Abstract
Background
The link between concentrations of particulate matter (PM) and respiratory morbidity has been investigated in numerous studies.
Objectives
The aim of this study was to analyze the role of different particle size fractions with respect to respiratory health in Beijing, China.
Methods
Data on particle size distributions from 3 nm to 1 μm; PM10 (PM ≤ 10 μm), nitrogen dioxide (NO2), and sulfur dioxide concentrations; and meteorologic variables were collected daily from March 2004 to December 2006. Concurrently, daily counts of emergency room visits (ERV) for respiratory diseases were obtained from the Peking University Third Hospital. We estimated pollutant effects in single- and two-pollutant generalized additive models, controlling for meteorologic and other time-varying covariates. Time-delayed associations were estimated using polynomial distributed lag, cumulative effects, and single lag models.
Results
Associations of respiratory ERV with NO2 concentrations and 100–1,000 nm particle number or surface area concentrations were of similar magnitude—that is, approximately 5% increase in respiratory ERV with an interquartile range increase in air pollution concentration. In general, particles < 50 nm were not positively associated with ERV, whereas particles 50–100 nm were adversely associated with respiratory ERV, both being fractions of ultrafine particles. Effect estimates from two-pollutant models were most consistent for NO2.
Conclusions
Present levels of air pollution in Beijing were adversely associated with respiratory ERV. NO2 concentrations seemed to be a better surrogate for evaluating overall respiratory health effects of ambient air pollution than PM10 or particle number concentrations in Beijing.
Keywords: emergency room visits, particle number concentration, particle surface area concentration, particulate matter, short-term effects, time-series analyses, ultrafine particles
There is consistent evidence that particulate matter (PM) with an aerodynamic diameter < 10 μm or 2.5 μm (PM10 or PM2.5, respectively) is adversely associated with respiratory morbidity and mortality (Alfaro-Moreno et al. 2007; Health Effects Institute 2003; Pope and Dockery 2006). Some findings suggest that associations are stronger for finer than for coarser particles (Kan et al. 2007; Peng et al. 2008; Wichmann et al. 2000) and that there are qualitative differences between the health effects of different particle size fractions (Samuelsen et al. 2009). Physicochemical parameters such as size, shape, distribution, number, and volume of airborne particles determine the potential to induce inflammatory injury, oxidative damage, and other biological effects, which are all stronger for smaller-size fractions of particles (Oberdörster et al. 2005; Valavanidis et al. 2008). Ultrafine particles (UFP; particles with a diameter < 100 nm) may contribute more than other particle size fractions to the observed health effects, because they dominate total particle number and surface area concentrations and have a high deposition efficiency in the pulmonary region (Delfino et al. 2005; Frampton 2001; Wichmann 2007).
Because of the limited availability of measurement data, few epidemiologic studies have investigated associations between particle number concentrations in different size ranges and daily respiratory morbidity or mortality, and their findings have been inconsistent. Wichmann et al. (2000) reported in a study conducted in Erfurt, Germany, with a population of approximately 200,000, that associations with total mortality were comparable for an interquartile range (IQR) increase in UFP [relative risk (RR) = 4.1%; 95% confidence interval (CI), 0.1–8.2] and PM2.5 (RR = 4.9%; 95% CI,1.1–8.8), whereas cause-specific mortality showed a somewhat stronger association with respiratory diseases compared with cardiovascular diseases and other causes. Peters et al. (1997) reported that inverse associations with peak expiratory flow (PEF) in asthmatic patients were stronger for an IQR increase in particle number concentrations of UFP than mass concentrations of fine particles (aerodynamic diameter 0.1–0.5 μm). Penttinen et al. (2001) reported that daily mean particle number concentrations, but not particle mass concentrations, were negatively associated with daily PEF deviations in adult asthmatics, whereas the strongest associations were found with particles in the ultrafine range. In a study conducted in Copenhagen, Denmark, Andersen et al. (2008) reported associations between pediatric asthma hospital admissions and IQR increases in accumulation-mode particles (0.1–1 μm), UFP (< 0.1 μm), and nitrogen oxide (NOx) concentrations.
