Abstract
Background. The role of toxins secreted by the type II secretion system (T2SS) of Pseudomonas aeruginosa during lung infection has been uncertain despite decades of research.
Methods. Using a model of pneumonia in Toll-like receptor (TLR) 2,4−/− mice, we reexamined the role of the T2SS system. Flagellin-deficient mutants of P. aeruginosa, with mutations in the T2SS and/or T3SS, were used to infect mice. Mice were followed up for survival, with some killed at different intervals to study bacterial clearance, inflammatory responses, and lung pathology.
Results. Strains carrying either secretion system were lethal for mice. Double mutants were avirulent. The T3SS+ strains killed mice within a day, and the T2SS+ strains killed them later. Mice infected with a strain that had only the T2SS were unable to eradicate the organism from the lungs, whereas those infected with a T2SS-T3SS double deletion were able to clear this mutant. Death caused by the T2SS+ strain was accompanied by a >50-fold increase in bacterial counts and higher numbers of viable intracellular bacteria.
Conclusions. The T2SS of P. aeruginosa may play a role in death from pneumonia, but its action is delayed. These data suggest that antitoxin strategies against this organism will require measures against the toxins secreted by both T2SS and T3SS.
Pneumonia due to Pseudomonas aeruginosa carries a high mortality rate [1] attributed to its secreted exotoxins [2] and possibly the inflammatory response to bacterial products [3]. This bacterium is known to possess 5 protein secretion systems of which the type II secretion system (T2SS) and type III secretion system (T3SS) secrete the majority of known toxins. The T2SS secretes exotoxin A, LasA and LasB proteases, type IV protease, and phospholipase H, as well as lipolytic enzymes [4]. The T3SS secretes exotoxins U, S, T, and Y [5]. The latter system uses a membrane-spanning structure and needle to inject toxins into mammalian cells [6] whereas the T2SS is composed of multiprotein secretons encoded by the xcp and hxc operons [7] as well as an additional secretin, XqhA, that functions in T2 secretion when xcpQ, the major secretin, is mutated [8].
The biologic roles of the exoproducts of these systems have been under study for decades with clarity only about the role of the T3SS. Its major toxins, ExoU and ExoS cause death in animal models of pulmonary infection [9] and possible humans [10]. However, most studies with few exceptions have failed to demonstrate that the T2SS toxins are important virulence factors during pulmonary infections [11–13]. There are several possible reasons for this: (1) no studies have addressed the role of the T2SS as a whole in an acute pneumonia model; (2) multiple toxins may be involved in death confounding mutational analysis when the roles of single toxins were addressed; (3) the full repertoire of T2 secreted toxins is not known, and other unidentified toxins may be effectors in mortality; (4) studies were done in the presence of a fully functional T3SS; and (5) any contribution of inflammation as a cause of death was not circumvented.
In prior studies, we demonstrated that Toll-like receptor (TLR) 2,4−/− mice are hypersusceptible to low inocula of a flagellin-negative mutant of P. aeruginosa, owing to the lack of recognition of lipopolysaccharide and flagellin [14]. In those studies, bronchoalveolar lavage (BAL) samples of infected mice demonstrated a grossly defective early inflammatory response [14], Thus, we hypothesized that death was due to bacterial toxins and not inflammation. Using this model, we conclusively demonstrate that both the T2SS and the T3SS play independent roles in death due to Pseudomonas lung infection and that the XcpQ secretin is the major outer membrane pathway used by the T2SS effectors.
MATERIALS AND METHODS
Animals
TLR2−/− and TLR4−/− mice obtained from S. Akira were backcrossed 8 times with C57BL/6 to ensure similar genetic backgrounds. TLR2,4−/− mice were generated by breeding TLR2−/− and TLR4−/− mice. Male mice were used for the experiments. Mice were fed and housed under standard conditions with air filtration and cared for in accordance with Pasteur Institute guidelines and in compliance with European Animal Welfare regulations.
Animal Infection
Mice were anesthetized by intraperitoneal injection of a mixture of ketamine-xylazine, and infected via the intratracheal route, as described elsewhere [15]. A 50-μL bacterial suspension containing 0.5–1.0 × 107 colony-forming units (CFU) was administered. In some mice survival was observed for 1 week after infection, and in others BAL was performed on groups of three mice after pentobarbital euthanasia at 6, 24, or 44 h after infection. The BAL fluids were diluted and plated on LB agar plates to obtain total viable bacterial counts. In some animals viable intracellular bacteria were enumerated by incubating washed BAL cells in tobramycin for 30 min, to kill extracellular bacteria, and lysing the cells with .1% triton X - 100 before plating on L-agar plates. Cell counts were measured in the BAL fluids and cell differential counts were determined after cytospin centrifugation and staining with Diff-Quik products. Murine cytokine concentrations in BAL fluid were determined using DuoSet enzyme-linked immunosorbent assay kits (R&D Systems).
