Abstract
Cerebral malaria (CM) is a leading cause of death in Plasmodium falciparum infections. In the Plasmodium berghei ANKA (PbA) murine model, CM pathogenesis is associated with low nitric oxide (NO) bioavailability and brain microcirculatory complications, with a marked decrease in cerebral blood flow, vasoconstriction, vascular plugging by adherent cells, and hemorrhages. Using intravital microscopy through a closed cranial window, here we show that NO supplementation in the form of a NO donor (dipropylenetriamine NONOate [DPTA-NO]) prevented vasoconstriction and improved blood flow in pial vessels of PbA-infected mice. Arterioles and venules of smaller diameters (20–35.5 μm) showed better response to treatment than vessels of larger diameters (36–63 μm). Exogenous NO provided protection against brain hemorrhages (mean, 1.4 vs 24.5 hemorrhagic foci per section) and inflammation (mean, 2.5 vs 10.9 adherent leukocytes per 100 μm vessel length) compared with saline treatment. In conclusion, NO protection against CM is associated with improved brain microcirculatory hemodynamics and decreased vascular pathology.
Cerebral malaria (CM), a complication of malaria infection by Plasmodium falciparum, is a leading cause of mortality and neurologic impairment in endemic areas, especially in sub-Saharan Africa [1]. Although prompt antimalarial treatment is able to rescue most CM patients from death, still 10%–20% die and 25% of survivors may develop neurologic deficits [2]. Typical brain histopathologic findings of patients who died of CM include vascular plugging by parasitized red blood cells (RBCs), diffuse microhemorrhages, and the presence of leukocytes in blood vessels [3]. In vivo microvasculature studies of patients with severe malaria and particularly CM confirmed that vascular occlusion causes disturbance of blood flow, impaired perfusion, and ischemia [4, 5]. Human severe malaria has been reported to be associated with endothelial dysfunction related to low nitric oxide (NO) bioavailability, hypoarginemia, and elevated levels of cell-free hemoglobin [6]. In these patients, intravenous infusion of arginine improved endothelial function and NO production [6, 7]. CM patients also present systemic inflammation [8] and commonly develop acidosis attributable to poor tissue oxygenation [9].
The murine model of CM by Plasmodium berghei ANKA (PbA) shares many characteristics with human CM [10–12], including brain microhemorrhages, vascular plugging and occlusion predominantly by adherent leukocytes, systemic inflammation [8], acidosis, and brain ischemia [13, 14]. Murine CM is also associated with low NO bioavailability, hypoargininemia, and high levels of cell-free hemoglobin, and administration of exogenous NO prevented the development of the syndrome [15]. Although hypoargininemia may limit the capacity of the NO synthases to produce NO, the major cause for low NO bioavailability in malaria seems to be the NO-scavenging activity of cell-free hemoglobin [15] resulting from the destruction of parasitized RBCs. In this regard, severe malaria shares pathophysiologic features with other hemolytic states, such as the sickle cell vaso-occlusive crisis [16].
A major physiologic role of NO is as regulator of vascular tone [17]. Low NO bioavailability induces vasoconstriction and limits blood flow and oxygenation [16]. We have recently shown that murine CM is associated with constriction of pial vessels, marked decreases in cerebral blood flow, and eventually vascular collapse [18]. These findings show similarities with the vasospasm phenomenon observed after subarachnoid hemorrhage, in which hemoglobin derived from the blood clot induces vasoconstriction, and it is associated with poor outcome [19]. In addition, increased expression of endothelial cell adhesion molecules in the brain vasculature during PbA infection [20] leads to leukocyte sequestration that can cause vascular occlusion further impairing blood flow [18], as well as vascular damage [21] causing blood-brain barrier disruption and disseminated brain microhemorrhages. We hypothesize that the brain microcirculatory dysfunction observed in murine CM is linked to the low NO bioavailability and should be prevented by exogenous NO supplementation. In the present work we show indeed that administration of the NO donor dipropylenetriamine NONOate (DPTA-NO) ameliorates cerebral vascular and hemodynamic performance in PbA-infected mice, attenuating the decrease in pial blood flow, improving RBC velocities, and reducing vasoconstriction, in addition to affording marked protection against leukocyte accumulation in the brain and against brain hemorrhages.
METHODS
Mice, Infection, and DPTA-NO Treatment
Animal handling and care followed the National Institutes of Health Guide for Care and Use of Laboratory Animals. All protocols were approved by the La Jolla Bioengineering Institutional Animal Care and Use Committee. Eight- to 12-week-old C57Bl/6 (Jackson Laboratories) were inoculated intraperitoneally with 1 × 106 PbA parasites expressing the green fluorescent protein (PbA-GFP, a donation from the Malaria Research and Reference Reagent Resource Center—MR4; deposited by C.J. Janse and A.P. Waters; MR4 number: MRA-865). Parasitemia, body weight, and rectal temperature were checked daily from day 4. Parasitemia was checked by flow cytometry by detecting the number of fluorescent GFP-expressing parasitized RBCs in relation to 10,000 RBCs. CM was defined as the presentation of ≥1 of the following clinical signs of neurologic involvement: ataxia, limb paralysis, poor righting reflex, seizures, roll-over, and coma. In addition, a set of 6 simple behavioral tests (transfer arousal, locomotor activity, tail elevation, wire maneuver, contact righting reflex, and righting in arena) adapted from the SHIRPA protocol [22, 23] was used to provide a better estimate of the overall clinical status of the mice during infection. The performance in each test was assessed using a modified scoring system: 0 to 5 (transfer arousal), 0 to 4 (locomotor activity), 0 to 4 (tail elevation), 0 to 4 (wire maneuver), 0 to 3 (contact righting reflex), and 0 to 3 (righting in arena), and a composite score was built (scores ranging from 0 to 23, where 23 indicates maximum performance and 0 indicates complete impairment—usually coma). PbA-infected mice were treated with either saline or dipropylenetriamine NONOate (DPTA-NO; Cayman Chemical) 1mg per mouse in saline, intraperitoneally, twice a day starting on day 0. Levels of exhaled NO of saline-treated and DPTA-NO–treated mice were measured using a NOA 280i NO analyzer (Sievers).
