Abstract
It has been reported that levels of viremia reflect the severity of illness in dengue virus infection. We assessed the levels of viremia in patients with primary and secondary infections, using 2 cell lines: FcγR-expressing BHK cells and FcγR-negative cells. In primary infection, virus titers were at similar levels between FcγR-expressing and FcγR-negative cells. In secondary infection, however, virus titers were ∼10 times higher in FcγR-expressing cells on days 1–6 when compared with FcγR-negative cells, indicating discrepancy in viremia titers between FcγR-expressing and FcγR-negative cells. The results suggest that dengue virus-antibody complexes with infectious capacity exist in patients with secondary infection, and these immune complexes can be detected by using FcγR-expressing cells. As it has been reported that principal target cells of dengue virus infection are FcγR-positive, monocyte/macrophage lineage cells, virus titers determined using FcγR-expressing cells may better reflect the actual viremic conditions in vivo.
Dengue fever (DF)/ dengue hemorrhagic fever (DHF) is endemic in tropical and subtropical regions of the world. DF/ DHF is caused by infection with dengue virus (DENV), a group of 4 serotypes: DENV-1, DENV-2, DENV-3, and DENV-4. Infection with 1 serotype confers lifelong immunity to infection with homologous serotypes, but immunity to heterologous serotypes is short-lived. Primary DENV infection induces antibodies that are cross-reactive for heterologous DENV serotypes, as well as those specific for the infecting serotype. It has been reported that DHF occurs at higher rates in secondary infection with heterologous DENV serotypes than in primary infection. DENV cross-reactive antibodies, when present at subneutralizing concentrations, have been proposed to play a major role in the development of DHF by facilitating viral entry into FcγR-expressing cells, which leads to higher viral progeny production [1,2]. This immune enhancement may result in increase in total viremia levels leading to DHF.
High viremia titers are associated with increased disease severity [3–5], and understanding of the detailed patterns of viremia is important for elucidating the pathogenesis of DF/DHF. The interpretation of viremia levels in the presence of antibodies, however, is complicated by several factors. Assessing virus levels by quantitative real-time PCR (RT-PCR) detects viral nucleic acids but not the infectious capability of virus particles. Interpretation of DENV viremia levels is further challenged by the ratio of flavivirus genomes to infectious particles, which could range from 1000:1 to 5000:1 [6]. Plaque titration methods that use FcγR-negative cell lines such as Vero and BHK-21 cell lines [7–9] lack the capability of detecting infectious DENV-antibody immune complexes. As a result of exclusive detection of viral genomes using RT-PCR and virus titers using FcγR-negative cells, the infectious capability of DENV-antibody immune complexes may not be accurately reflected.
DENV-antibody immune complexes are present in DHF and DSS patients [10], and DENV in immune complexes has been detected by quantitative RT-PCR [11]. However, the ability of DENV-antibody immune complexes to infect FcγR-expressing cells remains unclear. It is possible that DENV-antibody immune complexes have a different effect in FcγR-positive cells and FcγR-negative cells, leading to viremia levels that are different from those described in the literature. As the principal target cells of DENV are FcγR-expressing cells such as monocyte/macrophage lineage cells, we reasoned that viremia titers determined using FcγR-positive cells may better reflect viremia levels in vivo. In the present study, we determined whether the presence of DENV-antibody immune complexes leads to higher viremia titers in secondary infections, as determined using FcγR-expressing cells.
MATERIALS AND METHODS
Serum Samples
Seventy-three serum samples from 54 dengue cases were used. The serum samples consisted of 42 serum samples obtained from 33 individuals with primary dengue virus infection and 31 serum samples from 21 individuals with secondary infection. These serum samples were obtained in clinics and hospitals in Japan, from the year 2004 to 2010, and sent to the National Institute of Infectious Diseases, Japan, for laboratory diagnosis of dengue. As all serum samples were deidentified, patient consent was not required. The study protocol has been approved by the institutional review board of the National Institute of Diseases, Japan. Demographics of the patient population sampled are summarized in Table 1. Day after onset of disease is defined as the identification of first symptoms such as fever [12].
Table 1.
Characteristics of the Patient Population Sampled
| Variable | Primary infection | Secondary infection |
| No. of patients | 33 | 21 |
| Age, years, mean ± SD | 35.0 ± 12.8 | 33.9 ± 17.3a |
| Sex, no. of patients | ||
| Men | 18 | 15 |
| Women | 15 | 6 |
| No. of days illb, mean ± SD | 3.3 ± 2.0 | 3.5 ± 1.7 |
| Infecting serotypes | ||
| DENV-1 | 10 | 6 |
| DENV-2 | 6 | 4 |
| DENV-3 | 13 | 7 |
| DENV-4 | 4 | 4 |
NOTE. a Data on the age of a patient with secondary infection (S9) was not available.
Indicates the no. of days after onset of disease at the time of blood collection.
Virologic and Serologic Studies
Virus serotypes were determined by a serotype-specific reverse transcriptase polymerase chain reaction (RT-PCR), and the virus RNA copy number was determined by quantitative fluorogenic RT-PCR [13]. The limit for detection for DENV-1 is ≤9.5 × 102 genome copies/mL; DENV-2, ≤4.7 × 102 genome copies/mL; DENV-3, ≤4.7 × 104 genome copies/mL and DENV-4, ≤6.5 × 104 genome copies/mL.