Bacterial Strains
All bacterial strains and plasmid vectors used in this study are described in Table1. For mutant construction, P. aeruginosa was grown in Luria broth at 37°C with shaking at 250 rpm or on 1.5% L-agar plates with or without antibiotics. For sucrose selection, plates containing 10% sucrose were used. To detect secretion of proteases, wild-type or mutant bacteria were spotted on 1.5% Casein-Milk agar plates. For the animal challenges, bacteria were grown overnight in Luria broth, transferred to fresh medium and grown for 4–5 h to mid–log phase. The cultures were centrifuged at 4000 g for 15 min, and the cell pellets were washed twice with phosphate-buffered saline and suspended in its original volume. The optical density was adjusted to give the approximate desired inoculum and verified by plate counts.
Table 1.
Bacterial Strains and Plasmids Used in Study
| Strain or plasmid | Relevant characteristics | Source or reference |
| Strains | ||
| Pseudomonas aeruginosa | ||
| PAK | Wild-type clinical isolate | D. Bradley |
| PAK ΔpscC | Deletion of bp 30–1701 in pscC in strain PAK | Wolfgang et al [16] |
| PAK ΔfliC-ΔpscC | fliC mutation in PAK ΔpscC | Current study |
| PAK Δxcp | Deletion in xcp operon in strain PAK | Lee et al [17] |
| PAK ΔfliC-Δxcp | fliC mutation in PAK Δxcp | Current study |
| PAK ΔpscC-Δxcp | Double mutant of strain PAK carrying both pscC and xcp operon deletions | Lee et al [17] |
| PAK ΔfliC | In-frame deletion of fliC gene in strain PAK | Dasgupta et al [18] |
| PAK ΔfliC-ΔpscC-Δxcp | fliC mutation in PAK ΔpscC-Δxcp | Current study |
| PAK ΔxcpQ | Partial deletion in xcpQ gene in strain PAK | Current study |
| PAK ΔfliC-ΔxcpQ | fliC mutation in PAK ΔxcpQ | Current study |
| PAK ΔpscF | In-frame partial deletion of pscF from bp 78–213 in strain PAK | Current study |
| PAK ΔfliC-ΔpscF | pscF mutation in PAK ΔfliC | Current study |
| PAK ΔxcpQ-ΔpscF | pscF mutation in PAK ΔxcpQ | Current study |
| PAK ΔfliC-ΔpscF-ΔxcpQ | fliC mutation in PAK ΔxcpQ-ΔpscF | Current study |
| PA14 | Wild-type strain | F. Ausubel |
| PA14ΔfliC | fliC mutation in PA14 | Current study |
| PA14ΔxcpQ | Partial deletion in xcpQ gene in PA14 | Current study |
| PA14ΔfliC-ΔxcpQ | fliC mutation in PA14ΔxcpQ | Current study |
| PA14ΔpscF | pscF mutation in PA14 | Current study |
| PA14ΔfliC-ΔpscF | fliC mutation in PA14ΔpscF | Current study |
| PA14-ΔxcpQ-ΔpscF | pscF mutation in PA14ΔxcpQ | Current study |
| PA14ΔfliC-ΔpscF-ΔxcpQ | fliC mutation in PA14ΔxcpQ-ΔpscF | Current study |
| Plasmid | ||
| pEX18Gm | GmR; oriT+ sacB+, gene replacement vector with MCS from pUC18 | Hoang et al [19] |
| PAX24 | P. aeruginosa xcpP to –Z cluster in pLAFR3 (IncP Tcr) | Filloux et al [20] |
| Mini-CTX1 | Tcr; self-proficient integration vector with tet, Ω-FRT-attP MCS, ori, int, and oriT | Hoang et al [21] |
| Mini-CTX-5.2xcp | P. aeruginosa xcpPQ on a 5.2-kb BamHI fragment from PAX24 cloned in mini-CTX1 | Current study |
| pEXpΔpscF | pEX18Gm containing an in-frame deletion of pscF gene from bp 78–213 of strain PAK | Current study |
| pEXpΔxcpQ | pEX18Gm containing a 1109-bp Sal I fragment deletion in xcpQ of strain PAK | Current study |
CONSTRUCTION OF MUTANTS.
fliC Mutants
P. aeruginosa mutant strains PAKΔpscC (T3SS−), PAKΔxcp (T2SS−), and PAKΔpscCΔxcp (T2SS−/T3SS−) were obtained from Stephen Lory [16, 17]. These well-characterized mutants were used in studies of the role of the T3SS in toxicity to cells [17]. The fliC mutants of these strains were engineered as described elsewhere, using a disrupted allele of the fliC gene of strain PAK [18]. This provided 1 set of fliC mutant strains for the first series of experiments.
pscF and xcpQ Mutants
An in-frame partial gene deletion of 138 bp was made in pscF, the gene encoding the major needle protein of the T3SS, by cloning 1-kb DNA fragments up and downstream of the region to be deleted into the vector pEX18Gm [19]. Plasmid pEXpΔpscF was used to introduce a partial deletion of the pscF gene in strain PAKΔfliC, using sucrose selection, as described elsewhere [19]. An in-frame partial deletion in xcpQ was made by first cloning a 3.2-kb DNA fragment containing the entire xcpQ gene into pEX18Gm and then excising a 1109-bp Sal I fragment from xcpQ. This plasmid, pEXpΔxcpQ, was used to generate PAK mutants in PAKΔfliC and PAKΔfliCΔpscF, providing a second independent mutation in the T2SS (PAKΔfliCΔxcpQ) as well as a T2SS-T3SS mutant (PAKΔfliCΔpscFΔxcpQ). Next, we engineered these latter mutations in strain PA14 to examine whether our observations held with another P. aeruginosa strain, and lastly, the same T2SS and T3SS mutations were engineered in the wild-type flagellated strain PAK.