Mice Cranial Window Preparation
The closed cranial window model was used as previously described [18, 24]. Briefly, mice were anesthetized with ketamine-xylazine and were administered dexamethasone 0.2mg/kg, carprofen 5mg/kg, and ampicillin 6mg/kg, subcutaneously, then placed on a stereotaxic frame (Stoelting) and the head immobilized using ear bars. The scalp was removed, lidocaine-epinephrine was applied on the periosteum, which was then retracted exposing the skull. A 3–4-mm diameter skull opening was made in the left parietal bone using a surgical drill (F.S.T.). The craniotomy was lifted away and saline-soaked gelfoam (Pfizer) was applied to the dura mater to stop any eventual small bleeding. The exposed area was covered with a 5-mm glass coverslip (EMS) secured with cyanocrylate-based glue (3M) and dental acrylic (Lang Dental). Carprofen and ampicillin were given daily for 3–5 days after recovery from surgery. Mice presenting signs of pain or discomfort were euthanized with 100 mg/kg of euthasol intraperitoneally.
Microvascular Experimental Setup
Two to three weeks after surgery, mice were lightly anesthetized with isoflurane (4% for induction and 1%–2% for maintenance) and held on a stereotaxic frame. A panoramic picture of the vessels under the window was taken, and then mice were transferred to an intravital microscope stage (customized Leica-McBain). Body temperature was maintained using a heating pad. By means of water-immersion objectives (×20), blood vessel images were captured (COHU 4815) and recorded on videotape.
Microhemodynamics
An image shear device (Image Shear; Vista Electronics) was used to measure vessel diameters (D), and RBC velocities (V) were measured off-line by cross-correlation (Photo Diode/Velocity Tracker Model 102B; Vista Electronics). Arterioles and venules were discriminated on the basis that arterioles show a divergent branching pattern, meaning that blood flows from one arteriole into two branches at each bifurcation; venules, conversely, collect blood and two branches meet at a confluent junction. Venules have a larger cross-sectional area than the corresponding arterioles, which results in a lower blood flow velocity and wall shear stress. Measurements of 6–10 pial venules (diameter range, 20–65 μm; velocity range, 2-4 mm/s) and 2–6 pial arterioles (diameter range, 20–64 μm; velocity range, 4–6 mm/s) were performed in each animal 5 hours after saline or DPTA-NO had been injected, and blood flow (Q) in each individual vessel was calculated using the equation: Q = V × (D/2)2. The intravital microscopy procedure was performed on day 0 and daily from day 4 of infection until the mice died or were euthanized.
Leukocyte-Endothelium Interaction
To quantify adherent and rolling leukocytes, anti-CD45-TxR antibodies (CalTag) were infused intravenously through the tail vein on day 6 of infection. Adherence was defined as cells remaining static for 30 seconds, and rolling as cells flowing at a velocity significantly lower than the centerline velocity of that vessel. For each selected vessel, leukocytes were quantified in a 100-μm–long section and, because leukocyte adherence affects vessels heterogeneously, to avoid bias we used for leukocyte quantification vessels preselected on day 0 of infection.
Brain Hemorrhage Quantification
On day 6 of infection, saline-treated and DPTA-NO-treated PbA-infected mice, as well as uninfected control mice, were anesthetized with ketamine-xylazine as described above. After bleeding by the retro-orbital sinus, the mouse was euthanized with euthasol 100 mg/kg intraperitoneally. The brain was carefully removed and conserved in formalin 10% until processing. Brains were cut in four slices (coronal plane) of 2-3 mm using a mouse brain blocker (David Kopf Instruments), and each slice was defined as frontal lobe/olfactory bulb, midbrain anterior, midbrain posterior, or cerebellum-brainstem. Each slice was embedded in paraffin, and 5-μm–thick sections were obtained at approximate 400-μm intervals (five sections per slice). Therefore, 20 sections were obtained and analyzed for each brain. Sections were mounted in glass slides and stained with hematoxylin-eosin, and the number and area of hemorrhages were quantified using an ocular grid calibrated with a ×400 magnification (field dimensions, 200 × 180 μm). The whole area of each section was similarly quantified with the grid calibrated at ×40 magnification.
Statistical Analysis
Results are presented as mean and standard error of the mean (SEM), unless otherwise noted. Results were considered statistically significant if P < .05. All parameters are reported as absolute values, except for blood flow, for which the values are presented as relative to baseline. The product limit method (Kaplan-Meier) was used to produce survival curves, and analysis of survival was conducted using the log-rank test. Hematocrit and adherent and rolling leukocytes were analyzed at a single time-point (day 6) using one-way analysis of variance (ANOVA) for nonparametric repeated measurements (Kruskal-Wallis test), and post hoc analyses were performed with the Bonferroni posttests. Parasitemia, exhaled NO, blood flow, vessel diameter, RBC velocity, and hemorrhages were analyzed at multiple time points using two-way ANOVA nonparametric repeated measurements and, when appropriate, post hoc analyses to baseline were performed with the Bonferroni posttests. Changes in vessel diameter and RBC velocity over time in individual groups (controls, saline-treated, or DPTA-NO-treated) were analyzed using one-way ANOVA for nonparametric repeated measurements, and post hoc analyses to baseline were performed using Bonferroni posttests. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software).
