Abstract
A major goal of the worldwide malaria eradication program is the reduction and eventual elimination of malaria transmission. All currently available antimalarial compounds were discovered on the basis of their activity against the asexually reproducing red blood cell stages of the parasite, which are responsible for the morbidity and mortality of human malaria. Resistance against these compounds is widespread, and there is an urgent need for novel approaches to reduce the emergence of resistance to new antimalarials as they are introduced. We have established and validated the first high-throughput assay targeting the red blood cell parasite stage required for transmission, the sexually reproducing gametocyte. This assay will permit identification of compounds specifically targeting the transmission stages in addition to the asexual stage parasites. Such stage-specific compounds may be used in a combination therapy, reducing the emergence of resistance by blocking transmission of resistant parasites that may be selected in a patient.
Efforts for the development of vaccines and antimalarial drugs have traditionally targeted asexual stages of Plasmodium falciparum while virtually neglecting transmission stages. Asexual stages are the proliferative stages (generation time is ∼48 h) during the parasite cycle in the human host, and organisms in this stage can infect up to 20% of red blood cells (RBCs) in the body. Current evidence suggests that a small subset of late asexual RBC stages convert into sexually committed schizont stages [1]. After invasion of an RBC, the invasive daughter cells develop into male and female sexual stage parasites, termed gametocytes, which undergo fertilization after transmission to a mosquito vector. Although the molecular mechanisms leading to sexual conversion in vivo have not been characterized, it is assumed that multiple external stimuli activate a signaling cascade that feeds into some as-yet unknown master regulator determining the ratio of asexual versus sexual stages. After invasion of sexually committed parasites into RBCs, parasites differentiate into mature gametocytes over the course of 8–10 days.
The emergence of resistance against all currently used drugs and unsuccessful attempts to develop vaccines based on asexual blood stage antigens highlight the importance of targeting transmission stages. Indeed, the global campaign to eradicate malaria, initiated by The Bill and Melinda Gates Foundation in 2007, has recognized that inhibiting transmission needs to be a top priority in the eradication effort. In addition to a major focus on insecticide research, drugs and vaccines designed to block transmission of malaria from an infected person by killing or preventing maturation of the sexual form of the parasite are key new approaches. Current transmission blocking vaccine strategies are designed to induce human antibodies targeted against parasite antigens expressed during the early development of mosquito-stage parasites, thereby effectively blocking parasite development. Naturally acquired transmission-blocking immunity by this mechanism is well described (eg, in [2]). Alternatively, transmission-blocking strategies could be based on treating infected individuals with small molecules that inhibit sexual stage parasite development in the human host. To date, because of the technical difficulties in studying this stage, minimal effort has been invested in the development of drugs specifically active against transmission stages. Although artemisinin combination therapies can reduce transmission, other treatments are less effective. For example, sulphadoxine-pyrimethamine can increase the fraction of gametocytes in patients with malaria [3]. In addition, mature transmission stages are refractory to most antimalarial compounds [4]. Development of novel agents that work against transmission stages has been hindered by lack of an assay that would allow large-scale compound screening against sexual stages. This lack has been due to difficulties in achieving reproducible sexual conversion rates in vitro and to a lack of tools for the detection and quantification of individual gametocyte stages. The aim of this study is to overcome these experimental limitations through establishment of an in vitro assay for sexual conversion and early development with high-throughput capacity. We chose a whole cell assay to ensure that potential hits have at least minimal drug-like properties and acceptable solubility and cell permeability.
MATERIAL AND METHODS
P. falciparum In vitro Culture
The gametocyte-producing 3D7 parasite strain, the P2G12 clone and transgenic parasites derived from this clone were cultured in vitro essentially as described elsewhere [5]. Parasites were maintained in fresh type 0+ human erythrocytes (Research Blood Components) suspended at 4% hematocrit in complete medium containing 1% (w/v) AlbuMAX II (Invitrogen), 0.5 mL of gentamycin, 5.94 g of HEPES, 2.01 g of sodium bicarbonate, 0.05 g of hypoxanthine, and 10.44 g Roswell Park Memorial Institute 1640 per liter at a pH of 6.74. Cultures were kept in a controlled environment at 37°C in a gassed chamber at 5% CO2 and 1% O2.
Plasmid Construction and Generation of Transgenic Parasite Lines
DNA fragments spanning ∼2000 bp immediately upstream of the translational start site of the 3 selected gametocyte-specific markers were amplified from 3D7 genomic DNA template using primer pairs PF14_0744-s CctcgaggtcgacGTACAATCGTTATATTTG, PF14_0744-as CggatccgcggccgcGGAAGGAGGTATCCCGATATTG, PF14_0748-s CctcgaggtcgacCTTCATAGAACCGCCCTATAC, PF14_0748-as CggatccgcggccgcCCTCCTTGCTTCCTCTAC, PF10_0164-s CctcgaggtcgacGCATGAACGTTTTGTAAAC, and PF10_0164-as CggatccgcggccgcGTCGGAAATCGGATAAGAAG and were subcloned into the XhoI and BamHI sites in pGEM7z. After sequence confirmation, fragments were ligated into the SalI and NotI sites of the P. falciparum expression plasmid pHHK(-)-green fluorescent protein (GFP) [6] by replacing the HSP86 promoter upstream of the KAHRP signal sequence fused to green fluorescent protein (GFP). The resulting expression vectors pH744K(-)-GFP, pH748K(-)-GFP and pH164K(-)-GFP were transfected into highly synchronized 5% ring stage parasites of the P2G12 clone and selected using 10 nM WR99210 (kindly provided by Jacobus Pharmaceuticals), as described elsewhere [7].
