Abstract
Background. Elevated immune activation persists during treated human immunodeficiency virus (HIV) infection and is associated with blunted CD4 recovery and premature mortality, but its causes remain incompletely characterized. We hypothesized that asymptomatic cytomegalovirus (CMV) replication might contribute to immune activation in this setting.
Methods. Thirty antiretroviral therapy–treated HIV-infected CMV-seropositive participants with CD4 counts <350 cells/mm3 were randomized to receive valganciclovir 900 mg daily or placebo for 8 weeks, followed by an additional 4-week observation period. The primary outcome was the week 8 change in percentage of activated (CD38+ HLA-DR+) CD8+ T cells.
Results. Fourteen participants were randomized to valganciclovir and 16 to placebo. Most participants (21 [70%] of 30) had plasma HIV RNA levels <75 copies/mL. The median CD4 count was 190 (IQR: 134–232) cells/mm3, and 12 (40%) of 30 had detectable CMV DNA in saliva, plasma, or semen at baseline. CMV DNA continued to be detectable at weeks 4–12 in 7 (44%) of 16 placebo-treated participants, but in none of the valganciclovir-treated participants (P = .007). Valganciclovir-treated participants had significantly greater reductions in CD8 activation at weeks 8 (P = .03) and 12 (P = .02) than did placebo-treated participants. These trends were significant even among those with undetectable plasma HIV RNA levels.
Conclusions. CMV (and/or other herpesvirus) replication is a significant cause of immune activation in HIV-infected individuals with incomplete antiretroviral therapy–mediated CD4+ T cell recovery.
Clinical Trials Registration. NCT00264290.
Despite clear improvements in morbidity and mortality in the modern antiretroviral therapy era, treated human immunodeficiency virus (HIV)–infected individuals continue to have at least a 10-year shorter life expectancy than the general population and remain at higher risk for many non–AIDS-associated morbidities associated with aging [1–3]. This risk is greatest in those with persistently low CD4+ T cell counts [4–6]. This is of major clinical relevance, since up to one-third of HIV-infected individuals initiating antiretroviral therapy at CD4+ T cell counts <200 cells/mm3 fail to restore normal CD4+ T cell counts, even after 10 years of viral suppression [7–9]. We and others have observed that most HIV-infected individuals continue to have abnormal T cell activation despite years of treatment-mediated viral suppression [10–13], which is associated with blunted CD4+ T cell recovery [11, 14]. Higher plasma levels of the inflammatory cytokine interleukin-6 (IL-6) are also associated with increased all-cause mortality and cardiovascular disease events among treated HIV-infected individuals, which further suggests a negative effect of inflammation and immune activation on clinical outcomes among these patients [15].
Although the causes of persistent immune activation during suppressive antiretroviral therapy remain incompletely characterized, several observations suggest that asymptomatic cytomegalovirus (CMV) replication may be an important contributor. First, the prevalence of CMV coinfection approaches 90% among HIV-infected individuals and perhaps even higher among homosexual men [16–18]. Second, among healthy HIV-uninfected individuals, asymptomatic CMV coinfection is associated with higher T cell activation and is responsible for nearly 10% of the entire circulating memory T cell repertoire [19, 20]. CMV-specific T cell responses are even higher in HIV-infected individuals, particularly those receiving antiretroviral therapy [21]. Third, CMV infection is associated with more rapid progression to non-CMV AIDS events and mortality in both untreated and treated HIV infection [22–26]. Last, higher CMV-specific immune responses have been strongly associated with greater atherosclerosis in both HIV-infected and -uninfected individuals [27, 28], and treatment of CMV reduces the risk of atherosclerosis in solid-organ transplant recipients [29]. Chronic immune activation from asymptomatic CMV infection may also have long-term effects on the immune system. For example, asymptomatic CMV coinfection is associated with accelerated immunosenescence and mortality among elderly HIV-uninfected individuals, which suggests a major role of this virus in aging [30–36]. Although the long-term effects of CMV in HIV-infected individuals have not been well studied, there is a growing appreciation for accelerated immunosenescence and aging in HIV-infected individuals [37]. It remains unclear, however, whether CMV replication increases HIV disease progression and risks of age-associated diseases by increasing immune activation or whether it is simply a marker for immunodeficiency.
To determine whether asymptomatic CMV (and/or other herpesvirus) replication contributes to immune activation in patients with treated HIV infection, we conducted a randomized placebo-controlled trial of valganciclovir in HIV-infected individuals with incomplete CD4+ T cell recovery during antiretroviral therapy. Valganciclovir has potent activity against CMV and most other herpesviruses and is safe in patients with HIV infection [38]. Furthermore, a prophylactic regimen of 900 mg per day taken for 8 weeks decreases salivary CMV DNA levels by 80% in asymptomatic HIV-infected and -uninfected individuals [39].