On the other hand, associations between PM and hospital admissions due to cardiovascular and respiratory disease in the elderly in Copenhagen appeared to be mediated mainly by PM10 or accumulation mode particles rather than urban background UFP (Andersen et al. 2008). In addition, Osunsanya et al. (2001) found no association between UFP and respiratory symptoms or PEF in adult patients with chronic airflow obstruction in Aberdeen, Scotland.
Most studies of air pollution and health have been conducted in Western Europe or North America. Differences between Asian and Western populations in overall health status, lifestyle and age structure of the populations, and exposures to different air pollution mixtures and levels might influence associations between human health and air pollution. Studies conducted in Asian countries indicate that associations are similar to those in Western countries (Health Effects Institute 2004). Nevertheless, local authorities need population-specific information on current health risks of air pollution to develop air pollution control strategies adapted to local conditions.
The aim of this study was to analyze the role of different particle size fractions in the size range of 3 nm–1 μm with respect to respiratory health in Beijing, China.
Data and Methods
The study was conducted from March 2004 to December 2006 (1,036 days) in Beijing, China.
Data
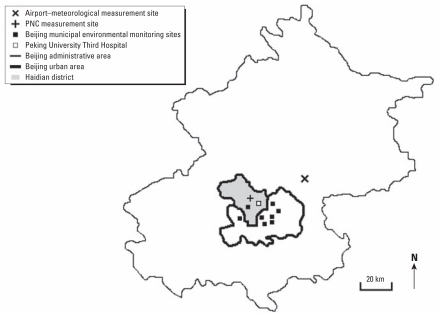
Particle measurements were performed at a background measuring station on the campus of the Peking University (PKU) located in the northwestern part of Beijing in the Haidian district (Figure 1). The campus area is primarily residential and commercial, without heavy traffic or industrial sources. The inlet of the sampling system was placed 20 m above ground on top of a six-floor building located > 500 m away from major roads. Local emission sources within a 1-km radius include vehicular traffic and fuel combustion for domestic cooking, heating, and construction. A study of the spatial variability of PM2.5 mass and chemical composition in 1999–2002 showed only minor differences between a PKU campus site and an urban measurement site located approximately 10 km southeast from the PKU measurement site. The setup of the measurement station is described in detail elsewhere (e.g., Wehner et al. 2004, 2008).
Figure 1.
Map of Beijing, the study area, with administrative (outline) and urban (inner area) area, Haidian district (grey), and measurement stations.
We continuously measured aerosol number size distributions between 3 nm and 1 μm. Sampling was done using a Twin Differential Mobility Particle Sizer (TDMPS; Birmili et al. 1999), consisting of two Hauke-type differential mobility analyzers and two condensation particle counters (models 3010 and 3025; TSI Inc., St. Paul, MN, USA) which covered the size range from 3 nm to 800 nm (mobility diameter) and an Aerodynamic Particle Sizer (APS; model 3321; TSI Inc.) which measured particles between 800 nm and 1 μm (aerodynamic diameter). To combine both measurements, APS data were transformed from aerodynamic diameter to Stokes diameter assuming an effective particle density of 1.7 g/cm3 for the particles > 800 nm, because these particles are dominated by sulfate and crustal material in Beijing (Yao et al. 2003). A low-flow PM10 inlet was used to minimize contamination by large dust particles. The ambient aerosol was dried in a diffusion drier before entering the air-conditioned laboratory to avoid condensation of water in the inlet systems during warm and humid days in summertime. The data were corrected for losses due to diffusion and sedimentation within the inlet line. Size-dependent losses for the TDMPS inlet line were estimated using empirical particle loss corrections (diffusion and gravitation) from Willeke and Baron (1993). Losses of 4-nm and 10-nm particles were estimated to be 35% and approximately 10%, respectively.
