Abstract
Background. Viral load may influence the course of human papillomavirus type 16 (HPV-16) infection.
Methods. This case-control study was nested within the 2-year Atypical Squamous Cells of Undetermined Significance and Low-Grade Squamous Intraepithelial Lesion Triage Study, in which women were followed semiannually for HPV and cervical intraepithelial neoplasia (CIN). Case patients (n = 62) were women diagnosed with CIN3 following HPV-16–positive detection at a follow-up visit. HPV-16–positive controls (n = 152) without CIN2 or CIN3 were matched to cases based on the follow-up visit in which viral load was measured. Real-time polymerase chain reaction was used for HPV-16 DNA quantification.
Results. The risk of CIN3 increased with increasing HPV-16 DNA load at the follow-up visit (odds ratio, 1.63; 95% confidence interval, 1.33–1.99 per 1 log10 unit increase); the association was not affected by whether HPV-16 was present at enrollment. When HPV-16 was present at both enrollment and follow-up, viral load remained high among cases (P = .77) but decreased substantially among controls (P = .004). Among women with HPV-16 found initially during follow-up, viral load in the first HPV-16–positive sample was associated with short-term persistence; load was higher in those with infection, compared with those without infection, 1 visit after the initial positivity (P = .001).
Conclusions. Viral load of newly detected infections and changes in viral load predict persistence and progression of HPV-16 infections.
Human papillomavirus type 16 (HPV-16) is the most carcinogenic type of HPV. In prospective studies, infection with HPV-16 as compared with other types leads to the highest risk of cervical intraepithelial neoplasia grade 3 (CIN3), the precursor of cervical cancer. In worldwide cross-sectional studies, HPV-16 is responsible for approximately 50% of cases of invasive cervical cancer [1, 2]. Most HPV-16 infections are, however, transient [3–5], with only a small fraction leading to viral persistence and the development of CIN3 or cervical cancer. It is important to understand aspects of host, environment, and virus that increase the eventual risk of cancer among infected women. Given that viral load reflects the productivity of DNA replication in the HPV life cycle, its level may play a role in defining the course of HPV infection.
Positive associations of HPV-16 DNA load with risk of persistent infection and CIN2/3 have been reported in some studies [6–22] but not others [23–27]. In almost all of these studies, however, the analysis was based on a single measurement of viral load for prevalent infections found at the baseline screening phase of a study. Prevalently detected infections are a mixture of preexisting and recently acquired infections; longer-duration infections are overrepresented, especially among older women. The risk posed by the increasing viral load of newly detected infections remains largely undefined. In addition, although viral load is known to fluctuate over time [28], except for findings from a few small studies [28–31], there is little known about such changes in viral load and risk of subsequent CIN3.
Thus, there is a need for longitudinal studies of viral load in the course of HPV infections. In this study, we assess the impact of the viral load of newly detected HPV-16 infections on retaining HPV-16 positivity and risk (and timing) of development of CIN3. Among women with a prevalent HPV-16 infection, we describe changes in viral load between those with and without a subsequent diagnosis of CIN3.
STUDY SUBJECTS AND METHODS
Study Design and Subjects
The case-control study was nested in the Atypical Squamous Cells of Undetermined Significance (ASCUS) and Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS), a randomized trial designed to evaluate strategies for management of women with a referral of equivocal/mildly abnormal cervical cytology. A detailed description of the ALTS design is presented elsewhere [32, 33]. Briefly, participants were randomly assigned into 1 of 3 arms: immediate colposcopy (referral of all women to colposcopy at enrollment), HPV triage (referral to colposcopy if the enrollment testing result was high-risk HPV positive or the enrollment cytology was high-grade squamous intraepithelial lesion [HSIL]), or conservative management (referral to colposcopy if the enrollment cytology was HSIL). All subjects regardless of arm were scheduled for liquid-based cytology and HPV testing every 6 months for 2 years. Women with HSIL cytology during follow-up were referred for colposcopy. At exit, participants were asked to undergo an exit procedure including cytology, HPV testing, and colposcopic examination with biopsy of any visible lesion.
Case patients for the present study were women who had HPV-16 DNA detected by polymerase chain reaction (PCR)–based reverse line blot [34] at the follow-up visit, at which they were referred to colposcopy and CIN3 was initially histologically confirmed by a panel of expert pathologists, regardless of whether HPV-16 was detected at enrollment (prevalent versus new). Control subjects were selected from a pool of women who had HPV-16 DNA detected at 1 or more follow-up visits but did not have a diagnosis of CIN2 or CIN3 in the 2-year study period. Controls were frequency matched to cases on the timing of the HPV-16–positive follow-up sample for viral load measurement in a ratio of ∼1:1 for those with an enrollment HPV-16 infection and 2:1 for those without.
