155–4160. doi: 10.4049/jimmunol.170.8.4155. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura K, McLaughlin KA, Weissert R, Forsthuber TG. Myelin-reactive type B T cells and T cells specific for low-affinity MHC-binding myelin peptides escape tolerance in HLA-DR transgenic mice. J Immunol. 2008;181:3202–3211. doi: 10.4049/jimmunol.181.5.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci U S A. 2008;105:6121–6126. doi: 10.1073/pnas.0801973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutton JC. The insulin secretory granule. Diabetologia. 1989;32:271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- 30.Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol. 2005;87:123–162. doi: 10.1016/S0065-2776(05)87004-X. [DOI] [PubMed] [Google Scholar]
- 31.Dadaglio G, et al. Characterization and quantification of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity. 1997;6:727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 32.Hoglund P, et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph Nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catalfamo M, et al. HLA-DM and invariant chain are expressed by thyroid follicular cells, enabling the expression of compact DR molecules. Int Immunol. 1999;11:269–277. doi: 10.1093/intimm/11.2.269. [DOI] [PubMed] [Google Scholar]
- 34.Croizet K, et al. Culture of dendritic cells from a nonlymphoid organ, the thyroid gland: evidence for TNF alpha dependent phenotypic changes of thyroid-derived dendritic cells. Lab Invest. 2000;80:1215–1225. doi: 10.1038/labinvest.3780129. [DOI] [PubMed] [Google Scholar]
- 35.Gray D, et al. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods. 2008;329:56–66. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Brunner Y, et al. Proteomics analysis of insulin secretory granules. Mol Cell Proteomics. 2007;6:1007–1017. doi: 10.1074/mcp.M600443-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.