RESULTS
DPTA-NO Treatment Decreases CM Incidence in PbA-Infected Mice
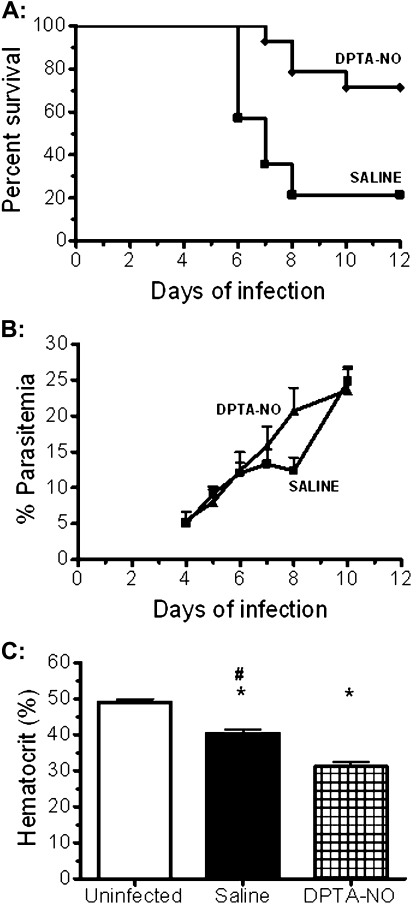
We initially performed an assessment of the protective effect of DPTA-NO on CM development. Although 11 (78%) of 14 saline-treated animals developed CM and died between days 6 and 8, only 4 (29%) of 14 DPTA-NO-treated mice died between days 7 and 10 (Figure 1A). The course of parasitemia in both groups was similar despite an apparent transient control of parasitemia on day 8 in the few survivor saline-treated mice (Figure 1B). DPTA-NO-treated mice showed lower hematocrits on day 6 of infection (Figure 1C). DPTA-NO-treated mice presented increased levels of exhaled NO between 1 and 5 hours after injection (data not shown), showing that NO was biologically available upon treatment.
Figure 1.
Incidence of cerebral malaria (CM), course of parasitemia and hematocrit in nitric oxide [NO] donor dipropylenetriamine NONOate (DPTA-NO)–treated Plasmodium berghei ANKA (PbA)-infected mice. A, Incidence of CM in saline-treated mice (n = 14) was 79%, compared with 29% in DPTA-NO–treated mice (n = 14) (P < .05). Statistical analysis was performed by the log-rank test. B, Although saline-treated mice that did not develop CM showed apparently lower parasitemia levels on day 8, the course of parasitemia was not significantly different between groups (statistical analysis performed by 2-way analysis of variance [ANOVA] with a post hoc Bonferroni test). C, Hematocrit levels in noninfected control mice (n = 8), and saline-treated (n = 14) and DPTA-NO-treated (n = 14) PbA-infected mice on day 6 of infection. *: P < .05 compared with control; #: P < .05 compared with DPTA-NO (statistical analysis performed by 1-way ANOVA and post hoc analyses performed using Bonferroni test). Data are from 3 experiments.
DPTA-NO Treatment Improves Cerebral Hemodynamics
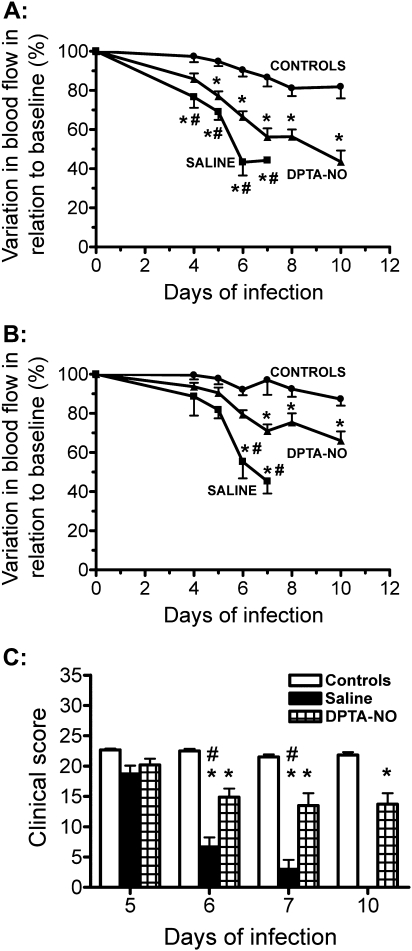
Cerebral hemodynamics were evaluated in 10 saline-treated (42 arterioles and 56 venules) and 16 DPTA-NO-treated (51 arterioles and 128 venules) PbA-infected mice, and in 10 uninfected control (36 arterioles and 66 venules) mice bearing implanted cranial windows. CM incidence was 90% (9 of 10) in the saline-treated group, with mice dying on days 6 and 7, versus 37% (6 of 16) in the DPTA-NO-treated group, with mice dying between days 6 and 10 (P < .05). DPTA-NO treatment did not prevent but significantly attenuated the decrease in blood flow in relation to saline-treated PbA-infected mice, particularly on days 6 and 7 when CM develops in most animals (Figure 2A and B). DPTA-NO-treated mice presented significant decreases in the composite clinical scores on day 6 and afterward in relation to uninfected control mice, but performance was significantly improved in relation to saline-treated mice (Figure 2C).
Figure 2.
