Abstract
The sensitivity limitations for magnetic resonance imaging of organic molecules have recently been addressed by hyperpolarization methods, which prepare excess nuclear spin polarization. This approach can increase sensitivity by orders of magnitude, but the enhanced signal relaxes away in tens of seconds, even in favorable cases. Here we show theoretically that singlet states between strongly coupled spins in molecules can be used to store and retrieve population in very-long-lived disconnected eigenstates, as long as the coupling between the spins substantially exceeds both the couplings to other spins and the resonance frequency difference between them. Experimentally, 2,3-carbon-13–labeled diacetyl has a disconnected eigenstate that can store population for minutes and is read out by hydration to make the two spins inequivalent.
Magnetic resonance imaging of different endogenous and exogenous molecules in tissue and living organisms holds the promise of resolving metabolic pathways and diagnosing early disease states, thus broadly affecting pharmaceutical development and molecular medicine. However, magnetic resonance is extremely insensitive, mainly because even very large magnetic fields induce only a very small nuclear magnetization at room temperature. For example, hydrogen atoms in a 7-T imager have ~0.0001 of the bulk magnetization they would have if all spins were aligned in the same direction. As a result, virtually all clinical magnetic resonance images measure water signal, and localized magnetic resonance spectroscopy is challenging and slow.
This signal limitation has spurred the development of so-called hyperpolarization methods, which commonly produce bulk magnetizations of 20% or more. Although the first applications used spin-polarized noble gases (1) (for instance, 3He and 129Xe), in recent years attention has turned to two methods that can hyperpolarize molecules: (i) para-H2 addition across double bonds (2–4) and (ii) dynamic nuclear polarization (DNP) (5–13). The DNP methodology in particular is very versatile, and hundreds of different molecules have been polarized, but virtually all in vivo work has focused on one molecule (13C-labeled pyruvate), largely because the population relaxation time T1 is relatively long (40 s at 14.1 T). The polarized nuclei can undergo metabolic reactions before the nuclear magnetic resonance (NMR) signal returns to thermal equilibrium and becomes undetectable. Generically, 13C T1 values are tens of seconds for carbons without attached protons (and much shorter with attached protons), and hydrogen T1 values are shorter still. This lifetime permits some important metabolic processes to be studied, but it is vastly shorter than the lifetimes associated with other molecular imaging modalities (e.g., 18F positron emission tomography, which decays in ~2 hours).
We present a practical approach to storing and retrieving population from very-long-lived spin states in a fairly wide range of molecular targets. The key is understanding and exploiting symmetry effects and the way they are altered by chemical reactions. To begin, suppose a molecule has two spin-1/2 nuclei that are isolated (meaning that they are not coupled to any other spins) and are chemically equivalent (14, 15) (meaning that they have the same resonance frequency, usually because of symmetry). The molecule then has what we will call a “disconnected eigenstate.” The singlet energy level [2−1/2(α1β2 − β1α2), where αn and βn are the two possible states of spin n] is an eigenstate of the internal molecular Hamiltonian and has no dipole moment connecting it to the other three triplet levels [α1α2, β1β2, and 2−1/2(α1β2 + β1α2)]. The simplest example is H2 itself, where molecules in the singlet state (called para-H2) can actually be chemically separated from the other (ortho) form and can persist almost indefinitely in the gas phase.
The triplet levels support two allowed transitions with the same energy, making the scalar spin-spin “J” coupling unobservable in this molecule (or for that matter in water, which has the same symmetry). Singlet states also have no substantial interactions with external magnetic fields, as long as the field does not break the symmetry between the spins, so they are protected from the interactions that cause T1 relaxation. Of course, pairs of isolated, chemically equivalent spins are found in only a handful of molecules; in addition, the lack of interaction with external fields implies that the signal we need to measure is unobservable.