METHODS
Study Participants
CMV-seropositive adults with chronic HIV infection who had been receiving a stable antiretroviral therapy regimen for at least 6 months and with CD4+ T cell counts <350 cells/mm3 for ≥1 year were eligible to participate in the study. We chose to enroll individuals with persistently low CD4+ T cell counts to enrich for higher T cell activation levels [11, 14]. Patients were ineligible if they reported <90% adherence to their antiretroviral regimen; had any serious illness requiring hospitalization or intravenous antibiotics during the preceding 3 months; had active CMV end-organ disease necessitating treatment; had received ganciclovir or valganciclovir during the previous month; were receiving concurrent nephrotoxic, immunosuppressive, or immunomodulatory drugs; were pregnant or breastfeeding; or had any of the following abnormal laboratory values: absolute neutrophil count, <1000 cells/mm3; platelet count, <100,000 cells/mm3; hemoglobin, <8 mg/dL; or creatinine clearance, <50 mL/min. To enrich for participants with high T cell activation, the percentage of activated (CD38+ HLA-DR+) CD8+ T cells was measured at screening on fresh whole blood and potential participants with <10% activated CD8+ T cells were excluded. All participants provided written informed consent for the study, and the study was approved by the Committee on Human Research at the University of California San Francisco.
Study Procedures
Enrolled participants were randomized to receive oral valganciclovir or placebo, 900 mg daily for 8 weeks, followed by a 4-week observation period. Randomization was stratified by plasma HIV RNA level (<75 vs ≥75 copies/mL). Participants, clinicians, and laboratory personnel were blinded to treatment assignment. Participants were monitored biweekly during the treatment period for CD4+ T cell counts and plasma HIV RNA levels and screened for evidence of toxic effects (complete blood count and renal function tests). Saliva, peripheral blood mononuclear cells (PBMCs), and plasma were cryopreserved at baseline and every 4 weeks for immunological and virological testing at the end of the study. Semen was also cryopreserved at baseline and at week 8 from consenting male participants.
Laboratory Measurements
Screening for T Cell Activation.
The percentage of activated (CD38+ HLA-DR+) CD8+ T cells was measured on fresh whole blood at screening to exclude participants with <10% activated CD8+ T cells using methods described elsewhere [14].
T Cell Activation on Trial Samples.
T cell activation was measured on PBMCs in batch at the end of the study using methods that have been optimized and validated for PBMCs. Cryopreserved PBMCs were rapidly thawed in warm media, washed, stained with Viacount (Millipore), and run on a Guava PCA (Millipore) for cell count and viability. One sample was excluded for low cell viability (23%), but the remaining samples ranged from 70% to 97% viability (median, 92%). PBMCs were stained with AquaAmine Reactive Dye (Invitrogen) to exclude nonviable cells and for CD3, CD4, CD38, HLA-DR (BD Biosciences), and CD8 (Invitrogen). Stained cells were run on a BD LSR II and data analyzed using FlowJo (Tree Star). The frequencies of CD3+CD4+ and CD3+CD8+ T cells expressing both CD38 and HLA-DR were determined using fluorescent minus 1 gates to define double-positive cells.
Herpesvirus DNA Levels.
DNA was extracted from cryopreserved saliva, plasma, and seminal plasma for the quantification of CMV with a double-primer set to the UL55 and UL123-exon 4 as reported elsewhere [39]. Assays for Epstein-Barr virus (EBV), human herpesvirus type 8 (HHV-8), and HHV-6 were also performed on the same samples with the same extraction methods as previously described [39–41]. Samples with ≥3 copies/reaction and >150 copies/mL of sample were considered to have a positive result. Several negative and positive controls were run with each reaction, including 2 reaction mixtures without DNA (negative controls) and ≥1 sample with a known quantity of CMV DNA. An internal control was amplified with each specimen to ensure that negative results were not attributable to PCR inhibition.
Herpes Simplex Virus Type 2 Serological Characteristics.
Baseline serum samples were tested using a gG-2-specific serology kit (HerpeSelect Herpes Simplex Virus Type 2 [HSV-2] enzyme-linked immunosorbent assay; Focus Diagnostics).
Inflammatory Biomarkers.
Plasma samples from weeks 0, 4, 8, and 12 were assessed for biomarkers, including levels of high-sensitivity C reactive protein (hs-CRP), IL-6, D-dimer, soluble CD14, and cystatin C, by means of immunoassay at the Laboratory for Clinical Biochemistry Research at the University of Vermont [15, 42, 43]. Coefficients of variation were 5.1%, 12.2%, 15.0%, 6.7%, and 2.5%, respectively.
