Abstract
Objective
To assess the effect of Osmotic-Release Oral System (OROS) methylphenidate (MPH) on a variety of measures evaluating academic performance, cognition, and social behavior in children with attention-deficit/hyperactivity disorder (ADHD).
Methods
This double-blind, randomized, placebo-controlled, crossover laboratory school study enrolled 78 children aged 9–12 years with ADHD who responded to OROS MPH. After determining individualized OROS MPH dosing (18–54 mg/day), 71 subjects received blinded treatment (OROS MPH or placebo then vice versa) on each of 2 laboratory school days, separated by 1 week. Primary efficacy was measured by Permanent Product Measure of Performance at 4 hours after study drug administration.
Results
Treatment with OROS MPH resulted in statistically significant improvement in Permanent Product Measure of Performance and Swanson, Kotkin, Agler, M-Flynn, and Pelham scores, measures of response time, and of working memory compared to placebo. Other measures did not meet all pre-established criteria for significance (maintenance of the overall type I error rate at 5%). Adverse events were consistent with previous reports of stimulant medications used in the management of ADHD. There were no discontinuations due to adverse events, and no serious adverse events or deaths.
Conclusions
OROS MPH dosed to reduce core symptoms of ADHD to within the normal range also improved performance on a variety of academic tasks in school-aged children compared to placebo. Adverse effects reported were consistent with prior studies.
Clinical Trial Registry Information
Double-Blind, Randomized, Placebo-Controlled, Crossover Study Evaluating the Academic, Behavioral and Cognitive Effects of Concerta on Older Children with ADHD, URL: http://clinicaltrials.gov/ct2/show/NCT00799409, unique identifier: NCT00799409.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common psychiatric disorder of childhood, affecting 8% to 10% of school-aged children (American Psychiatric Association 2000). Features of inattention and hyperactivity/impulsivity present before 7 years of age and lead to impairment across multiple settings, including academic and social situations (American Psychiatric Association 2000). Children with ADHD frequently display academic problems, with estimated rates between 25% and 40% (Dykman and Ackerman 1991; Semrud-Clikeman et al. 1992; Faraone et al. 2001; DeShazo et al. 2002; Loe and Feldman 2007). Behavioral problems that affect relationships with peers, family members, and teachers contribute to these difficulties, as do learning disabilities (LD): ∼30% of children with ADHD have comorbid LD in reading or math (Faraone et al. 2001). For the purpose of simplicity, the term “LD” is used throughout this article without distinction between this traditionally legal term and the purely diagnostic term, learning disorder (American Psychiatric Association 2000). Despite the association of LD with ADHD, clinical trial measures of performance in children with ADHD typically relate to effort and symptoms of the disorder rather than academic skills.
Specific academic difficulties noted in children with ADHD include slower reading fluency (Ghelani et al. 2004), weaker reading comprehension (Ghelani et al. 2004), and poor penmanship (Racine et al. 2008). Mastery of academic skills can also be hampered by the secondary effects (impulsivity, inattention, and disorganization) that ADHD can have upon a child's ability to practice newly learned skills or study recently presented material in the homework setting.
One of the issues facing researchers investigating these treatments is establishing a target for ADHD symptom improvement that will generalize to clinical gains that are meaningful to patients and their families. Achieving a 25% to 30% improvement in symptom severity on parent-, clinician-, or teacher-rated symptom checklists is generally considered a positive response; however, this level of symptom reduction may still leave some children with clinically significant impairment. A recent proposal suggested that optimal treatment (remission) is achieved by lowering ADHD symptom scores to within the range of those of typically developing children (Steele et al. 2006). The present inclusion of such an analysis of the goals of medication titration is important to better understand the clinical interpretation of treatment effects.
The laboratory school setting provides a simulation of the classroom environment, including potential for interaction and distraction among subjects. Such studies typically use objective and subjective measures of performance for comparing effects of treatment to those of placebo. The common training and experience of the laboratory schools staff offers consistency of evaluation compared to independent evaluations by a teacher in each subject's community school, an advantage particularly for subjective measures. The setting allows accurate timing of measurements in relationship to when the study medication was taken (Wigal and Wigal 2006).
The laboratory school as a setting has provided dosing, delivery, and safety profile information for novel stimulant and nonstimulant medications and, in the present study, allows the development of a more comprehensive understanding of the effects of Osmotic-Release Oral System (OROS) methylphenidate (MPH) HCl extended-release tablets (Concerta® CII, McNeil Pediatrics™, Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.) on the academic, behavioral, and cognitive difficulties experienced by older school-aged children with ADHD. It incorporates assessments commonly used in clinical trials of ADHD, such as ratings by parents and teachers, persistent effort on grade-level math tests, and observer ratings of attention and deportment. In this study, we seek to capitalize upon the strengths of the laboratory school design, which has contributed to the clinical understanding of medicines approved for the treatment of ADHD (Swanson et al. 2000, 2002; McCracken et al. 2003; Wigal et al. 2003, 2006, 2009).
This study also extends the scope of inquiry to include standardized measures of core cognitive deficits associated with ADHD (sustaining attention and inhibiting impulsive responses), reading proficiency (reading fluency and reading comprehension), and handwriting. In addition, we examine the child's ability to complete a set of homework-like tasks around the time of day that many children would be expected to complete such tasks. Thus, this investigation includes a broader range of cognitive and academically oriented measures within a laboratory school context than previously published.
Eligibility criteria for this study allowed for evaluation of a heterogeneous group. OROS MPH is an extended-release MPH formulation, designed to provide a 12-hour duration of effect allowing coverage throughout the hours during which children are typically at school and early evening, when they are working on their homework or in an afterschool setting (Pelham et al. 2001). Previous studies have already demonstrated the effect of OROS MPH upon the behavior of children with ADHD as assessed by teacher ratings of behavior and upon a child's ability to persistently put forth effort on a grade-level math test (Swanson et al. 2004; McGough et al. 2006).
The objective of this study was to expand on previously demonstrated effects of OROS MPH by further evaluating the effect of OROS MPH versus placebo on a variety of measures assessing academic performance using The Permanent Product Measure of Performance (PERMP) as the primary outcome measure, with measures of cognition and social behavior as secondary outcomes in 9- to 12-year-old children with ADHD who responded to OROS MPH.
Methods
This randomized, double-blind, within-subject, crossover-design laboratory school study of OROS MPH was conducted at the University of California, Irvine, California, and Bayou City Research, Houston, Texas. Both sites had extensive experience in the conduct of laboratory school studies. Study staff, including clinical and activity teachers, behavioral raters, and study coordinators, along with mental health professionals, participated in training sessions for this study during a 2-day investigator meeting, and each laboratory school cohort included a laboratory school practice day, allowing staff additional opportunities to practice the administration and scoring of most of the measures (Swanson et al. 2000; Wigal and Wigal 2006).
Nine- to 12-year-old children with symptoms of ADHD, with and without prior formal ADHD diagnosis, were recruited from December 2008 through April 2009 from area pediatric and psychiatric practices, and from the surrounding communities. All study activities were performed in accordance with the principles of the International Conference on Harmonization Good Clinical Practice, including written consent and Authorization of Release of Personal Health Information for Research Purposes form (for Health Insurance Portability and Accountability Act compliance) from a parent or legally authorized representative, as well as written assent from the subject when it was the policy of the institutional review board.