As an alternative, several groups (16–18) have elegantly demonstrated lengthening of singlet-state lifetimes in molecules with inequivalent spins. In these experiments, the singlet state is not an eigenstate, so without any intervention it would rapidly evolve into unprotected states. However, it can be forced to be a pseudo-eigenstate either by removing frequency differences with multiple spin echoes or by lowering the magnetic field to such an extent that the resonance frequencies are essentially the same. The signal can then be observed by permitting free evolution in a high field. Both approaches give clear lifetime increases, but neither is practical for magnetic resonance imaging. Multiple spin-echo sequences rapidly exceed safe power dissipation limits in tissue, and rapidly shuttling patients in and out of a magnet induces vertigo and nausea. In addition, at the microscopic level, both of these approaches must have their limitations. Relaxation is dominated by the local components of the magnetic field fluctuating near the resonance frequency, and if two sites are physically inequivalent, these fluctuations are expected to be poorly correlated, even if the resonance frequencies are nearly the same.
Here, we show that, in many cases, we can take advantage of chemistry to access and retrieve population from long-lived states and that disconnected eigenstates suitable for this storage exist in a fairly wide range of molecules. A test system that demonstrates both concepts is 2,3-13C–labeled diacetyl [CH3(13C=O)(13C=O)CH3]. Diacetyl is formed in butter during ripening by the organism Streptococcus lactis cremoris, it is the source of the buttery flavor in wine and beer (at typical concentrations of a few micrograms per milliliter) (19), and it is added to soft margarines, producing concentrations in air commonly of ~100 parts per million (ppm) during cooking (20). However, there are concerns about extremely long-term inhalation (at chemical manufacturing plants), which has recently been implicated in bronchiolitis obliterans (21).
13C-labeled diacetyl was prepared from 13C2 oxalate, which was reacted with N,N′-dimethylethylenediamine to afford 2,3-13C2-1,4-dimethylpiperazine-2,3-dione (97% yield). Two equivalents of methyl magnesium bromide were added to the piperazine dione to produce the dimethyl piperazine N,O-acetal. Crude acetal was hydrolyzed with 10% aqueous HCl to yield 2,3-13C2 diacetyl, which was purified by fractional distillation. The carbonyl carbons in diacetyl are chemically equivalent, so at modest resolution (as is commonly achieved in an imaging system), the spectrum is expected to have only a single line. Neat 2,3-13C diacetyl does have a single-line carbon spectrum, but the carbon spectrum in water has five lines. The monohydrate CH3(13C=O)[13C(OH)2CH3] (Fig. 1), with two inequivalent carbons and a scalar coupling JC–C = 45 Hz, is the majority species (22); the dihydrate is undetectable. Equilibrium can be shifted back to diacetyl by changing the solvent, and the rate of interconversion is pH-dependent. At pH 7, inversion of the diacetyl alone causes recovery in 8 s, which gives the rate of dehydration; inversion of all lines causes diacetyl to recover in 22 s.
Fig. 1.

The hydrate of 2,3-13C diacetyl (left) has two inequivalent carbons (denoted by asterisks) whose resonance frequency differs by 110 ppm (~8 kHz in our magnet). The 45-Hz C–C scalar coupling splits both carbon lines into doublets, making all four energy levels accessible. The dehydrated version (right) has the same resonance frequency for both carbons and has a disconnected eigenstate. The αβ and βα populations in the hydrate are thus readily perturbed by radiofrequency pulses. Dehydration locks part of this population in the long-lived disconnected state; rehydration makes it observable again.
Preparation of the singlet state requires perturbation of the αβ and βα populations from their equilibrium 25%. Hyperpolarization does not do this efficiently by itself. For example, 20% nuclear polarization (60% α, 40% β) would imply α1β2 and β1α2 populations of 24% (only 1% from equilibrium) and would waste most of the potential signal. However, because all the energy levels in the hydrate are accessible, suitable pulse sequences can manipulate the αβ and βα populations. The simplest is inversion of one line in one of the doublets (e.g., α1α2 → α1β2), which in this example would interchange the 36% α1α2 and 24% α1β2 populations. Dehydration converts the sum of the α1β2 and β1α2 populations (in this case, 60%) evenly among the singlet α1β2 − β1α2 and triplet α1β2 + β1α2 of diacetyl; the singlet population in this case is 30%, six times farther from equilibrium than that produced by DNP alone. After this dehydration step, the population should be locked for a very long time, as discussed later, unless it exchanges back to the hydrate.