xcpQ Complementation of PAK ΔfliCΔpscFΔxcpQ
Chromosomal complementation of the PAKΔfliCΔpscFΔxcpQ mutant was achieved at the att site by cloning a 5.2-kb BamHI fragment from cosmid PAX24 that contains the xcp operon of P. aeruginosa strain PAO1 [20] into the mini-CTX1 vector [21]. This construct was transformed into Escherichia coli S17 and mated into PAKΔfliCΔpscFΔxcpQ mutant. The tetracycline-resistant colonies were resolved through flp excision [21]. The mutant with an insertion of the mini-CTX1 vector into the att site was also constructed and used as a control. Complementation was verified by polymerase chain reaction and examining for restoration of protease secretion on casein-milk agar plates.
Histologic Studies
Mice were infected with 0.5–1 × 107 CFU of the mutants to be studied and euthanized with pentobarbital at different intervals after infection, and their lungs were fixed in formalin, sectioned, and stained with hematoxylin-eosin.
Statistical Calculations
Cytokine levels, polymorphonuclear neutrophil (PMN) counts, and pathogen counts were expressed as means ± standard errors of the mean. Differences between groups were assessed for statistical significance, using analysis of variance followed by Fisher's test or t test, as appropriate. Differences were considered statistically significant at P < .05.
RESULTS
Role of T2SS and T3SS in Death Due to Lung Infection
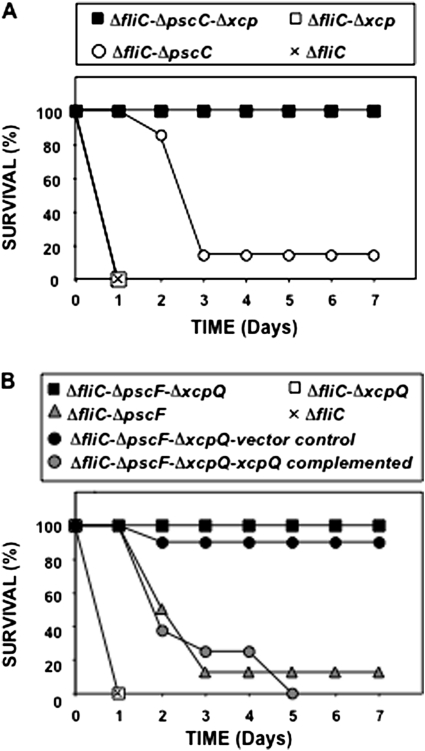
TLR2,4−/− mice were infected with the T2SS− and/or T3SS−defective strains and observed for mortality. A PAKΔfliC mutant that caused 100% mortality [14] served as the control. In most experiments, PAK mutants were flagellin defective; therefore, the notation “PAKΔfliC” will not be repeated unless required for clarification. Δxcp is a T2SS mutant, in which there is a deletion of the xcp operon, including xcpQ encoding the secretin XcpQ. ΔpscC is a T3SS mutant, in which part of the gene encoding PscC, an outer membrane protein, is deleted. ΔpscCΔxcp is a double mutant of the 2 secretion systems. The PAKΔfliC mutant rapidly killed all mice within 24 h (Figure 1A). This dose of bacteria, 0.5–1.0 × 107 CFU, does not cause death in wild-type mice (data not shown), because the innate host defense eradicates the infection [15]. Unequivocally, the Δxcp mutant (T3SS+) killed all the mice within 24 h. The ΔpscC mutant (T2SS+) killed 8 of 9 mice, but deaths began after 24 h, with most occurring between days 2 and 3. In contrast, ΔpscCΔxcp (T3SS−/T2SS−) was avirulent (Figure 1A). Thus, each toxin secretion system played an independent role in causing death.
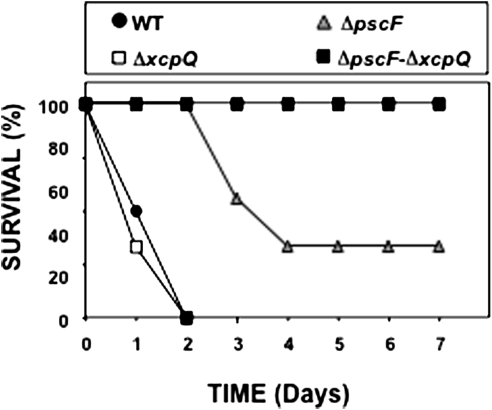
Figure 1.
A, Survival of Toll-like receptor (TLR) 2,4 −/− mice infected with type II secretion system (T2SS) and type III secretion system (T3SS) mutants of Pseudomonas aeruginosa strain PAK. Mice were infected with the P. aeruginosa mutants, and their survival was monitored for ≥1 week. PAKΔfliC is shown as a positive control strain. B, Survival of TLR2,4 −/− mice infected with mutants in the T2SS secretin, XcpQ, and the T3SS needle of P. aeruginosa. Mice were infected with P. aeruginosa PAK mutants, and their survival monitored for ≥1 week. Mutant PAKΔfliCΔpscFΔxcpQ was complemented by insertion of a mini-CTX vector (control) or the same vector with a DNA fragment that carries the xcpQ gene and its promoter. PAKΔfliC is shown as a positive control strain.