Study participants
This laboratory school study enrolled children aged 9 to 12 years with ADHD to evaluate an individually determined dose of OROS MPH (18, 36, or 54 mg/day) in a setting where it was possible to ensure exact timing of assessments for treatment effect (Wigal and Wigal 2006). Children were included in the study if they satisfied Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision criteria for a primary diagnosis of any of the three subtypes of ADHD (American Psychiatric Association 2000). The diagnosis of ADHD was based on semistructured interviews with parents (Kaufman Revised Schedule for Affective Disorders and Schizophrenia for School Age Children: Present and Lifetime Version [K-SADS-PL]) (Kaufman et al. 1997). Subjects also were required to have a baseline ADHD Rating Scale–IV (ADHD-RS-IV) score in the 90th percentile or greater relative to the general population of children of the same age and gender (DuPaul et al. 1998). Subjects receiving medication to treat their ADHD at the time of study enrollment exhibited an inadequate response to their then-current stimulant dose and completed a washout equivalent to 5 half-lives of the given medication before completing baseline assessments. Additional requirements included attendance of regular school and the ability to read and understand English.
Participants were excluded for a history or current diagnosis of epilepsy, severe anxiety, conduct, or psychotic disorders. In addition, pervasive developmental, eating, obsessive compulsive, sleep, major depressive, bipolar, chronic tic, or disorders. Tourette's disorders, as determined by intake psychosocial history and supported by the K-SADS-PL clinical interview. Known cardiac structural abnormalities; clinically significant abnormalities in electrocardiogram results; family history of sudden death or ventricular arrhythmia; or inability to take or tolerate OROS MPH were also excluding factors. Other key exclusion criteria were allergies to MPH or other ingredients of OROS MPH; known gastrointestinal narrowing or significant gastrointestinal problems; glaucoma; use of medication with central nervous system effects (excluding bronchodilators); or clinically significant laboratory and electrocardiogram abnormalities and blood pressure in the 95th percentile or greater for age, gender, and height. Study participants were prohibited from using any caffeine-containing products on study visit days or laboratory assessment days and were limited to 1 (12 ounces) caffeinated beverage a day during study participation.
In this study, we chose to include and specifically identify children with LD in reading and math. In this study, learning disabled children were defined by lower-than-expected achievement scores in the absence of mental retardation, a grouping criterion that has demonstrated utility in differentiating groups in a valid manner according to both test performances and academic achievement (Fletcher et al. 2007). For the purposes of this study, the terms LD, reading disability (RD), and mathematics disability (MD) are used to provide readers with common terminology for subjects with lower-than-expected achievement scores on the screening measures.
Participants were screened for RD, MD, and low intelligence. Participants were classified in the moderate to mild RD category if the Oral Reading Fluency Composite of the Gray Oral Reading Test-4 (GORT) and/or Elision subtest scores of the Comprehensive Test of Phonological Processing (CTOPP) were between 5 and 7, inclusive (i.e., between 1 and 2 standard deviations less than mean scores for age). Similarly, moderate to mild MD was assessed if the standard score on the Numerical Operations subtest of the Weschler Individual Achievement Test (WIAT-II AB) was between 71 and 85 (i.e., between 1 and 2 standard deviations less than the mean). Subjects with an estimated full-scale intelligence quotient (IQ) <80, as measured by the Wechsler Abbreviated Scale of Intelligence, were excluded (Wechsler 1999) from participation in the study. Participants were excluded if their scores were two or more standard deviations less than mean scores for age on the GORT, CTOPP, or WIAT-II AB, as these scores were considered in the severe range (Wagner et al. 1999; Wechsler 2001; Wiederholt and Bryant 2001).
Study design
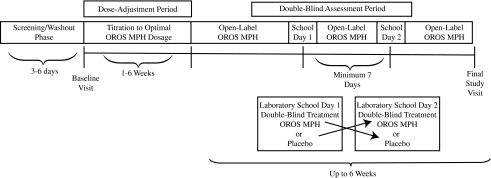
Subjects were permitted up to 28 days for screening/washout and then participated in a baseline/qualification visit, followed by an open-label phase with OROS MPH dosage initiation starting at 18 mg/day with permitted increases of 18-mg/day increments at clinic visits every 3 to 7 days for up to 6 weeks, allowing ample time to achieve a clinical response with optimal dosage up to a maximum of 54 mg/day by day 42 (the laboratory school practice day). After dose optimization, the double-blind assessment period began and included two laboratory school assessment days, with each laboratory school day separated by at least 7 days. Subjects received double-blind treatment on the laboratory school visit days and open-label OROS MPH on the nonlaboratory school days. The final study visit occurred within 2 weeks of completing the last of two laboratory school visits (Fig. 1).
FIG. 1.
Study design. MPH = methylphenidate; OROS = Osmotic-Release Oral System.
All subjects initiated treatment with open-label OROS MPH 18 mg in the morning and received an increased dose until meeting one of the following criteria: (1) ADHD-RS-IV Home Version total and subscale scores less than or equal to the 75th percentile based on gender and age norms and a Clinical Global Impressions–Improvement rating score of “very much improved” or “much improved”; Clinical Global Impressions–Improvement was assessed as a dose-finding measure and not as a measure of treatment response. (2) an adverse event (AE) profile that was not tolerable; or (3) a daily dose reaching 54 mg, the maximum approved dose of OROS MPH for this age group. In the standardization of the ADHD-RS-IV, scores greater than or equal to the 80th percentile clearly discriminated children with ADHD from their peers given age and gender (DuPaul et al. 1998). Scores less than or equal to the 75th percentile were the targeted treatment goal because this was felt to be the range in which children with ADHD would exhibit minimal symptoms and be indistinguishable from their peers without ADHD.
One decrease in dosage by 18 mg during the dose adjustment period was permitted to assure tolerability. Up to 6 weeks were allowed for the dose-adjustment period, including a practice session designed to familiarize subjects with the procedures of the laboratory school study days. Cohorts ranging in size from 7 to 12 subjects were scheduled for two laboratory school days (subsequent Saturday sessions if possible) separated by at least 7 days.
Administration of study drug
In the crossover design, subjects who completed both laboratory school assessments served as their own control and provided data for both OROS MPH and placebo; this design reduces potential for an unequal distribution in a relatively small sample to favor either treatment over the other. Subjects were randomized to 1 of 2 treatment sequences (OROS MPH followed by placebo or vice versa) based on a computer-generated randomization schedule prepared by the sponsor before the study. The double-blind treatments were identical in appearance and were packaged in bulk supplies from which all subjects were dispensed tablets for use. Each site had an unblinded pharmacist who administered the appropriate study medication according to the randomization sequence to maintain blinding of the investigators and subjects throughout the study.
AEs were recorded from the signing of consent until the last study procedure was completed. Serious AEs are defined as any untoward medical occurrence that is life-threatening, requires hospitalization or prolongation of hospitalization, results in persistent or significant disability/incapacity, is a congenital anomaly or birth defect, or results in death. Additional safety evaluations included vital signs and body weight measurements at each visit. Parents were re-interviewed using the K-SADS-PL, including the section on suicidal ideation, at the final visit after the subject's last dose of study drug to collect information on any ongoing or new AEs, serious AEs, and concomitant medications.
Outcome measures
Permanent Product Measure of Performance
The PERMP is a math test that measures effortful performance without a learning curve (Wigal and Wigal 2006). The test determines the number of problems attempted and the number of problems correctly answered. In this study, the primary efficacy measure was a PERMP evaluation done 4 hours after taking study medication, a time selected a priori to coincide with the known pharmacodynamic effects based on prior research and to minimize the impact of repeated measures on adjustment of multiplicity (Wigal et al. 1998; Pelham et al. 2001). A secondary efficacy measure was the determination of the time course of treatment effect for OROS MPH compared to placebo, based on PERMP evaluations performed 30 minutes before and at 1, 2, 4, 10, 11, and 12.5 hours after taking study medication. Data on the time course are not reported in this article but rather will be presented separately.