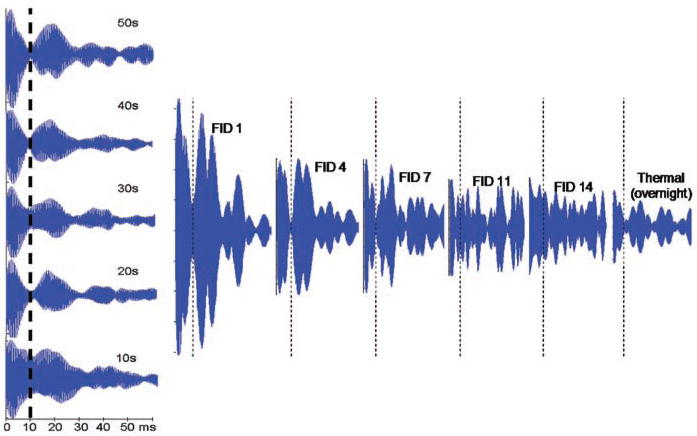
Figure 2 demonstrates this lifetime extension, both with and without hyperpolarization. The left side of Fig. 2 shows the result of selectively inverting only one line in thermally polarized monohydrate, then checking populations with a small flip-angle probe pulse after a variable delay. With a very long delay (50 s, top) the free induction decay (FID) from the monohydrate looks like the normal FID, which has a null at t = 1/2J (10 ms), because each pair of lines separated by frequency J in Fig. 1 destructively interferes at that time. However, deviations of the αβ and βα population from their equilibrium values cause a characteristic alternation of the peak intensities [or, equivalently, FID signal at t = 1/(2J)]. For short delays between the selective inversion pulse and the probe pulse (10 s), the signal is large at t = 1/2J, also as expected. Figure 2A shows that population then flows rapidly into diacetyl as expected, but when it returns to the monohydrate (~30 s), it has excess αβ and βα population consistent only with a long-lived state (the singlet).
Fig. 2.
Inverting the highest field monohydrate line locks population into a long-lived singlet state of diacetyl after dehydration, thus perturbing populations as the diacetyl gets rehydrated. Thermal (left) and hyperpolarized (right) FIDs are shown of only the portion of the spectrum around the monohydrate peaks. For equilibrium magnetization, the line splitting implies that the FID should vanish at t = 1/2J (vertical dotted lines). The signal at that point directly measures excess population in α1β2 and β1α2 states, which is perturbed here by population flow from α1β2 − β1α2 in diacetyl.
With hyperpolarization, this population could be readily converted back to observable signal in the hydrate (excess population in α1β2 and β1α2 implies dipolar order, which can be converted to observable signal with a 45° pulse and delay), dramatically extending the effective hyperpolarized lifetime. This procedure differs from most hyperpolarization experiments, where small-pulse flip angles are used to conserve the signal for multiple shots; singlet diacetyl is unaffected by radio frequency pulses, so large flip angles do not deplete the stored population. Equilibrium is readily shifted away from the hydrate by the addition of acetone, thus permitting the singlet state to last longer as a spin reservoir.
For the right side of Fig. 2, we started by hyperpolarizing diacetyl in water, achieving ~20% nuclear polarization. We then inverted one line of the monohydrate and followed with immediate 3:1 dilution with acetone. Dilution slowed the rate of hydration and helped to preserve any locked population produced by dehydration of the monohydrate. Individual spectra were acquired by 45° pulses with 10-s time separation. The dynamics are complex (for example, the equilibrium is being shifted), but useful insights can be gained by following only at the FID from hydrate peaks (Fig. 2B). The first FIDs have excess signal at t = 1/2J (vertical dotted lines), as expected from the selective inversion. At intermediate times (sufficient for dehydration and mixing of the acetone), the FIDs look similar to the thermal ones (averaged over 360 shots). Most interestingly, the later FIDs have signal higher than thermal, and the structure is very complex. In the case of FID 14, for example, the preceding 45° pulses should have depleted all but 1% of the hyperpolarized signal, and relaxation should have depleted all but 0.1%, so less than 10−5 should remain in the absence of singlet effects.
