Abstract
We describe a 72-year-old man, who had been suffered from Henoch-Schönlein purpura (HSP) several times, presented with hematoproteinuria with granular cast, and general lymphadenopathy. The immunological examination of the serum showed polyclonal hypergammagloburinemia with high value of IgG4. The renal biopsy revealed interstitial inflammatory cell infiltration, including infiltration of lymphocytes and plasma cells, and segmental glomerulonephritis. Direct immunofluorescence microscopy revealed apparent positive staining with anti-human IgA, and anti-human IgG in glomeruli, anti-human IgG4 antibody staining showed many positive plasma cells in the interstitium. The patient was diagnosed with HSP nephritis that was complicated by IgG4-related nephropathy. As a result of the treatment with 30mg prednisolone, the swelling of the LNs decreased, but the patient continued to have persistent hematoproteinuria.
Introduction
A novel clinicopathological entity of IgG4-related autoimmune disease characterized by extensive IgG4-positive plasma cell infiltration of organs together with CD4- or CD8-positive T lymphocytes is proposed [1]. Renal involvement in this entity was also suggested, and three patterns of renal involvement have been described: 1) extraparenchymal involvement such as hydronephrosis associated with retroperitoneal lesions; 2) diffuse tubulointerstitial nephritis (TIN); and 3) renal lesions composed of focal lymphoplasmacytic infiltration of the renal interstitium [2]. In this report we describe a rare case diagnosed with HSP nephritis that was complicated by IgG4-related nephropathy.
Case report
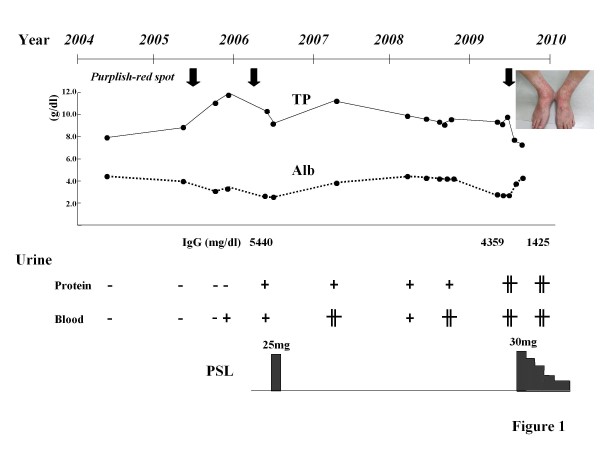
A 72-year-old man presented with cervical, axillary, left subclavian, and inguinal lymph nodes (LNs) swelling. The LNs gradually increased in size for 1 month. During this period, the patient often had a low-grade fever and arthralgia. He also experienced a marked weight loss of 7 kg in 3 months. In June 2009, he developed an erythematous rash predominantly on his lower legs and was admitted to the hospital. In 2005, he had developed similar erythematous rashes in the lower extremities several times. In 2006, the patient was diagnosed with Henoch-Schönlein purpura (HSP) on the basis of histological examination of skin biopsy samples, which showed leukocytoclastic vasculitis. Immunohistochemical study with anti-IgA antibody was not performed. A treatment with prednisolone (PSL; 25 mg) had been effective (Figure 1). He had no history of allergic diseases such as bronchial asthma, atopic dermatitis, and allergic rhinitis. In 2002, he underwent gastrectomy for gastric cancer.
Figure 1.
Clinical course of the patient. Purplish-red spot in the lower extremities as a picture had been developed 3 times in 6 years (downward bald arrow). Hematoproteinuria has been detected since 2006. TP; serum total protein, Alb; serum albumin, PSL; prednisolone.