Dengue-virus specific immunoglobulin M (IgM) antibodies in serum samples were determined using IgM capture enzyme-linked immunosorbent assay (ELISA) (Dengue Fever Virus IgM Capture ELISA, Focus Diagnostics) according to the manufacturer's instructions. Dengue indirect immunoglobulin G (IgG) ELISA (Panbio Ltd) was used for anti DENV-IgG antibodies according to the manufacturer's instructions. Primary infection (P) was defined by the absence of DENV-specific anti-DENV IgG antibodies at the stage of the absence or presence of anti-DENV IgM antibodies, and at the stage of virus isolation or viral RNA detection. Secondary infection (S) was defined by the presence of anti-DENV IgG antibodies at the stage of absence of anti-DENV IgM antibodies, and at the stage of virus isolation or viral RNA detection [14]. The definition of acute primary and secondary flavivirus infections was supported by DENV E/ M protein-specific IgM/ IgG OD ratios of ≥1.2 (primary infection) and <1.2 (secondary infection) [15]. All the serum samples used were positive for DENV as detected by RT-PCR.
BHK-21 cells, a hamster kidney cell line, and, FcγRIIA-expressing BHK-21 cells were used [16,17]. BHK-21 cells were cultured in Eagles Minimum Essential Medium (EMEM, Sigma), supplemented with heat-inactivated 10% fetal calf serum (FCS) (Sigma), without antibiotics at 37 °C in 5% CO2. FcγRIIA expressing BHK-21 cells were cultured in EMEM (Sigma), supplemented with heat-inactivated 10% FCS (Sigma) and 0.5 mg/mL neomycin (G418, PAA Laboratories GmbH) at 37 °C in 5% CO2.
Quantification of the Viremia Titer by Plaque Assay
Human serum samples were serially diluted 10-fold from 1:101 to 1:106 with EMEM supplemented with 10% FCS. Serially diluted serum samples were then incubated at 37°C for 1 h and used in infection experiments. Fifty microliters of diluted serum samples were inoculated onto BHK-21 monolayers in 12-well plates. The plates were incubated for 1 h at 37 °C in 5% CO2. After virus absorption, the cells were overlaid with maintenance medium containing 1% methylcellulose (Wako Pure Chemical Industry). The plates were incubated at 37 °C in 5% CO2 until visible plaques could be observed (5 to 7 days of incubation). The cells were fixed with 3.7% (vol/vol) formaldehyde for 1 h at room temperature and washed with water. The cells were then stained with methylene blue solution for 1 h at room temperature and washed with water. Plaques were counted by naked eye. The viral titers were expressed as plaque-forming units per millimeter (PFU/mL), using the following formula: (number of plaques per well) × (dilution) / (inoculum volume).
Levels of Progeny Virus by Quantitative RT-PCR in Culture Supernatant From FcγR-expressing Cells and FcγR-negative BHK Cells
Twenty-five microliters of diluted serum samples were inoculated onto BHK-21 monolayers in 24-well plates. The plates were incubated for 1 h at 37 °C in 5% CO2. After virus absorption, the cells were washed once with 0.5 mL EMEM and overlaid with 0.5 mL EMEM containing 10% FCS. The plates were incubated at 37 °C in 5% CO2 for 5 days. After 5 days of incubation, 200 μL of culture supernatant were collected and assayed by quantitative fluorogenic RT-PCR. Viral RNA was extracted from culture medium using a High Pure RNA extraction kit (Roche Diagnostics, Mannheim, Germany) and 5 μl of RNA solution was applied to TaqMan RT-PCR as previously described [13].
Assessment of Contribution of Virus-immune Complex to Viremia Titers
Heat-aggregated IgG was prepared by diluting whole IgG (Human IgG, Sigma) to 10 mg/mL in 0.9% saline and then heating at 65°C for 60 minutes [18]. One hundred micrograms of heat-aggregated IgG was then incubated onto each well of BHK-21 monolayers in 12-well plates. The cells were then incubated at 4°C for 20 minutes and washed once with EMEM. Serially diluted human serum samples were then applied to the cells and viremia titers were quantified by using plaque assay.
To react serum samples with (Fab')2 anti-human IgG Fc antibody, ten micrograms of the antibody (goat anti-human IgG, Fc Fragment specific, Pierce) were added to each of the serially diluted human serum samples and incubated at 37°C for 1 h. Viremia titer was then quantified by plaque assay.
Statistics
Results were expressed as mean ± SD The statistical significance of differences was determined by Students t test to compare the mean titers. The difference was considered to be significant when the P-value was <.05. The fold increase in virus titer or VN/VO ratio was calculated by the formula: (Average virus titer assessed with FcγR-expressing BHK cells, VN)/(Average virus titer assessed with FcγR-negative BHK cells, VO).
RESULTS
DENV Titers in Serum Samples from Dengue Patients with Primary Infection Determined Using FcγR-negative and FcγRIIA-positive BHK Cells
DENV titers in serum samples from dengue patients with primary infection were assessed, using FcγR-negative BHK cells and FcγRIIA-expressing BHK cells, by a conventional plaque titration method. Virus titers were at similar levels in both FcγRIIA-negative BHK cells and FcγRIIA-expressing BHK cells, for 14 of 15 anti-DENV IgM-negative, IgG-negative samples, and all the 10 anti-DENV IgM-positive, IgG-negative samples (Table 2). As an indicator of infection enhancement, the ratios of virus titers using FcγR-expressing BHK cells (VN) against those obtained using FcγR-negative BHK cells (VO) were calculated. The ratio ranged from 0.8 – 1.5, suggesting no difference in titers except for P10a. No infectious virus was detected using either FcγRIIA-expressing cells or FcγR-negative cells in serum samples P6b, P17, P21, P22, P3b, P27, P29, and P30, and in 9 anti-DENV IgM-positive and IgG-positive serum samples tested (Table 2).
Table 2.