Figure 3 shows the result of an experiment similar to Fig. 2, except that this time we used perdeuterated diacetyl. We generated excess α1β2 and β1α2 population, diluted with acetone, then applied a series of 45° pulses, both before and after the addition of excess water. Before the water addition, hyperpolarization goes away because of the ~30-s T1 relaxation time and population depletion by the pulses themselves; at the time of the water injection, ~3% of the initial hyperpolarized signal is still present. The water injection permits rehydration, thus making the stored singlet population accessible. Within seconds after the injection, the signal more than triples and, in this case, is still readily observable for minutes.
Fig. 3.

Hyperpolarized perdeuterated diacetyl monohydrate was pulsed to perturb the α1β2 and β1α2 populations, diluted in acetone, then probed with a series of 45° pulses (both before and after addition of excess water). The dramatic increase in signal with rehydration reflects the unlocking of long-lived population stored in the diacetyl disconnected eigenstate.
Shifting the equilibrium with acetone in vivo would not be feasible. As diacetyl does not have a dipole moment, it can migrate to a nonpolar phase [for instance, it is mostly found in the fatty phase in butter (23)], and we picture one ultimate application of this agent as a “reporter molecule” in a delivery system [for example, functionalized or temperature-sensitive liposomes or functionalized ultrasound contrast agents, including commercially available agents that are based on encapsulated perfluorocarbons (24)].
To understand how general these results would be, it is important to recognize that the high-resolution spectrum of diacetyl is more complicated than that of para-H2 or water because the two carbons are chemically equivalent but not isolated. Each carbon is coupled differently to the two methyl groups (the C–H couplings are 6.4 Hz and −1.1 Hz). Such couplings generate simple splitting patterns in most molecules. For example, Fig. 4A shows the carbonyl region 13C NMR spectrum of the singly carbon-labeled species (2-13C diacetyl), where the two different couplings to three hydrogens each generate a quartet of quartets. Fig. 4B also shows the spectrum of acetone [CH3(13C=O)CH3], which leads to a septet because the six hydrogens are equivalent. Both of these results would be obvious to a practicing organic chemist. However, Fig. 4C shows the superficially very complicated spectrum of 2,3-13C diacetyl. The complexity arises from addition of the C–C coupling (~45 Hz) and hides a secret: The carbon singlet state is nearly a disconnected eigenstate.
Fig. 4.

(A to F) Experimental and theoretical spectra validating the presence of disconnected eigenstates (even in molecules such as diacetyl) without magnetically equivalent spins.
The bottom panels of Fig. 4 show simulations (using WindNMR-Pro) that unravel the complexity by varying the coupling JC–C between the two carbons. If JC–C = 0 (Fig. 4D), the doubly labeled and singly labeled spectra are identical, and inspection of the energy levels shows that the carbon singlet is not an eigenstate: The two different scalar couplings readily connect this state to others with the same overall symmetry, but with (αβ + βα) as the carbon component. However, if JC–C is much larger than all other couplings (Fig. 4E), the spectrum changes dramatically. It collapses back into a septet, similar to the acetone spectrum, except that the splitting is not a real coupling; rather, it is the 2.65-Hz average of the couplings between the near and far methyl groups. This is an example of a “deceptively simple spectrum” (25–27). It can be explored by exact calculations, which readily show that the spectrum comes entirely from transitions involving αβ + βα as a carbon state, which is delocalized over the two carbons and is coupled equally to each hydrogen. The αβ − βα state is a disconnected eigenstate; hence, it is long-lived.
Smaller spin systems that would have similar properties (e.g., the case of two coupled pairs of chemically equivalent spins) (28) were solved analytically decades ago, and it would have been possible to draw the same conclusions about disconnected eigenstates from those analytic solutions. For example, consider 2,3-13C diacetylene (H–C≡13C–13C≡C–H). This would be called an AA′XX′ system in standard NMR parlance, where A and X stand for the two different NMR-active nuclei (here 13C and H) and the primes indicate that there are two different couplings between A and X. We expect 12 lines associated with the carbon (A) transitions, not 1, and the singlet state is generally mixed with many other states. Careful inspection of (28) shows that the critical parameters are the ratios (J ± J′)/(JA–A − JX–X), where J and J′ are the two different A–X couplings. Here this ratio would be small, and the exact result (or a simple perturbation theory analysis) would show the worst overlap of a carbon singlet state with a true spin eigenstate to be {1 − 0.25[(J − J′)/(JA–A − JX–X)]2}. We then expect the singlet lifetime lengthening to be on the order of [(JA–A − JX–X)/(J − J′)]2. However, the importance of disconnected eigenstates was apparently not appreciated, in part because, in the absence of hyperpolarization and a mechanism to populate the state, the associated state has no obvious applications.