Statistical Analysis
The primary analysis compared the change in percentage of activated CD8+ T cells from baseline to week 8 between valganciclovir- and placebo-treated participants. Changes in the percentage of activated T cells across all time points were assessed with generalized estimating equations, and differences in the change from baseline between groups at each time point were assessed with interaction terms. The percentage of activated T cells was log-transformed to satisfy model assumptions. Linear predictions from the models were back-transformed for graphical representation of the data. Changes in the proportion of participants with positive herpesvirus DNA levels over time were assessed with the Cochran Q test. Between-group comparisons of proportions were assessed with the Fisher exact test.
The power calculations were as follows. We assumed a standard deviation of 5% in the week 8 change in percentage of activated CD8+ T cells from baseline, a likely rate of study noncompletion of 2 enrolled participants per study arm, and a Type I error rate of 5%, yielding 80% power to detect a difference between groups as small as 5.5% with 15 planned participants in each arm.
RESULTS
Characteristics of Participants
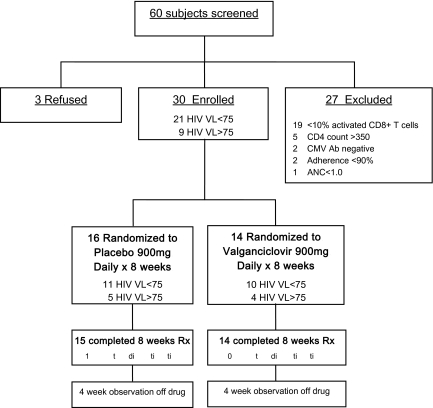
Of 60 screened subjects, 3 refused participation, 27 were excluded, and 30 met inclusion criteria and were enrolled (Figure 1). The most common reason for exclusion was <10% activated CD8+ T cells (n = 19; median, 5.9%, range: 3.7% to 7.6%). Most enrolled participants (93%) were men, the median age was 49 (IQR: 44 to 56), and the median duration of antiretroviral therapy was 27 (IQR: 18 to 38) months (Table 1). Most had current CD4+ T cell counts of <200 cells/mm3 and self-reported pretreatment nadir CD4 counts of <50 cells/mm3, and there was no evidence for a difference between treatment arms (P > .18 for both comparisons). Among the 9 participants with detectable viremia at baseline, the median plasma HIV RNA level was 4.1 log10 copies/mL (range, 3.4–5.7 log copies/mL) and there was no evidence for a difference between arms. The majority of participants randomized to placebo and valganciclovir were HSV-2 seropositive (63% and 86%, respectively), and approximately one-third in each group were receiving daily acyclovir prophylaxis at enrollment, which continued throughout the study. All 14 participants randomized to valganciclovir completed the trial, but 1 of the 16 placebo-treated participants discontinued the study medication prematurely (congestive heart failure exacerbation).
Figure 1.
Screening and enrollment status. The disposition of all screened subjects is outlined. Of 60 screened subjects, 27 were excluded for the reasons noted, 3 refused participation, and 30 were enrolled. Randomization was stratified by plasma human immunodeficiency virus (HIV) RNA level (<75 or ≥75 copies/mL) in block sizes of 2. All 14 participants randomized to valganciclovir completed the trial, whereas 1 of the 16 placebo-treated participants discontinued study medication prematurely (congestive heart failure exacerbation). ANC, absolute neutrophil count; CMV, cytomegalovirus; VL, virus level.
Table 1.