Plasmodium berghei ANKA (PbA) infection leads to decreased blood flow in pial vessels, which is partially prevented by nitric oxide [NO] donor dipropylenetriamine NONOate (DPTA-NO) treatment. A, Arteriolar and B, venular blood flow in saline-treated (n = 10) or DPTA-NO-treated (n = 16) PbA-infected mice and in uninfected control mice (n = 10). Results are expressed as the percent change in relation to baseline measurements performed before infection. Number of vessels analyzed: uninfected controls: 36 arterioles and 66 venules; saline-treated: 42 arterioles and 56 venules; DPTA-NO–treated: 51 arterioles and 128 venules. *: P < .05 compared with controls; #: P < .05 compared with DPTA-NO. Statistical analysis was performed by 2-way analysis of variance (ANOVA) with a post hoc Bonferroni test. Data are from 3 experiments. C, Clinical composite scores in saline-treated and DPTA-NO–treated PbA-infected mice. *: P < .05 compared with controls; #: P < .05 compared with DPTA-NO. Statistical analysis was performed by 2-way ANOVA with a post hoc Bonferroni test. Data are from 2 experiments.
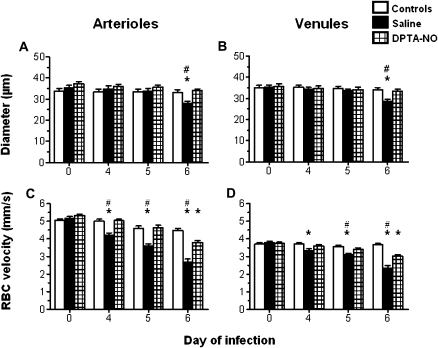
DPTA-NO Treatment Positively Impacts on Both Vessel Diameters and RBC Velocities
The decrease in blood flow in saline-treated PbA-infected mice was due to decreased arteriolar and venular diameters as well as to low RBC velocities (Figure 3). By contrast, vascular diameters were stable over time in DPTA-NO-treated PbA-infected mice (Figure 3A and B), and although RBC velocities showed a significant decrease only on day 6 the figures were still significantly improved in relation to saline-treated animals (Figure 3C and D). Arteriolar and venular diameters, as well as venular RBC velocities, were stable over time in uninfected control mice, whereas arteriolar RBC velocities presented a slight but significant decrease on day 6 in relation to day 0 (Figure 5C). In uninfected control mice and in DPTA-NO-treated mice that survived, arteriolar and venular diameters remained stable up to day 10 of infection (data not shown). Further decreases in arteriolar blood flow observed in these animals on days 8 and 10 (Figure 2) were due to additional decreases in RBC velocities.
Figure 3.
Nitric oxide [NO] donor dipropylenetriamine NONOate (DPTA-NO) treatment prevents vasoconstriction and attenuates the decrease in red blood cell (RBC) velocities in pial vessels during Plasmodium berghei ANKA (PbA) infection. Changes in arteriolar and venular diameters (A, B) and RBC velocities (C, D) in uninfected controls, and saline-treated and DPTA-NO-treated PbA-infected mice during PbA infection. Number of vessels analyzed: uninfected controls: 36 arterioles and 66 venules; saline-treated: 42 arterioles and 56 venules; DPTA-NO–treated: 51 arterioles and 128 venules. *: P < .05 compared with controls; #: P < .05 compared with DPTA-NO. Statistical analysis was performed by 2-way analysis of variance (ANOVA) for nonparametric repeated measurements, and post hoc analyses were performed using Bonferroni test. Data are from 3 experiments.
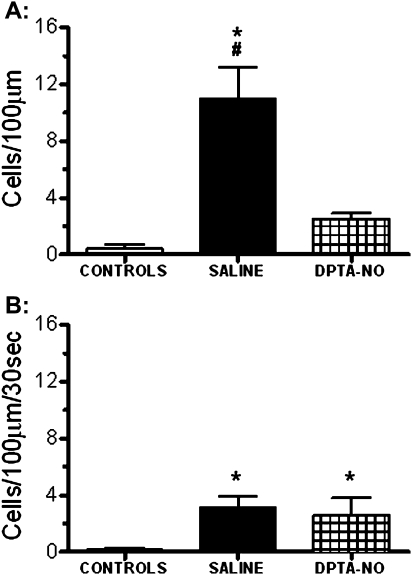
Figure 5.
Nitric oxide [NO] donor dipropylenetriamine NONOate (DPTA-NO) treatment decreases brain inflammation in Plasmodium berghei ANKA (PbA)-infected mice. Mean number of adherent (A) and rolling (B) leukocytes in pial venules of saline-treated (n = 6) and DPTA-NO–treated (n = 10) PbA-infected mice on day 6 of infection and in uninfected control mice (n = 7). Six to 10 pial venules were analyzed per animal. Saline-treated mice showed a large number of adherent cells, and DPTA-NO treatment markedly inhibited leukocyte adherence, which was not significantly different from uninfected controls. Rolling was less pronounced and not modified by DPTA-NO treatment at this stage. *: P < .05 compared with controls; #: P < .05 compared with DPTA-NO. Statistical analysis was performed by 1-way analysis of variance (ANOVA), and post hoc analyses were performed using Bonferroni test. Data are from 2 experiments.
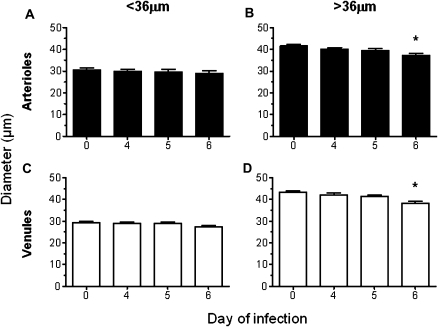
Effect of DPTA-NO Is Greater on Smaller Vessels
In the present study, the vessels analyzed ranged in diameter from 20 to 65 μm (mean, 36 μm). A more detailed analysis was conducted in the subgroups of vessels smaller or larger than 36 μm and revealed that smaller arterioles and venules of DPTA-NO-treated animals showed no significant variations in diameter between day 0 and day 6 (Figure 4A and C). Interestingly, larger arterioles and venules presented significantly decreased diameters on day 6, compared with those of day 0 (Figure 4B and D). Nevertheless, the decrease of 11% in mean diameter observed in larger vessels in the DPTA-NO-treated group was milder than that observed in the same group of vessels in saline-treated mice (19% for arterioles and 24% for venules).