Role of XcpQ Secretin in T2SS-Mediated Death in Lung Acute Infections
P. aeruginosa possesses 3 secretins, XcpQ, HxcQ and XqhA, that are known to be involved in T2 secretion [22]. We deleted xcpQ to provide another mutation in the T2SS as well as to ascertain whether this was the secretin involved in secretion of the factors responsible for death. To test whether another independent mutation in the T3SS demonstrated a similar phenotype as the pscC mutant, we engineered a mutation in pscF, encoding the major protein of the T3SS needle and a double-secretion mutant, ΔpscFΔxcpQ. TLR2,4−/− mice were infected as described earlier. This second set of mutants had the same virulence phenotypes as the first, demonstrating that each secretion system had an independent effect (Figure 1B), and that XcpQ was the critical secretin, with no measurable contribution from the alternate secretion pathway through HxcQ or XqhA. The ΔxcpQ mutant (T3SS+) rapidly killed all mice, the ΔpscF mutant (T2SS+) killed 9 of 10 mice, again with the delay seen with the previous T2SS+ strain, and ΔpscFΔxcpQ (T3SS−/T2SS−) was avirulent (Figure 1B). To confirm that this specific secretin deletion led to the loss of virulence rather than a secondary mutation, we complemented xcpQ in ΔpscFΔxcpQ and challenged mice with this strain. As a control for these experiments, the mini-CTX vector was inserted into the att site of ΔpscFΔxcpQ. The complemented secretin mutation restored virulence, confirming the important role of the T2SS and XcpQ in virulence (Figure 1B).
Effect of T2SS and T3SS Mutations in P. aeruginosa Strain PA14
Strain PA14 is believed to be one of the most virulent P. aeruginosa strain studied. We therefore engineered flagellin and secretion system mutants in this strain and examined the virulence of these mutants at 2 × 107 CFU per animal. Again, we observed the same independent effects of the T2SS and T3S, with the double mutant being less avirulent (Figure 2).
Figure 2.
Survival of Toll-like receptor 2,4−/− mice infected with secretion mutants of Pseudomonas aeruginosa strain PA14. Mice were infected with P. aeruginosa PA14 mutants similar to those used for strain PAK, and their survival was monitored for ≥1 week. The type II secretion system of this strain also killed mice, and its deletion reduced mortality due to this strain.
Gross and Microscopic Pathologic Lung Findings in Mice Infected with T2SS and T3SS Mutants
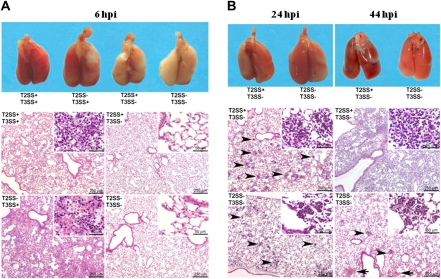
To characterize the role of the toxin secretion systems at the pathologic level, histopathologic analysis of the lungs was performed in mice 6, 24, or 44 h after infection with strains having mutations in the secretion systems, as indicated in Figure 3. Gross examination of the lungs at 6 h after infection (Figure 3A top panel) showed an intense inflammatory response among mice infected with strains that had a functioning T3SS (both T2SS+/T3SS+ and T2SS−/T3SS+). No data were obtained beyond this time with these 2 strains, because mice were dead within 24 h. In contrast, infection with a strain having only a functioning T2SS demonstrated gross hemorrhagic lesions only at 24 h after infection, which progressed with time (Figure 3B top panel). Mice infected with the T2SS−/T3SS− mutant demonstrated some gross inflammation on the surface of the lungs at 24 h, but this did not progress. Microscopic examination of the lungs (Figure 3 bottom panels) demonstrated changes that correlated with the survival data and the gross pathologic findings. At 6 h after infection only mice infected with strains having an intact T3SS (T2SS+/T3SS+ and T2SS−/T3SS+) showed pathologic changes, characterized by randomly distributed infiltrates of neutrophils, located in bronchiolar and alveolar spaces, often associated with focal necrosis of the bronchiolar overlying epithelium and alveolar walls. At this time, mice that were infected with the strain having only the T2SS did not demonstrate histopathologic changes, nor did mice infected with the T2SS−/T3SS− strain. At 24 and 44 h after infection, mice infected with T2SS+/T3SS− and T2SS−/T3SS− strains displayed similar inflammatory lesions—infiltrates of neutrophils located in the bronchiolar and alveolar spaces—but the extent and severity of the lesions differed between the 2 mutants (Figure 3B bottom panel). At 44 h after infection, the changes were more extensive and severe for the T2SS+/T3SS− mutant, whereas the mild inflammatory response seen in the double mutant was receding. Thus, the pathologic effects of the T2SS on the lungs are delayed, and they correlated with the survival studies, whereas the effects of the T3SS were seen 6 h after the challenge, and the animals died within 24 h.
Figure 3.