Swanson, Kotkin, Agler, M-Flynn, and Pelham Scales of Attention and Deportment
Behavioral manifestations of ADHD are based on subjective assessment using the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) scales of attention and deportment, which can also be combined into a composite score (Wigal et al. 1998; Wigal and Wigal 2006). The SKAMP has become a standard measure in laboratory school studies for the assessment of medication treatment effects on classroom behaviors and attention to tasks in children with ADHD. Its reliability and validity has been repeatedly demonstrated in the analog classroom. In this study, the SKAMP was administered during both laboratory school days on the same schedule as the PERMP. This article presents the results of the SKAMP evaluation at 4 hours, and the time course will be presented separately.
The Test of Variables of Attention
The Test of Variables of Attention (TOVA) is a computerized, visual, continuous-performance test that provides an objective assessment of sustained attention and impulsivity (Baren and Swanson 1996; Leark et al. 2007). Subtests were first presented on the practice day and then administered on the laboratory school days. The TOVA has been demonstrated to be sensitive to the effects of stimulant medication (Huang et al. 2007). The test is administered individually, with the child instructed to respond only when the target stimulus appears on the screen. Targets are present on 22.5% of trials during the first half of the test and 77.5% of the trials during the last half of the test (Forbes 1998). In this way, the first half of the test requires sustained attention, whereas the second half requires an inhibition of a response to a nontarget (Schatz et al. 2001). The variables measured are omissions (number of targets that were missed), commissions (number of responses to nontargets), response time (mean response latency measured in milliseconds), response time variability (the standard deviation of response times), the number of multiple responses (number of stimuli to which the subject responded more than once), and the number of anticipatory responses (very short latency responses that probably represent guessing) (Forbes 1998).
Finger Windows subtest of the Wide Range Assessment of Memory and Learning
The Finger Windows subtest of the Wide Range Assessment of Memory and Learning and a modified backward version were administered to each child individually once on each of the laboratory school study days to assess visual working memory. The Wide Range Assessment of Memory and Learning consists of nine subtests designed to evaluate a child's ability to learn and memorize information. The Finger Windows subtest requires the child to watch the examiner point to a longer and longer series of windows on a card and then reproduce the sequence exactly. On the backward version of the test developed by Bedard et al. (2007), children are asked to reverse the sequence shown to them by the examiner. The forward and backward trials are scored separately. The forward version of the task assesses storage in visuospatial working memory, whereas the backward version adds the requirement for manipulation of the visuospatial information in working memory (Bedard et al. 2007). One point is given for each correctly recalled sequence, and each test is discontinued after 3 consecutive errors. The total possible number of correct trials is 28 (Bedard and Tannock 2008).
Digit Span subtest of the Wechsler Intelligence Scale for Children (third edition)
The Digit Span subtest of the Wechsler Intelligence Scale for Children (third edition) was administered to all subjects to assess the impact of OROS MPH on auditory working memory. Several studies have found that children with ADHD have deficits in multiple components of working memory (Hechtman et al. 2004; Martinussen et al. 2005; Castellanos et al. 2006; Flake et al. 2007). The ability to recall strings of digits verbatim and in reverse order has been widely used both clinically and empirically to examine verbal working memory. Bedard and colleagues (2007) investigated the utility of the Digit Span subtest in clarifying the nature of this deficit in children found to have ADHD, and have quantified the effects of treatment with stimulant medication. For this task, each child individually was given a sequence of numbers orally and asked to repeat them, in order as heard or in reverse order.
Test of Handwriting Skills, Revised
The Test of Handwriting Skills, Revised (THS-R), is a standardized, untimed assessment used to evaluate optimal handwriting following dictation that was included to assess the impact of MPH on handwriting (Milone 2007). The test was administered in a group only once during each laboratory school day. The THS-R has been validated and it has been demonstrated that students with confirmed ADHD scored significantly lower than children of the same age without a diagnosis of ADHD. Consistent with the validation in children with ADHD, the children were directed to respond to test items in cursive writing only using a pen.
Dynamic Indicators of Basic Early Iiteracy Skills
The Dynamic Indicators of Basic Early Literacy Skills (DIBELS) was administered to determine the impact of OROS MPH on reading rate and accuracy. The DIBELS consists of a set of standardized, individually administered measures of early literacy development. The responses are designed to be short (1-minute) fluency measures used to regularly monitor the development of reading skills. These measures were developed based on essential domains discussed in the National Reading Panel 2000 reports (National Institute of Child Health and Human Development 2000) to assess student development of phonological awareness, alphabetic understanding, and automaticity and fluency with the code. Each measure has been researched thoroughly and demonstrated to be a reliable and valid indicator of early literacy development and predictive of later reading proficiency to aid in the early identification of students who are not progressing as expected (Good and Kaminski 2002). Considering the ages of children assessed in this study, only the paragraph fluency component of the DIBELS was used. The DIBELS (up to 6th grade level) was individually administered once each laboratory school day. Subjects who were above the 6th grade level still were tested at the 6th grade level. Subjects read three stories on each laboratory school day as determined by their GORT fluency grade level at screening and completed the parallel forms. Thus, each subject who completed all study days read a total of nine unique stories. The DIBELS Oral Reading Fluency Total, the total number of words minus errors, was scored using the scoring manual for the DIBELS (Good and Kaminski 2002). DIBELS Oral Reading Fluency scores were not converted to percentiles in this analysis, and no norms were used, as each child was compared against himself or herself. Age, time of year, actual grade level, and gender were not taken into account. As such, only qualified statements about statistical significance or actual reading fluency are made. Additional analyses are expected to be performed and presented in later articles.
Gray Silent Reading Test
The Gray Silent Reading Test (GSRT) was administered to determine the effect of OROS MPH on reading comprehension. The GSRT was administered in the laboratory school group setting per the published instructions for group administration (Wiederholt and Bryant 2001).
Schoolwork productivity during afterschool hours
To assess ability to perform late in the afternoon, a simulated homework activity packet was administered on each laboratory school day, with 30 minutes given for completion. An abbreviated version was used for practice during the practice visit. During this session, children were asked to complete assignments in their packet. The homework session was designed to assess each child's ability to listen to instructions, organize materials, and initiate and complete tasks during the time point in the day when children might be likely to complete homework assignments in their homes or after-school programs. Tasks in the packet included short stories with questions on the content, base words or vocabulary assessments, alphabetization, homophones or multiple meanings, sentence completion in fill-in-the-blank format, word search, and message decoding. Materials were provided on individual clipboards located with written instructions for each child. A coordinator for the homework session provided instructions aloud, after which children picked up and began work on their packets when they chose to initiate, as is typical of afterschool child-care settings that permit homework completion. Observers recorded the time that each child began the assignment, the time of completion, and the number of times each child left his or her seat. Scoring also included number of items attempted, number of items correct, and totals completed.
In many laboratory school studies, efficacy measurements would be repeated to represent cycles of pharmacodynamic effects as they relate to study drug (Swanson et al. 2000; Wigal et al. 2006). Due to the large number of efficacy measures included in this study, investigators scheduled exploratory assessments during the time period when maximum drug effects would be expected based on previously conducted pharmacodynamic and pharmacokinetic studies (Pelham et al. 2001; Swanson et al. 2003). During the laboratory school day, there was also a need to balance the large number of academic measurements in this study with other components of the day, including meals and fun/recess activities, within a prolonged evaluation day in a young age group. For these reasons, all components of the TOVA, the Finger Windows subtests, the Digit Span subtests, the THS-R, DIBELS, and grammar test were done during the period 3 to 7.5 hours after taking study medication. In the period 8 to 10 hours after taking study medication, the GSRT was administered, followed by the exercises in the homework simulation activity packet. The simulated homework tasks were scheduled to occur at a time corresponding to the time period when homework would typically be completed in an afterschool setting or in the home.