Baseline Characteristics of Participants
| Characteristic | Placebo group | Valganciclovir group |
| (n = 16) | (n = 14) | |
| Age, years | 50 (44–59) | 48 (43–55) |
| Male sex, no. (%) | 16 (100) | 12 (86) |
| ART regimen, no. (%) | ||
| PI-based | 11 (69) | 11 (79) |
| NNRTI-based | 3 (19) | 2 (14) |
| NRTIs only | 2 (13) | 1 (7) |
| Duration of ART regimen, months | 27 (17–35) | 28 (16–40) |
| Self-reported ART adherence past 30 days, % | 100 (96–100) | 100 (96–100) |
| CD4+ T cell count, cells/mm3 | 187 (108–218) | 207 (176–249) |
| Self-reported CD4 count nadir, cells/mm3 | 64 (20–114) | 18 (8–54) |
| Plasma HIV RNA level, copies/mL, no. (%) | ||
| <75 | 11 (69) | 10 (71) |
| ≥75 | 5 (31) | 4 (29) |
| HSV-2 seropositive, no. (%) | 10 (63) | 12 (86) |
| Currently taking acyclovir, no. (%) | 6 (38) | 5 (36) |
| Estimated creatinine clearance, mL/min | 85 (73–101) | 94 (82–108) |
| Absolute neutrophil count, ×103 cells/mm3 | 2.2 (1.9–3.3) | 2.9 (1.9–3.5) |
| Hemoglobin, mg/dL | 14.6 (13.3–15.2) | 14.4 (13.4–15.4) |
| Platelet count, ×103/mm3 | 215 (178–270) | 255 (223–304) |
Changes in Herpesvirus DNA Levels
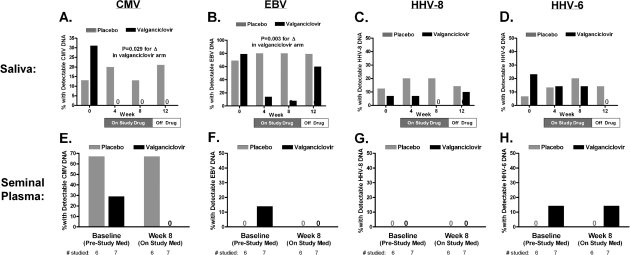
At baseline, 7 (44%) of 16 placebo-treated participants and 5 (36%) of 14 valganciclovir-treated participants had detectable CMV DNA levels in saliva, seminal plasma, or peripheral blood plasma. Median (range) titers in those with detectable levels were 3737 (218 to 28,255), 15,541 (153 to 862,223), and 159 (158 to 160) copies/mL, respectively. CMV DNA levels were most commonly detectable in seminal plasma and saliva (Figure 2), with only 2 participants having detectable peripheral blood plasma CMV DNA levels at baseline (both placebo-treated). Whereas there was no evidence for a change in CMV DNA levels in the placebo arm (titers or proportion positive), valganciclovir significantly reduced CMV DNA levels such that no valganciclovir-treated participant had a detectable CMV DNA level at any site after baseline, even 4 weeks after stopping therapy (P = .007 for between-group comparison of proportions with a detectable level at any postbaseline time point).
Figure 2.
Changes in herpesvirus DNA levels in saliva and semen with valganciclovir therapy. Herpesvirus DNA levels were assessed by polymerase chain reaction (lower limit of detection, 150 copies/mL) on saliva (at weeks 0, 4, 8, and 12) and seminal plasma (at weeks 0 and 8 only). The proportion of participants with a positive herpesvirus DNA level result is plotted for both valganciclovir and placebo groups in saliva and seminal plasma for cytomegalovirus (CMV) (A and E), Epstein-Barr virus (EBV) (B and F), human herpesvirus (HHV) type 8 (C and G), and HHV type 6 (D and H). Plotted P values test whether the proportion of participants with a positive DNA level result changes over time within each treatment group (Cochran Q test). The proportion of participants with positive CMV and EBV DNA levels in saliva declined significantly with valganciclovir therapy but not with placebo. The proportion of participants with detectable salivary CMV DNA levels remained suppressed 4 weeks after study drug discontinuation, whereas the proportion with detectable EBV levels rebounded to pretreatment levels. Whereas 2 (29%) of 7 valganciclovir-treated participants had detectable CMV DNA plasma at baseline, none had it at week 8.
We also assessed the effect of valganciclovir on other herpesvirus DNA levels. Salivary EBV DNA was detectable in 11 (69%) of 16 placebo-treated participants and 11 (79%) of 14 valganciclovir-treated participants at baseline. Whereas there was no evidence for a change in the proportion of placebo-treated participants with detectable EBV DNA levels, the proportion of valganciclovir-treated participants with detectable salivary EBV DNA levels declined significantly through week 8, then rebounded to near pretreatment levels by week 12 (P = .003 for changes within valganciclovir-treated participants). A much lower proportion of participants in each group had detectable HHV-8 or HHV-6 DNA levels in either saliva or semen, and there was no evidence for a change in this proportion in either treatment group over time. There was also no evidence for a change in salivary HHV-8 or HHV-6 DNA levels over time in valganciclovir-treated participants with detectable levels at baseline.