Number size distributions were converted to particle number concentrations (PNC) and particle surface area concentrations (PSC) assuming spherical particles. For our analysis, we calculated daily means for 3–10 nm, 10–30 nm, 30–50 nm, 50–100 nm, 100–300 nm, and 300–1,000 nm size fractions. Total particle number or surface area concentration were computed as the sum of all fractions, and UFP as the sum of particle number concentrations < 100 nm.
In addition, daily particle mass (PM10), sulfur dioxide (SO2), and nitrogen dioxide (NO2) concentrations were gathered from the monitoring network of the Beijing Environmental Protection Bureau. Measurements were obtained as averages of eight fixed monitoring sites located in different parts of the urban area (Figure 1).
Daily mean meteorologic data from a measurement station near the Beijing Capital International Airport (Figure 1) were gathered from an internet weather service (Weather Underground 2009) because measurements from the meteorologic station closest to the particulate measurement station were incomplete. The Pearson correlation coefficients for valid days between the two data sources were 0.99 and 0.95 for daily air temperature and relative humidity, respectively, indicating a good agreement.
We collected data on hospital emergency room visits (ERV) from the Peking University Third Hospital, located in the Haidian district (Figure 1), where patients within 10 km of the measurement site were likely to be treated (personal communication with hospital doctors). The data acquisition system of the hospital recorded only patients who did not stay longer than 1 day. A standardized form was completed by medically trained study personnel at PKU, School of Public Health, by abstracting the data from the medical records. A database was built on the basis of these files, and respiratory clinical end points were coded according to the International Classification of Diseases, 10th Revision (ICD-10) [World Health Organization (WHO) 1993] for respiratory diseases (ICD-10 codes J00–J99). Total respiratory ERV comprise acute upper respiratory infections (J00–J06), pneumonia (J18), acute bronchitis (J20), other diseases of the upper respiratory tract (J30–J39), and chronic lower respiratory diseases (J40–J47). In addition, location of permanent residence was recorded for each case; based on this information, only patients from Haidian district were considered for further analysis. Because of an outbreak of the severe acute respiratory syndrome in China in 2003, all patients with body temperature > 38°C were separately treated in the hospital, and these cases were excluded. Assignment of the diagnoses to ICD-10 disease categories were quality-assured by a nosological expert from the Third Hospital, resulting in a good agreement, as the percentage of misclassification was about 4%.
Statistical analysis
We applied a time-series analysis using generalized additive Poisson regression models (Hastie and Tibshirani 1987) to estimate associations between air pollutants and respiratory ERV.
In the first step, a base model without pollutants was built for identifying pollutant-independent variability. Long-term trends and seasonality were represented by a smooth function of calendar time, and a categorical variable for day of the week was forced into the model as hospital emergency room admissions showed weekly and yearly patterns. As further potential confounders, we considered the meteorologic parameters air temperature, air pressure, and relative humidity. We accounted for time-delayed effects of these variables using arithmetic means for days up to 7 days before the visit. Additional influences of the year, season (December–February, March–May, June–August, September–November), month, day of the month and holiday, represented by categorical variables were investigated as well. The base model was selected according to Akaike’s information criterion (Akaike 1974) and statistical significance of the covariates (p < 0.05). Nonlinear effects were modeled by regression splines with an automatic smoothness selection (Wood 2006). The smoothness of the trend function was manually adjusted based on the absolute value of the sum of the partial autocorrelation function (Touloumi et al. 2006).
The final base model consisted of the categorical variables holiday, weekend, and season, the linear influence of same-day relative air humidity, and the smooth functions of calendar time, air temperature, represented by the mean of the present and two previous days, and same-day air pressure.
