Abstract
Pneumonitis is a rare but serious complication associated with paclitaxel and/or trastuzumab treatment. We report a 51-year-old female patient with locally advanced breast cancer who presented with shortness of breath, fever, dry cough and pulmonary infiltrates. She had been treated without complications for 10 weeks with paclitaxel (Taxol®) and trastuzumab (Herceptin®) as neoadjuvant therapy, with complete clinical and pathological response. Infections and cardiomyopathy were excluded as causes of her symptoms. Bronchoscopy and biopsy were performed and a diagnosis of drug-induced interstitial pneumonitis was made. After treatment with steroids, the patient showed a significant response in less than 24 h; she was discharged home without the need for oxygen less than 48 h after therapy initiation. Although no causative association could be found between either trastuzumab or paclitaxel and this patient's pulmonary syndrome, the potential for such toxicity should be considered, especially as paclitaxel/trastuzumab is a vey common combination therapy for breast cancer.
Key Words: Paclitaxel, Trastuzumab, Interstitial pneumonitis, Drug-induced infiltrative lung disease, Breast cancer
Introduction
Paclitaxel and trastuzumab combination therapy is a well-known treatment for breast cancer [1]. Major adverse effects include peripheral neuropathy, myelotoxicity, bradycardia, hypotension, arthralgia, myalgia, granulocytopenia and hypersensitivity [2, 3]. To date, several cases of patients developing paclitaxel-induced interstitial pneumonitis have been reported, with estimated frequencies of 0.73–12% [4]. Trastuzumab-induced pneumonitis may present as rapidly progressive pulmonary infiltrate respiratory failure after the administration of 1 dose of trastuzumab or after 6 weeks of therapy. The incidence of trastuzumab-induced pneumonitis is 0.4–0.6% [4].
Although there is one report of fatal pneumonitis associated with docetaxel (Taxotere®)/trastuzumab therapy, no fatalities associated with paclitaxel/trastuzumab combination therapy have been reported to date. However, awareness of the potential for such fatal toxicity is important.
Case Report
A 51-year-old female patient, nonsmoker and with no comorbid disease, was diagnosed with locally advanced left breast cancer (T2N1M0). HER2/neu was overexpressed in the primary tumor. Estrogen and progesterone receptors were negative. Neoadjuvant treatment comprised paclitaxel 80 mg/m2 weekly for 12 weeks concurrent with trastuzumab 4 mg/m2 loading dose in week 1, then 2 mg/m2 weekly for 11 weeks. As per protocol, the patient was given dexamethasone prior to chemotherapy. The patient had completed 9 doses of both therapies with no complications. When she was due for week 10 of the therapy, she complained of shortness of breath, dry cough and fever. Her clinical examination revealed the following: temperature 39.6°C, heart rate 104 bpm, BP 96/33 mm Hg, respiratory rate 30 cycles/min and SpO2 84% on room air.
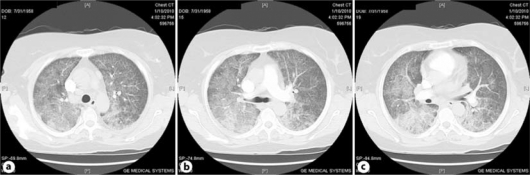
Chest examination showed bilateral diffuse crepitation and wheezing. The patient was admitted to hospital and started on therapy with 4 liters O2 per nasal canula, and Tazocin (piperacillin sodium- tazobactam sodium) 4.5 g every 8 h and vancomycin 1 g every 12 h empirically after drawing blood for culture and sensitivity. At this time, the patient was not neutropenic and the following laboratory values were noted: WBC 5.5 × 109/l, ANC 4.3 × 109/l, Hb 91 g/l and platelets 315 × 109/l. Her renal and liver functions were within normal range, echocardiography was normal, both blood and sputum cultures were negative for acid-fast bacteria and fungal infection, and urine culture showed no growth. X-ray showed ground-glass opacification of the lungs (fig. 1) and spiral chest CT showed satisfactory opacification of the pulmonary trunk, and right and left main pulmonary arteries, with no evidence of central pulmonary embolism. Bilateral airspace disease consistent with pulmonary edema was noted, along with no detectable lung nodules, and no evidence of either pleural or pericardial effusion (fig. 2a, b, c). Special stains for AFB, fungus, Pneumonitis jirovecii (carinii) pneumonia and CMV immunohistochemistry were all negative.