Statistical analyses
Power and design
A review of multiple laboratory school studies suggested that a mean point difference of 28 points on the number of PERMP questions attempted (PERMP-A) would sufficiently demonstrate a clinically relevant difference in treatment conditions (Pelham et al. 2001; McGough et al. 2006). Based on a power analysis that assumed a mean difference of 28 points on the PERMP-A between the OROS MPH (least squares mean = 114) and placebo (least squares mean = 86) groups with an associated standard deviation of 69 points, type I error of 5%, and 90% power, 33 randomized subjects were required for each sequence (i.e., a total of 66 subjects). Allowing for 10% unusable data (violation of Intent-To-Treat [ITT] definition), the number of randomized subjects required per sequence was increased to 37 (total ∼75 randomized in both sequences combined).
The ITT analysis set was defined as all randomized subjects who received at least 1 dose of the study medication and had efficacy data in a randomized sequence. All efficacy analyses were done using the ITT analysis set. The safety analysis set was defined as all subjects who took at least 1 dose of study medication and for whom safety data were available.
Subject disposition in this study tabulated the number of subjects who (1) entered into the open-label phase; (2) took study drug; (3) discontinued treatment, including their reason; (4) were randomized into each treatment sequence; (5) were in the safety analysis set; and (6) were in the efficacy analysis set. A descriptive summary was performed for demographic (e.g., age, gender, body mass index, and race) and baseline characteristics (e.g., depression, LD) by providing the number, mean, standard deviation, minimum, and maximum for each continuous variable and frequencies and percents for each categorical variable.
Efficacy measures
The primary academic outcomes were based on the PERMP, specifically PERMP-A and the number of correct (PERMP-C) math problems scored 4 hours postdose. A higher PERMP score indicates better performance. PERMP-A scores at 4 hours were tested by using appropriate contrasts in repeated-measures mixed model with terms for time, treatment, and time-by-treatment interaction, subject, and period. Subjects were considered a random effect nestled within the sequence. The test for sequence effect in the model was tested for carryover effect. This analysis was done for subjects in the ITT analysis set. A similar analysis was done for the PERMP-C, SKAMP-Deportment, SKAMP-Attention, and SKAMP-Composite scores. All other endpoints were analyzed by using a general linear mixed-model approach, as these efficacy measures were administered only once during each laboratory school day. The following independent (fixed) variables were assessed: Treatment, period, and sequence; subject was a random effect nestled within the sequence. The test for sequence effect in the model tested for equal carryover effect. The primary efficacy endpoints, PERMP-A and PERMP-C, are analyzed using Hochberg's step-up multiple-comparison procedure, to maintain the overall type I error rate at a 0.05 level of significance. The study required that both hypotheses be rejected to be considered positive.
An additional 23 efficacy endpoints were tested using a fixed-sequence gatekeeper approach to maintain the overall type I error rate at 5% once the primary hypotheses had been rejected as previously described. With a fixed-sequence gatekeeper approach, a test is performed only if all the preceding tests were rejected. Testing of additional endpoints in the sequence may still be performed, but no unqualified statements about statistical significance may be posited.
The sequence for testing endpoints using the gatekeeper approach was determined in the final protocol, a priori. Because the sample size was determined based on experience with the PERMP, we recognized that adequate statistical power for each endpoint was not assured. Consequently, we based the order for testing these endpoints empirically, using our best estimates of the nominal p value that might be achieved for each, rather than using related content as the basis for the sequence of testing. The prespecified sequence is reproduced in the results tables, which are presented separately as results for the endpoints that fulfilled the gatekeeper criteria for maintaining type I error at the 0.05 level and for the endpoints that did not fulfill the gatekeeper criteria.
The prospective statistical analysis plan provided for efficacy and safety data obtained from this study (NCT00799409) to be combined with data from a second clinical study (NCT00799487) with an identical design; this article presents only the data from the NCT00799409 study.
Results
Subjects
A total of 78 children (70% male, 30% female) aged 9 to 12 years (mean, 10.1 years) diagnosed with ADHD enrolled in this study. The demographic characteristics of the patients in the 2 treatment sequences are compared in Table 1. The distribution by ADHD subtype showed that 19% were inattentive, 81% were combined, and none was hyperactive/impulsive. Of these subjects, 32% also presented with features of LD; no children met the criteria for anxiety or depressive disorders. The ADHD-RS-IV scores averaged 18.3 on the hyperactivity/impulsivity scale and 22.5 on the inattentive scale.
Table 1.
Demographic Data
| Demographic data | All patients (n = 78)a |
|---|---|
| Gender, n (%) | |
| Boys | 55 (70) |
| Girls | 23 (30) |
| Age (years) | |
| Mean | 10.1 |
| SD | 1.08 |
| Median | 10.0 |
| Range (minimum, maximum) | 9, 12 |
| ADHD subtype, n (%) | |
| Inattentive | 15 (19) |
| Hyperactive-impulsive | 0 |
| Combined | 63 (81) |
| Race, n (%) | |
| Black or African American | 22 (28) |
| White | 45 (58) |
| Other | 11 (14) |
| Body mass index | |
| Mean | 19.5 |
| SD | 4.998 |
| Median | 18.2 |
| Range (minimum, maximum) | 13.5, 44.9 |
| Concurrent diagnosis, n (%) | |
| Anxiety | 0 (0) |
| Depressive disorders | 0 (0) |
| Learning disability | 25 (32) |
| Baseline ADHD-RS-IV hyperactivity-impulsivity score | |
| Mean | 18.3 |
| SD | 6.46 |
| Median | 19 |
| Range (minimum, maximum) | 3, 27 |
| Baseline ADHD-RS-IV inattentive score | |
| Mean | 22.5 |
| SD | 3.26 |
| Median | 23 |
| Range (minimum, maximum) | 11, 27 |
| Distribution by OROS MPH dose,bn (%) | |
| 18 mg | 10 (12.8) |
| 36 mg | 34 (43.6) |
| 54 mg | 27 (34.6) |
Seven patients withdrew during dose adjustment.
Includes subjects from the Intent-To-Treat population (n = 71) for whom an optimized OROS MPH dose was reached.
ADHD = attention-deficit/hyperactivity disorder; ADHD-RS-IV = ADHD Rating Scale-IV; MPH = methylphenidate; OROS = Osmotic-Release Oral System.
The safety population included all 78 patients who enrolled in the study. The ITT population included 71 (91%) patients who completed dose adjustment and presented for randomization on their first day of laboratory school assessment with at least 1 dose of study medication and efficacy data in the randomized sequence; 92% of these patients had achieved an ADHD-RS-IV Home Version below the 75th percentile for age and gender, with 13% taking 18 mg/day, 49% taking 36 mg/day, and 38% taking 54 mg/day of study medication. Of the 71 patients, 35 were assigned the sequence of OROS MPH followed by placebo and 36 the sequence of placebo followed by OROS MPH on the laboratory school days.
Of the seven children who dropped out before randomization on the first laboratory school assessment day, two cited insufficient efficacy, one elected to withdraw, one was lost to follow-up, and three cited other reasons (one subject failed to follow the protocol; one did not attend the first laboratory school day; and the third subject was noncompliant with OROS MPH).
The two laboratory school assessments were done on successive Saturdays. Patients who were randomized took OROS MPH daily throughout the study (average duration 40 days) except for the one laboratory school day when placebo was assigned. None of the patients dropped out during the laboratory school assessment.
Efficacy
Results of double-blind efficacy endpoints assessed during the two laboratory school days are presented in Table 2, which includes results for the endpoints that fulfilled the gatekeeper criteria for maintaining type I error at the 0.05 level, and Table 3, which presents the results for the endpoints that did not fulfill the gatekeeper criteria.