Virus Titers in Primary DENV Infection Determined Using BHK and BHK- FcγRIIA Cell Lines
| Serum | DENV | Log10 genome | Virus titers (log10 PFU/mL) |
||||
| ID no. | Type | Daysa | equivalents/mL | BHK (VO) | FcγRIIA (VN) | Ratio (VN/VO) | P-value (VN,VO) |
| Anti-DENV IgM -, IgG - | |||||||
| P1ac | DENV-1 | 0 | 6.56 | 6.45 ± 0.10 | 6.49 ± 0.03 | 1.1 | .227 |
| P2a | DENV-1 | 1 | 8.60 | 7.32 ± 0.03 | 7.34 ± 0.10 | 1.0 | .449 |
| P3a | DENV-1 | 1 | 7.04 | 6.59 ± 0.01 | 6.50 ± 0.07 | 1.2 | .070 |
| P4a | DENV-1 | 1 | 7.38 | 5.41 ± 0.02 | 5.36 ± 0.15 | 1.0 | .488 |
| P5 | DENV-1 | 1 | 6.27 | 3.95 ± 0.21 | 4.00 ± 0.19 | 1.1 | .364 |
| P6a | DENV-2 | 1 | 7.63 | 6.90 ± 0.33 | 6.90 ± 0.22 | 1.0 | .458 |
| P7 | DENV-2 | 1 | 7.48 | 5.32 ± 0.03 | 5.35 ± 0.09 | 1.0 | .388 |
| P8 | DENV-3 | 1 | 9.38 | 5.93 ± 0.04 | 6.03 ± 0.08 | 1.3 | .105 |
| P9 | DENV-3 | 1 | 8.92 | 6.74 ± 0.01 | 6.72 ± 0.06 | 0.9 | .320 |
| P10a | DENV-1 | 2 | 7.48 | 6.66 ± 0.08 | 6.80 ± 0.04 | 1.3 | .040 |
| P11a | DENV-1 | 2 | 7.93 | 6.10 ± 0.02 | 6.22 ± 0.09 | 1.1 | .241 |
| P12a | DENV-3 | 2 | 8.49 | 6.70 ± 0.12 | 6.90 ± 0.04 | 1.5 | .078 |
| P13 | DENV-4 | 2 | 8.76 | 5.45 ± 0.01 | 5.38 ± 0.05 | 0.8 | .060 |
| P14 | DENV-2 | 3 | 6.75 | 5.15 ± 0.09 | 5.10 ± 0.13 | 0.8 | .303 |
| P15 | DENV-2 | 4 | 4.81 | 3.08 ± 0.21 | 3.04 ± 0.13 | 0.9 | .425 |
| P6b | DENV-2 | 5 | 6.36 | <2.30 | <2.30 | -b | -b |
| Anti-DENV IgM +, IgG - | |||||||
| P16 | DENV-3 | 1 | 7.76 | 6.74 ± 0.01 | 6.72 ± 0.06 | 1.2 | .332 |
| P17 | DENV-3 | 1 | 5.55 | <2.30 | <2.30 | - | - |
| P18 | DENV-1 | 2 | 6.00 | 4.15 ± 0.28 | 4.09 ± 0.14 | 0.8 | .447 |
| P19 | DENV-2 | 3 | 5.67 | 4.38 ± 0.05 | 4.39 ± 0.15 | 1.0 | .473 |
| P20 | DENV-3 | 3 | 9.20 | 6.61 ± 0.31 | 6.57 ± 0.14 | 0.9 | .494 |
| P12b | DENV-3 | 3 | 7.82 | 5.19 ± 0.02 | 5.16 ± 0.04 | 0.9 | .330 |
| P21 | DENV-3 | 3 | 6.68 | <2.30 | <2.30 | - | - |
| P22 | DENV-4 | 3 | 7.51 | <2.30 | <2.30 | - | - |
| P3b | DENV-1 | 4 | 4.64 | <2.30 | <2.30 | - | - |
| P23 | DENV-2 | 4 | 6.15 | 4.41 ± 0.05 | 4.38 ± 0.10 | 0.9 | .311 |
| P24 | DENV-3 | 4 | 8.46 | 5.11 ± 0.09 | 5.11 ± 0.07 | 1.0 | .458 |
| P25 | DENV-4 | 4 | 8.23 | 3.88 ± 0.04 | 3.98 ± 0.18 | 1.2 | .311 |
| P26 | DENV-1 | 5 | 6.00 | 4.13 ± 0.05 | 4.19 ± 0.05 | 1.1 | .277 |
| P27 | DENV-1 | 5 | 5.73 | <2.30 | <2.30 | - | - |
| P28 | DENV-3 | 5 | 6.28 | 3.30 ± 0.19 | 3.19 ± 0.23 | 0.7 | .259 |
| P29 | DENV-3 | 5 | 7.08 | <2.30 | <2.30 | - | - |
| P30 | DENV-4 | 5 | 6.97 | <2.30 | <2.30 | - | - |
| Anti-DENV IgM +, IgG+ | |||||||
| P1b | DENV-1 | 5 | - | <2.30 | <2.30 | - | - |
| P11b | DENV-1 | 5 | - | <2.30 | <2.30 | - | - |
| P2b | DENV-1 | 5 | - | <2.30 | <2.30 | - | - |
| P10b | DENV-1 | 6 | - | <2.30 | <2.30 | - | - |
| P31d | DENV-3 | 6 | - | <2.30 | <2.30 | - | - |
| P32d | DENV-3 | 6 | 5.48 | <2.30 | <2.30 | - | - |
| P33d | DENV-3 | 6 | - | <2.30 | <2.30 | - | - |
| P4b | DENV-1 | 7 | - | <2.30 | <2.30 | - | - |
| P10c | DENV-1 | 7 | - | <2.30 | <2.30 | - | - |
NOTE. a Days indicates days after onset of disease.
– indicates that calculation is not possible or below detection limits.
“a”, “b” and “c” indicates serial serum samples collected from a single patient
Patients P31, P32, and P33 were confirmed anti-DENV IgG negative during the early phase of the disease or 1–3 days after onset of the disease.