Changes in T Cell Activation
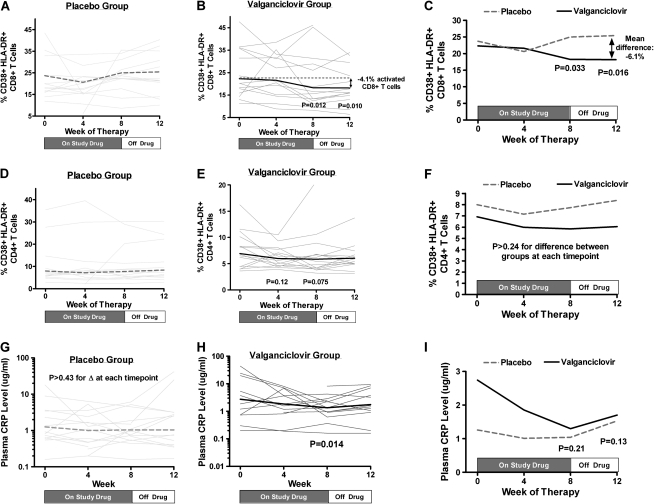
At baseline, the median percentage of activated (CD38+ HLA-DR+) CD8+ T cells was 20.0% in the placebo arm (interquartile range [IQR], 16.4%–35.5%) and 20.2% (IQR, 15.9%–35.8%) in the valganciclovir arm. Whereas there was no evidence for a change in the percentage of activated CD8+ T cells at any time point in placebo-treated participants, the percentage of activated CD8+ T cells declined by a mean 4.0% at week 8 (95% CI, −0.9% to −5.7%) and 4.1% at week 12 (95% CI, −1.1% to −6.8%) in valganciclovir-treated participants, representing an approximately 20% relative decrease from baseline levels (Figures 3A and 3B). Compared with placebo-treated participants, valganciclovir-treated participants experienced a greater reduction in CD8+ T cell activation at weeks 8 (P = .03) and 12 (P = .02) (Figure 3C), even when analysis was restricted to those with plasma HIV RNA levels <75 copies/mL (P = .04 and P = .006, respectively) or those with <20.2% activated CD8+ T cells at baseline (P = .01 and P = .01, respectively). This valganciclovir-mediated reduction in T cell activation is comparable to roughly 40% of the observed increase in CD8+ T cell activation in treated HIV-infected individuals relative to HIV-uninfected control participants studied in the same laboratory (median, 10% [IQR: 6% to 14%] activated CD8+ T cells; data not shown). When the analysis was restricted to those with negative HSV-2 serology or those continuing to receive acyclovir prophylaxis (n = 16), valganciclovir-treated participants continued to have a greater median reduction in the percentage of activated CD8+ T cells at week 8 (-3.2%, IQR: -4.8% to -1.0%) than did placebo-treated participants (+0.8%, IQR: -0.7 to +3.3%) but this difference was not statistically significant (P = .07). Whereas there was no evidence for a change in CD4+ T cell activation levels in the placebo arm (P > .36 for all time points), there were trends toward a decrease in CD4+ T cell activation levels at weeks 4 (P = .12) and 8 (P = .08) in the valganciclovir arm, but the differences between arms were not significant (P > .24 for all time points) (Figure 3D–F). There was no evidence for a relationship between the presence of detectable CMV DNA at baseline and either the baseline frequency of activated T cells or the extent of reduction in T cell activation during valganciclovir therapy.
Figure 3.
Changes in T cell activation and C reactive protein (CRP) levels with valganciclovir therapy. The percentage of activated (CD38+ HLA-DR+) CD8+ T cells, percentage of activated CD4+ T cells, and plasma CRP levels were assessed over time in both placebo-treated (A, D, G) and valganciclovir-treated participants (B, E, H) with generalized estimating equations. The thin gray lines (A, B, D, E, G, H) indicate individual participant changes, and the thick lines represent the estimated mean changes at each time point within each treatment arm. Mean changes from baseline at each time point were also plotted and compared between placebo- and valganciclovir-treated participants with generalized estimating equations (C, F, I), with P values referring to differences in the change from baseline between treatment arms at each time point. Whereas there was no evidence for a change from baseline in the percentage of activated CD8+ T cells at any time point for placebo-treated participants (A), valganciclovir-treated participants experienced a mean decline of 4% activated CD8+ T cells by week 8 (P = .01) and continued to have a mean 4.1% fewer activated CD8+ T cells than baseline at week 12 (P = .01, B). Compared with placebo-treated participants, those receiving valganciclovir experienced a greater decline in CD8+ T cell activation from baseline at week 8 (P = .03) and from baseline to week 12 (P = .02, C). Whereas there was no evidence for a change in CD4+ T cell activation levels in the placebo arm (P > .36 for all time points, D), there were trends toward a decrease in CD4+ T cell activation levels at weeks 4 (P = .12) and 8 (P = .08) in the valganciclovir arm (E), but the differences between arms were not significant (P > .24 for all time points, F). Whereas there was no evidence for a change in the placebo arm (P > .43 for all time points, G), CRP levels declined significantly by week 8 in the valganciclovir arm (P = .01, H), but the difference in CRP changes between arms was not significant (P ≥ .13 for all time points, I).