Table 2.
Efficacy Outcomes for Academic and Behavioral Measures in Prespecified Rank Order During Double-Blind Laboratory School Study Days: Efficacy Endpoints That Fulfilled the Gatekeeper Criteria to Maintain Overall Type I Error at 0.05
| |
Least-squares mean (standard error) |
|
|
|
|
|---|---|---|---|---|---|
| Efficacy measures | Placebo | OROS MPH | Difference | p value | Cohen's effect sizea |
| Primary efficacy endpointsb | |||||
| PERMP-attempted | 74.9 (2.41) | 103.5 (2.41) | 28.6 | <0.0001 | 1.4 |
| PERMP-correct | 69.0 (2.32) | 97.3 (2.32) | 28.3 | <0.0001 | 1.5 |
| Secondary efficacy endpointsc | |||||
| SKAMP-deportment | 9.0 (0.57) | 3.1 (0.57) | 5.9 | <0.0001 | 1.2 |
| SKAMP-composite | 20.8 (0.88) | 9.8 (0.88) | 11.0 | <0.0001 | 1.5 |
| SKAMP-attention | 11.8 (0.56) | 6.7 (0.56) | 5.1 | <0.0001 | 1.1 |
| TOVA ADHD score | −4.62 (0.451) | −1.46 (0.446) | −3.16 | <0.0001 | 0.93 |
| TOVA reaction time | 73.67 (2.604) | 89.70 (2.586) | 16.03 | <0.0001 | 0.74 |
| TOVA reaction time variability | 62.69 (4.095) | 85.14 (4.076) | −22.45 | <0.0001 | 0.66 |
| Finger windows backward | 11.24 (0.463) | 12.17 (0.463) | −0.93 | 0.018 | 0.24 |
| Finger windows forward | 12.93 (0.454) | 14.25 (0.454) | −1.32 | 0.0057 | 0.35 |
Based on least-squares mean difference.
Primary efficacy endpoints analyzed using the Hochberg's step-up multiple-comparison procedure in order to maintain the overall type I error at a 0.05 significance level. PERMP results are based on testing 4 hours post dose.
Secondary endpoints analyzed and tested at the 5% significance level using the fixed-sequence gatekeeper approach. SKAMP results are based on testing 4 hours post dose.
PERMP = Permanent Product Measure of Performance; SKAMP = Swanson, Kotkin, Agler, M-Flynn, and Pelham; TOVA = Test of Variables of Attention.
Table 3.
Efficacy Outcomes for Academic and Behavioral Measures in Prespecified Rank Order During Double-Blind Laboratory School Study Days: Efficacy Endpoints That Did Not Fulfill Gatekeeper Criteria to Maintain Overall Type I Error at 0.05
| |
Least-squares mean (standard error) |
|
|
|
|
|---|---|---|---|---|---|
| Efficacy measures | Placebo | OROS MPH | Difference | p value | Cohen's effect sizea |
| TOVA Commissions subtest | 86.31 (4.084) | 92.61 (4.011) | −6.30 | 0.1091 | 0.19 |
| Digit Span backward | 5.10 (0.243) | 5.35 (0.243) | 0.25 | 0.2653 | 0.12 |
| Gray Silent Reading Test | 85.49 (2.522) | 91.98 (2.521) | −6.49 | 0.0038 | 0.31 |
| Test of Handwriting Skills, Revised | 93.56 (2.541) | 98.31 (2.541) | −4.75 | 0.0001 | 0.22 |
| Dynamic Indicators of Basic Early Literacy Skills ORF | 106.15 (4.179) | 111.91 (4.179) | −5.76 | 0.0092 | 0.16 |
| Digit Span forward (subtest of WISC) | 9.24 (0.213) | 9.25 (0.213) | −0.02 | 0.9195 | 0.01 |
| TOVA Omissions subtest | 24.58 (13.08) | 64.13 (12.896) | −39.55 | 0.0002 | 0.37 |
| Homework tasks | |||||
| Grammar task | 0.24 (0.023) | 0.33 (0.023) | −0.08 | 0.0002 | 0.45 |
| Short story with questions for comprehension | 0.61 (0.031) | 0.63 (0.031) | −0.02 | 0.4625 | 0.08 |
| Identify root word | 0.74 (0.037) | 0.76 (0.3054) | −0.02 | 0.6262 | 0.06 |
| Alphabetize list of words | 0.67 (0.042) | 0.67 (0.041) | 0.00 | 0.9980 | 0.00 |
| Identify multiple meanings for words | 0.69 (0.036) | 0.80 (0.035) | −0.10 | 0.0151 | 0.35 |
| Complete sentences using words from list provided | 0.65 (0.037) | 0.74 (0.037) | −0.09 | 0.0043 | 0.030 |
| Word search | 0.94 (0.019) | 0.98 (.019) | −0.04 | 0.0371 | 0.28 |
| Decode a mystery sentence | 0.96 (0.016) | 0.99 (0.015) | −0.03 | 0.0965 | 0.27 |
Based on least-squares mean difference.
ORF = Oral Reading Fluency; WISC = Wechsler Intelligence Scale for Children.
As shown in Table 2, OROS MPH significantly improved performance on the number of problems attempted and number of problems correctly answered on the PERMP at 4 hours postdose as well as on the inattention, deportment, and total behavior ratings of the SKAMP at 4 hours postdose (p < 0.0001 for all). The complete presentation of PERMP and SKAMP results from measurements collected at other time points will be presented in a separate publication.
In addition to improvement on these traditional laboratory school measures, children taking OROS MPH also obtained statistically significantly better scores than placebo-treated children on the ADHD, Reaction Time, and Reaction Time Variability scores of the TOVA (p < 0.0001 for all). Finally, OROS MPH significantly improved performance on tests of visual working memory as demonstrated on both the Finger Windows forward and backward subtests. Raw scores are presented for DIBELS, Digit Span, and Finger Windows subtests; these results are not based on age- or gender-related norms.
The fixed gatekeeper sequence was used in the statistical analysis of study results. Although the remaining endpoints were still tested, the next endpoint in the sequence failed to meet the overall type I error rate at the 0.05 level of significance. Thus, no unqualified statements about statistical significance can be made for these remaining endpoints (Table 3).
Adverse events
AEs are presented for all patients because the two treatment sequences differed only in the order in which a patient took placebo on either laboratory school day. A total of 39 subjects (50%) reported at least one treatment-emergent AE during the study. The types of AEs reported were consistent with those previously reported with the use of stimulant medications in the management of ADHD. The more commonly reported AEs (≥5%) in this study are reported in Table 4. There were no deaths or serious AEs, and no subject discontinued treatment because of an AE.
Table 4.
Adverse Events Reported by 5% or More of Subjects in Either Treatment Group
| |
|
Number of adverse events by severity ratinga |
||
|---|---|---|---|---|
| Description of adverse event (n = 78) | n (%) | Mild | Moderate | Severe |
| Decreased appetite | 20 (25.6) | 17 | 3 | 0 |
| Abdominal pain (upper) | 13 (16.7) | 11 | 2 | 0 |
| Headache | 13 (16.7) | 8 | 4 | 1 |
| Irritability | 12 (15.4) | 9 | 3 | 0 |
| Initial insomnia | 6 (7.7) | 3 | 2 | 1 |
| Nasal congestion | 4 (5.1) | 2 | 2 | 0 |
| Pyrexia (fever) | 4 (5.1) | 2 | 2 | 0 |
| Dizziness | 4 (5.1) | 4 | 0 | 0 |
If a subject reported the same event more than once, the subject is counted once at the maximum severity.