DENV Titers in Serum Samples from Dengue Patients with Secondary Infection Determined Using FcγR-negative and FcγRIIA-positive BHK Cells
Similar assays were performed for serum samples from dengue patients with secondary infection. Twenty of 22 serum samples that had viremia levels above the detection limit of the plaque assay demonstrated higher viremia titers using FcγRIIA-expressing BHK cells as compared with FcγRIIA-negative BHK-21 cell lines (Table 3). To assess the fold increase in virus titers, the VN/VO ratios were calculated as described in the Materials and Methods section. The VN/VO ratio of the 20 serum samples obtained from patients with secondary infection ranged from a 3.0-fold to as high as a 40-fold increase. Levels of viremia determined by using FcγR-expressing cells were higher than those determined using FcγR-negative cells, both in IgM-negative and IgG-positive samples, and IgM-positive and IgG-positive serum samples from patients with secondary infection. Two serum samples (S7 and S14), however, demonstrated similar levels of viremia both in FcγR-expressing and FcγR-negative cells. The virus titer of S16 was 4.85 log10 PFU/mL when assayed using FcγR-expressing BHK cells, but it was below the detection limit when assayed with FcγR-negative cells. The virus titer of serum sample S16 was 8.11 log10 genome equivalents/mL when determined by quantitative RT-PCR (Table 3). The results indicate that higher virus titers were detected using FcγR-expressing BHK cells as compared with FcγR-negative BHK cells, and this was observed in the presence or absence of anti-DENV IgM.
Table 3.
Virus Titers in Secondary DENV Infection Determined Using BHK and BHK- FcγRIIA Cell Lines
| Serum | DENV | Log 10 Genome | Virus titers (log10 PFU/mL) |
||||
| ID no. | Type | Daysa | equivalents/mL | BHK (VO) | FcγRIIA (VN) | Ratio (VN/VO) | P-value(VN,VO) |
| Anti-DENV IgM -, IgG+ | |||||||
| S1ac | DENV-2 | 1 | 8.40 | 7.00 ± 0.12 | 8.17 ± 0.15 | 14.0 | .002 |
| S2a | DENV-2 | 1 | 8.57 | 6.95 ± 0.38 | 8.56 ± 0.07 | 40.0 | .049 |
| S3a | DENV-2 | 1 | 9.11 | 6.81 ± 0.24 | 7.51 ± 0.14 | 4.9 | .021 |
| S4 | DENV-3 | 1 | 7.50 | 7.06 ± 0.19 | 8.26 ± 0.12 | 16.4 | .019 |
| S5a | DENV-2 | 2 | 7.83 | 6.29 ± 0.04 | 6.76 ± 0.07 | 3.0 | .001 |
| S6a | DENV-3 | 2 | 7.88 | <2.30 | <2.30 | -b | -b |
| S7 | DENV-4 | 2 | 8.45 | 5.18 ± 0.21 | 5.27 ± 0.13 | 1.2 | .306 |
| S8 | DENV-1 | 3 | 6.83 | 5.76 ± 0.03 | 6.77 ± 0.07 | 10.3 | <.001 |
| S9 | DENV-2 | 3 | 9.30 | 6.59 ± 0.14 | 7.71 ± 0.05 | 10.0 | .019 |
| S2b | DENV-2 | 3 | 8.04 | 6.53 ± 0.07 | 8.01 ± 0.04 | 38.2 | .004 |
| S10 | DENV-4 | 3 | 7.87 | 3.49 ± 0.02 | 4.70 ± 0.22 | 16.1 | <.001 |
| S11 | DENV-1 | 4 | 6.28 | 5.11 ± 0.37 | 5.92 ± 0.12 | 4.1 | .004 |
| S12a | DENV-1 | 4 | 7.66 | <2.30 | <2.30 | - | - |
| S13 | DENV-1 | 5 | 6.55 | 5.04 ± 0.05 | 5.93 ± 0.15 | 7.7 | <.001 |
| S14 | DENV-3 | 5 | 5.99 | 4.20 ± 0.15 | 4.26 ± 0.07 | 1.1 | .341 |
| Anti-DENV IgM +, IgG+ | |||||||
| S15a | DENV-4 | 1 | 8.76 | 6.08 ± 0.21 | 7.53 ± 0.06 | 27.5 | .028 |
| S1b | DENV-2 | 3 | 8.75 | 5.77 ± 0.01 | 6.70 ± 0.10 | 8.3 | <.001 |
| S5b | DENV-2 | 3 | 6.20 | 5.11 ± 0.04 | 6.10 ± 0.06 | 9.2 | <.001 |
| S16 | DENV-3 | 3 | 8.11 | <2.30 | 4.85 ± 0.33 | >3500 | .021 |
| S17 | DENV-1 | 4 | 5.18 | <2.30 | <2.30 | - | - |
| S2c | DENV-2 | 4 | 7.23 | 6.15 ± 0.04 | 7.18 ± 0.11 | 10.7 | <.001 |
| S3b | DENV-2 | 4 | 4.00 | <2.30 | <2.30 | - | - |
| S18 | DENV-3 | 4 | 6.68 | 3.20 ± 0.24 | 4.10 ± 0.15 | 7.5 | .037 |
| S19 | DENV-4 | 4 | 8.76 | 5.15 ± 0.09 | 6.24 ± 0.18 | 12.1 | <.001 |
| S15b | DENV-4 | 4 | 7.67 | 3.60 ± 0.34 | 4.65 ± 0.25 | 11.2 | .042 |
| S1c | DENV-2 | 5 | 4.30 | <2.30 | <2.30 | - | - |
| S12b | DENV-1 | 6 | - | <2.30 | <2.30 | - | - |
| S3c | DENV-2 | 6 | 3.74 | <2.30 | <2.30 | - | - |
| S20a | DENV-3 | 6 | 7.48 | 2.95 ± 0.07 | 4.26 ± 0.05 | 20.0 | .002 |
| S21 | DENV-3 | 6 | - | <2.30 | <2.30 | - | - |
| S20b | DENV-3 | 7 | 5.46 | <2.30 | <2.30 | - | - |
NOTE. a Days indicates days after onset of disease.