The base model was extended by including air pollutants. Delayed effects up to a maximum of 5 days were estimated by polynomial distributed lag (PDL) models (Zanobetti et al. 2003), cumulative effects models that represented the delayed effect as a moving average, and single lag models that incorporated the delayed effect as a lagged variable into the model. We constrained the lag coefficients in PDL models to follow a third-degree polynomial of the lag number. Two-pollutant cumulative effects models included PM10 or NO2 in addition to another air pollution variable, with each modeled according to the same lag (e.g., same-day total particle number concentration and same-day NO2 concentration).
To explore the robustness of the models, we performed several sensitivity analyses for associations with particle number concentrations from 100 to 300 nm (PNC100–300) that varied the lag pattern of temperature, relative humidity, and air pressure, and used different degrees of smoothness for the time trend function. Additionally, for PDL models we varied the number of the maximum lag and the degree of the polynomial order.
All statistical analyses were done using the mgcv package in the R software (R Development Core Team 2003), version 2.90.
Results
In total, we identified 15,981 cases of respiratory ERV in the study period, including 12,798 (80%) due to acute upper respiratory infections followed by 1,954 (12%) due to lower respiratory diseases. The daily mean of respiratory ERV was 15, with a minimum of 0 and a maximum of 88 patients per day (Table 1). Because of technical difficulties and maintenance, 218 days of particle size–segregated measurements were missing. Consequently, effect estimates for particulate air pollutants other than PM10 are based on 818 days of data (13,055 respiratory ERV were recorded in this period). Particle number concentrations were dominated by particles < 300 nm and particle surface area concentrations by particles in the range of 50–1,000 nm (Table 1). The time series of respiratory ERV showed a seasonal and weekly pattern, with more cases during the cold season and a peak on the weekend and on holidays (data not shown).
Table 1.
Descriptive statistics for daily mean values of respiratory ERV, meteorologic variables, and air pollution variables.
| Variable | No. of valid days | Mean ± SD | Minimum | Maximum | IQR |
|---|---|---|---|---|---|
| Respiratory ERV | 1,036 | 15 ± 10 | 0 | 88 | 12 |
| Air temperature (°C) | 1,036 | 13 ± 11 | −10 | 31 | 18 |
| Relative humidity (%) | 1,036 | 58 ± 22 | 9 | 100 | 37 |
| Air pressure (hPa) | 1,036 | 1,017 ± 10 | 992 | 1,048 | 16 |
| SO2 (μg/m3) | 1,036 | 87 ± 88 | 6 | 600 | 100 |
| NO2 (μg/m3) | 1,036 | 63 ± 38 | 5 | 290 | 40 |
| PM10 (μg/m3) | 1,036 | 120 ± 83 | 10 | 570 | 90 |
| UFP (1/cm3) | 818 | 22,000 ± 9,800 | 5,600 | 76,000 | 11,000 |
| PNCtotal (1/cm3) | 818 | 29,000 ± 10,000 | 7,400 | 87,000 | 12,600 |
| PNC3–10 (1/cm3) | 818 | 3,600 ± 4,400 | 85 | 41,000 | 3,880 |
| PNC10–30 (1/cm3) | 818 | 6,900 ± 3,800 | 1,200 | 30,000 | 4,300 |
| PNC30–50 (1/cm3) | 818 | 4,900 ± 1,800 | 890 | 14,000 | 2,300 |
| PNC50–100 (1/cm3) | 818 | 6,700 ± 2,800 | 630 | 19,000 | 3,600 |
| PNC100–300 (1/cm3) | 818 | 6,300 ± 3,500 | 340 | 21,000 | 4,400 |
| PNC300–1,000 (1/cm3) | 818 | 870 ± 710 | 28 | 4,800 | 830 |
| PSCtotal (μm2/cm3) | 818 | 1,300 ± 800 | 78 | 5,100 | 960 |
| PSC50–100 (μm2/cm3) | 818 | 110 ± 49 | 10 | 330 | 60 |
| PSC100–300 (μm2/cm3) | 818 | 580 ± 350 | 29 | 2,100 | 440 |
| PSC300–1,000 (μm2/cm3) | 818 | 510 ± 430 | 17 | 2,700 | 490 |
Abbreviations: PNCx, particle number concentration in the given (x nm) or total size range (3 nm–1 μm); PSCx, particle surface area concentration in the given (x nm) or total size range (3 nm–1 μm).