Discussion
This study assessed the effects of OROS MPH compared with placebo on the core symptoms of ADHD, as well as performance in a variety of skills thought to underlie aspects of academic performance in a group of older children with ADHD. As expected and documented in the research literature (Pelham et al. 2001; Swanson et al. 2003), the primary efficacy endpoints that the PERMP and SKAMP measured at 4 hours were statistically significant for the comparison between OROS MPH and placebo. These results demonstrate improvement in classroom behaviors, productivity, attention to task, and other core symptoms of ADHD with OROS MPH.
This study examined children with inattentive and combined subtypes of ADHD, as well as children with comorbid LDs, in numbers that appear consistent with those seen in other ADHD populations studied. For example, in the Multimodal Treatment Study, approximately one-third of the sample had comorbid anxiety, almost 40% had comorbid oppositional defiant disorder, and 6% had depression (The MTA Cooperative Group 1999). Whereas the MTA only included children with ADHD combined subtype, other researchers have reported that 50% to 60% of children with ADHD have combined subtype, <15% have hyperactive-impulsive subtype, and 20% to 30% demonstrate the inattentive subtype (Spencer et al. 2007). In our study, the majority of children (80.8%) demonstrated symptoms of combined inattention and hyperactivity/impulsivity, whereas a smaller proportion demonstrated predominantly inattentive symptoms (19.2%). Approximately one-third of the sample identified their race as black or African American (28.2%) and 17.9% identified their ethnicity as Hispanic or Latino. These numbers are greater than those historically seen in laboratory school studies. It is unclear whether this represents a general increase in diagnosis and participation in clinical trials for ADHD among racial and ethnic minorities, an isolated increase in this sample, a result of the specific geographic location of study sites (Los Angeles, CA; Houston, TX), or a combination of these and/or other factors.
One third of our sample (32.5%) had low achievement scores in word manipulation or phonologic processing, reading fluency, and/or numerical operations (i.e., the Elision subtest of the CTOPP, GORT fluency score, and/or numerical operations score on the WIAT-II-A, respectively), while demonstrating intellectual abilities within normal range (i.e., Wechsler Abbreviated Scale of Intelligence 4-subtest full-scale IQ score). Although the use of low achievement scores does not establish a formal diagnosis of LD, these findings are consistent with the prevalence of co-occurring ADHD and LD reported in the most recent national survey conducted by the Centers for Disease Control and Prevention (Pastor and Reuben 2008). Additional analyses of these results will be useful in better defining the effects of treatment with OROS MPH in the population of children with ADHD and comorbidities.
To our knowledge, this is the first use of this group of assessments in a large sample of children with ADHD to evaluate the effects of a stimulant on working memory and other cognitive skills thought to be related to academic success in a placebo-controlled laboratory school study. Our findings confirm those of Bedard et al. (2007) in documenting the selective effects of MPH on working memory in children with ADHD. Of note, this study also found statistically significant improvement in a task of visual working memory, specifically visual-spatial manipulation and storage (Finger Windows backward and forward, respectively), with OROS MPH compared with placebo. Although these same effects were not seen in the audio-verbal working memory domains (Digit Span backward and forward), it is meaningful that skills of visual working memory were improved, as they are thought to be more strongly related to reading development. These findings are particularly noteworthy, as the more complex working memory processes that improved with treatment are known to play a significant role in academic achievement (Gathercole et al. 2004).
Significant differences were demonstrated on several subtests of the TOVA (i.e., ADHD Score, Response Time, and Response Time Variability); the subjects' scores on the laboratory school day when they received OROS MPH were closer to those of children without ADHD than the scores on the day they received placebo. These findings are similar to those demonstrated in an earlier study (Huang et al. 2007), although the more robust results seen in this study may be due to differences in study design. For the present study, improvements shown in response time and variability of response time may represent improved consistency of response. It is unclear why there was no differentiation between OROS MPH and placebo conditions on TOVA commission errors, particularly given the large portion of children in this sample (80.8%) with ADHD combined subtype. As the Commissions score is meant to evaluate impulsivity, one would expect a group of children with impulsive symptoms to have higher scores. Finally, a robust response was seen in the OROS MPH group compared with placebo in the TOVA Omissions subtest evaluating sustained attention, although the assigned sequence of this measure in the gatekeeper analysis fell after a tested endpoint failed to be rejected. Although the results of these subtests cannot be generalized to other settings and treatment conditions, these findings may have implications for future assessments of treatment response and require further study. Additional analyses are planned using the pooled data from this study and its companion study and may provide more information on the use of this tool in the evaluation of treatment response.
The role of attention in academic work has not been definitively characterized, but an impact of ADHD on academic achievement has been documented (Barbaresi et al. 2006). Teacher ratings of attention problems in early childhood have been implicated in low math and reading achievement in late adolescence, even after adjusting for childhood IQ and family factors (Breslau 2009). Stimulant treatment has been associated with improved scores in reading in a large, population-based study (Barbaresi et al. 2007) and with improved written language usage, note-taking quality, homework completion, and accuracy on reading and math tasks (Elia et al. 1993; Evans et al. 2001; Vance et al. 2003). Although some of these measures failed to reach statistical significance in the predetermined sequence specified for the statistical analysis, this study demonstrated positive effects of OROS MPH compared with placebo in some domains noted to be critical to academic progress.
A robust effect was demonstrated on handwriting as measured by the THS-R with OROS MPH treatment compared with placebo. Although many children now use computers for school assignments, few schools are currently able to offer every child full-day use of a computer to complete all of their classroom tasks. Moreover, handwriting continues to be important for other communications. To our knowledge, this is the first evaluation of a large sample of children with ADHD using a validated tool to assess the impact of a stimulant medication on handwriting in a laboratory school setting.
Reading skills are a central component of academic success. In this study OROS MPH improved reading comprehension scores in comparison to placebo. Although the specific mechanisms associated with this improvement remain unknown, improved reading scores have also been documented in children with ADHD with extended use of MPH (Scheffler et al. 2009).
One-half of the sample experienced an AE (50%). Although the types of AEs experienced in this study are consistent with those previously reported with stimulant medication, the frequency of AEs reported in this study (Table 4) occurred at a higher incidence than has previously been observed. However, a formal assessment of causality has not yet been completed. This evaluation will assist in determining the potential role of dosing and other factors on the emergence of AEs in this study.
The severity of reported AEs is also an important consideration. Of the 76 AEs reported in this study, 56% were rated as mild, 18% were rated as moderate, and 2% were rated as severe in intensity. None of the subjects in the study cited an AE as the reason for dropping out of the study, no unexpected treatment-emergent AEs or serious AEs were reported during the study, and there were no deaths.
The results of this study should be viewed in the context of the potential limitations. First, only children demonstrating the requisite decrease in ADHD symptoms with OROS MPH within the labeled dosing range were included in the study. We accepted the possibility that nonresponders to MPH were not included, as our goal was to explore potential effects of OROS MPH, whose efficacy has already been well established. Children who may have required a dose >54 mg to achieve full therapeutic effect may have also been excluded.
There are several additional limitations to this study. Recognized limitations inherent in the highly controlled environment of the laboratory school setting include the following: The atypical structure of the school day; a class entirely composed of students found to have ADHD; the exclusion of nonaffected peers; and a smaller class size (20 or less pupils) than seen in traditional school programs.
The number of subjects planned for this study was based on experience with PERMP. There was limited information on the response and variability for some of the efficacy endpoints measured, and it was anticipated that the study might prove underpowered to achieve statistical significance for some of those endpoints. Several of the efficacy endpoints were piloted in this study and have not been previously validated. Thus, subjects may not have been representative of all endpoints to complete some of the tasks, and the tasks not yet established might not have been sensitive or reliable for the purposes of this study. The absence of a drug effect on some tasks is also a possibility. Finally, in developing the gatekeeper sequence, the order of endpoints was determined based on published data of prior use and our estimate of the likelihood of achieving a nominal p value ≤0.05. Future studies can make more accurate sample size determinations with the benefit of the results demonstrated in this study. Anticipating these limitations, the statistical analysis plan for this study provided for combining data from it with data from a concurrent companion study that had an identical design and that was conducted independently.