Data on HPV typing results, cervical lesions, and characteristics of study subjects were from the ALTS database. We measured HPV-16 DNA load in 1 matched follow-up sample for each subject. Of the 100 case patients and 155 control subjects initially selected, 5 (2 cases and 3 controls) were excluded because their follow-up samples were insufficient for viral load quantification. We additionally excluded 18 cases with a final diagnosis of CIN2: 6 cases whose HPV-16–positive follow-up visit was after their CIN3 diagnosis and 12 cases who, in final results, were HPV-16 negative at the screening visit directly before the CIN3 diagnosis. This left 62 cases and 152 controls in the analysis.
For women with an HPV-16 infection initially detected during follow-up, those with viral load tested on the first positive sample were included in the analysis of the initial viral load–related persistence and progression of HPV-16 infections. For women with an HPV-16 infection at enrollment into ALTS, viral load was measured on a follow-up sample from the visit prior to or at the time of CIN3 diagnosis for cases and a visit number–matched sample for controls. Because we previously measured viral load on all HPV-16–positive enrollment samples [20], this allows us to use both enrollment and follow-up sample for a pair-wise comparison of viral loads for those who were selected for the present study.
The ALTS protocol was approved by the institutional review boards at the National Cancer Institute and all other collaborating institutions. The protocol for this study was approved by the Institutional Review Board at the University of Washington.
Quantification of HPV-16 DNA Load
HPV-16 E7 copy number and cellular DNA amount (estimated by testing for β-actin gene) in cervical swab samples were measured by multiplex real-time PCR, as described previously [20]. Two log-phase 5-point standard curves, one for HPV-16 and the other for cellular DNA, were implemented in each set of the assay for absolute quantification. The viral load was normalized to the input amount of cellular DNA and then log10-transformed. Each sample was assayed in triplicate, and the mean value of the triplicate measurements, expressed as the mean of log10 (HPV-16 E7 copies per 1 ng of cellular DNA), was used for statistical analyses. HPV-16 E7 DNA was undetectable by real-time PCR in 27 samples that were previously positive by PCR-based reverse line blot. Considering that the negative result might be due to a tiny amount of viral DNA, a value of 1 viral copy per nanogram of cellular DNA was assigned to each sample. Similar conclusions were obtained when these samples were excluded from the analysis (data not shown).
Statistical Analyses
The main goal of the analyses is to examine the relationship of viral load with the persistence and progression of HPV-16 infections. To help with a selection of appropriate covariates for adjustment, we assessed distributions of subjects’ characteristics by case/control status and associations of these factors with viral load in the control group. Characteristics of the case patients versus control subjects were evaluated by χ2 tests. Among control subjects, a Student t-test or 1-way analysis of variance, whichever was appropriate, was used to compare follow-up viral load by age (18–24 or ≥25 years), race (white or nonwhite), lifetime number of male sex partners (0–5 or ≥6), current use of hormonal contraceptives (yes or no), cigarette smoking (never, current, or former), Pap tests per year in the last 5 years (<1 or ≥1), follow-up visit number for viral load measurement (1, 2, 3, or 4), study arm (immediate colposcopy, HPV triage, or conservative management), cervical cytology (within normal limits, ASCUS, or squamous intraepithelial lesion [SIL]), and coinfection with other high-risk HPV types (yes or no) including HPV-18/31/33/35/39/45/51/52/56/58/59/68. Characteristics were based on information at enrollment, with the exception of coinfection with other high-risk HPV types and cytologic findings, which were based on information at the follow-up visits from which samples were assayed for viral load.
A logistic regression model [35] was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association of risk of CIN3 per 1 log10 unit increase in follow-up viral load. The ORs were adjusted for smoking status at enrollment due to its association with both case status and viral load in the control group. Among women with an HPV-16 infection initially detected during follow-up, a trend of decrease in viral load in the first HPV-16–positive sample with increase in time from CIN3 diagnosis (ie, diagnosed at the first HPV-16–positive visit, 1 visit later, and 2 or 3 visits later) was examined by a trend test.
Among women with HPV-16 infection initially detected during follow-up, we used a linear regression model [36] to compare viral load in the first HPV-16–positive sample between women who remained HPV-16 positive and those who became negative at subsequent visits, while adjusting for smoking status at enrollment and cytologic findings at the follow-up visits from which samples were assayed for viral load. Cases were censored at the time of CIN3 diagnosis. In ALTS, the events of becoming HPV negative were ascertained by 6-month intervals. A woman was eligible for analysis of the initial viral load–related HPV-16 status at a given visit if she had HPV-16 detected at all scheduled previous visits. For example, a woman with an HPV-16 infection at month 6 was eligible for analysis of HPV-16 status at the month 12 visit; if she continued to be HPV-16 positive at month 12, she would be eligible for the analysis at month 18. While this analysis is straightforward, it excluded all visits after an initial missing visit. Thus, a second analysis was performed to examine the relationship between viral load in the first HPV-16–positive sample and the number of subsequent HPV-16–positive visits (including the one with a newly detected infection). P values were additionally adjusted for number of visits seen after the newly detected infection.