The perturbation calculation is easily extended to diacetyl, where the ratio (J ± J′)/(JA–A − JX–X) is also small (the minus sign gives the larger value in cases such as ours, where the couplings have opposite signs). Although the spectra in Fig. 4, C and F, are quite complex, assuming the couplings have the same value as in the hydrate (which gives the excellent spectral fit in Fig. 4F) shows that the overlap of the singlet state with an eigenstate is better than 0.96. This result is also readily verified by precise numerical analysis of this eight-spin system, and thus we predict more than an order-of-magnitude lengthening of the spin lifetime. In effect, the strong coupling between the two carbons quenches communication with other spins—virtually all the spectral complexity comes from the other three carbon states, and singlet-to-triplet interconversion is slow. Of course, perdeuteration dramatically reduces even this limited singlet-triplet mixing and further increases the lifetime.
The advantage of the perturbation theory calculation is that it lets us discuss the generic case. The generalization is more subtle than one might expect. The common case of magnetic equivalence [where the two spins have the same resonance frequency and each of the two spins is coupled identically to every other spin (12)] does not necessarily produce a disconnected eigenstate. For example, any two of the spins in a freely rotating methyl group are magnetically equivalent, but the energy level diagram for three equivalent spins produces no fully disconnected states, so the immunity to environmental perturbations is not present. The only possible disconnected eigenstate for two spins is the singlet; for a larger even number of spins with enough symmetry [e.g., benzene (29)] other disconnected states exist, although they might be difficult to access in practice.
The critical constraint for producing a disconnected eigenstate is that the coupling between two spins substantially exceeds both the couplings to other spins and the resonance frequency difference between the spins. Systems of interest as hyperpolarized contrast agents have two nearby H, 13C, 15N, 19F, or 31P atoms that satisfy this constraint. They have a precursor where the two atoms are inequivalent (hence permitting hyperpolarization of the α1β2 population), which can be converted to the contrast agent by chemical manipulation in a time that is short compared with the normal T1. Finally, they have a biological pathway that again makes them inequivalent, permitting detection of the hyperpolarization. Diacetyl in vivo satisfies these conditions. Partition in vivo between hydrophobic and hydrophilic phases would modulate the exchange rate (drastically reducing the water concentration and, hence, lengthening the time to convert from singlet); even ignoring hydration, the first metabolite is acetoin with inequivalent carbons. More generally, the simplest case is two equivalent adjacent carbons or nitrogens without directly bonded hydrogens, as in diacetyl, naphthalene, and oxolin (an antiviral compound) or in many derivatives of pyridazine or phthalazine, which have recently been shown to have vascular endothelial growth factor receptor–2 inhibitory activity (30). In other cases, deuteration can essentially eliminate the coupling to outside nuclei, as could very weak irradiation (far less than is necessary when the spin systems differ in their chemical shift frequency). At moderate fields, even molecules with not-quite-equivalent spins (such as the 3,4-13C versions of L-dopa or dopa-mine) might be usable, as the degradation pathway leads to compounds such as HVA with substantial asymmetry.
Acknowledgments
This work was supported by the NIH under grant EB02122 and by the North Carolina Biotechnology Center. We thank D. Bhattacharyya for his help in determining optimal conditions to hyperpolarize diacetyl; M. Jenista for help with calculations; T. Ribiero for his assistance in running NMR spectra; L. Bouchard for discussions of singlet character in strongly coupled systems; and S. Craig, E. Toone, D. Coltart, and M. S. Warren for particularly useful discussions on the chemistry of these compounds. A provisional patent has been submitted on this work by W.S.W. and Duke University.