Fig. 1.
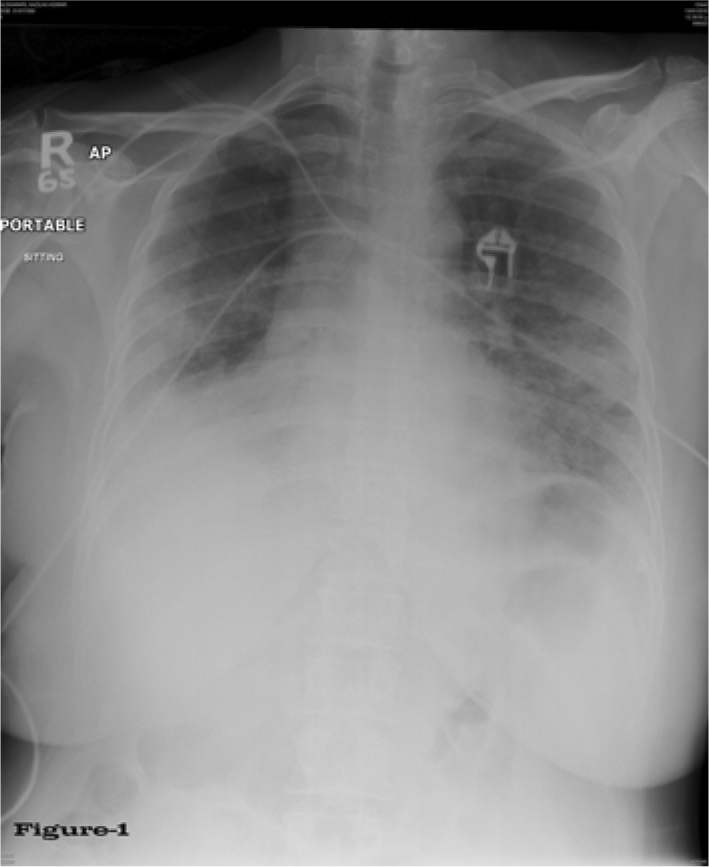
Chest X-ray during acute disease.
Fig. 2.
Chest CT during acute disease.
Bronchoscopic lung biopsy showed moderate interstitial inflammatory infiltrate composed of polymorphonuclear leukocytes, lymphocytes and some histiocytes forming occasional aggregates. The alveolar spaces showed focal deposits of fibrin; there was focal pneumocyte atypia and hyperplasia, as well as focal intra-alveolar hemorrhage. Bronchoalveolar lavage was negative for malignant cells and viral inclusion bodies; however, reactive lymphocytes were present. Gram stain culture findings were negative. Scattered pulmonary macrophages were noted against a background of reactive lymphocytes, and only occasional eosinophils were seen. Based on the above-mentioned findings, a diagnosis of acute interstitial pneumonitis was made. Although the patient's fever had subsided and her blood pressure had begun to normalize, lung auscultation revealed the same initial crepitations and the patient was still in need of O2 supply (4 l/h).
A pulmonary function test (spirometry) yielded the following prebronchodilator results: FEV1 49.9% and FVC 43.5%; the postbronchodilator differences were 16% for FEV1 and 27.6% for FVC. Due to a very short exhalation time of 1–2 s max., the patient was unable to exhale maximally in order to record total lung capacity Furthermore, the patient refused to complete the postbronchodilator spirometry test due to shortness of breath. Given the data at hand, the best possible flow volume loop was chosen.
As there was no indication of any type of infection, the case was diagnosed as drug-induced interstitial pneumonitis, antibiotics were withdrawn and treatment with prednisone 40 mg orally once daily was begun within 18 h of initial presentation.