Fluorescence Microscopy Analysis of Transgenic Lines
The stage-specific GFP expression pattern in the 3 transgenic lines 744/GFP, 748/GFP, and 164/GFP was analyzed using an inverted epifluorescence microscope (Zeiss). Immunofluorescence assays were performed in fixed cells using a mixture of 4% paraformaldehyde and 0.0075% glutaraldehyde, permeabilized with 0.1% Triton X - 100, as described elsewhere [8]. This gentle fixation method preserves GFP fluorescence and at the same time allows antibody binding. For colocalization experiments, we used polyclonal mouse antibodies against Pfs16 (1/5000). Parasite nuclei were labeled with DAPI and analyzed in mounting solution (Vectashield).
Flow Cytometry
Cytometry data were collected with a Beckman Coulter Cell Lab Quanta SC Flow Cytometry System with 2 different sources of excitation wavelength, a 488-nm laser and an ultraviolet (UV) light source optimized for excitation at 355/37 nm. The following band pass filters were used: 465/30 nm for the UV channel and 525 nm for the fluorescein isothiocyanate (FITC) channel. Initial gating in the UV channel for quantification of the parasite load was performed with unstained, uninfected RBCs incubated for the entire assay period. For this purpose, the nuclear dye Hoechst 33342 (Invitrogen) was titrated to a final concentration of 4 μmol/L. At this concentration, single (rings and gametocytes) and multinucleated parasites (trophozoites and schizonts) can be differentiated, whereas the background is minimal (see also Figure 3). Gating in the FITC channel for the quantification of gametocytemia was performed with fresh, uninfected RBCs (uRBCs) and uRBCs incubated for the entire assay period. The minimal autofluorescence present in fresh uRBCs thereby provided a clean control for initial gating (see also Figure 3). The used voltage settings are 6.17 for Hoechst detection and 5.68 for GFP detection. Data were collected with an acquisition of 50,000 events (Hoechst readout) and 400,000 events (GFP readout) per sample. Raw flow cytometry (FCM) data were processed with Quanta software and analyzed for half maximal inhibitory concentration (IC50) calculations using GraphPad Prism.
Figure 3.
Establishment of Hoechst and GFP detection by cytometry. Upper panel, Hoechst staining of infected red blood cells (iRBCs) at day 2 of the assay demonstrates that the majority of parasites have 1 nucleus (ie, rings and gametocytes [R + G]), whereas a subset is multinucleated (trophozoites and schizonts [T + S]). Both fresh uninfected red blood cells (uRBCs; from 4°C) and uRBCs incubated along for the assay period and stained with Hoechst were used as a negative control for gating. Lower panel, iRBCs at day 2 of the assay show GFP–positive population. This population is significantly higher than the background signal represented by autofluorescence in uRBC control samples that were either fresh or were incubated for the entire assay period.
Definition of Assay Parameters
For the calculation of signal-to-noise ratios, uRBCs and the RBCs infected with the parental P2G12 line were used as potential negative controls. P2G12 was seeded into 96 well plates and induced simultaneously with the positive control 164/GFP. Signal to noise ratios were calculated as the ratio of 164/GFP signal at day 2 divided by the uRBC or P2G12 signal from the FITC channel at each time point during the assay period. The sensitivity of microscopy and FCM to quantify parasitemia and gametocytemia in serial dilutions was compared as follows. 164/GFP-infected erythrocytes were seeded at 1% parasitemia in a 96-well plate (without drug) and handled as described below for the drug assay. On the day of analysis, these positive control wells were pooled and stained with 4 μmol/L Hoechst. The infected RBCs were subsequently diluted with uninfected RBCs in a one-sixth serial dilution. Each data point of 3 independent experiments was prepared in duplicates and analyzed in parallel by FCM (GFP and Hoechst) as well as fluorescence microscopy (GFP and Giemsa). The microscopy counting was performed blinded. The resulting measured parasite and gametocyte levels were plotted against the calculated values of the serial dilution and analyzed with GraphPad Prism (San Diego, CA) for corresponding R square values.