Size-segregated particle number and surface area concentrations were correlated with meteorologic and other air pollution variables [Table 2; see also Supplemental Material, Table 1 (doi:10.1289/ehp.1002203)]. Particle number concentrations of smaller particles (< 50 nm) and UFP were negatively correlated with air temperature, relative air humidity, and SO2. UFP and NO2 concentrations were not correlated when all days were included in the analysis (Table 2) but were moderately correlated when restricted to summer (r = 0.45), winter (r = −0.33), or transitional months (r = 0.16). There were small negative correlations between NO2 and PNC3–10 (−0.16) and PNC10–30 (−0.09) and a moderate positive correlation between NO2 and PNC30–50 (0.22) and PNC50–100 (r = 0.43). Particle number and surface area concentrations of larger particles (> 100 nm) as well as PSCtotal were moderately correlated with relative air humidity, NO2, and PM10. PNCtotal was moderately correlated with all meteorologic (air temperature, relative air humidity, and air pressure) and air pollutant (SO2, NO2, and PM10) variables. In addition, correlations between adjacent particle size classes were high.
Table 2.
Correlation coefficients for daily mean values of meteorologic and air pollution variables.
| Particle fraction | Air temperature (°C) | Relative humidity (%) | Air pressure (hPa) | SO2 (μg/m3) | NO2 (μg/m3) | PM10 (μg/m3) |
|---|---|---|---|---|---|---|
| UFP (1/cm3) | −0.22 | −0.39 | 0.18 | −0.26 | 0.06 | 0.05 |
| PNCtotal (1/cm3) | −0.25 | −0.21 | 0.18 | −0.18 | 0.27 | 0.23 |
| PNC3–10 (1/cm3) | −0.22 | −0.51 | 0.19 | −0.19 | −0.16 | −0.09 |
| PNC10–30 (1/cm3) | −0.02 | −0.40 | 0.02 | −0.32 | −0.09 | 0.01 |
| PNC30–50 (1/cm3) | −0.17 | −0.15 | 0.16 | −0.23 | 0.22 | 0.12 |
| PNC50–100 (1/cm3) | −0.26 | 0.08 | 0.19 | −0.03 | 0.43 | 0.23 |
| PNC100–300 (1/cm3) | −0.13 | 0.36 | 0.05 | 0.15 | 0.55 | 0.44 |
| PNC300–1,000 (1/cm3) | −0.02 | 0.47 | −0.04 | 0.25 | 0.56 | 0.55 |
| PSCtotal (μm2/cm3) | −0.06 | 0.44 | −0.02 | 0.23 | 0.58 | 0.55 |
| PSC50–100 (μm2/cm3) | −0.26 | 0.12 | 0.18 | −0.01 | 0.45 | 0.24 |
| PSC100–300 (μm2/cm3) | −0.10 | 0.39 | 0.02 | 0.18 | 0.55 | 0.47 |
| PSC300–1,000 (μm2/cm3) | −0.01 | 0.48 | −0.05 | 0.25 | 0.56 | 0.56 |
Respiratory ERV increased by 5% [RR = 1.05 (95% CI, 1.02–1.08)] with an IQR increase (4,400 cm−3) in PNC100–300 with a 1-day lag (PDL model) [Table 3; see also Supplemental Material, Table 2 (doi:10.1289/ehp.1002203)]. Effect estimates for an IQR increase in NO2 were similar in magnitude to estimates for IQR increases in particle number and surface area concentrations of particles > 100 nm. Some inverse associations were observed with UFP and particle number concentrations of fractions < 50 nm. In most cases PNC50–100 was positively associated with respiratory ERV based on PDL models, although some single lag model associations were inverse.