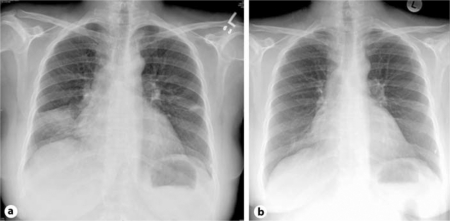
The patient's crepitations disappeared and her oxygen saturation reached 95% on room air. A chest X-ray taken 18 h after initiation of prednisone (fig. 3a) showed improvement in the lung picture compared to the initial radiograph. A follow-up X-ray taken 48 h after initiation of prednisone (fig. 3b) was completely normal, so the patient was discharged home in very good general condition without the need for O2 supply. Follow-up continued in the outpatient clinic, including the completion of a 10-day course of prednisone.
Fig. 3.
Chest X-ray 18 h (a) and 48 h (b) after start of prednisone treatment.
Discussion
Drug-induced infiltrative lung disease (DI-ILD) is the most common form of anti-neoplastic agent-induced respiratory disease. Patterns of DI-ILD are non-specific interstitial pneumonia, eosinophilic pneumonia, hypersensitivity pneumonitis, pulmonary fibrosis, or organizing pneumonia [5]. Early withdrawal of the causative drug will often lead to improvement or even cure of the ILD. Corticosteroids may suppress the inflammatory reaction [6, 7].
In several series, trastuzumab has been well tolerated, and its addition to chemotherapy does not significantly increase the frequency of side effects [8]. Infusion-related events are common but rarely severe, and more common during the first administration of the drug [9]. In an analysis of the safety of trastuzumab administered to 25,000 patients, the only respiratory-associated serious adverse event was bronchospasm [10]. This reaction usually occurred within 2.5 h of administration.
However, apart from the previously described infusion-related bronchospasms, rare cases of interstitial pneumonitis have been reported. One patient with organizing pneumonia and another with pneumonitis were reported; both events were probably due to trastuzumab administration [11, 12]. In trial B-31, four patients in the trastuzumab group had interstitial pneumonitis, one of whom died [13]. In the N9831 trial, five patients in the trastuzumab group had grade 3+ pneumonitis or pulmonary infiltrates, one of whom died [13].
Paclitaxel acts against tumors through both promotion of microtubule assembly and inhibition of microtubule disassembly, activities that result in disruption of cell division and cell death [14]. Hypersensitivity reactions are well-recognized complications of paclitaxel therapy and typically occur with the first or second dose [15]. These reactions are characterized by dyspnea, hypotension, bronchospasm, urticaria, and erythematous rashes. Respiratory symptoms may develop hours to weeks after paclitaxel administration. Severity of these symptoms ranges from mild dyspnea to respiratory failure requiring mechanical ventilation [15, 16]. The mechanism of paclitaxel-induced hypersensitivity reactions remains unclear. It may be mediated by the release of histamines or other vasoactive substances similar to anaphylactoid reactions encountered with IV contrast agents [15], or it may result from a delayed hypersensitivity response [17].
Kuip and Muller [18] reported a case in which a patient presented with fever and dyspnea after a second dose of docetaxel/trastuzumab. The patient died due to respiratory failure 3 weeks later and interstitial pneumonitis was diagnosed at autopsy. In spite of accompanying dexamethasone and antihistamines given concurrently with paclitaxel/trastuzumab, our patient, like that of Kuip and Muller [18], developed interstitial pneumonitis with concomitant complaint of dry cough, fever and dyspnea after 9 weeks of therapy. Our patient's symptoms appeared later than what has been reported in the literature for each medication separately. Fortunately, the diagnosis of interstitial pneumonitis was expected and confirmed and our patient recovered completely.
This case raises another question: which drug is responsible for this lung complication? Paclitaxel or trastuzumab? Or both? Since they have synergistic efficacy against the tumor, could they be synergistic in the side effects as well? As the two drugs were given concurrently and the patient had no known risk factors to anticipate this reaction, and there is no clear diagnostic test to confirm which drug caused the pneumonitis, it is difficult to be sure of the offensive drug. It is well known that trastuzumab infusion-related events are common but rarely severe, and that they are more common during the first administration [19], usually within 2.5 h. Trastuzumab-associated pneumonitis may develop many months after initiation of treatment and may run a more insidious course.