Macroscopic and microscopic pathologic findings in the lungs of Toll-like receptor (TLR) 2,4−/− mice infected with secretion mutants of Pseudomonas aeruginosa. Groups of 3 mice were infected with type II secretion system (T2SS) and type III secretion system (T3SS) mutants, as indicated, and were euthanized at different intervals after infection. Mice that were infected with mutants PAKΔfliC and PAKΔfliCΔxcpQ, both having a competent T3SS, could be studied only at 6 h after infection (hpi), because they died before 24 h. A, At 6 h, lesions were already visible grossly (Fig. 3A top) and microscopically (Fig. 3A bottom) in the lungs of mice infected with mutants possessing a competent T3SS (description in text). By contrast, at this time point, the lungs of the mice infected with the T2SS+ mutant or the double-secretion mutant T2SS−/T3SS− did not show significant macroscopic (Fig. 3A top) or microscopic lesions (Fig. 3A bottom). B, At 24 h, both gross (Fig. 3B top) and histologic (Fig. 3B bottom) changes were apparent in animals infected with both the T2SS+ and the double-secretion mutant but were more severe with the T2SS+ strain, with findings characterized by randomly distributed intra-alveolar neutrophils infiltrates (arrowheads, insets). At 44 h, mice infected with the double-secretion mutant showed minimal gross pathologic changes (Fig. 3B top) and a decrease in cell infiltrates (Fig. 3B bottom) with rare hemorrhages (arrowheads). By contrast, lesions were more severe and extensive, and progressed to hemorrhage and consolidation with the T2SS+ strain. This coincided with deaths that began on day 2 after infection.
Effect of the T2SS on Bacterial Clearance and Host Innate Responses
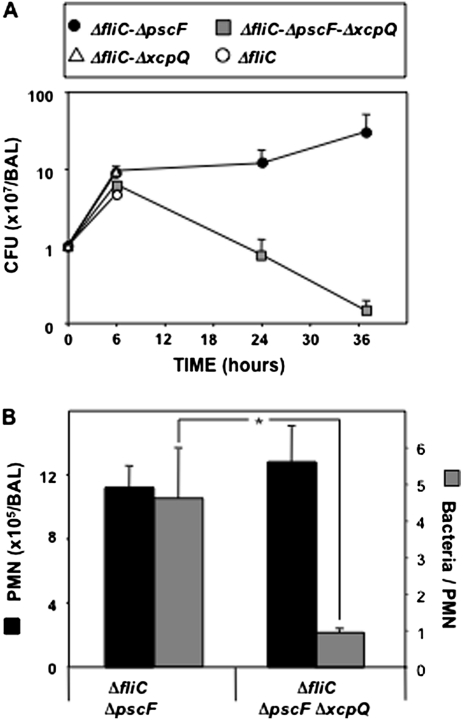
Infected mice were euthanized, and BAL performed. Mice infected with T3SS+ mutants could be studied only at 6 h after infection. Mice infected with mutants that were T2SS+/T3SS− or T2SS−/T3SS− were studied at 6, 24, and 44 h after infection. Regardless of the mutant used, the number of bacteria in the BAL increased 5–10-fold at 6 h after challenge, compared with the administered inoculum (Figure 4A). Interesting differences were observed at later time points between mice infected with ΔpscFΔxcpQ (T2SS−/T3SS−) and those infected with ΔpscF (T2SS+/T3SS−). In mice infected with ΔpscFΔxcpQ, BAL bacterial counts fell rapidly in the next 20 h, but in those infected with ΔpscF, counts rose to >50-fold more than the initial inoculum (Figure 4A), implying a defect in the host defenses in the presence of the T2SS. The number of PMNs recovered from the BAL fluids did not differ significantly at 24 h (Figure 4B) from that at other time points (data not shown). Thus, the difference in colony counts cannot be explained by differences in the number of PMNs recruited. We therefore examined whether there was a defect in phagocytic killing when the T2SS was present by measuring viable intracellular bacterial counts in total BAL PMNs (Figure 4B). There were 4-fold more viable intracellular bacteria in the cells of the T2SS+ infected group than in the T2SS− group (P = .0186).
Figure 4.
A, Bacterial proliferation in the bronchoalveolar lavage (BAL) fluid of Toll-like receptor (TLR) 2,4−/− mice infected with type II secretion system (T2SS) mutant strains of Pseudomonas aeruginosa strain PAK (T2SS+ and T2SS−). Groups of 5–6 mice were infected with T2SS+ and T2SS− strains and were euthanized at 6, 24 and 36 h after infection. BAL was performed, and samples were processed to obtain viable bacterial counts. Data are presented as means ± standard errors of the mean for 5–6 mice per time point. Differences between groups were assessed 36 h after infection for statistical significance, using analysis of variance followed by Fisher's test; *P < .05. CFU, colony-forming units. B, Polymorphonuclear neutrophil (PMN) and intracellular bacterial counts in the BAL fluid of TLR2,4−/− mice infected with T2SS+ and T2SS− strains of P. aeruginosa strain PAK. Groups of 5–6 mice were infected with T2SS and T3SS mutants and were euthanized at 24 h after infection. BAL was performed, and samples were processed to obtain PMN counts. Left ordinate shows the PMN counts; right ordinate, number of viable bacteria per PMN. Data are presented as means ± standard errors of the mean for 5–6 mice per time point. Differences between groups were assessed for statistical significance, using the t test; *P = .0186.