Among women who were positive for HPV-16 at enrollment into ALTS, a paired t-test was used for a pairwise comparison of enrollment and follow-up HPV-16 DNA load, stratified by case/control status. All statistical tests were conducted at the 5% 2-sided significance level.
RESULTS
The mean age at enrollment into ALTS was 23.9 years (SD, 6.6) for 62 case patients and 23.0 (SD, 4.8) for 152 control subjects. Characteristics of the study subjects are summarized in Table 1. Cases, as compared with controls, were more likely to self-report as current or former smokers at enrollment (P < .001) and have a cytologic interpretation of ASCUS or SIL (P < .001) at the follow-up visit from which a sample was assayed for viral load. Among controls, higher viral load was related to current or former cigarette smoking (P = .01) and abnormalities of cervical cytology (P < .001) but not other characteristics listed in Table 1 (data not shown).
Table 1.
Characteristics of the Case Patients and Control Subjects
| Characteristic a | Case Patients (no. [%]) | Control Subjects (no. [%]) | P |
| Age at study entry, years | .44 | ||
| 18–24 | 42 (67.7) | 111 (73.0) | |
| ≥25 | 20 (32.3) | 41 (27.0) | |
| Raceb | .25 | ||
| White | 44 (72.1) | 97 (63.8) | |
| Nonwhite | 17 (27.9) | 55 (36.2) | |
| Lifetime no. of male sex partnersc | .99 | ||
| 0–5 | 32 (52.5) | 79 (52.3) | |
| ≥6 | 29 (47.5) | 72 (47.7) | |
| Current hormonal contraceptive used | .23 | ||
| No | 37 (59.7) | 75 (50.7) | |
| Yes | 25 (40.3) | 73 (49.3) | |
| Smoking status | <.001 | ||
| Never | 16 (25.8) | 85 (55.9) | |
| Current | 34 (54.8) | 52 (34.2) | |
| Former | 12 (19.4) | 15 (9.9) | |
| No. of Pap tests/year in the past 5 years | .95 | ||
| <1 | 36 (58.1) | 89 (58.6) | |
| ≥1 | 26 (41.9) | 63 (41.4) | |
| Coinfection with other high-risk HPV types | .61 | ||
| No | 27 (43.5) | 72 (47.4) | |
| Yes | 35 (56.5) | 80 (52.6) | |
| Cytologic findings | <.001 | ||
| Within normal limits | 8 (12.9) | 77 (50.7) | |
| ASCUS | 13 (21.0) | 38 (25.0) | |
| SIL | 41 (66.1) | 37 (24.3) | |
| Study arm | .82 | ||
| Immediate colposcopy | 19 (30.6) | 46 (30.3) | |
| HPV triage | 7 (11.3) | 22 (14.5) | |
| Conservative management | 36 (58.1) | 84 (55.2) | |
| Follow-up visit no. for viral load analysis | .37 | ||
| 1 | 17 (27.4) | 58 (38.2) | |
| 2 | 19 (30.7) | 35 (23.0) | |
| 3 | 10 (16.1) | 28 (18.4) | |
| 4 | 16 (25.8) | 31 (20.4) |
NOTE. a Characteristics were based on information at enrollment, with the exceptions of coinfection with other high-risk HPV types and cytologic findings, which were based on information at follow-up visits where HPV-16 DNA load was measured.
One case patient who did not provide information on race was excluded. The category of “Nonwhite” race includes African American, American Indian/Alaskan, and Asian/Pacific Islander women.
One case patient and one control subject who did not provide information on lifetime number of male sex partners were excluded.
Four control subjects who did not provide information on hormonal contraceptive use were excluded.
HPV-16 DNA Load and Risk of CIN3
The mean value of log10-transformed HPV-16 E7 copy number per 1 ng of cellular DNA was 3.09 (SD, 1.50) for cases at the visit prior to or at the time of CIN3 diagnosis and 1.60 (SD, 1.63) for controls at the corresponding visit. Adjusting for smoking status at enrollment, the risk of CIN3 increased by 63% per one unit increase in log10-transformed HPV-16 DNA load (Table 2; ORadjusted, 1.63; 95% CI, 1.33–1.99). Biopsy was performed in 29 of 62 cases and 70 of 152 controls at visits prior to measurement of follow-up viral load. An additional adjustment for having biopsy or age at enrollment did not alter the associations appreciably (data not shown).
Table 2.