– indicates that calculation is not possible or below detection limits.
“a”, “b” and “c” indicates serial serum samples collected from a single patient.
Higher Levels of Progeny Virus Production With Serum Samples of Secondary Infection in FcγRIIA-expressing Cells
Genome copies of DENV were assessed by quantitative RT-PCR in culture supernatant from FcγR-expressing BHK cells and FcγR-negative BHK cells after inoculation with 1:101 to 1:104 serially diluted serum samples. Serum samples from patients with primary DENV infection did not consistently demonstrate higher levels of virus RNA copies in culture supernatant of FcγR-expressing BHK cells as compared to FcγR-negative BHK-21 cells (Figure 1A). In contrast, all 4 serum samples from patients with secondary infection (S4, S8, S9, and, S15a) consistently demonstrated higher levels of virus RNA copies in culture supernatant of FcγR-positive cells as compared with those of FcγR-negative cells using 1:101 to 1:104 serially diluted serum samples (P < .05; Figure 1).
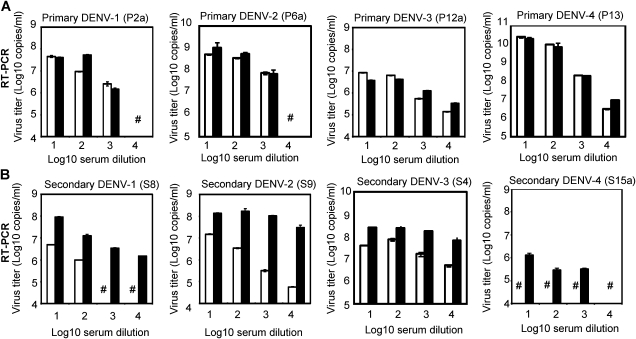
Figure 1.
Virus titers by quantitative RT-PCR in culture supernatant of FcγR-expressing and FcγR-negative cells that were inoculated with serially diluted serum samples. A, Serum samples from patients with primary DENV infection (DENV-1, DENV-2, DENV-3, and DENV-4 for P2a, P6a, P12a, and P13, respectively) were diluted from 1:101 to 1:104 and inoculated onto BHK cells and FcγRIIA-expressing BHK cells. B, Serum samples from patients with secondary DENV infection (DENV-1, DENV-2, DENV-3, and DENV-4 for S8, S9, S4, and S15a, respectively, as secondary DENV infection) were diluted from 1:101 to 1:104 and inoculated onto BHK cells and FcγRIIA-expressing BHK cells. Culture supernatant was collected on day 5, and virus titers were determined by quantitative RT-PCR. Using 4 serial dilutions (101–104) of serum samples from secondary infection (S4, S8, S9, and S15a) virus titer in culture supernatant fluids were consistently higher in FcγR-expressing cells as compared with FcγR-negative cells (P < .05). Virus titers were, however, not consistently higher in FcγR-expressing cells than FcγR-negative cells when primary infection serum samples were used. Thus, statistical analysis was performed using secondary infection serum samples. Results indicate geometric mean virus titer (genome copies/ mL) ± SD of 2 replicates. Open bars indicate FcγR-negative BHK cells, closed bars indicate FcγR-expressing BHK cells, and (#) indicates virus titers below detection limits as in materials and methods.
Time Course of Virus Titers
Virus titers were also determined in sequential serum samples from 4 patients with secondary DENV infection using FcγR-expressing BHK cells and FcγR-negative BHK cells. Higher virus titers were detected on disease days 1–5 using FcγR-expressing cells as compared with FcγR-negative BHK cells (Figures 2A– 2D). The VN/VO ratios of these serum samples ranged from 3.0 to 40.0 (P < .05) between FcγR-expressing BHK cells and FcγR-negative BHK cells.
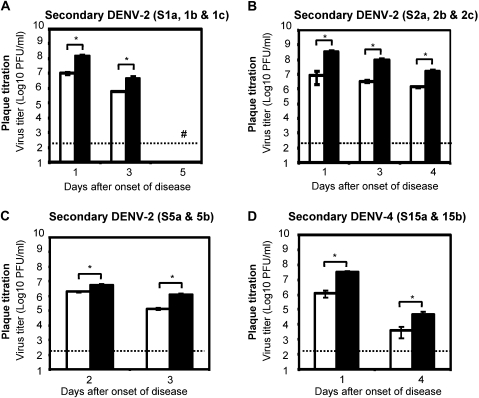
Figure 2.
Changes in viremia levels on days after onset of disease in individual patients with secondary dengue infection. Viremia levels in serum samples collected on 1–5 days after onset of disease from patients with secondary infection were determined. Panels A—D show viremia titers from patients with secondary infection determined using FcγR-negative BHK cells and FcγR-expressing BHK cells. Results indicate geometric mean virus titer ± SD of a minimum of 2 replicates determined using FcγR-negative BHK cells and 4 replicates from 2 experiments by using FcγR-expressing BHK cells. Open bars indicate FcγR-negative BHK cells, closed bars indicate FcγR-expressing BHK cells, and (#) indicates virus titers below detection limits (<2.30 log10 PFU /mL), and (*) indicates P < .05. P values indicate comparison between viremia titers determined by using FcγR-negative BHK cells and FcγR-expressing BHK cells.