Figure 4.
Beneficial effect of nitric oxide [NO] donor dipropylenetriamine NONOate (DPTA-NO) treatment on preventing vasoconstriction is more robust in smaller pial vessels. Arterioles and venules with diameters smaller than 36 μm (A, C) do not constrict during Plasmodium berghei ANKA (PbA) infection in DPTA-NO–treated mice, whereas those with diameter larger than 36 μm (B, D) showed a mean 11% decrease in diameter on day 6 of infection. Notably, PbA-infected mice treated with saline showed constriction in vessels of all sizes and, in those larger than 36 μm, the decrease in diameter was more severe (19%–24%) than in DPTA-NO–treated mice. Number of vessels analyzed: arterioles <36 μm: 20; arterioles >36 μm: 31; venules <36 μm: 65; venules >36 μm: 63. *: P < .05 compared with baseline (day 0). Statistical analysis was performed by 1-way analysis of variance (ANOVA) for nonparametric repeated measurements, and post hoc analyses to baseline were performed using Bonferroni test. Data are from 3 experiments.
DPTA-NO Treatment Decreases Leukocyte Accumulation in Pial Vessels
Saline-treated PbA-infected mice showed a high number of adherent leukocytes in pial vessels, which was markedly reduced by DPTA-NO treatment (Figure 5A; Figure 7D–I). Rolling leukocytes were also observed in infected animals but in a less prominent fashion than adherence (Figure 5B). A trend for decreased rolling observed in DPTA-NO-treated mice was offset by one of 10 mice presenting a high number of rolling leukocytes, resulting in no difference with saline-treated mice.
Figure 7.
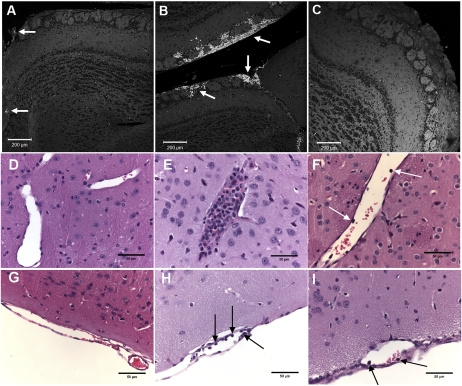
Prevention of brain hemorrhages and inflammation in Plasmodium berghei ANKA (PbA)-infected mice by nitric oxide [NO] donor dipropylenetriamine NONOate (DPTA-NO) treatment. A-C, Hemorrhages: pictures of hematoxylin-eosin–stained brain (olfactory bulb) sections of PbA-infected and control mice taken on a confocal microscope (Zeiss LSM 5 Pascal) using the HeNe laser 543nm and a ×10 objective, with red blood cells (RBCs) exhibiting autofluorescence (bright white dots). A, Olfactory bulb section of an uninfected control mouse; the tiny bright spots correspond to RBC inside microvessels; two larger vessels with several RBC are indicated by arrows. B, Extensive hemorrhages (arrows) in the olfactory bulb of a saline-treated mouse with cerebral malaria (CM), occupying the subarachnoid space and penetrating into the brain parenchyma. C, Olfactory bulb section of a DPTA-NO–treated mouse on day 6 of infection showing no hemorrhages. Magnification: ×100. D-I, Inflammation: D and G: parenchimal (D) and pial (G) vessels of uninfected control mice, showing no accumulation or adhesion of leukocytes. E and H: parenchimal (E) and pial (H) vessels of saline-treated PbA-infected mice; the parenchimal vessel is plugged with a large number of leukocytes, and the pial vessel shows several endothelium-adherent leukocytes (arrows). F and I: parenchimal (F) and pial (I) vessels of DPTA-NO–treated PbA-infected mice, showing reduced accumulation of leukocytes (arrows). Magnitude of leukocyte accumulation and adherence was heterogeneous among vessels in both saline-treated and DPTA-NO–treated mice (quantification of pial adherence is shown on Figure 5). All sections were stained with hematoxylin-eosin, magnification ×400.
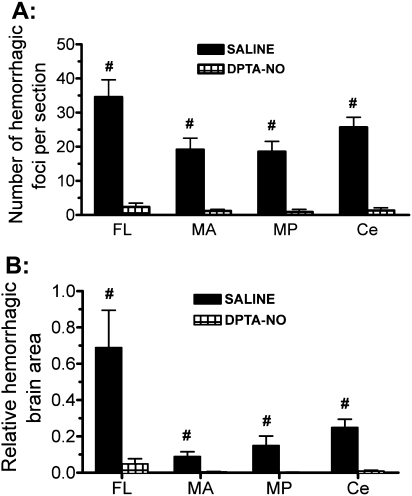
DPTA-NO Treatment Causes a Marked Decrease in Brain Microhemorrhage Incidence in PbA-Infected Mice
We quantified the number of, and the area affected by, microhemorrhages in saline-treated mice with CM and the effect of DPTA-NO treatment on microhemorrhage incidence in 20 serial sections spanning the whole brain. Mice with CM showed a large number of hemorrhages throughout the brain, being most numerous in the olfactory bulb and the cerebellum (Figures 6A and 7B). When adjusted by the area affected, the predominance of hemorrhages in the olfactory bulb was even more evident (Figure 6B). Treatment with DPTA-NO caused a marked reduction in microhemorrhage incidence, with some mice presenting no hemorrhages at all (Figures 6 and 7C).