Odds Ratios and 95% Confidence Intervals for the Association of Cervical Intraepithelial Neoplasia Grade 3 per 1 Unit Increase in Log10-transformed Follow-up Human Papillomavirus Type 16 DNA Load
| Group | Total Assessed (no.) | Log10 Viral Copies/ng of Cellular DNA (Mean Load ± SD) | Adjusted OR (95% CI)a |
| Control subjects | 152 | 1.60 ±1.63 | 1.00 |
| Case patients | 62 | 3.09 ±1.50 | 1.63 (1.33–1.99) |
NOTE. a Adjusted for smoking status at enrollment.
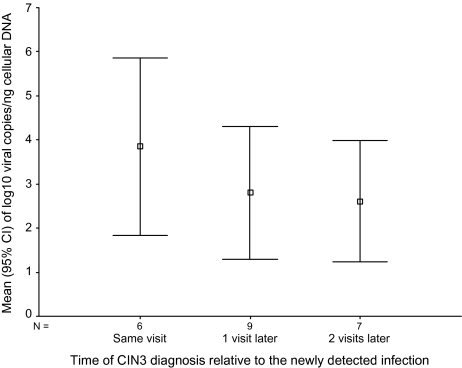
The HPV-16 infection was initially detected during follow-up in 24 of 62 cases and 108 of 152 controls. Of these, 22 cases and 101 controls had HPV-16 DNA load measured on the first HPV-16–positive sample. The risk of CIN3 increased by 62% per 1 unit increase in log10-transformed viral load in the first positive samples (Table 3; ORadjusted, 1.62; 95% CI, 1.21–2.17). The association was stronger when controls were compared with cases with the lesion diagnosed at visits coincident with the newly detected HPV-16 infection (ORadjusted, 2.09; 95% CI, 1.25–3.50). Viral load in the first HPV-16–positive sample marginally decreased with increasing time until diagnosis of HPV-16–positive CIN3, although the trend was not statistically significant (Figure 1; Pfor trend = .24). Among women with a detectable HPV-16 infection at enrollment into ALTS who were selected for the present study (38 cases and 44 controls), the adjusted OR associating CIN3 with 1 log10 unit increase of viral load was 1.62 (95% CI, 1.19–2.20) for the follow-up specimen and 1.38 (95% CI, .91–2.11) for the enrollment specimen.
Table 3.
Odds Ratios and 95% Confidence Intervals for the Association of Cervical Intraepithelial Neoplasia Grade 3 per 1 Unit Increase in Log10-transformed Human Papillomavirus Type 16 DNA Load in the First Positive Sample Among Women With a Newly Detected Infection
| Group | Total Assessed (no.) | Log10 Viral Copies/ng of Cellular DNA (Mean Load ± SD) | Adjusted OR (95% CI)a |
| Control subjects | 101 | 1.52 ±1.57 | 1.00 |
| Case patients | 22 | 3.03 ±1.80 | 1.62 (1.21–2.17) |
| Cases by time of diagnosis relative to the newly detected infection | |||
| The same visit | 6 | 3.85 ±1.92 | 2.09 (1.25–3.50) |
| 1 visit later | 9 | 2.80 ±1.96 | 1.53 (1.03–2.28) |
| 2 or 3 visits later | 7 | 2.61 ±1.49 | 1.46 (.94–2.26) |
NOTE. aAdjusted for smoking status at enrollment.
Figure 1.
Mean (square) and 95% confidence interval (upper and lower bound) of log10 human papillomavirus type 16 E7 copies per 1 nanogram (ng) of cellular DNA detected in the first positive sample, stratified by the time to diagnosis of cervical intraepithelial neoplasia 3 (CIN3) from the newly detected infection.
Changes in HPV-16 DNA Load Between Women With and Without Subsequent CIN3
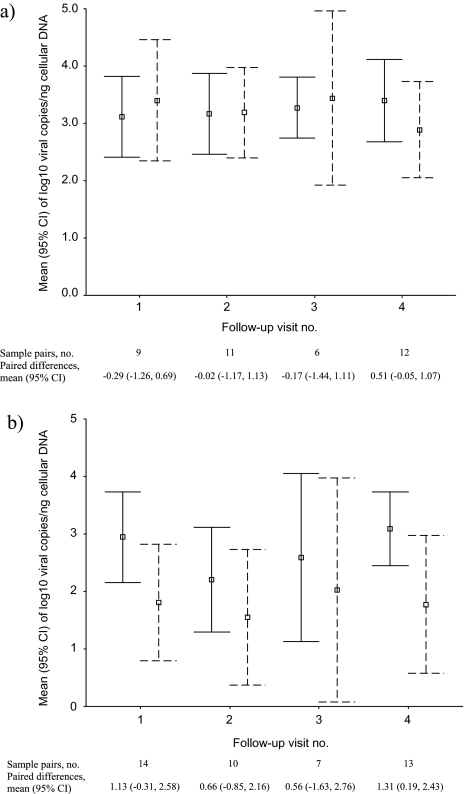
Eighty-two pairs of enrollment and follow-up samples from women with baseline HPV-16 infections were available for a pairwise comparison of HPV-16 DNA load. The mean length of the time span between the 2 samples was 15.5 months (SD, 7.0) for cases and 15.1 months (SD, 7.4) for controls (P = .80). In contrast to consistently high HPV-16 DNA load in the paired samples among cases (P = .77), the viral load among controls decreased substantially, from a mean value of 2.76 log10 units at enrollment to a mean value of 1.78 at the follow-up visit (Table 4; P = .004).