Higher Viremia Titers in Patients With Secondary Infection Determined by FcγR-expressing Cells but not in Primary Infection
Average viremia titers were calculated from days 1–7 after onset of disease in 73 serum samples from 33 patients with primary infection (Figure 3A) and 21 patients with secondary infection (Figure 3B). Serum samples from primary infection did not exhibit higher virus titers when determined using FcγR-expressing BHK cells as compared with FcγR-negative BHK cells from disease days 1–5. Viremia was not detected for most of the samples tested on disease days 6 and 7. The rate of decrease in virus titers in patients with primary infection detected using FcγR-negative BHK cells and FcγR-expressing BHK cells (mean clearance rate, –0.92 vs –0.94 log10 PFU/ day) were similar to those with secondary infection using FcγR-negative BHK cells and FcγR-expressing BHK cells (mean clearance rate, –1.07 vs –1.10 log10 PFU/ day). There was no difference in the rates of decrease in virus titers using FcγR-negative BHK cells in primary and secondary infection (primary infection vs secondary infection, P = .465), and when assayed with FcγR-expressing BHK cells (primary infection vs secondary infection, P = .451). The estimated duration of detectable levels of viremia was longer using FcγR-expressing cells in patients with secondary infection (mean duration detected using BHK cells vs FcγR-expressing BHK cells, 6.9 days vs 7.4 days), but not in patients with primary infection (mean duration detected using BHK cells vs FcγR-expressing BHK cells, 6.3 days vs 6.3 days). Using FcγR-expressing BHK cells, the difference in virus titer was significant between primary and secondary infection on illness day 1 (P < .001), day 3 (P = .003), day 4 (P = .011), day 5 (P ≤ .010), and, day 6 (P = .041). Using FcγR-negative BHK cells, no significant difference in virus titer between primary and secondary infection was found on illness day 1 (P = .110), day 3 (P = .052), day 4 (P = .089), day 5 (P = .354), and day 6 (P = .087). Comparison of virus titer on illness day 2 was not performed due to insufficient number of secondary infection serum samples.
Reduction of Virus Titer in Serum Samples of Secondary Infection by Blockade of Immune Complex Binding to FcγR
To examine the contribution of infectious virus-antibody immune complex to viremia titers, FcγR-expressing cells were exposed to heat-aggregated IgG antibodies prior to inoculation with viremic serum samples. Alternatively, human serum samples were allowed to react with F(ab')2 anti-IgG Fc antibodies to block binding of the Fc-portion of IgG to FcγR.
Two serum samples, S4 and S9, from patients with secondary infection were used. Higher virus titers were not detected using heat-aggregated (HA) IgG treated FcγR-expressing cells and serum samples treated with anti-human IgG Fc fragment specific antibodies. Virus titers of serum sample S4 determined using FcγR-BHK with HA-IgG = 6.78 ± 0.49 vs BHK = 6.69 ± 0.12 (P = .415), FcγR-BHK with anti-IgG Fc antibody treated S4 = 6.87 ± 0.04 vs BHK = 6.69 ± 0.12 (P = .077) and untreated-control FcγR-BHK = 7.90 ± 0.15 versus BHK = 6.69 ± 0.12 (P = .032). Virus titers of serum sample S9 determined using FcγR-BHK with HA-IgG = 6.77 ± 0.21 versus BHK = 6.60 ± 0.34 (P = .276), FcγR-BHK with anti-IgG Fc antibody treated S9 = 6.70 ± 0.12 versus BHK = 6.60 ± 0.34 (P = .349) and untreated-control FcγR-BHK = 7.34 ± 0.05 versus BHK = 6.60 ± 0.34 (P = .012). Virus titers were significantly lower in treated samples as compared with untreated samples for S4 using FcγR-expressing BHK cells: FcγR-BHK with HA-IgG versus untreated-control FcγR-BHK (P = .034), and, FcγR-BHK with anti-IgG Fc antibody treated S4 versus FcγR-BHK (P = .033). Similar results were obtained using serum sample S9: FcγR-BHK with HA-IgG versus FcγR-BHK (P = .015), and FcγR-BHK with anti-IgG Fc antibody treated S9 versus FcγR-BHK (P = .008). Values are expressed as mean log10 virus titer PFU/mL ± SD (Table 4).
Table 4.
Effect of Aggregated IgG and Goat F(ab')2 Anti-IgG Fc Antibody on Virus Titers in Secondary DENV Infection
| Serum ID no. | Cell Line | Treatment | Virus Titer (log10 PFU/mL) | P-valuea (BHK) | P-valueb (FcγR-BHK) |
| S4 | FcγR-BHK | None | 7.90 ± 0.15 | .032 | c |
| BHK | None | 6.69 ± 0.12 | - | .032 | |
| FcγR-BHK | Aggregated IgG | 6.78 ± 0.49 | .415 | .034 | |
| FcγR-BHK | F(ab')2 anti-IgG Fc antibody | 6.87 ± 0.04 | .077 | .033 | |
| S9 | FcγR-BHK | None | 7.34 ± 0.05 | .012 | - |
| BHK | None | 6.60 ± 0.34 | - | .012 | |
| FcγR-BHK | Aggregated IgG | 6.77 ± 0.21 | .276 | .015 | |
| FcγR-BHK | F(ab')2 anti-IgG Fc antibody | 6.70 ± 0.12 | .349 | .008 |
NOTE. aP-value of virus titer compared with those determined using BHK cells in the absence of any treatment.
P-value of virus titer compared with those determined using FcγR-expressing BHK cells in the absence of any treatment.
- Indicates that calculation is not possible.
DISCUSSION
The extent to which the DENV-antibody immune-complex contributes to viremia titers has not been fully defined. In the present study, we demonstrated increased viremia titers and slower virus clearance using FcγRIIA-expressing BHK cells in patients with secondary DENV infection. Higher viremia titers were detected using FcγR-expressing BHK cells from 1 to 6 days after onset of disease in serum samples obtained from patients with secondary DENV infection as compared with viremia titers detected using FcγR-negative BHK cells. There was no difference in the levels of viremia in serum samples from patients with primary DENV infection between FcγR-expressing BHK cells and FcγR-negative BHK cells. Constant detection of higher levels of viremia in secondary infection using FcγR-expressing cells suggests the presence of DENV-antibody complexes that were infectious to FcγR-expressing cells but not to FcγR-negative cells. The observed higher levels of viremia in secondary infection suggest that infectious virus titers of ∼10 folds higher than those described in the literature exist in patients with secondary DENV infection.