Drug Assays
Highly synchronous 3–4-h old ring–stage parasites were seeded at 1% parasitemia and 4% hematocrit in 110 μL of medium into flat-bottom 96-well plates (Microtest Tissue Culture Plates; Becton Dickinson). Twenty-four hours later, the culture medium was increased to 220 μL to trigger sexual conversion by a decrease in the hematocrit level from 4% to 2%. Serial dilutions of compounds were either added at this point of the assay (compound incubation period, 72 h) or 24 h later (incubation period, 48 h). Compounds were dissolved in dimethyl sulfoxide (DMSO), with the exception of chloroquine and methylene blue, which were dissolved in water. DMSO alone was also tested to account for possible DMSO-specific effects. The final concentration of DMSO ranged from 0.3% (Primaquine) to 0.002%, depending on the concentration of the used drug stock. For each compound, 2 separate experiments were performed. On the day of analysis, cells were incubated for 20 min at 37°C with the permeable nuclear dye Hoechst 33342 at a final concentration of 4 μmol/L.
RESULTS
Generation of Transgenic Parasites Expressing a Fluorescent Reporter Only in Gametocytes
There is great variability between different in vitro adapted cell lines in their capability to produce transmission stages. We screened individual clones of the reference strain 3D7 to identify parasites that are capable of sexual conversion (data not shown). Several gametocyte-producing clones were identified, and one of those, here termed P2G12, was selected for further studies. We introduced a reporter gene into the P2G12 background for quantification of sexual conversion and early gametocyte development in live cells. The morphological differentiation associated with sexual development allows identification of gametocytes only after 36 h of development. Parasites in earlier stages can only be distinguished from asexual parasites using specific antibodies, precluding studies with live parasites. To establish unambiguous identification of early gametocyte stages in live cells, we generated a series of transgenic parasite lines expressing a fluorescent reporter under a gametocyte-specific promoter. Several global transcriptional analyses have identified and validated markers of early sexual development in P. falciparum [9–11]. We amplified 2000 bp of the upstream regions of 3 such markers (PF14_0744 [9], PF14_0748 [9], and PF10_0164 [10]), cloned them into a P. falciparum GFP expression vector, and transfected the P2G12 line with each of these plasmids (Figure 1A), generating parasite lines 744/GFP, 748/GFP, and 164/GFP, respectively. Live fluorescence microscopy experiments demonstrate that GFP is expressed in these lines during sexual development. These experiments also demonstrated an expression peak at stage I for 744/GFP (Figure 1B) and 748/GFP (Figure 1B) and at stage II or later for 164/GFP (Figure 1C). We selected the 164/GFP line for additional analysis and confirmed that the stage specificity of GFP expression matched that of the transmission stage marker Pfs16 using specific antibodies for detection (Figure 1D).
Figure 1.
Characterization of transgenic parasite lines expressing GFP under different gametocyte-specific promoters. A—C, GFP expression during sexual development. Transgenic parasites were cultured in T75 flasks, induced for in vitro sexual development by reduction of hematocrit as described elsewhere [13], and analyzed live by fluorescence microscopy. Morphological differentiation of individual gametocyte stages (stage I–V) was based on the classification by Hawking et al [43]. GFP was expressed throughout sexual development starting at sexually committed schizont stages. In 744/GFP (A) and 748/GFP (B), fluorescence peaks during early development. In 164/GFP (C), fluorescence intensity increases during development, either through elevated expression levels and/or accumulation of GFP over time. Stage I gametocytes are indistinguishable from asexual trophozoites except for the presence of dispersed hemozoin crystals in the former. Stage II gametocytes adopt a characteristic morphology, with an elongated wheat-shaped body and subpellicular extensions. SI/SII, gametocyte stage I and II. D, Co-localization of GFP fluorescence in 164/GFP with a transmission stage marker. To determine whether GFP expression was indeed gametocyte specific, we performed immunofluorescence assay in induced cultures using antibodies against the sexual stage antigen Pfs16 for co-localization. Two representative panels are shown (D), with a stage I gametocyte (top) and a stage III gametocyte (bottom).
Establishment of a Gametocyte Induction Protocol With High-Throughput Capacity
The 164/GFP line was used to develop a gametocyte induction protocol that provides a high-throughput readout by flow cytometry. Sexual conversion can be induced in vitro by a sudden decrease in hematocrit concentration and a high parasite load, reflecting conditions of physiological stress in the human host that are known to correlate with increased numbers of transmission stages in the blood circulation [12]. However, conversion rates can vary widely between experiments. We aimed to reduce this variation and increase reproducibility by starting with a highly synchronized parasite culture, using more defined media components, and optimizing the sexual conversion protocol. In a series of experiments, we altered individual parameters of the original protocol and quantified the increase in total parasite load and sexual stages over time by live fluorescence microscopy. First, we replaced human serum, a standard component of the culture medium in current gametocyte induction protocols [13, 14], with a lipid-rich bovine serum albumin concentrate (AlbuMAX II) that is commonly used for asexual parasite culture [15]. We determined that a concentration of 1% AlbuMAX (w/v; compared with 0.5% for asexual culture) in the complete medium results in sexual conversion rates superior to human serum without negatively affecting asexual growth rates (Figure S1A). We then evaluated different starting parasite loads to unlink anemia and high parasite loads as combined triggers of sexual conversion, and reduce between-assay variability (Figure S1B). We also varied the time point of the decrease in hematocrit concentration on the sexual conversion rate to optimize the signal-to-background ratio. The final assay protocol is shown in Figure 2.