Changes in Soluble Biomarkers
Whereas we observed no evidence for a change in plasma hs-CRP levels at any time point in placebo-treated participants (P > .44 for all time points), hs-CRP levels declined by week 8 in valganciclovir-treated participants (P = .02), but the difference in week 8 changes between treatment groups was not statistically significant (P = .21) (Figure 3G–I). There was no evidence for a change in plasma IL-6, d-Dimer, soluble CD14, or cystatin C levels at any time point within either treatment group.
Changes in CD4+ T Cell Counts and Plasma HIV RNA Levels
There was no evidence for a change from baseline in either CD4+ T cell counts or plasma HIV RNA levels in either valganciclovir- or placebo-treated participants at any time point, nor was there evidence for changes over time in either measure as assessed by linear mixed models (Figures 4A and 4B).
Figure 4.
Changes in plasma human immunodeficiency virus (HIV) RNA levels and CD4 counts during valganciclovir therapy. Changes in plasma HIV RNA levels (A) and CD4+ T cell counts (B) were assessed over time in valganciclovir- and placebo-treated participants. Each line represents an individual participant's trajectory. Dark thick lines in panel B represent the estimated mean changes over time within each treatment group from a linear mixed model. There was no evidence for changes over time in either plasma HIV RNA levels or CD4+ T cell counts in either treatment group.
Adverse Events
There was only 1 grade 3 adverse event during the trial: a placebo-treated patient was hospitalized for congestive heart failure prior to the scheduled week 4 visit. There were no other serious adverse events. Specifically among valganciclovir-treated participants, there was no evidence for a change in absolute neutrophil counts (P = .61), hemoglobin levels (P = .37), platelet counts (P = .67), or serum creatinine concentrations (mean, −0.006 [95% CI: -.012 to +.001] mg/dL per week through week 8; P = .12) during the 8-week treatment interval.
DISCUSSION
Although antiretroviral therapy has dramatically improved the health of HIV-infected individuals, abnormal immune activation persists in most of these individuals and predicts subsequent morbidity and earlier mortality. To assess whether low-level asymptomatic CMV replication contributes to persistent immune activation in these patients, we conducted a randomized placebo-controlled trial of valganciclovir among HIV-infected individuals with persistently low CD4+ T cell counts despite antiretroviral therapy. Eight weeks of valganciclovir therapy resulted in potent suppression of CMV DNA levels, which persisted for at least 4 weeks after therapy was discontinued. Valganciclovir also resulted in significantly greater declines in the frequency of activated CD8+ T cells than placebo, which persisted for at least 4 weeks after treatment cessation. Reductions in hs-CRP levels were also observed with valganciclovir. Because we observed no change in plasma HIV RNA levels among viremic participants and the reduction in T cell activation remained significant when analysis was restricted to those with undetectable plasma HIV RNA levels, the reduction in T cell activation with valganciclovir therapy does not seem to be explained by a direct effect on HIV replication. These results suggest that CMV and/or other herpesvirus coinfections are a substantial cause of in vivo T cell activation among treated HIV- and CMV-coinfected individuals.
Treating herpesvirus coinfections has long been pursued as a potential strategy to delay HIV disease progression. Prior to the introduction of protease inhibitors in the mid-1990s, several small trials established a mortality benefit to high-dose acyclovir when used in combination with 1 or 2 antiretroviral drugs [44]. More recently, standard prophylactic doses of acyclovir to prevent genital ulcer disease have been shown to decrease the rate of CD4+ T cell decline in untreated HIV- and HSV-2–coinfected individuals in a large international trial [45]. Although some research groups have suggested that acyclovir might exhibit a direct antiviral effect on HIV replication [46, 47], these in vitro studies have used much higher concentrations of acyclovir than used in current dosing regimens and evidence of acyclovir-associated resistance mutations in HIV-1 reverse transcriptase has not been demonstrated in vivo. This has led many to hypothesize that the beneficial effect of acyclovir on HIV progression is mediated by a reduction in HSV-2–induced immune activation [45].
Because asymptomatic chronic CMV infection elicits massive immune responses in HIV-uninfected individuals [20] and even higher responses in HIV-infected individuals receiving antiretroviral therapy [21], we suspect that the valganciclovir-mediated reductions in T cell activation that we observed were largely mediated by reductions in CMV replication. While we have not seen a relationship between detectable salivary CMV DNA levels and T cell activation in this trial or in a recent observational study [48], the relationship between CMV replication and immune activation is likely to be complex. For example, individuals with the strongest immune responses to very low amounts of CMV replication are likely to have the strongest antiviral responses, limiting the detection of CMV in bodily fluids.