Table 4.
Pair-wise Comparisons of Human Papillomavirus Type 16 DNA Load at Enrollment Versus During Follow-up, by Case/Control Status
| Group | Sample Pairs (no.) | Log10 Viral Copies/ng of Cellular DNA at Enrollment (Mean Load ± SD) | Log10 Viral Copies/ng of Cellular DNA During Follow-up (Mean Load ± SD) | Paired Differences (Mean [95% CI]) | P |
| Case patients | 38 | 3.25 ±.95 | 3.18 ±1.28 | .06 (–.36–.49) | .77 |
| Control subjects | 44 | 2.76 ±1.30 | 1.78 ±1.80 | .99 (.33–1.64) | .004 |
Figure 2 shows viral load in paired samples, stratified by the time span between the 2 samples. The reduction of viral load over time in controls, but not in cases, was observed regardless of the follow-up visit at which the second viral load measurement was obtained.
Figure 2.
Mean (square) and 95% confidence interval (upper and lower bound) of log10 human papillomavirus type 16 E7 copies per 1 nanogram (ng) of cellular DNA detected in enrollment (solid line) and follow-up (dashed line) samples among case patients (Figure 1A) and control subjects (Figure 1B), stratified by the follow-up visits from which samples were assayed for viral load.
HPV-16 DNA Load of the Newly Detected Infection and Positivity at Subsequent Visits
Among women without a detectable HPV-16 infection at enrollment into ALTS, those with HPV-16 DNA newly detected before their last follow-up visit (19 cases and 75 controls) were followed for a mean of 2 visits after their first positive detection (1 visit, n = 33; 2 visits, n = 29; and 3 visits, n = 32). With adjustment for smoking status at enrollment and cytologic findings at the first HPV-16–positive follow-up visit, viral load in the first positive sample was significantly higher among women who remained HPV-16 positive (determined by PCR-based line blot) as compared with those who became negative at the next visit immediately following the newly detected infection (Table 5; P = .001). Again, an additional adjustment for having biopsy or age at enrollment did not alter the result appreciably (data not shown). Among those who remained HPV-16 positive at month 6 (1 visit) after the newly detected infection, there was no appreciable difference in viral load in the first positive sample by positivity at the subsequent follow-up visit at month 12 (P = .79). Similarly, subsequent persistence of infections still present at month 12 was not predicted by viral load in the first positive sample (P = .45).
Table 5.
Human Papillomavirus Type 16 DNA Load of the Newly Detected Infection in Women Who Continued to Be Positive Compared With Those Who Became Negative at Subsequent Visit
| No. of Visits Subsequent to Newly Detected Infection | No. Excluded a | Remaining Positive |
Becoming Negative |
Pb | ||
| no. | Log10 Viral Copies/ng of Cellular DNA (Mean Load ± SD) | no. | Log10 Viral Copies/ng of Cellular DNA (Mean Load ± SD) | |||
| 1 | 13 | 47 | 2.26 ±1.71 | 34 | .76 ±1.14 | .001 |
| 2 | 22 | 17 | 1.79 ±1.36 | 8 | 1.92 ±1.54 | .79 |
| 3 | 7 | 3 | 2.06 ±2.27 | 7 | 1.25 ±1.08 | .45 |
NOTE. Data are from 94 women with a newly detected infection prior to the last follow-up visit.
Excluded were women who did not return for follow-up at the visit evaluated or who had CIN3 diagnosed one visit before.
Adjusted for smoking status at enrollment and cytologic findings at the follow-up visits from which samples were assayed for viral load for analyses of retaining viral load–related positivity at subsequent visits 1 and 2 but not visit 3 (due to the small number of women seen at this visit).
We further assessed viral load in the first HPV-16–positive sample by the number of HPV-16–positive visits. With an additional adjustment for the number of visits following the first positive detection, viral load in the first positive sample was significantly higher in women with 2 (n = 35; mean = 2.52 log10 units; Padjusted = .02) or 3–4 positive visits (n = 22; mean = 1.91 log10 units; Padjusted = .09) as compared with those with a single positive visit (n = 37; mean = 1.03 log10 units). Results remained similar when the analysis was restricted to 61 women who had 2 or 3 follow-up visits after the newly detected infections or 75 controls (data not shown).