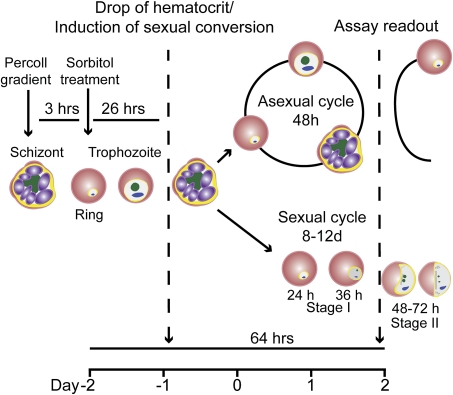
Figure 2.
Assay protocol in 96-well plates. Parasites were first treated with sorbitol to obtain a loosely synchronous ring stage population. After 1 cycle, sequential purification of late stages on a Percoll gradient and treatment with sorbitol 3 h later, after reinvasion, resulted in a highly synchronous population of ring-stage parasites. These were seeded at 1% parasitemia and 4% hematocrit into a 96-well plate. After 24 h, the hematocrit was reduced to 2% (ie, sexual conversion induced) by doubling the volume of culture medium from 110 μL to 220 μL. After another 72 h and 1 asexual replication cycle, cells from each well were analyzed to quantify total parasite and gametocyte loads. The timeline represents the assay period (96 h) performed in 96-well plates.
Quantification of Sexual Conversion by Flow Cytometry
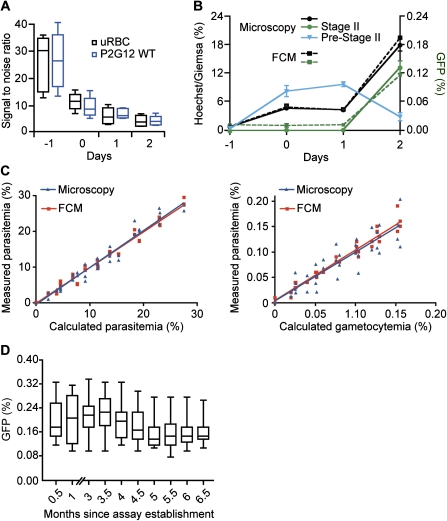
To make this protocol compatible for a high-throughput screen we established conditions for cytometry-based quantification of parasite and gametocyte loads (Figure 3). To quantify parasite loads in live cells by flow cytometry, we took advantage of the fact that mature RBCs lack a nucleus. Incubation with a membrane-permeable DNA dye, such as Hoechst 33342, therefore stains only parasite-infected erythrocytes [16]. Hoechst labeling as quantified by UV fluorescence intensity correlates with the number of nuclei per infected RBC and therefore allows differentiation between individual parasite stages (Figure 3, upper panel). Rings, early trophozoites, and gametocytes have 1 nucleus, whereas parasites in later asexual stages are undergoing mitosis and have up to 16 nuclei. The number of sexual stages was determined with the FITC channel to detect GFP fluorescence. To establish a negative control for assay purposes, we incubated uRBCs for the entire assay period. Although there is virtually no background in the UV channel, we observed significant background in the FITC channel (Figure 3, lower panel). Autofluorescence of RBCs is due to oxidative damage, hemoglobin degradation, and hemozoin formation in the infected RBC [17]. To define the negative control for GFP detection, we quantified fluorescence levels of uRBCs and P2G12-infected RBCs during the assay period and calculated signal–to-noise ratios (Figure 4A). These experiments demonstrated that uRBCs incubated for the entire assay period are the proper negative control for the assay. On the basis of this negative control, the z-factor (± standard deviation) [18] for Hoechst was determined to be 0.77 ± 0.01, and for GFP, it was 0.24 ± 0.03. To determine whether FCM quantification provides readout directly proportional to parasite growth and sexual conversion, we compared total parasite and gametocyte loads with microscopy counts (Figure 4B). Although total parasite counts correlated well during the entire assay period, the FITC channel only detects significant numbers of gametocytes at day 2. Stratifying the corresponding microscopy counts by stage revealed that FCM only detects stage II parasites, which represent the vast majority of counts at day 2 (Figure 4B). Earlier stages are not detected above the negative control, presumably due to the relatively weak GFP fluorescence (see also Figure 1C). Serial dilutions of infected red blood cells (iRBC) at day 2 of the assay demonstrated a linear relationship within the range of the assay between both stage II numbers and total parasite loads as determined by microscopy compared with FCM (Figure 4C). Importantly, sexual conversion rates did not decrease during the course of assay establishment and validation (Figure 4D), suggesting that the P2G12 clone and the transgenic versions thereof (eg, 164/GFP) harbor a stable genotype with respect to their capability to produce gametocytes.