In view of the extensive use of trastuzumab nowadays, trastuzumab-induced pneumonitis, if existent, seems rarer than paclitaxel-induced pneumonitis. This leads us to conclude that the pneumonitis in our patient was most likely due to paclitaxel.
Conclusion
Although pneumonitis is a rare side effect of paclitaxel/trastuzumab administration, it is important to be aware of this specific toxicity. Importantly, early recognition and appropriate therapy may be life saving.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Horiguchi J, Oyama T, Koibuchi Y, et al. Neoadjuvant weekly paclitaxel with and without trastuzumab in locally advanced or metastatic breast cancer. Anticancer Res. 2009;29:517–524. [PubMed] [Google Scholar]
- 2.Suzaki N, Hiraki A, Takigawa N, et al. Severe interstitial pneumonia induced by paclitaxel in a patient with adenocarcinoma of the lung. Acta Med. 2006;60:295–298. doi: 10.18926/AMO/30737. [DOI] [PubMed] [Google Scholar]
- 3.Sekine I, Nishiwaki Y, Watanabe K, Yoneda S, Saijo N. Phase II study of 3-hour infusion of paclitaxel in previous untreated non-small cell lung cancer. Clin Cancer Res. 1996;2:941–945. [PubMed] [Google Scholar]
- 4.Vahid B, Marik P. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528–538. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulou I, Bamias A, et al. Pulmonary toxicity from novel antineoplastic agents. Ann Oncol. 2006;17:372–379. doi: 10.1093/annonc/mdj057. [DOI] [PubMed] [Google Scholar]
- 6.Camus P, Fanton A, et al. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301–326. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 7.Camus PH, Foucher P, et al. Drug-induced infiltrative lung disease. Eur Respir J. 2001;18(suppl 32):93s–100s. [PubMed] [Google Scholar]
- 8.Marty M, Cogenitti F, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 Study Group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Carbonell X, et al. Phase II study of efficacy, safety and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–2171. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Cook-Burns N. Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology. 2001;61(suppl 2):58–66. doi: 10.1159/000055403. [DOI] [PubMed] [Google Scholar]
- 11.Vahid B, Mehrotra A. Trastuzumab (Herceptin)-associated lung injury. Respirology. 2006;11:655–658. doi: 10.1111/j.1440-1843.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 12.Radzikowska E, Szczepulska E, et al. Organising pneumonia caused by trastuzumab (Herceptin) therapy for breast cancer. Eur Respir J. 2003;21:552–555. doi: 10.1183/09031936.03.00035502. [DOI] [PubMed] [Google Scholar]
- 13.Romond E, Perez A, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 14.Pazdur R, Kudelka AP, Kavanagh JJ, Cohen PR, Raber MN. The taxoids: paclitaxel (Taxol) and docetaxel (Taxotere) Cancer Treat Rev. 1993;19:351–386. doi: 10.1016/0305-7372(93)90010-o. [DOI] [PubMed] [Google Scholar]
- 15.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 16.Sekine I, Nishiwaki Y, Watanabe K, Yoneda S, Saijo N. Phase II study of 3-hour infusion of paclitaxel in previously untreated non-small cell lung cancer. Clin Cancer Res. 1996;2:941–945. [PubMed] [Google Scholar]
- 17.Fujimori K, Yokoyama A, Kurita Y, Uno K, Saijo N. Paclitaxel-induced cell-mediated hypersensitivity pneumonitis: diagnosis using leukocyte migration test, bronchoalveolar lavage and transbronchial lung biopsy. Oncology. 1998;55:340–344. doi: 10.1159/000011873. [DOI] [PubMed] [Google Scholar]
- 18.Kuip E, Muller E. Fatal pneumonitis after treatment with docetaxel and trastuzumab. Neth J Med. 2009;67:237–239. [PubMed] [Google Scholar]
- 19.Baselga J, Carbonell X, et al. Phase II study of efficacy, safety and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–2171. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]