However, we cannot definitively exclude the possibility that valganciclovir's effect on T cell activation is mediated by suppression of other herpesviruses. EBV DNA levels also declined significantly during valganciclovir therapy but, unlike those of CMV DNA, rebounded to pretreatment levels 4 weeks after treatment cessation. Because CD8+ T cell activation remained suppressed at this time, it seems unlikely that valganciclovir's effect on CD8+ T cell activation is mediated by a reduction in EBV replication. Although we observed no evidence for a decline in HHV-8 or HHV-6 levels during valganciclovir therapy, a recent trial using the exact same dose and duration of valganciclovir—but more frequent sampling of the oropharynx and enrichment for participants with high salivary HHV-8 DNA levels—found significant reductions in HHV-8 DNA [39]. Valganciclovir also has known antiviral activity against HHV-6 [49]. Last, although there seemed to be reductions in CD8+ T cell activation even in valganciclovir-treated participants who were either HSV-2 seronegative or receiving concurrent acyclovir prophylaxis, we cannot rule out the possibility that reductions in HSV-2 replication may have partially contributed to the effects observed. A similar trial of acyclovir in HIV-infected individuals receiving antiretroviral therapy might help address this possibility, because acyclovir has excellent activity against HSV-2 but very little activity against CMV. Last, although acyclovir clearly reduces plasma HIV RNA levels in untreated HIV-infected individuals [45], we observed no evidence for a reduction in plasma HIV RNA levels in viremic valganciclovir-treated participants, and the effect of valganciclovir on CD8+ T cell activation remained significant even when analysis was restricted to participants with undetectable plasma HIV RNA levels.
While we did not observe any evidence for a change in CD4+ T cell counts during the 12-week observation period, our prior cross-sectional study suggests that each absolute 4% increase in the frequency of activated CD8+ T cells would be associated with just 28 fewer CD4+ T cells gained over a median of 2 years of suppressive antiretroviral therapy, so our trial was far too small and short to rule out an effect on CD4+ T cell counts [14]. However, it remains possible that T cell activation caused by CMV replication may have a lesser effect on CD4+ T cell recovery than T cell activation caused by other factors (ie, HIV release from latently infected cells, microbial translocation, and so forth). Similarly, although we observed some evidence for a valganciclovir-mediated decrease in hs-CRP levels, a larger trial would be necessary for adequate power to detect changes in other surrogate markers of interest, including the inflammatory biomarkers IL-6, D-dimer, and endothelial function, given the high within-subject variability of these measurements. Subsequent studies will also need to assess whether these findings are generalizable to individuals with higher CD4+ T cell counts and lower CD8+ T cell activation levels.
The clinical relevance of the observed valganciclovir-mediated reduction in immune activation is also unclear, and still larger studies would be necessary to establish a benefit of valganciclovir (or other anti-CMV therapy) in preventing AIDS- and non–AIDS-associated morbidity and mortality. Although we did not observe any toxic effects of valganciclovir in this study, the known marrow-suppressive and teratogenic effects of valganciclovir must be balanced against the potential for clinical benefit if such trials are to be conducted.
In summary, this randomized controlled trial of valganciclovir revealed that CMV and/or other herpesvirus replication is a significant cause of persistent T cell activation in patients with treated HIV infection. Although the clinical relevance of this finding remains unclear, persistent immune activation and inflammation is a major predictor of premature morbidity and mortality among these patients [15]. Furthermore, given persistent functional T cell defects and immunosenescence even in treated HIV-infected individuals with optimal CD4+ T cell recovery [50], and the clear associations between CMV and immunosenescence in HIV-uninfected individuals [30–36], a larger trial of anti-CMV therapy for treated HIV-infected patients is clearly warranted.
Funding
This work was supported in part by the University of California San Francisco (UCSF)/Gladstone Center for AIDS Research (P30 AI27763, P30 MH59037), UCSF-San Francisco General Hospital General Clinical Research Center (Clinical Research Feasibility Funds), NIAID (AI065244 and AI055273), the Center for AIDS Prevention Studies (P30 MH62246), the UCSF Clinical and Translational Science Institute (UL1 RR024131-01), and an investigator-initiated research grant, as well as donated drug and placebo from Roche Laboratories, Inc.