Figure 4.
Definition of assay parameters. A, Signal-to-noise ratios using uninfected red blood cells (uRBCs) and the parental P2G12 line as potential negative controls. Signal-to-noise ratios were calculated described in Materials and Methods. The ratio drops from 30-fold (when using cells from day -1 as negative control) to 5-fold (when using cells from day 2). Each data point represents at least 3 biological replicates done with technical duplicates. B, Comparison of microscopy and flow cytometry (FCM) for the quantification of parasite and gametocyte loads. An increase in parasite concentration from initially 1% to ∼18% during the assay period of 96 h is detected both by counting Giemsa-stained blood smears (circles, black solid line) and by quantifying a Hoechst-stained sample of the same culture by FCM (cubes, black dashed line). Sexual stages, defined as the GFP-positive subpopulation of infected red blood cells, increased from background levels of ∼0.005% to ∼0.15% at day 2, representing a 30-fold absolute or 5-fold relative increase (correcting for replication rate). FCM detects only GFP expression of stage II gametocytes (cubes, green dashed line), whereas the weaker fluorescence of earlier stages (ie, the sexually committed ring and stage I; termed Pre-Stage II in the graph) is only detectable by microscopy (triangle, solid blue). Stage II gametocyte numbers quantified by microscopy (circles, solid green line) correlate well with GFP-positive cells determined by FCM. Each data point represents at least 3 biological replicates done with technical triplicates. The graphs show mean values of the experiments, whereas the error bars represent the standard error of the mean. C, Sensitivity of microscopy and FCM to quantify parasite load (left panel) and gametocyte load (right panel). Each data point was prepared in duplicates as described in Materials and Methods and analyzed in parallel by FCM (GFP and Hoechst) as well as fluorescence microscopy (GFP and Giemsa). The resulting measured parasite and gametocyte loads were plotted against the calculated values of the serial dilution. The corresponding R2 values are as follows: 0.9659 by microscopy and 0.9798 by FCM for parasite load; 0.8345 by microscopy and 0.9610 by FCM for gametocyte load. D, The transgenic parasite line 164/GFP shows no loss of sexual conversion during continuous culture. The box-and-whisker plot represents all assay positive control wells over the time period when drug assays were performed. The sample sizes are as follows: month 0.5, n = 16; month 1, n = 18; month 3, n = 36; month 3.5, n = 22; month 4, n = 32; month 4.5, n = 39; month 5, n = 29; month 5.5, n = 45; month 6, n = 48; and month 6.5, n = 24. No experiments were performed in months 1.5–2.5.
Assay Validation With a Set of Known Antimalarial Compounds
The assay was validated by determining IC50 concentrations for some of the main antimalarial compounds that are currently in use. These are chloroquine and mefloquine (which are quinine based), 8-aminoquinolines (primaquine), and dihydroartemisinin, as well as the hydroxynaphthoquinone atovaquone, the phenothiazin dye methylene blue, and the antimicrobial biocide triclosan for comparison. To differentiate the effect of the drug against asexual stages, sexual conversion and early sexual stages, we used 2 different protocols for each experiment. Specifically, drugs were added either when the hematocrit was reduced at the late trophozoite stage (day -1) or 24 h later at the early ring stage (day 0). As shown in Table 1, IC50 values against asexual stages are in the same range as those from previous assays using either a hypoxanthine incorporation assay or cytometric assays with nuclear dyes [19–21]. However, we noted that IC50 values were generally higher when drugs are added at day 0 compared with drug addition at day -1. At least part of this reduced susceptibility is probably due the inoculum effect, which refers to an increase in the amount of drug necessary to inhibit growth in the presence of higher numbers of parasites [22]. Importantly, we provide the first experimental evidence that the quinine-based drugs as well as arteminisin are equally effective against asexual stages and early sexual stages (Table 1 and Figure 5A and B). Because these drugs (ie, artemisinin, chloroquine, and mefloquine) showed a similar effect on sexual stages when added either at day -1 or at day 0, it is likely that early sexual development rather than conversion is targeted. Functional and morphological similarities of early gametocytes with asexual stage parasites suggest that the effect of these drugs is due to the same mechanism through which they target asexual stages. Other compounds were significantly less active or ineffective against early sexual–stage parasites. For example, the presence of atovaquone or methylene blue, particularly at sublethal doses, appeared to increase sexual conversion rates (Table 1 and Figure 5C and D). This supports the hypothesis that activation of a stress response, either drug or host induced, is linked to sexual conversion [23–25]. Primaquine, on the other hand, is known to be highly active against hypnozoites and liver-stage schizonts in Plasmodium vivax and Plasmodium ovale, as well as gametocytes of all the 4 human malaria species [4]. However, primaquine is inactive under in vitro conditions while transformed to an active metabolite in the mammalian host [26].
Table 1.