Conclusions
This clinical trial demonstrated efficacy and safety of OROS MPH in the treatment of 9- to 12-year-old children with ADHD, 32% of whom had LDs. The trial was unique in that it incorporated both established and novel tests for both attention and behaviors associated with ADHD. OROS MPH demonstrated efficacy on novel tests such as the Finger Windows backward test, the TOVA Reaction Time Variability score, and THS-R. These results help prove the benefits of stimulant medication on executive functions such as working memory, sustaining attention on boring tasks, and impulsivity. These data, in addition to a well-tolerated AE profile, further support the use of OROS MPH as a first-line medication for the treatment of ADHD.
Most importantly, this study showed that secondary measures of novel tests for attention and behavioral manifestations of ADHD can be included in an analog classroom of children with ADHD and demonstrate differences due to drug condition. Further analog classroom studies are needed to replicate these results and to demonstrate the utility of these tests and of the analog classroom itself in the examination of onset of action, duration, efficacy, and effects of medication treating ADHD on comorbid conditions.
Disclosures
The study was supported by Ortho-McNeil-Janssen Scientific Affairs, LLC.
Sharon Wigal, Ph.D., is an Advisory Board Member/Consultant for Abbott, ALZA, Celgene, Celltech, Cephalon, Eli Lilly, McNeil Consumer & Specialty Pharmaceuticals, Next Wave Pharm., NIMH, Novartis, Shire US Inc., and TAISHO; receives research support from Addrenex, ALZA, Celgene, Celltech, Cephalon, Eisai, Eli Lilly and Company, Gliatech, McNeil Consumer & Specialty Pharmaceuticals, NIMH, Novartis, Psychogenics, Quintiles, Shionogi Pharma, Shire US Inc., and Sigma Tau; and is a member of Speaker's Bureaus of ALZA, Cephalon, McNeil Consumer & Specialty Pharmaceuticals, Novartis, and Shire US Inc.
Tim L. Wigal, Ph.D., receives research support from Cephalon, Eli Lilly, McNeil, New Rivers, NIH, Novartis, and Shire; has served as a consultant or advisor to McNeil, Novartis, and Shire; and has served on the speaker's bureau of McNeil and Shire.
Sabrina Schuck, Ph.D., received research support from Ortho-McNeil Janssen Scientific Affairs, LLC.
Matthew Brams, M.D., has been a speaker, consultant, and advisory board member for Novartis, McNeil Pediatrics, and Shire, and has received grant-research support from Novartis, Shire, Ortho-McNeil Janssen Scientific Affairs, LLC, and Eli Lilly for the treatment of ADHD in children.
David Williamson, Ph.D., Robert Armstrong, M.D., and H. Lynn Starr, M.D., are employees of Ortho-McNeil Janssen Scientific Affairs, LLC, and stockholders of Johnson & Johnson corporation.
Author Contributions
The following describes the contributions of the authors to the study: Sharon B. Wigal for contributions to protocol design, conduct of the study, interpretation of results, and writing of the article; Tim Wigal for contributions to protocol design, conduct of the study, interpretation of results, and writing of the article; Sabrina Schuck for contributions to protocol design, conduct of the study, interpretation of results, and writing of the article; David Williamson for contributions to protocol design, interpretation of results, and writing of the article; H. Lynn Starr for contributions to protocol design, oversight of the conduct of the trial, interpretation of results, and writing of the article; Robert B. Armstrong for contributions to protocol design, data analysis and interpretation, and writing of the article; Matthew Brams for contributions to the conduct of the study and writing of the article.
Footnotes
The study was supported by Ortho-McNeil-Janssen Scientific Affairs, LLC.
Acknowledgments
The authors would like to acknowledge Steve Ascher, Ph.D., and C.V. Damaraju, Ph.D., of Johnson and Johnson Pharmaceutical Research and Development, L.L.C., for supervision of the statistical analysis, and Michelle L. Metelo, PharmD, of JK Associates, Inc., for editorial assistance.
References
- American Psychiatric Association. Text Revision (DSM-IV-TR) 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Barbaresi WJ. Katusic SK. Colligan RC. Weaver AL. Jacobsen SJ. Modifiers of long-term school outcomes for children with attention-deficit/hyperactivity disorder: Does treatment with stimulant medication make a difference? Results from a population-based study. J Dev Behav Pediatr. 2007;28:274–287. doi: 10.1097/DBP.0b013e3180cabc28. [DOI] [PubMed] [Google Scholar]
- Barbaresi WJ. Katusic SK. Colligan RC. Weaver AL. Leibson CL. Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: Results from a population-based study. J Dev Behav Pediatr. 2006;27:1–10. doi: 10.1097/00004703-200602000-00001. [DOI] [PubMed] [Google Scholar]
- Baren M. Swanson J. How not to diagnose ADHD. Contemp Pediatr. 1996;13:53–64. [Google Scholar]
- Bedard AC. Jain U. Hogg-Johnson S. Tannock R. Effects of methylphenidate on working memory components: Influence of measurement. J Child Psychol Psychiatry. 2007;48:872–880. doi: 10.1111/j.1469-7610.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- Bedard AC. Tannock R. Anxiety, methylphenidate response, and working memory in children with ADHD. J Atten Disord. 2008;11:546–557. doi: 10.1177/1087054707311213. [DOI] [PubMed] [Google Scholar]
- Breslau J. The impact of early behavior disturbances on academic achievement in high school. Pediatrics. 2009;123:1472–1476. doi: 10.1542/peds.2008-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX. Sonuga-Barke EJ. Milham MP. Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- DeShazo-Barry T. Lyman RD. Klinger LG. Academic underachievement and attention/deficit hyperactivity disorder: the negative impact of symptom severity on school performance. J Sch Psychol. 2002;40:259–283. [Google Scholar]
- DuPaul G. Power T. Anastopolous A. Reid R. ADHD Rating Scale-IV: Checklists, Norms, Clinical Interpretation. New York: Guilford Publications; 1998. [Google Scholar]
- Dykman RA. Ackerman PT. Attention deficit disorder and specific reading disability: Separate but often overlapping disorders. J Learn Disabil. 1991;24:96–103. doi: 10.1177/002221949102400206. [DOI] [PubMed] [Google Scholar]
- Elia J. Welsh PA. Gulotta CS. Rapoport JL. Classroom academic performance: Improvement with both methylphenidate and dextroamphetamine in ADHD boys. J Child Psychol Psychiatry. 1993;34:785–804. doi: 10.1111/j.1469-7610.1993.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Evans SW. Pehlham WE. Smith BH. Bukstein O. Gnagy EM. Greiner AR. Altenderfer L. Baron-Myak C. Dose-response effects of methylphenidate on ecologically valid measures of academic performance and classroom behavior in adolescents with ADHD. Exp Clin Psychopharmacol. 2001;9:163–175. doi: 10.1037//1064-1297.9.2.163. [DOI] [PubMed] [Google Scholar]
- Faraone S. Biederman J. Monuteaux MC. Doyle A. Seidman L. A psychometric measure of learning disability predicts educational failure four years later in boys with attention-deficit/hyperactivity disorder. J Atten Disord. 2001;4:220–230. [Google Scholar]
- Flake RA. Lorch EP. Milich R. The effects of thematic importance on story recall among children with attention deficit hyperactivity disorder and comparison children. J Abnorm Child Psychol. 2007;35:43–53. doi: 10.1007/s10802-006-9078-z. [DOI] [PubMed] [Google Scholar]
- Fletcher JM. Lyon GR. Fuchs LS. Barnes MA. Learning Disabilities: From Identification to Intervention. New York: Guilford Publications; 2007. [Google Scholar]