Figure 6.
Nitric oxide [NO] donor dipropylenetriamine NONOate (DPTA-NO) treatment decreases brain hemorrhage incidence in Plasmodium berghei ANKA (PbA)-infected mice. Number of hemorrhages per section (A) and area occupied by hemorrhages in relation to the total area of the sections (B) in 4 regions of the brain in saline-treated (n = 9) and DPTA-NO–treated (n = 10) PbA-infected mice. After formalin fixation, the brain was cut (coronal plane) in 4 slices of 2–3mm each, and each region was defined as frontal lobe/olfactory bulb (FL); midbrain anterior (MA); midbrain posterior (MP); or cerebellum-brainstem (Ce). Five sections that were 400 μm apart were examined per region, with a total of 20 sections analyzed per mouse. #: P < .05 compared with DPTA-NO. Statistical analysis was performed by 1-way analysis of variance (ANOVA) for nonparametric repeated measurements, and post hoc analyses were performed using Bonferroni post tests. Data are from 2 experiments.
DISCUSSION
Murine CM is a complex syndrome with multifactorial pathogenesis. We have previously reported that murine CM is associated with a marked decrease in pial blood flow, due to vasoconstriction and low RBC velocities, with vascular collapse eventually occurring [18]. Low NO bioavailability has been shown to play a role in CM pathogenesis [15]. Here we show that exogenous NO administration, although not completely preventing microcirculatory dysfunction, significantly improved blood flow in PbA-infected mice. A major positive effect of exogenous NO was in preventing vasoconstriction.
Interestingly, the effect of DPTA-NO treatment on maintaining the vascular tone was more robust in smaller (20–35.5 μm) arterioles and venules, which largely preserved their baseline diameters during infection. Larger (36–63 μm) vessels of DPTA-NO-treated mice showed a significant decrease in diameters on day 6 of infection in relation to baseline, yet the intensity of this decrease was not as remarkable as that observed in vessels of similar diameters in saline-treated mice. The reason for this differential effect of exogenous NO in vessels of different sizes is not known. There are conflicting data on the differential sensitivity of vessels of different diameters to dilate or to constrict in response to NO stimulation or inhibition. Faraci [25] showed that larger rat brain arteries constricted more in response to L-NMMA than arterioles, but the dilatory effects of the NO-donor nitroprusside or acetylcholine were similar in large and small vessels. On the other hand, Kajita and coworkers [26] showed that, in rats, L-arginine caused stronger vasodilation and NG-Monomethyl-L-Arginine (L-NMMA) caused stronger vasoconstriction in smaller vessels originating from the basilar artery than in larger ones originating from the middle cerebral artery. You et al [27] suggested a greater sensitivity of larger rat brain arteries to NO-mediated dilation induced by ATP or constriction induced by nitro-L-arginine methyl ester. However, the NO-donor methylamine hexamethylene methylamine NONOate induced dilation in smaller, penetrating arterioles at a concentration lower than that needed to induce dilation in larger vessels. In addition, inhaled NO reversal of serotonin-induced constriction of pulmonary arteries was more efficient in smaller than in larger vessels [28]. Most of these studies were performed in ex vivo models, with vessel preparations from healthy animals, and analyzed the immediate effect of infusing NO stimulators or inhibitors; therefore, direct comparison with our findings is limited. The finding of greater effect of exogenous NO on smaller vessels in the specific setting of murine CM may have important implications for the understanding of pathogenesis and of the role of NO in different vascular beds in this model and therefore deserves further investigation.
Pial arterioles penetrate the cortex, supply blood to the whole brain, and are largely responsible for the cerebrovascular resistance, therefore playing a major role in the regulation of cerebral blood flow [29]. Relatively small decreases in pial arteriolar diameter can cause large increases in resistance and can dramatically decrease cerebral blood flow, leading to ischemia. The effect of exogenous NO in preventing pial vasoconstriction as well as the consequent increase in vascular resistance seems therefore to be a key event in the protective effect of DPTA-NO in preventing murine CM. DPTA-NO also ameliorated, but did not prevent, the decrease in RBC velocities, which was the main contributor for the decreased cerebral blood flow in DPTA-NO–treated mice. A number of factors may have contributed to the improved blood flow velocities in DPTA-NO–treated animals, including decreased vascular resistance and the lower hematocrit, which decrease shear stress and blood viscosity [30]. The reason that DPTA-NO–treated mice showed lower hematocrit levels than saline-treated mice on day 6 of infection is unclear. It has been previously shown that a short incubation of PbA-infected blood in medium with saturated concentrations of NO resulted in significant hemolysis mainly of noninfected RBCs [31]. Another potential explanation lies in the inhibitory effect of NO donors on erythropoiesis [32]. The effect of NO treatment on RBC velocities may also reflect a more systemic benefit of the treatment, for instance, on blood pressure. Indeed, DPTA-NO treatment, although causing a short-lived drop in blood pressure right after injection, in the long run actually offsets the hypotension observed during PbA infection [15].