Half Maximal Inhibitory Concentration (IC50) Values Representing the Effect of All Antimalarials Tested on Gametocytes and Parasitemia
| Drug addition |
||||||
| Asexual stages |
Sexual stages |
|||||
| Antimalarial classs | Compound | Day -1 | Day 0 | Day -1 | Day 0 | |
| IC50 | Pub. IC50 | IC50 | IC50 or effect | IC50 or effect | ||
| Quinoline-containing drugs | Chloroquine | 20 ± 8.5 | 10-30 | 40.4 ± 10.7 | 31 ± 5.8 | 42.2 ± 13.5 |
| Mefloquine | 36.3 ± 11.6 | 50 | 80 ± 24.8 | 42.4 ± 23.9 | 94.8 ± 34.8 | |
| Artemisinins | DHA | 6.4 ± 1.3 | 0.8–3 | 28.9 ± 2.6 | 10.2 ± 2.3 | 26.3 ± 9.9 |
| Naphthalenes | Atovaquone | 1.8 ± 0.2 | 0.9–3 | 1.66 ± 0.5 | Proinducing (188% at 15 nM) | Slight reduction |
| 8-Aminoquinolinea | Primaquine | 10.7 ± 4.8 | 15 | 30.5 ± 17.6 | No effect | No effect |
| Phenothiazine dye | Methylene blue | 16.5 ± 3.5 | 4 | 24.1 ± 7.4 | Proinducing (276% at 125 nM) | Proinducing (338% at 31 nM) |
| Antibiotic or antifungala | Triclosan | 5.3 ± 0.7 | 1.1 | 7.6 ± 0.7 | 4 ± 2.1 | 7.5 ± 4.7 |
NOTE. IC50 values are in nM, unless otherwise indicated. Each data point represents at least 3 biological replicates done in technical duplicates. Published IC50 data for comparison are derived from [19, 20, 44]. The data are represented as mean values (± standard deviation) for the biological replicates. The term “slight reduction” indicates minor reduction in gametocyte load, which is not sufficient to determine classic IC50 curves.
IC50 values are in μM.
Figure 5.
Half maximal inhibitory concentration curves representing the effect of chloroquine (A), DHA (B), atovaquone (C), and methylene dlue (D) on gametocyte and parasite loads. Each assay represents at least 3 biological replicates with 2 technical replicates per plate. The graphs show mean values of the experiments, whereas the error bars represent the standard error of the mean. conc., concentration.
DISCUSSION
The goal of eliminating and eventually eradicating human malaria will only be possible if the transmission cycle can successfully be interrupted. This requires radical cure in symptomatic patients and mass drug administration to eliminate multiple stages of the parasite on a population level [27, 28]. The only available compounds that are known to efficiently eliminate all gametocyte stages are 8-aminoquinolines, including primaquine and tafenoquine. However, they can cause hemolytic anemia in people with glucose-6-phosphate dehydrogenase deficiency [29]. It is therefore critical to develop a new generation of drugs with favorable toxicity profiles that specifically target malaria transmission stages. The recent establishment of cell-based high-throughput screens that quantify the effect of compounds on asexual parasite proliferation in RBCs has opened new avenues for drug development against human malaria [20, 30]. Moreover, the widespread application of transfection technology in the malaria field provides the basis for a next generation of cell-based assays targeting specific pathways or life cycle stages. We have established a reproducible sexual conversion protocol using a transgenic Plasmodium strain with a high and stable degree of conversion to gametocytes that expresses a gametocyte-specific reporter.
The observed differential in vitro activity against early gametocyte stages confirms data from field studies: for susceptible parasites, treatment with artemsinin and derivatives as well as chloroquine significantly reduces the prevalence and density of malaria transmission stages [31–34]. Emergence of resistance, however, is usually paralleled by increased transmission rates [35]. This is likely due to both selection on a genetic level and increased conversion rates at sublethal drug concentrations. Altogether, an initial screen of known antimalarials demonstrates that we have met the 2 main goals of this study: (1) we established a high-throughput screen that performs as well as published screen formats in quantifying the impact of drugs on asexual parasite growth, and (2) the same assay allows highly reproducible quantification of specific drug effects on sexual conversion and early sexual development.
To our knowledge this is the first targeted cell-based high throughput assay in a eukaryotic pathogen. It may serve as a template for the development of similar assays targeting either particular cellular pathways or differentiation processes for drug development purposes in human pathogens, such as Toxoplasma, Trypanosoma, and Leishmania species. In malaria, the assay will finally provide a basis for the development of drugs that are specifically targeted toward blocking the development of transmission stages. Because the fluorescent reporter is expressed throughout sexual development, it will be possible to perform secondary assays that quantify the effect of candidate compounds on the transmission-competent mature gametocytes. Identification of compounds that modulate sexual conversion will also provide biological probes to perform mechanistic studies on this important step in the parasite cycle, which is least understood. Several candidate pathways have been implicated in determining sexual conversion rates, however, a small-scale screen of activators and inhibitors of G protein-coupled receptor (GPCR)-dependent signaling could not confirm previous reports of an involvement of this pathway in conversion [36–42] (Figure S1C). Considering that current antimalarials have a very limited range of cellular targets, we anticipate that a large-scale high-throughput screen with structurally diverse small-molecule libraries have the potential to uncover a wealth of novel chemotypes and targets for future intervention strategies.