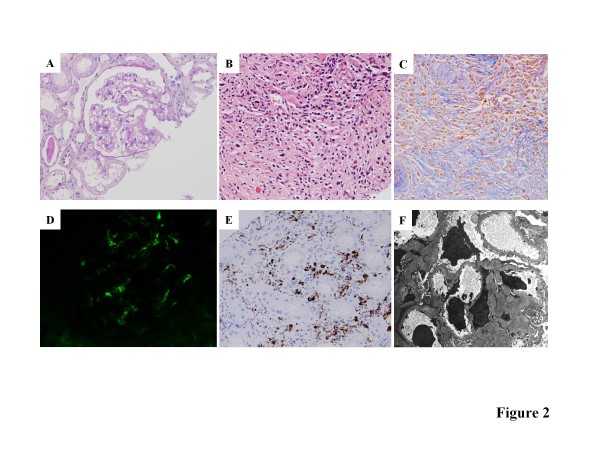
On admission, he was febrile, and the rash was palpable and purpuric in nature. A physical examination showed no abnormalities in the lungs, heart, abdomen, and central nervous system. Laboratory findings showed an increased erythrocyte sedimentation rate (73 mm/h) and the value of C-reactive protein was 0.22 mg/dL. The hemoglobin concentration was 11.0 g/dL, the white blood cell count was 8,900/mm3 (neutrophils 66.8%, lymphocytes 21.5%, monocytes 4.1%, eosinophils 7.0%, and basophils 0.6%), and the platelet count was 45.1 × 104/mm3. Hematuria and proteinuria with granular cast were detected. The results of the serum chemistry analyses are as follows: serum creatinine, 0.96 mg/dL (normal, 0.4-1.2 mg/dL); blood urea nitrogen, 16.7 mg/dL; total serum protein 8.6 mg/dL (normal, 6.5-8.2 g/dL); and serum albumin 3.6 g/dL (normal, 3.7-5.2 g/dL). Serum transaminase, amylase, and lactate dehydrogenase (LDH) levels were within normal limits. The immunological tests were positive for antinuclear antibody at a titer of 80 dil, and the immunofluorescence patterns were speckled and homogeneous. Anti-double-stranded DNA antibody, rheumatoid factor, anti-Sjögren's syndrome A (anti-SS-A), anti-SS-B antibodies, anti-Sm antibody, anti-Jo-1 antibody, and anti-RNP antibody were all absent. The serum level of immunoglobulin G (IgG) was abnormally high, but IgA and IgM were within normal limits (4,359 mg/dL, 242 mg/dL, and 64 mg/dL, respectively). The serum IgE level was elevated (537 U/mL). Molecules of the subclass IgG4 accounted for 25% (1,100 mg/dL) of the IgG molecules. Serum protein electrophoresis revealed polyclonal hypergammaglobulinemia. Serum levels of C3, C4, and total complement hemolytic activity (CH50) were 55 mg/dL (normal, 86-160 mg/dL), 3 mg/dL (normal, 17-45 mg/dL), and less than 12.0 U/mL (normal, 25-48 U/mL), respectively. Myeloperoxidase antineutrophil-cytoplasmic antibody (MPO-ANCA) was detected at a titer 22 EU (normal, <10EU), but proteinase-3 antineutrophil cytoplasmic antibody was not detected. Serologic specimens also tested negative for cytomegalovirus, herpes simplex virus, Epstein-Barr virus, mycoplasma, hepatitis C virus (HCV) antibody, and hepatitis B virus surface (HBs) antigen. The tuberculin skin test was negative for the purified protein derivative. Although several small LNs swelling in the para-aortic and bilateral renal artery branching area were detected in an abdominal CT scan, any abnormal finding was not confirmed in FDG-PET. Chest CT showed no finding such as interstitial pneumonia. Systemic lymphadenopathy, polyclonal hypergammaglobulinemia associated with IgE and IgG4 elevation, hypocomplementemia, and renal dysfunction reminded us of development of IgG4 related disease, and echo-guided percutaneous kidney biopsy was performed on the 7th hospital day. Four out of 28 glomeruli showed global sclerosis, and 2 glomeruli collapsed with periglomerular fibrosis. The other glomeruli showed mild or no mesangial proliferative change. The biopsy revealed interstitial inflammatory cell infiltration, including infiltration of lymphocytes and plasma cells, and concurrent segmental glomerulonephritis (Figure 2A, B and 2C). Direct immunofluorescence microscopy revealed apparent positive staining with anti-human IgA (Figure 2D) and anti-human IgG antibodies in the mesangium, Complement 3 deposition was also recognized. Anti-human IgG4 antibody staining revealed many positive plasma cells in the interstitium (Figure 2E). The ratio of IgG4-positive plasma cells to IgG-positive plasma cells was more than 50% (data not shown). Electron micrograph revealed numerous electron-dense deposits in the mesangium. Subepithelial electron-dense deposit in the capillary wall was not detected (Figure 2F).