It has been reported that higher viremia titers were detected in patients with secondary DENV-2 and dengue hemorrhagic fever (DHF), as compared with patients with dengue fever (DF) using RT-PCR [11]. Other investigators, however, reported no discrepancy in viremia between patients with secondary and primary infection [19,20], or, higher viremia in primary infection as compared with secondary infection [21]. In addition, due to the 2 competing antibody capacities against DENV (neutralization and enhancement), the antibodies may interfere in the interpretation of viremia levels [11]. However, other investigators reported that DENV were effectively recovered from patients with secondary infection using FcγR-expressing mouse macrophages P388D1 cells as compared with FcγR-negative C6/36 cells. The high rates of virus recovery using FcγR-expressing cells suggest the presence of infectious DENV-immune complex in secondary infection [22], which further supports our results.
One of the challenges encountered with textbook descriptions of dengue viremia levels is that the conventional titration method uses FcγR-negative cells. Our results demonstrated that this classical description of viremia titers, however, holds true for primary infection in particular. In contrast, omission of the infectious capacity of the DENV-antibody complex in FcγR-expressing cells has resulted in underestimated viremia levels during secondary infection, which consequently leads to a discrepancy in viremia titers of as much as one log PFU/mL (Table 3, Figure 3). The FcγR-expressing BHK cells used in the present study consistently detected the sum of enhancing and neutralizing capacities of antibodies in the form of immune complexes [16, 17]. Blockade of antibody binding to FcγR significantly reduced antibody-infection enhancement effect in FcγR-expressing BHK cells: indicating that infectious virus-antibody complexes are present during DENV infection and contribute to the enhanced infection of FcγR-expressing cells (Table 4). Overall, these data suggest the presence of circulating DENV-antibody immune complexes that are infectious to FcγR expressing cells, but not to FcγR-negative cells. Mammalian cells such as Vero, LLCMK, and BHK cells are commonly used in plaque assays as compared with C6/36 cells for virus titration [16, 23]. Virus titers in the cell culture supernatant fluids after DENV infection in the absence of antibodies in C6/36 cells were not higher than those of FcγR-expressing BHK cells and FcγR-negative BHK cells (author's unpublished data). As such, viremia titers detected using FcγR-expressing BHK cells may better reflect in vivo conditions as the major DENV target cells expresses the FcγR.
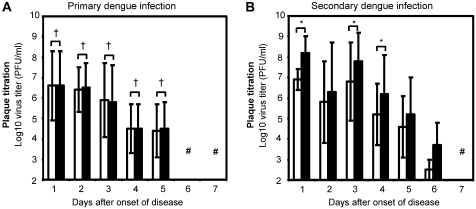
Figure 3.
Average viremia titers on 1–7 days after onset of disease in serum samples from primary and secondary DENV infections. Relationships between the mean levels of dengue virus titers, determined using FcγRIIA-expressing BHK cells and FcγRIIA-negative BHK cells in serum samples obtained from patients with (A) primary DENV infection (N = 33) and (B) secondary DENV infection (N = 21). Average serum viremia levels in individuals with primary dengue infection were (values in log10 PFU/mL): day 1 after onset of disease; BHK, 6.6 ± 1.6 vs FcγR-BHK, 6.7 ± 1.6, day 2; BHK, 6.4 ± 1.1 vs FcγR-BHK, 6.5 ± 1.2 (P = .330), day 3; BHK, 6.3 ± 1.8 vs FcγR-BHK, 6.5 ± 1.8 (P = .063), day 4; BHK, 4.5 ± 1.2 vs FcγR-BHK, 4.5 ± 1.2 (P = .482), day 5; BHK, 3.2 ± 1.2 vs FcγR-BHK, 3.2 ± 1.2 (P = .410), day 6; BHK < 2.3 vs FcγR-BHK < 2.3, day 7; BHK < 2.3 vs FcγR-BHK < 2.3. Individuals with secondary dengue infection consistently demonstrated higher virus titers in FcγR-expressing BHK cells than in FcγR-negative BHK cells from disease days 1–4 (values in log10 PFU/ mL): day 1 after onset of disease; BHK = 6.9 ± 0.4 vs FcγR-BHK, 8.2 ± 0.4 (P < .001), day 3; BHK, 6.8 ± 1.9 vs FcγR-BHK, 7.9 ± 1.5 (P < .001), day 4; BHK, 5.3 ± 1.5 vs FcγR-BHK, 6.3 ± 1.9 (P = .005). A similar tendency was detected on disease days 5 and 6, although the differences were not statistically significant: day 5; BHK, 4.5 ± 1.6 vs FcγR-BHK, 5.3 ± 1.9 (P = .187), day 6; BHK, 2.6 ± 0.6 vs FcγR-BHK, 3.8 ± 1.3 (P = .211). Virus titers were below detection limits using plaque titration methods for day 7; BHK < 2.3 vs FcγR-BHK < 2.3.Open bars indicate FcγRIIA-negative BHK cells, and closed bars indicate FcγRIIA-expressing BHK cells. Results indicate geometric mean virus titer ± SD.SD values were arithmetic SD derived from log10 transformed virus titers in PFU/mL. Error bars indicate ± 1 SD (#) indicates virus titer below detection limit using plaque titration (<2.30 log10 PFU/mL). P-value indicates comparison between virus titers determined by FcγR-negative BHK cells and FcγR-expressing BHK cells. (†) indicates P ≥ .05, and, (*) indicates P <.05.