Supplementary Data
Supplementary data are available at http://jid.oxfordjournals.org online.
Funding
A Grand Challenge Explorations grant from The Bill and Melinda Gates Foundation (M.M.), a New Investigator Award from the Massachusetts Life Science Center (M.M.), and a postdoctoral Feodor Lynen fellowship from the Alexander von Humboldt Foundation (K.B.).
Supplementary Material
Acknowledgments
We would like to thank Sergio Wittlin for critical reading of the manuscript and helpful discussions, and Natasha Barteneva and Howard Shapiro for technical help with flow cytometry.
References
- 1.Bruce MC, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100:191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 2.Healer J, McGuinness D, Carter R, Riley E. Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology. 1999;119:425–33. doi: 10.1017/s0031182099005041. [DOI] [PubMed] [Google Scholar]
- 3.Sowunmi A, Nkogho OO, Okuboyejo TM, Gbotosho GO, Happi CT, Adewoye EO. Effects of mefloquine and artesunate mefloquine on the emergence, clearance and sex ratio of Plasmodium falciparum gametocytes in malarious children. Malar J. 2009;8:297. doi: 10.1186/1475-2875-8-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher GA. Antimalarial drugs and the mosquito transmission of Plasmodium. Int J Parasitol. 1997;27:975–87. doi: 10.1016/s0020-7519(97)00079-9. [DOI] [PubMed] [Google Scholar]
- 5.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 6.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–3. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Kirkman LA, Wellems TE. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc Natl Acad Sci U S A. 1996;93:1130–4. doi: 10.1073/pnas.93.3.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonkin CJ, van Dooren GG, Spurck TP, et al. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol Biochem Parasitol. 2004;137:13–21. doi: 10.1016/j.molbiopara.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Eksi S, Haile Y, Furuya T, Ma L, Su X, Williamson KC. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:90–9. doi: 10.1016/j.molbiopara.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Silvestrini F, Bozdech Z, Lanfrancotti A, et al. Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:100–10. doi: 10.1016/j.molbiopara.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Young JA, Fivelman QL, Blair PL, et al. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Price R, Nosten F, Simpson JA, et al. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60:1019–23. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 13.Fivelman QL, McRobert L, Sharp S, et al. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol. 2007;154:119–23. doi: 10.1016/j.molbiopara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Ponnudurai T, Lensen AH, Meis JF, Meuwissen JH. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology. 1986;93:263–74. doi: 10.1017/s003118200005143x. [DOI] [PubMed] [Google Scholar]
- 15.Cranmer SL, Magowan C, Liang J, Coppel RL, Cooke BM. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1997;91:363–5. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- 16.Grimberg BT, Erickson JJ, Sramkoski RM, Jacobberger JW, Zimmerman PA. Monitoring Plasmodium falciparum growth and development by UV flow cytometry using an optimized Hoechst-thiazole orange staining strategy. Cytometry A. 2008;73:546–54. doi: 10.1002/cyto.a.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khandelwal S, Saxena RK. Age-dependent increase in green autofluorescence of blood erythrocytes. J Biosci. 2007;32:1139–45. doi: 10.1007/s12038-007-0115-z. [DOI] [PubMed] [Google Scholar]
- 18.Sui Y, Wu Z. Alternative statistical parameter for high-throughput screening assay quality assessment. J Biomol Screen. 2007;12:229–34. doi: 10.1177/1087057106296498. [DOI] [PubMed] [Google Scholar]
- 19.Wein S, Maynadier M, Tran Van Ba C, et al. Reliability of antimalarial sensitivity tests depends on drug mechanisms of action. J Clin Microbiol. 2010;48:1651–60. doi: 10.1128/JCM.02250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baniecki ML, Wirth DF, Clardy J. High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob Agents Chemother. 2007;51:716–23. doi: 10.1128/AAC.01144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–8. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gluzman IY, Schlesinger PH, Krogstad DJ. Inoculum effect with chloroquine and Plasmodium falciparum. Antimicrob Agents Chemother. 1987;31:32–6. doi: 10.1128/aac.31.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daily JP, Scanfeld D, Pochet N, et al. Distinct physiological states of Plasmodium falciparum in malaria-infected patients. Nature. 2007;450:1091–5. doi: 10.1038/nature06311. [DOI] [PubMed] [Google Scholar]