DISCUSSION
In this nested case-control study, we found that elevation in HPV-16 DNA load was associated with an increased risk of CIN3. The association cannot be explained by potential ascertainment biases, as viral load was assessed without knowledge of case/control status and the diagnosis of cervical lesion was extensively reviewed by the ALTS quality control group. Our results confirm those of other studies [6–15] that suggest that among HPV-16–positive women, those with higher, compared with lower, viral load are more likely to progress to high-grade CIN. The present study extends previous observations by showing the association of CIN3 with the viral load of newly detected HPV-16 infections. Although the temporal relationship between exposure and outcome for cases with the lesion diagnosed concurrently with a newly detected HPV-16 infection could not be demonstrated, data on those with the lesion diagnosed after support the notion that higher HPV-16 DNA load is linked to rapid development of CIN3.
The analysis of viral load in the first HPV-16–positive sample from a limited number of cases suggests a trend of decrease in viral load with increasing time until CIN3 diagnosis. This trend, although not statistically significant, somewhat agrees with a previous report [19] that showed an association between CIN2/3 and viral load 0–12 months before diagnosis (but not 13–24 months before). While there is a widely accepted view that persistent infection is a prerequisite for the development of cervical lesion [37–43], the length of persistence is not defined. Our results suggest that a sufficiently higher viral load might signal the development of CIN3 as an early outcome following a newly detected HPV-16 infection. For those with initially relatively low viral load, a sustained productive infection period is under way that permits the virus to replicate, produce a sufficient amount of oncoproteins, and eventually lead to the development of CIN3. But it is also possible that the growth of a small CIN3 clone could be causing, instead, increasing viral load that we assessed on cytologic specimens.
By analyzing HPV-16 DNA load in paired samples, we demonstrated that changes of viral load differed substantially by whether there was a subsequent diagnosis of CIN3. In contrast to a substantial reduction among control subjects, the viral load was consistently high among case patients. This pattern reinforces a previous observation that cases stayed HPV positive while controls cleared [42]. The observed differences cannot be explained by confounding by the length of the time span of sample pairs, because controls were matched to cases based on the timing of the follow-up sample; patterns of viral load changes were similar when data were stratified by visit number for the second viral load measurement. However, the time of onset of the infection for women included in this analysis was unknown; it is possible that infections in cases as compared with controls had already lasted longer prior to enrollment. The different dynamics of HPV-16 DNA load between women with and without cervical lesions were previously noted by a study with a small sample size and unmatched positive duration [29]. Our findings suggest that for women who do not have an immediate diagnosis of CIN3 at the time of HPV-16 infection, a sustained higher viral load might be informative for progression to CIN3 or having a missing/delayed diagnosis of CIN3. Among those who do not develop CIN2/3, a substantial reduction of viral load suggests a spontaneous regression, perhaps due to an efficient immune response against the virus.
The findings of the association between higher HPV-16 DNA load of the newly detected infection and 6 months of persistence, but not longer, agree with what we observed in our previous study of the viral load–associated persistence of prevalent HPV infection [21]. In that study, however, it was unknown whether the association between viral load and short-term persistence was due to a subset of prevalent infections close to the end of the course of HPV infection. By focusing on newly detected infections, it became clear that the association of viral load with short-term, but not longer-term, persistence was driven mainly by the lower viral load of infections that cleared within the first 6 months. Previous studies of viral load in relationship to persistence are rare, with inconsistent findings reported [16–19, 26, 27]. Recognition of the viral load–associated duration-specific persistent infection may help explain these discrepant results.
Several limitations of the study should be addressed. We used the term newly detected infection rather than incident infection because new acquisition can never be distinguished from reactivation among sexually active women. It is possible that some of the so-called newly detected infections might not be “new,” possibly due to an inadequate sampling, fluctuation of viral DNA below the detectable threshold, or missing detection at enrollment. However, there is no evidence that such a misclassification would be differential. Second, HPV was tested every 6 months. Thus, the disappearance and reoccurrence of the infection within the interval were possible. This may lead to a misclassification of transient infection as persistent infection and consequently an overestimation of length of persistence. Given the association between lower viral load and single positive visit, however, these misclassified infections were likely to be featured with lower viral load. Therefore, such a misclassification tended to drive the association between viral load and short-term persistence toward null. Third, a maximum of 3 visits (∼18 months) subsequent to the newly detected infection was available for analysis. It is unclear whether the association observed would still hold for a prolonged time of follow-up. Finally, because of known differences in the natural history of HPV infections between young and old women, we are not in a position to extrapolate the findings of this study to other age groups.
In summary, our results indicate that viral load of the newly detected HPV-16 infection correlated to consequences of the infection, from a transient infection to a rapid progression to CIN3. Among those with a persistent HPV-16 infection, changes in viral load reflected risk of subsequent and/or underlying CIN3. These observational data, although not definitive, suggest a potential role of viral load in defining the course of the infection.