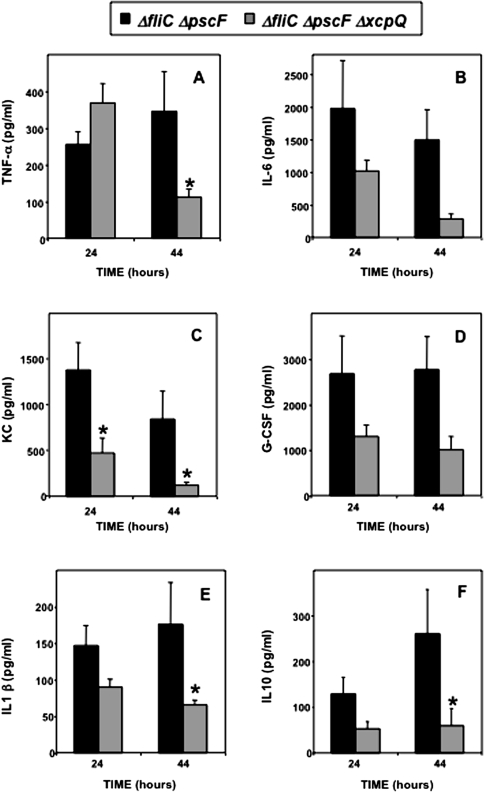
We also analyzed the synthesis of cytokines under the same experimental conditions (Figure 5 A–F). Globally, there was a tendency for a higher production in the presence of the T2SS, except for the tumor necrosis factor α response at 24 h, ruling out the subversion of these responses by the T2SS. However, there was one finding of possible significance in the host response to the T2SS competent mutant, elevated levels of the anti-inflammatory cytokine interleukin-10 (Figure 5F), which has been shown to inhibit P. aeruginosa clearance from the lungs and to worsen survival rates [23, 24], as well as affecting survival in other types of infections [25].
Figure 5.
Cytokine contents of the bronchoalveolar lavage (BAL) fluid of TLR2,4−/− mice resulting from infections with secretion mutants of Pseudomonas aeruginosa strain PAK. Groups of 5–6 mice were infected with type II secretion system (T2SS) and type III secretion system (T3SS) mutants, as indicated, and were euthanized at 24 or 44 h after infection. BAL was performed, and samples were processed to measure different cytokines, including granulocyte colony-stimulating factor (G-CSF), interleukin (IL), KC cytokine, and tumor necrosis factor (TNF). Data are presented as means ± standard errors of the mean for 5–6 mice per time point. Differences between groups were assessed for statistical significance at both 24 and 44 h after infection, using analysis of variance followed by Fisher's test; *P < .05.
Role of the T2SS and T3SS in Immunocompetent Mice
Given the clear-cut distinction between the action of the secretion systems in TLR2,4−/− mice, we examined whether this difference held for mice that had functioning TLRs 2, 4, and 5 by infecting wild-type mice with flagellated secretion system mutants (Figure 6). Even in these mice, we note that both secretion systems function independently, but 5–10-fold more bacteria were required to kill 100% of wild-type mice with the wild-type strain or the T3SS+ strain of P. aeruginosa. Deaths were even more delayed under these circumstances.
Figure 6.
Survival of wild-type (WT) mice infected with flagellated strain PAK carrying mutations in the type II secretion system (T2SS) and type III secretion system (T3SS). Mice were infected with 5 × 107 colony-forming units of each indicated strain, the dose of the wild-type strain required to kill 100% of wild-type mice [15]. The independent effects of the secretion systems are seen, but deaths caused by both secretion systems are delayed compared with outcomes in TLR 2,4−/− mice infected with the fliC mutant, in which the T3SS system kills all mice in <24 h at a lower dose of bacteria and deaths due to the T2SS begin after 24 h.
DISCUSSION
These studies demonstrate unequivocally that the T2SS of P. aeruginosa is capable of causing death in lung infections, an issue that has been unsettled. They also demonstrate that the onset of action differs between the T2SS and the T3SS , with the T3SS acting rapidly within 24 h, and the T2SS causing death at a slower rate, differences that were not hitherto appreciated and no doubt contributed to the lack of an appreciation of the role of the T2SS. It is not known whether this difference in the timing of lung injury is due to the fact that activation of the T2SS requires that bacteria reach high concentrations to achieve quorum sensing [26] or due to the fact that pathologic lesions are late in appearing; however, because the bacterial count in the lungs had increased significantly by 24 h, the observations are consistent with a role of quorum sensing in mediating lung injury and death.
These studies also narrow down the possible pathways used for T2 toxin secretion. It has long been suspected that the Xcp secreton was responsible for secretion of the better-known T2 secreted toxins of P. aeruginosa [27], but the discovery of the Hxc secreton [7] has raised the possibility that this pathway may also be used for novel toxin secretion. Additionally, there is XqhA [8], an alternative secretin that enables low-level secretion of XcpQ substrates. Neither of these, however, appear to play significant roles in virulence in this model, because deletion of xcpQ alone renders the organism avirulent in the absence of T3 secretion.
