Abstract
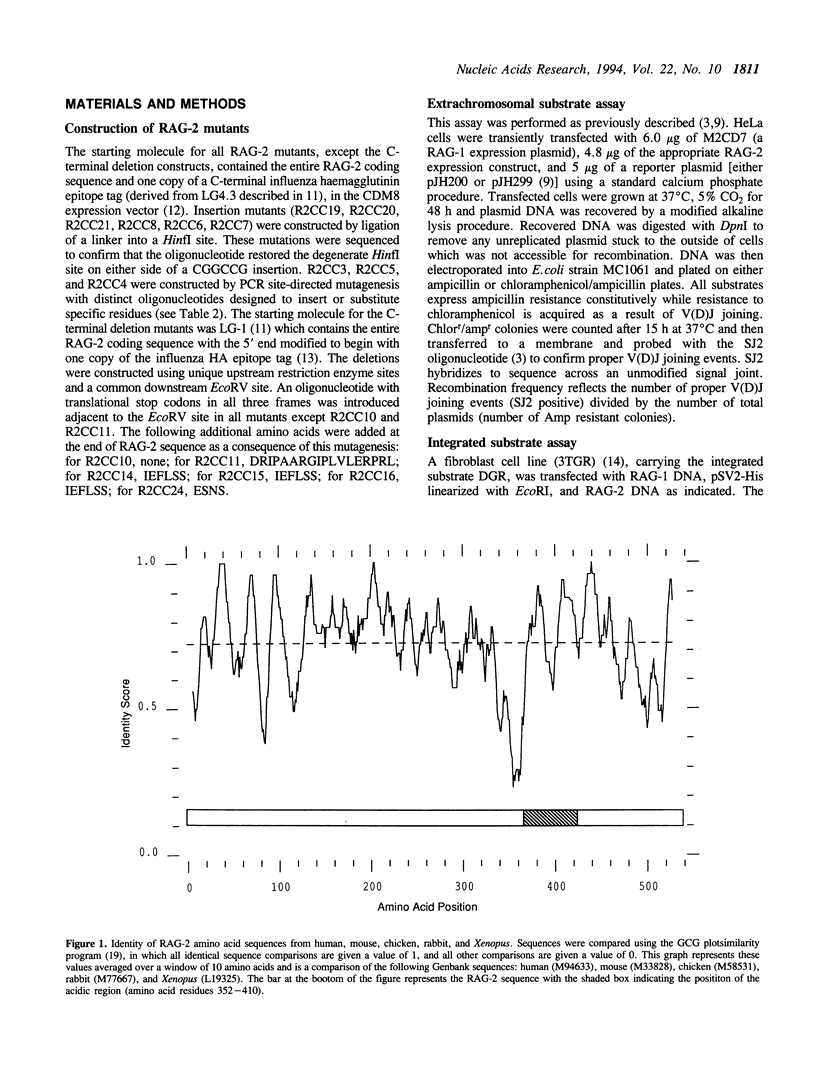
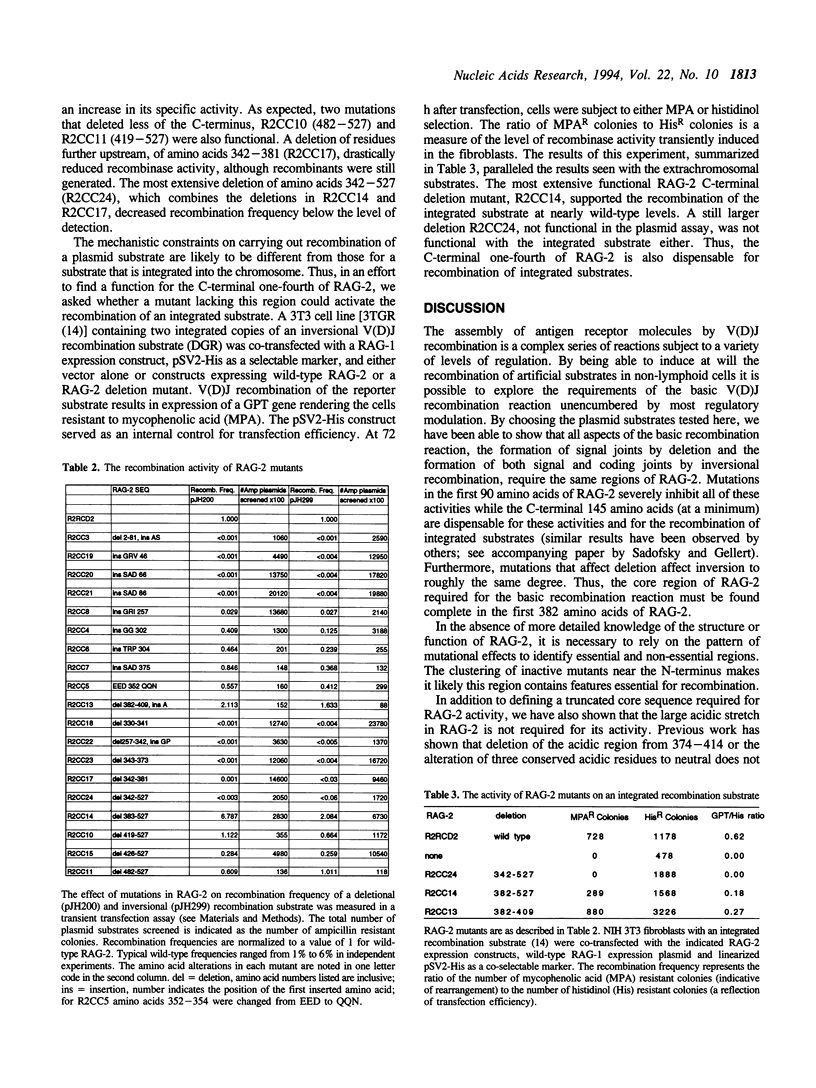
The recombinase activating genes RAG-1 and RAG-2 operate together to activate V(D)J recombination, and thus play an essential role in the generation of immune system diversity. As a first step in understanding the function of the RAG-2 protein, we have tested a series of deletion and insertion mutations for their ability to induce V(D)J joining of a variety of model substrates. Mutants were assayed for their ability to induce deletional and inversional V(D)J joining, thereby testing their proficiency at forming both signal and coding joints, and, in some cases, for their ability to carry out recombination of both extrachromosomal and integrated recombination substrates. All these reactions were affected similarly by any one mutation. Although the RAG-2 protein shows extensive evolutionary conservation across its length, we found that the carboxy-terminal portion of RAG-2, including an acidic region, is dispensable for all forms of recombination tested. In contrast, all mutations we created in the N-terminal region severely decreased recombination. Thus, the core active region required for V(D)J recombination is confined to the first three-quarters of the RAG-2 protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson L. M., Oettinger M. A., Schatz D. G., Masteller E. L., Hurley E. A., McCormack W. T., Baltimore D., Thompson C. B. Selective expression of RAG-2 in chicken B cells undergoing immunoglobulin gene conversion. Cell. 1991 Jan 11;64(1):201–208. doi: 10.1016/0092-8674(91)90221-j. [DOI] [PubMed] [Google Scholar]
- Craig N. L. The mechanism of conservative site-specific recombination. Annu Rev Genet. 1988;22:77–105. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- Fuschiotti P., Harindranath N., Mage R. G., McCormack W. T., Dhanarajan P., Roux K. H. Recombination activating genes-1 and -2 of the rabbit: cloning and characterization of germline and expressed genes. Mol Immunol. 1993 Aug;30(11):1021–1032. doi: 10.1016/0161-5890(93)90127-w. [DOI] [PubMed] [Google Scholar]