Funding
The work was supported by Public Health Service grant CA133569 to L.F.X.
Acknowledgments
This study was part of the project ancillary to the ALTS clinical trial but does not represent the ALTS Group. We would like to thank the ALTS Group for providing cervical samples for viral DNA quantification and the ALTS data for analyses.
References
- 1.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) study group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, et al. Cytological regression and clearance of high-risk human papillomavirus in women with an abnormal cervical smear. Lancet. 2001;358:1782–3. doi: 10.1016/S0140-6736(01)06809-X. [DOI] [PubMed] [Google Scholar]
- 4.Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–23. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 5.Syrjanen S, Shabalova IP, Petrovichev N, et al. Clearance of high-risk human papillomavirus (HPV) DNA and Pap smear abnormalities in a cohort of women subjected to HPV screening in the New Independent States of the former Soviet Union (the NIS cohort study) Eur J Obstet Gynecol Reprod Biol. 2005;119:219–27. doi: 10.1016/j.ejogrb.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 6.Swan DC, Tucker RA, Tortolero-Luna G, et al. Human papillomavirus (HPV) DNA copy number is dependent on grade of cervical disease and HPV type. J Clin Microbiol. 1999;37:1030–4. doi: 10.1128/jcm.37.4.1030-1034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ylitalo N, Sorensen P, Josefsson AM, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case–control study. Lancet. 2000;355:2194–8. doi: 10.1016/S0140-6736(00)02402-8. [DOI] [PubMed] [Google Scholar]
- 8.Josefsson AM, Magnusson PK, Ylitalo N, et al. Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case–control study. Lancet. 2000;355:2189–93. doi: 10.1016/S0140-6736(00)02401-6. [DOI] [PubMed] [Google Scholar]
- 9.Moberg M, Gustavsson I, Gyllensten U. Type-specific associations of human papillomavirus load with risk of developing cervical carcinoma in situ. Int J Cancer. 2004;112:854–9. doi: 10.1002/ijc.20480. [DOI] [PubMed] [Google Scholar]
- 10.Fiander AN, Hart KW, Hibbitts SJ, et al. Variation in human papillomavirus type-16 viral load within different histological grades of cervical neoplasia. J Med Virol. 2007;79:1366–9. doi: 10.1002/jmv.20875. [DOI] [PubMed] [Google Scholar]
- 11.Cricca M, Morselli-Labate AM, Venturoli S, et al. Viral DNA load, physical status and E2/E6 ratio as markers to grade HPV16 positive women for high-grade cervical lesions. Gynecol Oncol. 2007;106:549–57. doi: 10.1016/j.ygyno.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Moberg M, Gustavsson I, Wilander E, Gyllensten U. High viral loads of human papillomavirus predict risk of invasive cervical carcinoma. Br J Cancer. 2005;92:891–4. doi: 10.1038/sj.bjc.6602436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai HC, Peng MY, Nieh S, et al. Differential viral loads of human papillomavirus 16 and 58 infections in the spectrum of cervical carcinogenesis. Int J Gynecol Cancer. 2006;16:730–5. doi: 10.1111/j.1525-1438.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 14.Snijders PJ, Hogewoning CJ, Hesselink AT, et al. Determination of viral load thresholds in cervical scrapings to rule out CIN 3 in HPV 16, 18, 31 and 33–positive women with normal cytology. Int J Cancer. 2006;119:1102–7. doi: 10.1002/ijc.21956. [DOI] [PubMed] [Google Scholar]
- 15.Lo KW, Yeung SW, Cheung TH, Siu NS, Kahn T, Wong YF. Quantitative analysis of human papillomavirus type 16 in cervical neoplasm: a study in Chinese population. J Clin Virol. 2005;34:76–80. doi: 10.1016/j.jcv.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Lai CH, Chao A, Chang CJ, et al. Host and viral factors in relation to clearance of human papillomavirus infection: a cohort study in Taiwan. Int J Cancer. 2008;123:1685–92. doi: 10.1002/ijc.23679. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine J, Hankins C, Money D, et al. Human papillomavirus type 16 (HPV-16) viral load and persistence of HPV-16 infection in women infected or at risk for HIV. J Clin Virol. 2008;43:307–12. doi: 10.1016/j.jcv.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Dalstein V, Riethmuller D, Pretet JL, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 19.van Duin M, Snijders PJ, Schrijnemakers HF, et al. Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int J Cancer. 2002;98:590–5. doi: 10.1002/ijc.10232. [DOI] [PubMed] [Google Scholar]