References
- 1.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 2.Lewden C, Chene G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46:72–7. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 3.The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monforte A, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–53. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–8. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin B, Thiebaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–53. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byakwaga H, Murray JM, Petoumenos K, et al. Evolution of CD4+ T cell count in HIV-1-infected adults receiving antiretroviral therapy with sustained long-term virological suppression. AIDS Res Hum Retroviruses. 2009;25:756–76. doi: 10.1089/aid.2008.0149. [DOI] [PubMed] [Google Scholar]
- 8.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–94. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lok JJ, Bosch RJ, Benson CA, et al. Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS. 2010;24:1867–76. doi: 10.1097/QAD.0b013e32833adbcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–34. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 12.Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years' suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–66. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 13.Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 14.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 15.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang DJ, Kovacs AA, Zaia JA, et al. Seroepidemiologic studies of cytomegalovirus and Epstein-Barr virus infections in relation to human immunodeficiency virus type 1 infection in selected recipient populations. J Acquir Immune Defic Syndr. 1989;2:540–9. [PubMed] [Google Scholar]
- 17.Berry NJ, Burns DM, Wannamethee G, et al. Seroepidemiologic studies on the acquisition of antibodies to cytomegalovirus, herpes simplex virus, and human immunodeficiency virus among general hospital patients and those attending a clinic for sexually transmitted diseases. J Med Virol. 1988;24:385–93. doi: 10.1002/jmv.1890240405. [DOI] [PubMed] [Google Scholar]
- 18.Robain M, Carre N, Dussaix E, Salmon-Ceron D, Meyer L. Incidence and sexual risk factors of cytomegalovirus seroconversion in HIV-infected subjects. Sex Transm Dis. 1998;25:476–80. doi: 10.1097/00007435-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Lenkei R, Andersson B. High correlations of anti-CMV titers with lymphocyte activation status and CD57 antibody-binding capacity as estimated with three-color, quantitative flow cytometry in blood donors. Clin Immunol Immunopathol. 1995;77:131–8. doi: 10.1006/clin.1995.1136. [DOI] [PubMed] [Google Scholar]
- 20.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector SA, Wong R, Hsia K, Pilcher M, Stempien MJ. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest. 1998;101:497–502. doi: 10.1172/JCI1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery VC, Sabin C, Feinberg JE, Grywacz M, Knight S, Griffiths PD. Quantitative effects of valacyclovir on the replication of cytomegalovirus (CMV) in persons with advanced human immunodeficiency virus disease: baseline CMV load dictates time to disease and survival. J Infect Dis. 1999;180:695–701. doi: 10.1086/314936. [DOI] [PubMed] [Google Scholar]
- 24.Detels R, Leach CT, Hennessey K, et al. Persistent cytomegalovirus infection of semen increases risk of AIDS. J Infect Dis. 1994;169:766–8. doi: 10.1093/infdis/169.4.766. [DOI] [PubMed] [Google Scholar]
- 25.Deayton JR, Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet. 2004;363:2116–21. doi: 10.1016/S0140-6736(04)16500-8. [DOI] [PubMed] [Google Scholar]
- 26.Jabs DA, Holbrook JT, Van Natta ML, et al. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2005;112:771–9. doi: 10.1016/j.ophtha.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 27.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–83. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 28.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valantine HA, Gao SZ, Menon SG, et al. Impact of prophylactic immediate posttransplant ganciclovir on development of transplant atherosclerosis: a post hoc analysis of a randomized, placebo-controlled study. Circulation. 1999;100:61–6. doi: 10.1161/01.cir.100.1.61. [DOI] [PubMed] [Google Scholar]
- 30.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–53. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 31.Hadrup SR, Strindhall J, Kollgaard T, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–53. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang Q, Wagner WM, Wikby A, et al. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol. 2003;23:247–57. doi: 10.1023/a:1024580531705. [DOI] [PubMed] [Google Scholar]
- 33.Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–83. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauce D, Larsen M, Fastenackels S, et al. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–8. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–22. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vescovini R, Biasini C, Telera AR, et al. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol. 2010;184:3242–9. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- 37.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lalezari J, Lindley J, Walmsley S, et al. A safety study of oral valganciclovir maintenance treatment of cytomegalovirus retinitis. J Acquir Immune Defic Syndr. 2002;30:392–400. doi: 10.1097/00042560-200208010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Casper C, Krantz EM, Corey L, et al. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198:23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768–76. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 41.Kimura H, Morita M, Yabuta Y, et al. Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J Clin Microbiol. 1999;37:132–6. doi: 10.1128/jcm.37.1.132-136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 43.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–46. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 44.Ioannidis JP, Collier AC, Cooper DA, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349–59. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 45.Lingappa JR, Baeten JM, Wald A, et al. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–70. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMahon MA, Siliciano JD, Lai J, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283:31289–93. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobson MA, Ditmer DP, Sinclair E, et al. Human herpesvirus replication and abnormal CD8+ T cell activation and low CD4+ T cell counts in antiretroviral-suppressed HIV-infected patients. PLoS One. 2009;4:e5277. doi: 10.1371/journal.pone.0005277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burns WH, Sandford GR. Susceptibility of human herpesvirus 6 to antivirals in vitro. J Infect Dis. 1990;162:634–7. doi: 10.1093/infdis/162.3.634. [DOI] [PubMed] [Google Scholar]
- 50.Lange CG, Lederman MM, Medvik K, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–23. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]