The ability of P. aeruginosa to avoid phagocytic clearance by PMNs is a major virulence determinant. Successful evasion of phagocytosis by P. aeruginosa is believed to be primarily dependent on the presence of a functional T3SS, because this system has been demonstrated to kill neutrophils [28]. However, it also appears that a functional T2SS causes a clearance defect, as demonstrated in the present study by rising bacterial counts when the T2SS is present and more viable bacteria within the neutrophils. The sole possible insight into this defect involved the elevated concentrations of the anti-inflammatory cytokine interleukin-10 in BAL fluid. This cytokine has been demonstrated to impair both neutrophil and alveolar macrophage bactericidal activity [29, 30], and antibody against it improves survival in P. aeruginosa lung infection in a cecal ligation puncture model of immunosuppression [25]. Thus, there may be 2 independent actions of the T2SS as a whole that are not fully explained by our knowledge of the secreted products—a toxic effect on the mouse lung and an independent one on host defenses. In vitro, none of the major T2 secreted enzymes (ExoA, LasB, and phospholipase H) kill neutrophils [31], even though subtle effects on function have been reported [32]. In vivo, a mutant in a regulatory gene for ExoA, has been shown to impair host defenses in the lungs of wild-type mice by allowing bacterial counts to reach higher levels, but this mutation did not affect survival of mice and it had no effect on neutrophil migration into the airways [33]. Elucidating which toxins are having these effects(s) will require a detailed characterization of the secreted products that use the XcpQ secretin and testing of mutants in the secreted products.
It may be argued that the T3SS is the first system to act, and because it kills more rapidly, there may not be a role for the T2SS. However, not all P. aeruginosa strains are competent for T3 secretion. Sokol et al [34] examined 124 clinical isolates from burns and bacteremia and found that only 38% of the isolates secreted ExoS, whereas 80% secreted ExoA. Assuming that strains having ExoU were missed (approximately one-third of those that are ExoS positive), this suggests that only half of these strains were T3 secretion competent. Roy-Burman et al [10] examined 71 non CF lung isolates and found that only 66% secreted ExoS or ExoU. Hauser et al [35] examined 35 selected strains from patients with pneumonia and found that 74% were T3SS competent, but they further pointed out that among these same isolates, secretion of ExoS was not consistently associated with virulence in a mouse model of pneumonia [36], suggesting that other virulence factors played a role in death. We have also examined 100 blood isolates for T2 secretion, using elastolytic activity as a proxy for T2 secretion, and have found that 99% of these isolates are T2 secretion competent (data not shown). Thus, these 2 systems may be viewed together as comprising a fail-safe system for defense against whatever host the organism encounters and are both integral to pathogenesis, neither more important than the other.
The findings in this study are also of practical significance in the development of active or passive vaccines or treatments for P. aeruginosa if one wishes to target the toxin-producing systems. Targeting either the T3SS or the T2SS, or any of their products alone, will not be optimal. Although it has been demonstrated that targeting PcrV of strain PA103 is protective [37], it should be pointed out that this strain demonstrates defects in T2 secretion; it does not secrete the major protease LasB and lacks a flagellum [38] and is therefore not representative of a large number of P. aeruginosa strains. Thus, some surface-exposed component of the T2SS or a specific toxic secretion product needs to be included in a vaccine designed to target secretions.
Besides demonstrating a role for the T2SS, this study also demonstrates the potential of using TLR-knockout mice to elucidate pathogenesis under circumstances where there is host compromise. A reasonable assumption is that for a significant infection caused by this opportunistic bacterium [39] to occur there must be failure of innate immunity which we have replicated by using these mice. Although challenge with larger numbers of bacteria will overcome the innate immune response of normal mice, this is also likely to cause an inflammatory response that may confound an analysis of the effects on individual toxins, especially if multiple toxins act together to cause death.
Funding
This work was supported in part by grants from Vaincre la Mucoviscidose and Legs Poix to M.C. and National Institutes of Health grant 1R01AI078770-01A1 to R.R.