References and Notes
- 1.MacFall JR, et al. Radiology. 1996;200:553. doi: 10.1148/radiology.200.2.8685356. [DOI] [PubMed] [Google Scholar]
- 2.Bowers CR, Weitekamp DP. J Am Chem Soc. 1987;109:5541. doi: 10.1021/ja010572z. [DOI] [PubMed] [Google Scholar]
- 3.Natterer J, Bargon J. 1997;31:293. doi: 10.1006/jmre.1998.1421. [DOI] [PubMed] [Google Scholar]
- 4.Golman K, et al. Magn Reson Med. 2001;46:1. doi: 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- 5.Abragam A, Goldman M. Rep Prog Phys. 1978;41:395. [Google Scholar]
- 6.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Proc Natl Acad Sci USA. 2003;100:10435. doi: 10.1073/pnas.1733836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall DA, et al. Science. 1997;276:930. doi: 10.1126/science.276.5314.930. [DOI] [PubMed] [Google Scholar]
- 8.Kurhanewicz J, Bok R, Nelson SJ, Vigneron DB. J Nucl Med. 2008;49:341. doi: 10.2967/jnumed.107.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golman K, et al. Cancer Res. 2006;66:10855. doi: 10.1158/0008-5472.CAN-06-2564. [DOI] [PubMed] [Google Scholar]
- 10.Merritt ME, et al. Proc Natl Acad Sci USA. 2007;104:19773. doi: 10.1073/pnas.0706235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day IJ, Mitchell JC, Snowden MJ, Davis AL. Magn Reson Chem. 2007;45:1018. doi: 10.1002/mrc.2090. [DOI] [PubMed] [Google Scholar]
- 12.Gabellieri C, et al. J Am Chem Soc. 2008;130:4598. doi: 10.1021/ja8001293. [DOI] [PubMed] [Google Scholar]
- 13.McCarney ER, Armstrong BL, Lingwood MD, Han S. Proc Natl Acad Sci USA. 2007;104:1754. doi: 10.1073/pnas.0610540104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The nomenclature used here is explained more fully, with specific examples, in textbooks such as (15).
- 15.Becker ED. High Resolution NMR: Theory and Chemical Applications. Academic; San Diego: 2000. pp. 171–175. [Google Scholar]
- 16.Carravetta M, Johannessen OG, Levitt MH. Phys Rev Lett. 2004;92:153003. doi: 10.1103/PhysRevLett.92.153003. [DOI] [PubMed] [Google Scholar]
- 17.Carravetta M, Levitt MH. J Chem Phys. 2005;122:214505. doi: 10.1063/1.1893983. [DOI] [PubMed] [Google Scholar]
- 18.Ahuja P, Sarkar R, Vasos PR, Bodenhausen G. J Chem Phys. 2007;127:134112. doi: 10.1063/1.2778429. [DOI] [PubMed] [Google Scholar]
- 19.del Campo G, Carmen Lajo MC. Analyst (London) 1992;117:1343. [Google Scholar]
- 20.See http://seattlepi.nwsource.com/dayart/20071221/DiacetylProducts2.pdf for a study done by the Seattle Post-Intelligencer in December 2007.
- 21.van Rooy FGBGJ. Am J Respir Crit Care Med. 2007;176:498. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- 22.Bell RP. Adv Phys Org Chem. 1966;4:1. and references therein. [Google Scholar]
- 23.Hoecker WH, Hammer BW. J Dairy Sci. 1942;25:175. [Google Scholar]
- 24.Jakobsen JÅ, et al. Eur Radiol. 2005;15:941. doi: 10.1007/s00330-004-2601-0. [DOI] [PubMed] [Google Scholar]
- 25.Abraham RJ, Bernstein HJ. Can J Chem. 1961;39:216. [Google Scholar]
- 26.Anet FAL. Can J Chem. 1961;39:2262. [Google Scholar]
- 27.Musher JI, Corey EJ. Tetrahedron. 1962;18:791. [Google Scholar]
- 28.Pople JA, Schneider WG, Bernstein HJ. Can J Chem. 1957;35:1060. [Google Scholar]
- 29.Saupe A. Z Naturforsch. 1965;20a:572. [Google Scholar]
- 30.Kiselyov AS, Semenov VV, Milligan D. Chem Biol Drug Des. 2006;68:308. doi: 10.1111/j.1747-0285.2006.00456.x. [DOI] [PubMed] [Google Scholar]