Exogenous NO positively affected the arteriolar and venular vasculature. In addition, exogenous NO resulted in a marked decrease in leukocyte adherence to venules, therefore promoting venular perfusion. Indeed, adherent leukocytes in pial venules impair blood flow in PbA-infected mice with CM by further decreasing luminal diameters and blocking vessels [18]. The decreased leukocyte adherence in DPTA-NO–treated mice is likely due to the anti-inflammatory properties of NO, which modulates leukocyte-endothelial cell interactions and the expression of endothelial cell adhesion molecules [33, 34] and also decreases the levels of proinflammatory cytokine expression [15]. Improved vascular function and decreased intravascular inflammation help to explain the decreased vascular pathology, because the administration of exogenous NO showed a remarkable effect in preventing brain hemorrhages, showing that it protects the cerebral endothelium, preventing vascular damage. Additional mechanisms of endothelial protection may include prevention of apoptosis [35], as indeed PbA infection induces early apoptosis of endothelial cells in the brain [36]. We have previously proposed that brain hemorrhages may play an active role in CM pathogenesis by locally aggravating vasoconstriction and ischemia [18], in addition to other toxic actions of local release of free hemoglobin and heme on neurons [37]. Therefore, protection of the vascular endothelium in the brain and prevention of microhemorrhages are major pathways for the protection afforded by NO on CM.
DPTA-NO treatment did not completely prevent microvascular dysfunction, indicating that low NO bioavailability is not its sole cause. In fact, a number of mediators playing a role in CM pathogenesis are endowed with intrinsic vasoconstrictive properties, such as endothelin-1 [38–40], platelet factor 4 [41, 42], C5 [43–45], and even tumor necrosis factor α [46, 47], and other vasoactive molecules such as histamine [48], erythropoietin [49], and aspirin [41, 50] have also been shown to modify the expression of murine CM. However, the degree of vascular protection afforded by exogenous NO in the present study suggests that supplying NO by itself provides remarkable improvement in vascular function. The partial efficacy of DPTA-NO in reversing microcirculatory dysfunction may also be related to a suboptimal treatment protocol, with the need for adjustments to get a more effective improvement of the microcirculation. Indeed, the treatment protocol used (1mg of DPTA-NO every 12 hours) provides high levels of NO for a limited period of time. It is conceivable that NO-delivery systems providing lower doses of NO for extended periods of time would be more effective.
In summary, the decreased incidence of CM during exogenous NO therapy in the PbA-infected mice is associated with improved brain microvascular hemodynamics, particularly with decreased vasoconstriction and improved blood flow, decreased brain vascular inflammation, and decreased incidence of hemorrhages.
Funding
National Institutes of Health (grants R01-HL087290 and R01-AI082610 to L.J.M.C.); and Conselho Nacional de Desenvolvimento Científico e Tecnológico postdoctoral fellowship (Brazil; to G.M.Z.).
Acknowledgments
We thank Benoit Melchior of La Jolla Bioengineering Institute for capturing and processing the brain hemorrhage images in the confocal microscope.
References
- 1.Rowe AK, Rowe SY, Snow RW, et al. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol. 2006;35:691–704. doi: 10.1093/ije/dyl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pongponratn E, Turner GD, Day NP, et al. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:345–59. [PubMed] [Google Scholar]
- 4.Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis. 2009;199:263–71. doi: 10.1086/595735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 6.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo TW, Lampah DA, Tjitra E, et al. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis. 2009;200:1522–9. doi: 10.1086/644641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22:503–8. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–40. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 10.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;9:491–9. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 11.de Souza JB, Hafalla JC, Riley EM, Couper KN. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2009;137:755–72. doi: 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho LJM. Murine cerebral malaria: how far from human cerebral malaria? Trends Parasitol. 2010;26:271–2. doi: 10.1016/j.pt.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennan RP, Machado FS, Lee SC, et al. Reduced cerebral blood flow and N-acetyl aspartate in a murine model of cerebral malaria. Parasitol Res. 2005;96:302–7. doi: 10.1007/s00436-005-1349-z. [DOI] [PubMed] [Google Scholar]
- 14.Penet MF, Viola A, Confort-Gouny S, et al. Imaging experimental cerebral malaria in vivo: significant role of ischemic brain edema. J Neurosci. 2005;25:7352–8. doi: 10.1523/JNEUROSCI.1002-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gramaglia I, Sobolewski P, Meays D, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12:1417–22. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- 16.Conran N, Franco-Penteado CF, Costa FF. Newer aspects of the pathophysiology of sickle cell disease vaso-occlusion. Hemoglobin. 2009;33:1–16. doi: 10.1080/03630260802625709. [DOI] [PubMed] [Google Scholar]
- 17.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. 2009;61:62–97. doi: 10.1124/pr.108.000547. [DOI] [PubMed] [Google Scholar]
- 18.Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJM. Murine cerebral malaria is associated with a vasospasm-like microcirculatory dysfunction and survival upon rescue treatment is markedly increased by nimodipine. Am J Pathol. 2010;176:1306–15. doi: 10.2353/ajpath.2010.090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pluta RM, Hansen-Schwartz J, Dreier J, et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31:151–8. doi: 10.1179/174313209X393564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer PR, Van Der Heyde HC, Sun G, Specian RD, Granger DN. Regulation of endothelial cell adhesion molecule expression in an experimental model of cerebral malaria. Microcirculation. 2002;9:463–70. doi: 10.1038/sj.mn.7800159. [DOI] [PubMed] [Google Scholar]