References
- 1.Couch-Brewer C, Wunderink RG, Jones CB, Leeper KV., Jr Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–29. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 2.Kudoh I, Wiener-Kronish JP, Hashimoto S, Pittet JF, Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol. 1994;267:L551–6. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- 3.Wieland CW, Siegmund B, Senaldi G, Vasil ML, Dinarello CA, Fantuzzi G. Pulmonary inflammation induced by Pseudomonas aeruginosa lipopolysaccharide, phospholipase C, and exotoxin A: role of interferon regulatory factor 1. Infect Immun. 2002;70:1352–8. doi: 10.1128/IAI.70.3.1352-1358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cianciotto NP. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 2005;13:581–8. doi: 10.1016/j.tim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol. 2009;12:61–6. doi: 10.1016/j.mib.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Quinaud M, Chabert J, Faudry E, et al. The PscE-PscF-PscG complex controls type III secretion needle biogenesis in Pseudomonas aeruginosa. J Biol Chem. 2005;280:36293–300. doi: 10.1074/jbc.M508089200. [DOI] [PubMed] [Google Scholar]
- 7.Ball G, Durand E, Lazdunski A, Filloux A. A novel type II secretion system in Pseudomonas aeruginosa. Mol Microbiol. 2002;43:475–85. doi: 10.1046/j.1365-2958.2002.02759.x. [DOI] [PubMed] [Google Scholar]
- 8.Martínez A, Ostrovsky P, Nunn DN. Identification of an additional member of the secretin superfamily of proteins in Pseudomonas aeruginosa that is able to function in type II protein secretion. Mol Microbiol. 1998;28:1235–46. doi: 10.1046/j.1365-2958.1998.00888.x. [DOI] [PubMed] [Google Scholar]
- 9.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun. 2004;72:6969–77. doi: 10.1128/IAI.72.12.6969-6977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy-Burman A, Savel RH, Racine S, et al. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis. 2001;183:1767–74. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- 11.Elsheikh LE, Kronevi T, Wretlind B, Abaas S, Iglewski BH. Assessment of elastase as a Pseudomonas aeruginosa virulence factor in experimental lung infection in mink. Vet Microbiol. 1987;13:281–9. doi: 10.1016/0378-1135(87)90090-3. [DOI] [PubMed] [Google Scholar]
- 12.Blackwood LL, Stone RM, Iglewski BH, Pennington JE. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983;39:198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostroff RM, Wretlind B, Vasil ML. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect Immun. 1989;57:1369–73. doi: 10.1128/iai.57.5.1369-1373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramphal R, Balloy V, Jyot J, Verma A, Si-Tahar M, Chignard M. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J Immunol. 2008;181:586–92. doi: 10.4049/jimmunol.181.1.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramphal R, Balloy V, Huerre M, Si-Tahar M, Chignard M. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J Immunol. 2005;175:3927–34. doi: 10.4049/jimmunol.175.6.3927. [DOI] [PubMed] [Google Scholar]
- 16.Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell. 2003;4:253–63. doi: 10.1016/s1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee VT, Smith RS, Tümmler B, Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect Immun. 2005;73:1695–705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta N, Wolfgang MC, Goodman AL, et al. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol Microbiol. 2003;50:809–24. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 20.Filloux A, Bally M, Murgier M, Wretlind B, Lazdunski A. Cloning of xcp genes located at the 55 min region of the chromosome and involved in protein secretion in Pseudomonas aeruginosa. Mol Microbiol. 1989;3:261–5. doi: 10.1111/j.1365-2958.1989.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoang TT, Kutchma AJ, Becher A, Schweizer HP. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid. 2000;43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- 22.Bitter W. Secretins of Pseudomonas aeruginosa: large holes in the outer membrane. Arch Microbiol. 2003;179:307–14. doi: 10.1007/s00203-003-0541-8. [DOI] [PubMed] [Google Scholar]
- 23.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;62:392–9. [PubMed] [Google Scholar]
- 24.Muenzer JT, Davis CG, Chang K, et al. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78:1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 26.Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol. 2003;185:2080–95. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tommassen J, Filloux A, Bally M, Murgier M, Lazdunski A. Protein secretion in Pseudomonas aeruginosa. FEMS Microbiol Rev. 1992;9:73–90. doi: 10.1016/0378-1097(92)90336-m. [DOI] [PubMed] [Google Scholar]
- 28.Dacheux D, Attree I, Schneider C, Toussaint B. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect Immun. 1999;67:6164–7. doi: 10.1128/iai.67.11.6164-6167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laichalk LL, Danforth J, Standiford TJ. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol Med Microbiol. 1996;15:181–7. doi: 10.1111/j.1574-695X.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 30.Oswald I, Wynn TA, Sher A. Interleukin 10 inhibits macrophage microbicidal activity by blocking endogenous production of tumor necrosis factor-α required as a costimulatory factor for interferon-γ-induced activation. Proc Natl Acad Sci U S A. 1992;89:8676–80. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber B, Nickol MM, Jagger KS, Saelinger CB. Interaction of Pseudomonas exoproducts with phagocytic cells. Can J Microbiol. 1982;28:679–85. doi: 10.1139/m82-102. [DOI] [PubMed] [Google Scholar]
- 32.Terada LS, Johansen KA, Nowbar S, Vasil AI, Vasil ML. Pseudomonas aeruginosa hemolytic phospholipase C suppresses neutrophil respiratory burst activity. Infect Immun. 1999;67:2371–6. doi: 10.1128/iai.67.5.2371-2376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz MJ, Rijneveld AW, Florquin S, Speelman P, Van Deventer SJ, van der Poll T. Impairment of host defence by exotoxin A in Pseudomonas aeruginosa pneumonia in mice. J Med Microbiol. 2001;50:822–7. doi: 10.1099/0022-1317-50-9-822. [DOI] [PubMed] [Google Scholar]
- 34.Sokol PA, Iglewski BH, Hager TA, et al. Production of exoenzyme S by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1981;34:147–53. doi: 10.1128/iai.34.1.147-153.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauser AR, Cobb E, Bodi M, et al. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–8. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Schulert GS, Feltman H, Rabin SD, et al. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188:1695–706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- 37.Sawa T, Yahr TL, Ohara M, et al. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–8. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- 38.Liu PV. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973;128:506–13. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- 39.Ventura GM, Balloy V, Ramphal R, et al. Lack of MyD88 protects the immunodeficient host against fatal lung inflammation triggered by the opportunistic bacteria Burkholderia cenocepacia. J Immunol. 2009;183:670–6. doi: 10.4049/jimmunol.0801497. [DOI] [PubMed] [Google Scholar]