- 20.Xi LF, Kiviat NB, Galloway DA, Zhou XH, Ho J, Koutsky LA. Effect of cervical cytologic status on the association between human papillomavirus type 16 DNA load and the risk of cervical intraepithelial neoplasia grade 3. J Infect Dis. 2008;198:324–31. doi: 10.1086/589715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xi LF, Hughes JP, Edelstein ZR, et al. Human papillomavirus (HPV) type 16 and type 18 DNA loads at baseline and persistence of type-specific infection during a 2-year follow-up. J Infect Dis. 2009;200:1789–97. doi: 10.1086/647993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajeevan MS, Swan DC, Nisenbaum R, et al. Epidemiologic and viral factors associated with cervical neoplasia in HPV-16–positive women. Int J Cancer. 2005;115:114–20. doi: 10.1002/ijc.20894. [DOI] [PubMed] [Google Scholar]
- 23.Cheung JL, Lo KW, Cheung TH, Tang JW, Chan PK. Viral load, E2 gene disruption status, and lineage of human papillomavirus type 16 infection in cervical neoplasia. J Infect Dis. 2006;194:1706–12. doi: 10.1086/509622. [DOI] [PubMed] [Google Scholar]
- 24.Andersson S, Safari H, Mints M, Lewensohn-Fuchs I, Gyllensten U, Johansson B. Type distribution, viral load and integration status of high-risk human papillomaviruses in pre-stages of cervical cancer (CIN) Br J Cancer. 2005;92:2195–200. doi: 10.1038/sj.bjc.6602648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onan MA, Taskiran C, Bozdayi G, et al. Assessment of human papilloma viral load of archival cervical intraepithelial neoplasia by real-time polymerase chain reaction in a Turkish population. Eur J Gynaecol Oncol. 2005;26:632–5. [PubMed] [Google Scholar]
- 26.Molano M, Van den Brule A, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–94. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 27.Kulmala SM, Shabalova IP, Petrovitchev N, et al. Type-specific persistence of high-risk human papillomavirus infections in the New Independent States of the former Soviet Union cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16:17–22. doi: 10.1158/1055-9965.EPI-06-0649. [DOI] [PubMed] [Google Scholar]
- 28.Constandinou-Williams C, Collins SI, Roberts S, Young LS, Woodman CB, Murray PG. Is human papillomavirus viral load a clinically useful predictive marker? A longitudinal study. Cancer Epidemiol Biomarkers Prev. 2010;19:832–7. doi: 10.1158/1055-9965.EPI-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnier-Benoit S, Dalstein V, Riethmuller D, Lalaoui N, Mougin C, Pretet JL. Dynamics of HPV16 DNA load reflect the natural history of cervical HPV-associated lesions. J Clin Virol. 2006;35:270–7. doi: 10.1016/j.jcv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Crum CP, Beach KJ, Hedley ML, et al. Dynamics of human papillomavirus infection between biopsy and excision of cervical intraepithelial neoplasia: results from the ZYC101a protocol. J Infect Dis. 2004;189:1348–54. doi: 10.1086/382956. [DOI] [PubMed] [Google Scholar]
- 31.Ho CM, Cheng WF, Chu TY, et al. Human papillomaviral load changes in low-grade squamous intraepithelial lesions of the uterine cervix. Br J Cancer. 2006;95:1384–9. doi: 10.1038/sj.bjc.6603430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726–42. doi: 10.1159/000328554. [DOI] [PubMed] [Google Scholar]
- 33.ASCUS-LSIL Triage Study (ALTS) Group Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383–92. doi: 10.1067/mob.2003.457. [DOI] [PubMed] [Google Scholar]
- 34.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blizzard L, Hosmer DW. The log multinomial regression model for nominal outcomes with more than two attributes. Biom J. 2007;49:889–902. doi: 10.1002/bimj.200610377. [DOI] [PubMed] [Google Scholar]
- 36.Slinker BK, Glantz SA. Multiple linear regression is a useful alternative to traditional analyses of variance. Am J Physiol. 1988;255:R353–67. doi: 10.1152/ajpregu.1988.255.3.R353. [DOI] [PubMed] [Google Scholar]
- 37.Ho GY, Burk RD, Klein S, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 38.Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–14. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 39.Ylitalo N, Josefsson A, Melbye M, et al. A prospective study showing long-term infection with human papillomavirus 16 before the development of cervical carcinoma in situ. Cancer Res. 2000;60:6027–32. [PubMed] [Google Scholar]
- 40.Remmink AJ, Walboomers JM, Helmerhorst TJ, et al. The presence of persistent high-risk HPV genotypes in dysplastic cervical lesions is associated with progressive disease: natural history up to 36 months. Int J Cancer. 1995;61:306–11. doi: 10.1002/ijc.2910610305. [DOI] [PubMed] [Google Scholar]
- 41.Nobbenhuis MA, Walboomers JM, Helmerhorst TJ, et al. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20–5. doi: 10.1016/S0140-6736(98)12490-X. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100:513–7. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102:315–24. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]