Figure 2.
Representative images of the renal biopsy samples. A. Mild mesangial proliferation is observed. (PAS ×200) B. Obvious inflammatory cell infiltration including lymphocytes, plasma cells and eosinophils are found in the interstitium. (PAS ×200) C. Massive fibrosis and inflammatory cell infiltration are observed in the interstitium. (Masson Trichrome staining ×200) D. Immunofluorescence photomicrographs showing IgA (×400). E. Immunostaining reveals abundant IgG4-positive plasma cells in the interstitium. F. Electron micrograph shows numerous electron-dense deposits in the mesangium. ×3,000.
The patient was diagnosed with HSP nephritis that was complicated by IgG4-related TIN. The patient was treated with PSL (30 mg/day) for 14 days, followed by tapering of PSL. As a result of the treatment, the swelling of the LNs decreased, but the patient continued to have persistent hematoproteinuria.
Discussion
HSP has been recognized as a distinct clinical condition. The syndrome is also referred to as anaphylactoid purpura and allergic purpura because of circumstantial evidence implicating hypersensitivity to bacteria or viruses as a possible cause. Histopathological examinations revealed that the cutaneous lesions result from leukocytoclastic vasculitis. Immunofluorescence studies have revealed immunoglobulin (Ig) and complement component deposits in the cutaneous blood vessels and kidney, but serum complement levels are usually normal. IgA is the most abundant and sometimes the only Ig found in the skin and kidney lesions. The morphologic and immunopathologic features are similar in HSP nephritis and IgA nephropathy (IgAN), which is characterized by various degrees of focal or diffuse mesangial proliferation, diffuse deposition of IgA in the mesangium, and electron-dense deposits in the mesangium [3].
It has became well known that the elevation of serum IgG4 concentration and abundant IgG4-positive plasma cell infiltration in the pancreas are characteristic findings in autoimmune pancreatitis (AIP) [4], and IgG4-related TIN is also considered to belong to the same disease spectrum. Accordingly, the concept of IgG4-related systemic disease have not been established [1,5-11], the patients with this diseases share many common features; (1) elevated serum IgG4 level, (2) abundant IgG4-positive plasma cell infiltration in the affected organs, and (3) marked improvement with corticosteroid therapy [5,7,9,10,12-16]. Our patient exhibited these 3 features. Further, immunohistological studies revealed IgG, IgA, and C3 deposition in the glomeruli resembling IgAN. Although IgA nephropathy associated with MPO-ANCA positive glomerulonephritis has also been reported previously [17,18], renal biopsy of this case did not show any finding of necrotizing or crescentic glomerulonephritis. This patient had symptoms of HSP systemically. Therefore we made a diagnosis of concomitant HSP nephritis and IgG4-related TIN. Recently, it has been reported that several IgG4-related TIN complicated with glomerular disease [9,19]. However, this may be the first case of IgG4-related TIN with HSP nephritis.