- Forbes GB. Clinical utility of the Test of Variables of Attention (TOVA) in the diagnosis of attention-deficit/hyperactivity disorder. J Clin Psychol. 1998;54:461–476. doi: 10.1002/(sici)1097-4679(199806)54:4<461::aid-jclp8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Pickering SJ. Knight C. Stegmann Z. Working memory skills and educational attainment: Evidence from national curriculum assessments at 7 and 14 years of age. Appl Cogn Psychol. 2004;18:1–16. [Google Scholar]
- Ghelani K. Sidhu R. Jain U. Tannock R. Reading comprehension and reading related abilities in adolescents with reading disabilities and attention-deficit/hyperactivity disorder. Dyslexia. 2004;10:364–384. doi: 10.1002/dys.285. [DOI] [PubMed] [Google Scholar]
- Good RH, editor; Kaminski RA, editor. Dynamics Indicators of Basic Early Literacy Skills. 6th. Eugene, OR: Institute for the Development of Educational Achievement; 2002. [Google Scholar]
- Hechtman L. Abikoff H. Klein RG. Weiss G. Respitz C. Kouri J. Blum C. Greenfield B. Etcovitch J. Fleiss K. Pollack S. Academic achievement and emotional status of children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43:812–819. doi: 10.1097/01.chi.0000128796.84202.eb. [DOI] [PubMed] [Google Scholar]
- Huang YS. Chao CC. Wu YY. Chen YY. Chen CK. Acute effects of methylphenidate on performance during the Test of Variables of Attention in children with attention deficit/hyperactivity disorder. Psychiatry Clin Neurosci. 2007;61:219–225. doi: 10.1111/j.1440-1819.2007.01653.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-for School-Age Children—Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Leark RA. Greenberg LM. Kindschi CL. Dupuy TR. Hughes SJ. TOVA Professional Manual: Test of Variables of Attention Continuous Performance Test. Los Alamitos, CA: The TOVA Company; 2007. [Google Scholar]
- Loe IM. Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol. 2007;32:643–654. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- Martinussen R. Hayden J. Hogg-Johnson S. Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- McCracken JT. Biederman J. Greenhill LL. Swanson JM. McGough JJ. Spencer TJ. Posner K. Wigal S. Pataki C. Zhang Y. Tulloch S. Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:673–683. doi: 10.1097/01.CHI.0000046863.56865.FE. [DOI] [PubMed] [Google Scholar]
- McGough JJ. Wigal SB. Abikoff H. Turnbow JM. Posner K. Moon E. A randomized, double-blind, placebo-controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord. 2006;9:476–485. doi: 10.1177/1087054705284089. [DOI] [PubMed] [Google Scholar]
- Milone M. Test of Handwriting Skills, Revised Manual. Novato, CA: Academic Therapy Publications; 2007. [Google Scholar]
- National Institute of Child Health and Human Development. National Institutes of Health, Department of Health and Human Services: Report of the National Reading Panel: Teaching Children to Read (00-4769) Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- Pastor PN. Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital Health Stat. 2008;10:1–14. [PubMed] [Google Scholar]
- Pelham WE. Gnagy EM. Burrows-Maclean L. Williams A. Fabiano GA. Morrisey SM. Chronis AM. Forehand GL. Nguyen CA. Hoffman MT. Lock TM. Fielbelkorn K. Coles EK. Panahon CJ. Steiner RL. Meichenbaum DL. Onyango AN. Morse GD. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:E105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- Racine MB. Majnemer A. Shevell M. Snider L. Handwriting performance in children with attention deficit hyperactivity disorder (ADHD) J Child Neurol. 2008;23:399–406. doi: 10.1177/0883073807309244. [DOI] [PubMed] [Google Scholar]
- Schatz A. Ballantyne A. Trauner D. Sensitivity and specificity of a computerized test of attention in the diagnosis of attention-deficit/hyperactivity disorder. Assessment. 2001;8:357–365. doi: 10.1177/107319110100800401. [DOI] [PubMed] [Google Scholar]
- Scheffler RM. Brown TT. Fulton BD. Hinshaw SP. Levine P. Stone S. Positive association between attention-deficit/hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123:1273–1279. doi: 10.1542/peds.2008-1597. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M. Biederman J. Sprich-Buckminster S. Lehman B. Faraone S. Norman D. Comorbidity between ADHD and learning disability: A review and report in a clinically referred sample. J Am Acad Child Adolesc Psychiatry. 1992;31:439–448. doi: 10.1097/00004583-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Spencer TJ. Biederman J. Mick E. Attention-deficit/hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. J Ped Psychol. 2007;32:631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Steele M. Jensen PS. Quinn DM. Remission versus response as the goal of therapy in ADHD: A new standard for the field? Clin Ther. 2006;28:1892–1908. doi: 10.1016/j.clinthera.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Swanson J. Gupta S. Lam A. Shoulson I. Lerner M. Modi N. Lindemulder E. Wigal S. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder. Arch Gen Psychiatr. 2003;60:204–211. doi: 10.1001/archpsyc.60.2.204. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Agler D. Fineberg E UCI laboratory school protocol for PK/PD studies. In: Ritalin: Theory and Practice. 2nd. Greenhill L, editor; Osman B, editor. New Rochelle, NY: Mary Ann Liebert, Inc.; 2000. pp. 405–430. [Google Scholar]
- Swanson JM. Lerner M. Wigal T. Steinhoff K. Greenhill L. Posner K. Freid J. Wigal S. The use of a laboratory school protocol to evaluate concepts about efficacy and side effects of new formulations of stimulant medications. J Atten Disord. 2002;6:S73–S88. doi: 10.1177/070674370200601s10. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Wigal SB. Wigal T. Sonuga-Barke E. Greenhill LL. Biederman J. Kollins S. Nguyen AS. DeCory HH. Hirshe Dirksen SJ. Hatch SJ COMACS Study Group. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study) Pediatrics. 2004;113:e206–e216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Vance AL. Maruff P. Barnett R. Attention deficit hyperactivity disorder combined type: Better executive function performance with longer-term psychostimulant medication. Aust NZ J Psychiatry. 2003;37:570–576. doi: 10.1046/j.1440-1614.2003.01238.x. [DOI] [PubMed] [Google Scholar]
- Wagner RK. Torgesen JK. Rashotte CA. Comprehensive Test of Phonological Processing. Austin, TX: Pro-ed Publishing; 1999. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test. 2nd. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Wiederholt J. Bryant B. GORT 4: Gray Oral Reading Tests Examiner's Manual. Austin, TX: Pro-ed Publishing; 2001. [Google Scholar]
- Wigal S. Gupta S. Guinta D. Swanson JM. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacol Bull. 1998;34:47–53. [PubMed] [Google Scholar]
- Wigal S. Sanchez D. DeCory HH. D'Imperio J. Swanson J. Selection of the optimal dose ratio for a controlled-delivery formulation of methylphenidate. J Appl Res. 2003;3:46–63. [Google Scholar]
- Wigal SB. Wigal TL. The laboratory school protocol: Its origin, use, and new applications. J Atten Disord. 2006;10:92–111. doi: 10.1177/1087054705286049. [DOI] [PubMed] [Google Scholar]
- Wigal SB. Kollins S. Childress A. Squires L. A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2009;9:17–31. doi: 10.1186/1753-2000-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB. Wigal TL. Kollins SH. Advances in methylphenidate drug delivery systems for ADHD therapy. Adv ADHD. 2006;1:4–7. [Google Scholar]