- Gellert M. Molecular analysis of V(D)J recombination. Annu Rev Genet. 1992;26:425–446. doi: 10.1146/annurev.ge.26.120192.002233. [DOI] [PubMed] [Google Scholar]
- Greenhalgh P., Olesen C. E., Steiner L. A. Characterization and expression of recombination activating genes (RAG-1 and RAG-2) in Xenopus laevis. J Immunol. 1993 Sep 15;151(6):3100–3110. [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Hirai M., Kurosawa Y. Sequence and chromosome assignment to 11p13-p12 of human RAG genes. Immunol Lett. 1992 Aug;33(3):277–284. doi: 10.1016/0165-2478(92)90073-w. [DOI] [PubMed] [Google Scholar]
- Kallenbach S., Brinkmann T., Rougeon F. Rag-1: a topoisomerase? Int Immunol. 1993 Feb;5(2):231–232. doi: 10.1093/intimm/5.2.231. [DOI] [PubMed] [Google Scholar]
- Lin W. C., Desiderio S. Regulation of V(D)J recombination activator protein RAG-2 by phosphorylation. Science. 1993 May 14;260(5110):953–959. doi: 10.1126/science.8493533. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Sadofsky M. J., Hesse J. E., McBlane J. F., Gellert M. Expression and V(D)J recombination activity of mutated RAG-1 proteins. Nucleic Acids Res. 1993 Dec 11;21(24):5644–5650. doi: 10.1093/nar/21.24.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D. G., Baltimore D. Stable expression of immunoglobulin gene V(D)J recombinase activity by gene transfer into 3T3 fibroblasts. Cell. 1988 Apr 8;53(1):107–115. doi: 10.1016/0092-8674(88)90492-8. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Silver D. P., Spanopoulou E., Mulligan R. C., Baltimore D. Dispensable sequence motifs in the RAG-1 and RAG-2 genes for plasmid V(D)J recombination. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6100–6104. doi: 10.1073/pnas.90.13.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]