Allergy research has elucidated the relationship among IgG antibodies, allergens, and the IgG4 subclass in patients undergoing allergen-specific immunotherapy [20], [21], and it has been shown that extended and high-dose exposure to occupational or injected allergens can induce an increase in IgG and IgG4 antibodies and a decrease in IgE antibodies [22-24]. IgG4 is produced in response to repeated exposure to environmental antigens [25], [26]. Our patient had been experiencing relapsing HSP for 4 years, and this episode might indicate that he may have been repeatedly exposed to the allergen (Figure 1). Although the nature of the allergen that triggers HSP is unknown, the facts that amounts of IgG were extremely high at the point of purpura development indicate protection with the production of IgG4 might be induced by repetitive allergen exposures, and this hard protection may related with the development of IgG4 related TIN. Nevertheless, HSP developed 3 times, and therefore HSP nephritis might be complicated.
We described a rare case of HSP complicated by concurrent IgG4-related TIN. A biopsy of the collected specimens revealed IgG, IgA, and C3 deposition in the glomeruli and IgG4-producing plasma cell infiltration in the interstitium. We speculate that HSP resulting from repeated allergen exposure might induce the development of IgG4-related TIN and also HSP nephritis.
Competing Interests
The authors declare that they have no competing interests.
Authors' contributions
RT and YH provided clinical care, HN conceived the report, and SH performed all the immunochemistry. KM and TS participated in the design of this report. All authors have read and approved the final manuscript.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Contributor Information
Rukako Tamai, Email: rukako@dl.dion.ne.jp.
Yoshiyuki Hasegawa, Email: y-hasegawa@saiseikai-futsukaichi.org.
Satoshi Hisano, Email: hisanos1@cis.fukuoka-u.ac.jp.
Katsuhisa Miyake, Email: kmiyake@fukuoka-u.ac.jp.
Hitoshi Nakashima, Email: hnakashi@fukuoka-u.ac.jp.
Takao Saito, Email: tsaito@fukuoka-u.ac.jp.
Acknowledgements
This work was supported in part by grants from the Ministry of Education, Science, Technology, Sports and Culture of Japan (HN, KM, and TS) and also supported in part by a grant for the Progressive Renal Diseases Research Projects from the Ministry of Health, Labor and Welfare, Japan (TS).
References
- Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–4. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- Saeki T, Nishi S, Ito T, Yamazaki H, Miyamura S, Emura I, Imai N, Ueno M, Saito A, Gejyo F. Renal lesions in IgG4-related systemic disease. Intern Med. 2007;46:1365–71. doi: 10.2169/internalmedicine.46.0183. [DOI] [PubMed] [Google Scholar]
- Appel GBRJ, D'Agati V. Brenner and Rector's The Kidney. 8. 2006. Secondary glomerular disease; pp. 1094–7. [Google Scholar]
- Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- Kamisawa T. IgG4-related sclerosing disease. Intern Med. 2006;45:125–6. doi: 10.2169/internalmedicine.45.0137. [DOI] [PubMed] [Google Scholar]
- Hamano H, Kawa S. Are there any other organs in which autoimmune pancreatitis-associated lesions remain to be identified? Intern Med. 2006;45:883–4. doi: 10.2169/internalmedicine.45.0162. [DOI] [PubMed] [Google Scholar]
- Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, Watanabe K, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Tsuneyama K, Saito K, Haratake J, Takagawa K, Nakanuma Y. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Kuttner's tumor) Am J Surg Pathol. 2005;29:783–91. doi: 10.1097/01.pas.0000164031.59940.fc. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Harada S, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Takahashi H, Imai K. Clinical and pathological differences between Mikulicz's disease and Sjögren's syndrome. Rheumatology (Oxford) 2005;44:227–34. doi: 10.1093/rheumatology/keh447. [DOI] [PubMed] [Google Scholar]