- 21.Belnoue E, Kayibanda M, Vigario AM, et al. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol. 2002;169:6369–75. doi: 10.4049/jimmunol.169.11.6369. [DOI] [PubMed] [Google Scholar]
- 22.Lackner P, Beer R, Heussler V, et al. Behavioural and histopathological alterations in mice with cerebral malaria. Neuropathol Appl Neurobiol. 2006;32:177–88. doi: 10.1111/j.1365-2990.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- 23.Martins YC, Werneck GL, Carvalho LJ, et al. Algorithms to predict cerebral malaria in murine models using the SHIRPA protocol. Malar J. 2010;9:85–96. doi: 10.1186/1475-2875-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostany R, Portera-Cailliau C. A craniotomy surgery procedure for chronic brain imaging. J Vis Exp. 2008;15:680. doi: 10.3791/680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faraci FM. Role of endothelium-derived relaxing factor in cerebral circulation: large arteries vs. microcirculation. Am J Physiol. 1991;261:H1038–42. doi: 10.1152/ajpheart.1991.261.4.H1038. [DOI] [PubMed] [Google Scholar]
- 26.Kajita Y, Takayasu M, Suzuki Y, et al. Regional differences in cerebral vasomotor control by nitric oxide. Brain Res Bull. 1995;38:365–9. doi: 10.1016/0361-9230(95)02001-8. [DOI] [PubMed] [Google Scholar]
- 27.You J, Johnson TD, Marrelli SP, Bryan RM., Jr Functional heterogeneity of endothelial P2 purinoceptors in the cerebrovascular tree of the rat. Am J Physiol. 1999;277:H893–900. doi: 10.1152/ajpheart.1999.277.3.H893. [DOI] [PubMed] [Google Scholar]
- 28.Bentley J, Rickaby D, Haworth ST, Hanger CC, Dawson CA. Pulmonary arterial dilation by inhaled NO: arterial diameter, NO concentration relationship. J Appl Physiol. 2001;91:1948–54. doi: 10.1152/jappl.2001.91.5.1948. [DOI] [PubMed] [Google Scholar]
- 29.Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology. 2008;55:281–8. doi: 10.1016/j.neuropharm.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrales P, Tsai AG. Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. Am J Physiol Heart Circ Physiol. 2006;291:H2445–52. doi: 10.1152/ajpheart.00394.2006. [DOI] [PubMed] [Google Scholar]
- 31.Sobolewski P, Gramaglia I, Frangos JA, Intaglietta M, van der Heyde H. Plasmodium berghei resists killing by reactive oxygen species. Infect Immun. 2005;73:6704–10. doi: 10.1128/IAI.73.10.6704-6710.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsukahara H, Hori C, Hiraoka M, Mayumi M, Okada T, Gejyo F. Nitric oxide modulation of erythropoiesis in rats. Blood. 1997;90:473–4. [PubMed] [Google Scholar]
- 33.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zampolli A, Basta G, Lazzerini G, Feelisch M, De Caterina R. Inhibition of endothelial cell activation by nitric oxide donors. J Pharmacol Exp Ther. 2000;295:818–23. [PubMed] [Google Scholar]
- 35.Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr Mol Med. 2004;4:179–91. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]
- 36.Lackner P, Burger C, Pfaller K, et al. Apoptosis in experimental cerebral malaria: spatial profile of cleaved caspase-3 and ultrastructural alterations in different disease stages. Neuropathol Appl Neurobiol. 2007;33:560–71. doi: 10.1111/j.1365-2990.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 37.Lara FA, Kahn SA, da Fonseca AC, et al. On the fate of extracellular hemoglobin and heme in brain. J Cereb Blood Flow Metab. 2009;29:1109–20. doi: 10.1038/jcbfm.2009.34. [DOI] [PubMed] [Google Scholar]
- 38.Machado FS, Desruisseaux MS, Nagajyothi F, et al. Endothelin in a murine model of cerebral malaria. Exp Biol Med. 2006;231:1176–81. [PubMed] [Google Scholar]
- 39.Dietmann A, Lackner P, Helbok R, et al. Opposed circulating plasma levels of endothelin-1 and C-type natriuretic peptide in children with Plasmodium falciparum malaria. Malar J. 2008;7:253–9. doi: 10.1186/1475-2875-7-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desruisseaux MS, Machado FS, Weiss LM, Tanowitz HB, Golightly LM. Cerebral malaria: a vasculopathy. Am J Pathol. 2010;176:1075–8. doi: 10.2353/ajpath.2010.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava K, Cockburn IA, Swaim A, et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–87. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurrek MM, Winkler M, Robinson DR, Zapol WM. Platelet factor 4 injection produces acute pulmonary hypertension in the awake lamb. Anesthesiology. 1995;82:183–7. doi: 10.1097/00000542-199501000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Lackner P, Hametner C, Beer R, et al. Complement factors C1q, C3 and C5 in brain and serum of mice with cerebral malaria. Malar J. 2008;7:207–15. doi: 10.1186/1475-2875-7-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel SN, Berghout J, Lovegrove FE, et al. C5 deficiency and C5a or C5aR blockade protects against cerebral malaria. J Exp Med. 2008;205:1133–43. doi: 10.1084/jem.20072248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mügge A, Lopez JA, Heistad DD, Lichtlen PR. Vasoconstriction in response to activated leukocytes: implications for vasospasm. Eur Heart J. 1993;14(suppl I):87–92. [PubMed] [Google Scholar]
- 46.Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–12. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 47.Megyeri P, Abrahám CS, Temesvári P, Kovács J, Vas T, Speer CP. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci Lett. 1992;148:137–40. doi: 10.1016/0304-3940(92)90823-p. [DOI] [PubMed] [Google Scholar]
- 48.Beghdadi W, Porcherie A, Schneider BS, et al. Inhibition of histamine-mediated signaling confers significant protection against severe malaria in mouse models of disease. J Exp Med. 2008;205:395–408. doi: 10.1084/jem.20071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser K, Texier A, Ferrandiz J, et al. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J Infect Dis. 2006;193:987–95. doi: 10.1086/500844. [DOI] [PubMed] [Google Scholar]
- 50.Xiao L, Patterson PS, Yang C, Lal AA. Role of eicosanoids in the pathogenesis of murine cerebral malaria. Am J Trop Med Hyg. 1999;60:668–73. doi: 10.4269/ajtmh.1999.60.668. [DOI] [PubMed] [Google Scholar]