It has been speculated that the pathogenesis of severe DENV infection involves dengue serotype cross-reactive antibodies that enhance dengue infection resulting in higher viremia titers, one of the preludes for the development of severe dengue infection [24]. The observation of higher viremia titers using FcγRIIA-expressing cells in patients with secondary infection as compared with primary infection may suggest that immune complex with infectious capacity only for FcγR-expressing cells may, in part, contribute to severe dengue infection. These associations between higher viremia titers and the presence of infectious immune complexes during secondary dengue infection may be a key factor in understanding the pathogenesis of dengue infection.
Funding
This work was supported by funding from Research on Publicly Essential Drug and Medical Devices from the Japan Health Sciences Foundation (grants KH 53333 and KHC 3332) and Research on Emerging and Reemerging Infectious Diseases by the Ministry of Health, Labor and Welfare, Japan (grants H20-shinkou-ippan-013 and H20-shinkou-ippan-015).
Acknowledgments
We thank Dr. Jeffrey V. Ravetch, Rockerfeller University, New York, New York, for generously providing the FcγRIIA cDNA and Dr. Susheela Tridandapani, Ohio State University College of Medicine, Columbus, Ohio, for assistance in obtaining the FcγRIIA cDNA used in this work.
References
- 1.Kontny U, Kurane I, Ennis FA. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J Virol. 1988;62:3928–33. doi: 10.1128/jvi.62.11.3928-3933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Littaua R, Kurane I, Ennis FA. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol. 1990;144:3183–6. [PubMed] [Google Scholar]
- 3.Libraty DH, Endy TP, Houng HS, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis. 2002;185:1213–21. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 4.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–30. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 5.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 6.Bae HG, Nitsche A, Teichmann A, Biel SS, Niedrig M. Detection of yellow fever virus: a comparison of quantitative real-time PCR and plaque assay. J Virol Meth. 2003;110:185–91. doi: 10.1016/s0166-0934(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 7.Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 8.Morens DM, Halstead SB, Repik PM, Putvana R, Raybourne N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J Clin Microbiol. 1985;22:250–4. doi: 10.1128/jcm.22.2.250-254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roehrig JT. Guidelines for plaque reduction neutralization testing of human antibodies to dengue viruses. Geneva, Switzerland: World Health Organization; 2007. [DOI] [PubMed] [Google Scholar]
- 10.Ruangjirachuporn W, Boonpucknavig S, Nimmanitya S. Circulating immune complexes in serum from patients with dengue haemorrhagic fever. Clin Exp Immunol. 1979;36:46–53. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang WK, Chen HL, Yang CF, et al. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis. 2006;43:1023–30. doi: 10.1086/507635. [DOI] [PubMed] [Google Scholar]
- 12.Yamada K, Takasaki T, Nawa M, Kurane I. Virus isolation as one of the diagnostic methods for dengue virus infection. J Clin Virol. 1999;24:203–9. doi: 10.1016/s1386-6532(01)00250-5. [DOI] [PubMed] [Google Scholar]
- 13.Ito M, Takasaki T, Yamada K, et al. Development and evaluation of fluorogenic TaqMan reverse transcriptase PCR assays for detection of dengue virus types 1 to 4. J Clin Microbiol. 2004;42:5935–5937. doi: 10.1128/JCM.42.12.5935-5937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughn DW, Nisalak A, Kalayanarooj S, et al. Evaluation of a rapid immunochromatographic test for diagnosis of dengue virus infection. J Clin Microbiol. 1998;36:234–8. doi: 10.1128/jcm.36.1.234-238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization 2009. Dengue guidelines for diagnosis, treatment, prevention and control. http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. Accessed 17 December 2010. [PubMed] [Google Scholar]
- 16.Moi ML, Lim CK, Kotaki A, Takasaki T, Kurane I. Development of an antibody-dependent enhancement assay for dengue virus using stable BHK-21 cell lines expressing FcγRIIA. J Virol Meth. 2010;163:205–9. doi: 10.1016/j.jviromet.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Moi ML, Lim CK, Kotaki A, Takasaki T, Kurane I. Discrepancy in dengue virus neutralizing antibody titers between plaque reduction neutralizing tests with Fcγ receptor (FcγR)-negative and FcγR-expressing BHK-21 cells. Clin Vaccine Immunol. 2010;17:402–7. doi: 10.1128/CVI.00396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79:1242–51. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilarde AO, Turchi MD, Siqueira JB, et al. Dengue and dengue hemorrhagic fever among adults: clinical outcomes related to viremia, serotypes and antibody response. J Infect Dis. 2008;197:817–24. doi: 10.1086/528805. [DOI] [PubMed] [Google Scholar]
- 20.Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A. Viraemia in patients with naturally acquired dengue infection. Bull World Health Organ. 1981;59:623–30. [PMC free article] [PubMed] [Google Scholar]
- 21.Thai KT, Phuong HL, Thanh Nga TT, et al. Clinical, epidemiological and virological features of Dengue virus infections in Vietnamese patients presenting to primary care facilities with acute undifferentiated fever. J Infect. 2010;60:229–37. doi: 10.1016/j.jinf.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardosa MJ. Dengue virus isolation by antibody dependent enhancement in macrophages. Lancet. 1987;I1:193–94. doi: 10.1016/s0140-6736(87)90005-5. [DOI] [PubMed] [Google Scholar]
- 23.Zamree I, Drakes N, Rohani A, Lee HL. Sensitivity of Aedes albopictus C6/36 cells line for the detection and infectivity titration of dengue virus. Trop Biomed. 2005;22:217–19. [PubMed] [Google Scholar]
- 24.Wang WK, Chao DY, Kao CL, et al. High levels of plasma dengue viral load during defervescence in patients with dengue hemorrhagic fever: implications for pathogenesis. Virology. 2003;305:330–8. doi: 10.1006/viro.2002.1704. [DOI] [PubMed] [Google Scholar]