- Saeki T, Imai N, Ito T, Yamazaki H, Nishi S. Membranous nephropathy associated with IgG4-related systemic disease and without autoimmune pancreatitis. Clin Nephrol. 2009;71:173–8. doi: 10.5414/cnp71173. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Jenkins DA, Bellamy CO. Nephropathy in IgG4-related systemic disease. Am J Surg Pathol. 2006;30:1472–7. doi: 10.1097/01.pas.0000213308.43929.97. [DOI] [PubMed] [Google Scholar]
- Deshpande V, Chicano S, Finkelberg D, Selig MK, Mino-Kenudson M, Brugge WR, Colvin RB, Lauwers GY. Autoimmune pancreatitis: A systemic immune complex mediated disease. Am J Surg Pathol. 2006;30:1537–45. doi: 10.1097/01.pas.0000213331.09864.2c. [DOI] [PubMed] [Google Scholar]
- Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, Farnell MB. Diagnosis of autoimmune pancreatitis: The Mayo clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–6. doi: 10.1016/j.cgh.2006.05.017. quiz 934. [DOI] [PubMed] [Google Scholar]
- Saeki T, Saito A, Hiura T, Yamazaki H, Emura I, Ueno M, Miyamura S, Gejyo F. Lymphoplasmacytic infiltration of multiple organs with immunoreactivity for IgG4: IgG4-related systemic disease. Intern Med. 2006;45:163–7. doi: 10.2169/internalmedicine.45.1431. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. A new conceptualization for Mikulicz's disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335–40. doi: 10.1007/s10165-006-0518-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki T, Hamano H, Ochi Y, Komatsu K, Komiyama Y, Arakura N, Yoshizawa K, Ota M, Kawa S, Kiyosawa K. Autoimmune pancreatitis and complement activation system. Pancreas. 2006;32:16–21. doi: 10.1097/01.mpa.0000188308.75043.e4. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Jafri J, Bartosh SM, Karp SL, Adler SG, Meehan SM. ANCA-associated crescentic glomerulonephritis with mesangial IgA deposits. Am J Kidney Dis. 2000;36:709–18. doi: 10.1053/ajkd.2000.17615. [DOI] [PubMed] [Google Scholar]
- Allmaras E, Nowack R, Andrassy K, Waldherr R, van der Woude F, Ritz E. Rapidly progressive IgA nephropathy with anti-myeloperoxidase antibodies benefits from immunosuppression. Clin Nephrol. 1997;48:269–73. [PubMed] [Google Scholar]
- Morimoto J, Hasegawa Y, Fukushima H, Uesugi N, Hisano S, Saito T, Kaneoka H. Membranoproliferative glomerulonephritis-like glomerular disease and concurrent tubulointerstitial nephritis complicating IgG4-related autoimmune pancreatitis. Intern Med. 2009;48:157–62. doi: 10.2169/internalmedicine.48.1339. [DOI] [PubMed] [Google Scholar]
- Devey ME, Wilson DV, Wheeler AW. The IgG subclasses of antibodies to grass pollen allergens produced in hay fever patients during hyposensitization. Clin Allergy. 1976;6:227–36. doi: 10.1111/j.1365-2222.1976.tb01901.x. [DOI] [PubMed] [Google Scholar]
- van der Giessen M, Homan WL, van Kernbeek G, Aalberse RC, Dieges PH. Subclass typing of IgG antibodies formed by grass pollen-allergic patients during immunotherapy. Int Arch Allergy Appl Immunol. 1976;50:625–40. doi: 10.1159/000231566. [DOI] [PubMed] [Google Scholar]
- Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–6. [PubMed] [Google Scholar]
- Rowntree S, Platts-Mills TA, Cogswell JJ, Mitchell EB. A subclass IgG4-specific antigen-binding radioimmunoassay (RIA): Comparison between IgG and IgG4 antibodies to food and inhaled antigens in adult atopic dermatitis after desensitization treatment and during development of antibody responses in children. J Allergy Clin Immunol. 1987;80:622–30. doi: 10.1016/0091-6749(87)90017-0. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: A population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- Heiner DC. Significance of immunoglobulin G subclasses. Am J Med. 1984;76:1–6. doi: 10.1016/0002-9343(84)90313-9. [DOI] [PubMed] [Google Scholar]
- van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–22. [PMC free article] [PubMed] [Google Scholar]