- 24.Peatey CL, Skinner-Adams TS, Dixon MW, McCarthy JS, Gardiner DL, Trenholme KR. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J Infect Dis. 2009;200:1518–21. doi: 10.1086/644645. [DOI] [PubMed] [Google Scholar]
- 25.Le Roch KG, Johnson JR, Ahiboh H, et al. A systematic approach to understand the mechanism of action of the bisthiazolium compound T4 on the human malaria parasite, Plasmodium falciparum. MC Genomics. 2008;9:513. doi: 10.1186/1471-2164-9-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basco LK, Bickii J, Ringwald P. In-vitro activity of primaquine against the asexual blood stages of Plasmodium falciparum. Ann Trop Med Parasitol. 1999;93:179–82. doi: 10.1080/00034989958663. [DOI] [PubMed] [Google Scholar]
- 27.Crockett M, Kain KC. Tafenoquine: a promising new antimalarial agent. Expert Opin Investig Drugs. 2007;16:705–15. doi: 10.1517/13543784.16.5.705. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko A, Taleo G, Kalkoa M, Yamar S, Kobayakawa T, Bjorkman A. Malaria eradication on islands. Lancet. 2000;356:1560–4. doi: 10.1016/S0140-6736(00)03127-5. [DOI] [PubMed] [Google Scholar]
- 29.Beutler E, Duparc S, Group GPDW. Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77:779–89. [PubMed] [Google Scholar]
- 30.Plouffe D, Brinker A, McNamara C, et al. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A. 2008;105:9059–64. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okell LC, Drakeley CJ, Bousema T, Whitty CJ, Ghani AC. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med. 2008;5:e226. doi: 10.1371/journal.pmed.0050226. ; discussion e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider P, Bousema T, Omar S, et al. (Sub)microscopic Plasmodium falciparum gametocytaemia in Kenyan children after treatment with sulphadoxine-pyrimethamine monotherapy or in combination with artesunate. Int J Parasitol. 2006;36:403–8. doi: 10.1016/j.ijpara.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 33.von Seidlein L, Jawara M, Coleman R, Doherty T, Walraven G, Targett G. Parasitaemia and gametocytaemia after treatment with chloroquine, pyrimethamine/sulfadoxine, and pyrimethamine/sulfadoxine combined with artesunate in young Gambians with uncomplicated malaria. Trop Med Int Health. 2001;6:92–8. doi: 10.1046/j.1365-3156.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 34.Sutanto I, Supriyanto S, Ruckert P, Purnomo, Maguire JD, Bangs MJ. Comparative efficacy of chloroquine and sulfadoxine-pyrimethamine for uncomplicated Plasmodium falciparum malaria and impact on gametocyte carriage rates in the East Nusatenggara province of Indonesia. Am J Trop Med Hyg. 2004;70:467–73. [PubMed] [Google Scholar]
- 35.White NJ. The role of anti-malarial drugs in eliminating malaria. Malar J. 2008;7(Suppl 1):S8. doi: 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brockelman CR. Conditions favoring gametocytogenesis in the continuous culture of Plasmodium falciparum. J Protozool. 1982;29:454–8. doi: 10.1111/j.1550-7408.1982.tb05432.x. [DOI] [PubMed] [Google Scholar]
- 37.Inselburg J. Stage-specific inhibitory effect of cyclic AMP on asexual maturation and gametocyte formation of Plasmodium falciparum. J Parasitol. 1983;69:592–7. [PubMed] [Google Scholar]
- 38.Dyer M, Day K. Expression of Plasmodium falciparum trimeric G proteins and their involvement in switching to sexual development. Mol Biochem Parasitol. 2000;108:67–78. doi: 10.1016/s0166-6851(00)00205-x. [DOI] [PubMed] [Google Scholar]
- 39.Read LK, Mikkelsen RB. Comparison of adenylate cyclase and cAMP-dependent protein kinase in gametocytogenic and nongametocytogenic clones of Plasmodium falciparum. J Parasitol. 1991;77:346–52. [PubMed] [Google Scholar]
- 40.Trager W, Gill GS. Plasmodium falciparum gametocyte formation in vitro: its stimulation by phorbol diesters and by 8-bromo cyclic adenosine monophosphate. J Protozool. 1989;36:451–4. doi: 10.1111/j.1550-7408.1989.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 41.Dixon MW, Peatey CL, Gardiner DL, Trenholme KR. A green fluorescent protein-based assay for determining gametocyte production in Plasmodium falciparum. Mol Biochem Parasitol. 2009;163:123–6. doi: 10.1016/j.molbiopara.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Kaushal DC, Carter R, Miller LH, Krishna G. Gametocytogenesis by malaria parasites in continuous culture. Nature. 1980;286:490–2. doi: 10.1038/286490a0. [DOI] [PubMed] [Google Scholar]
- 43.Hawking F, Wilson ME, Gammage K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1971;65:549–59. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- 44.Abiodun OO, Gbotosho GO, Ajaiyeoba EO, et al. Comparison of SYBR Green I-, PicoGreen-, and [3H]-hypoxanthine-based assays for in vitro antimalarial screening of plants from Nigerian ethnomedicine. Parasitol Res. 2010;106:933–9. doi: 10.1007/s00436-010-1743-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.