Table 3.
Overview of RR (95% CIs) for respiratory ERV per IQR increment of air pollutant in single-pollutant models.a
| Pollutant | Time delay | IQRb | Cumulative effects modelc RR (95% CI) | PDL modeld RR (95% CI) | Single lag modele (95% CI) |
|---|---|---|---|---|---|
| SO2 | Same day | 100 | 1.01 (0.97–1.05) | 1.00 (0.96–1.04) | 1.01 (0.97–1.05) |
| 5 | 100 | 1.04 (0.97–1.12) | 1.01 (0.98–1.04) | 1.01 (0.98–1.05) | |
| NO2 | Same day | 40 | 1.02 (0.98–1.05) | 1.01 (0.97–1.05) | 1.02 (0.98–1.05) |
| 5 | 40 | 1.06 (1.00–1.12)* | 1.01 (0.98–1.04) | 1.02 (0.98–1.05) | |
| PM10 | Same day | 90 | 1.01 (0.98–1.05) | 1.01 (0.97–1.05) | 1.01 (0.98–1.05) |
| 5 | 90 | 1.00 (0.94–1.06) | 1.02 (0.99–1.06) | 1.02 (0.99–1.05) | |
| UFP | Same day | 11,000 | 1.01 (0.95–1.07) | 1.00 (0.94–1.07) | 1.01 (0.95–1.07) |
| 5 | 11,000 | 0.99 (0.89–1.09) | 0.93 (0.88–0.99)* | 0.93 (0.88–0.97)* | |
| PNCtotal | Same day | 12,600 | 1.03 (0.98–1.09) | 1.01 (0.95–1.08) | 1.03 (0.98–1.09) |
| 1 | 12,600 | 1.04 (0.97–1.12) | 1.06 (1.01–1.11)* | 1.02 (0.96–1.08) | |
| 2 | 12,600 | 1.07 (0.99–1.16) | 1.04 (1.00–1.08) | 1.03 (0.98–1.10) | |
| 3 | 12,600 | 1.05 (0.96–1.15) | 0.99 (0.95–1.03) | 0.96 (0.91–1.02) | |
| 4 | 12,600 | 1.04 (0.94–1.14) | 0.96 (0.92–1.00)* | 0.95 (0.90–1.00) | |
| 5 | 12,600 | 1.03 (0.93–1.15) | 0.97 (0.92–1.03) | 0.94 (0.89–0.99)* | |
| PNC3–10 | Same day | 3,880 | 0.97 (0.93–1.01) | 0.98 (0.93–1.03) | 0.97 (0.93–1.01) |
| 5 | 3,880 | 0.94 (0.86–1.02) | 0.96 (0.92–0.99)* | 0.96 (0.93–0.99)* | |
| PNC10–30 | Same day | 4,300 | 0.98 (0.93–1.04) | 0.99 (0.93–1.06) | 0.98 (0.93–1.04) |
| 5 | 4,300 | 0.98 (0.88–1.10) | 0.95 (0.90–1.00)* | 0.95 (0.90–0.99)* | |
| PNC30–50 | Same day | 2,300 | 1.03 (0.99–1.08) | 1.02 (0.98–1.07) | 1.03 (0.99–1.08) |
| 1 | 2,300 | 1.03 (0.97–1.09) | 1.04 (1.00–1.07)* | 1.00 (0.95–1.04) | |
| 2 | 2,300 | 1.05 (0.98–1.12) | 1.02 (0.99–1.05) | 1.01 (0.96–1.05) | |
| 3 | 2,300 | 1.04 (0.96–1.12) | 0.99 (0.96–1.02) | 0.98 (0.93–1.02) | |
| 4 | 2,300 | 1.03 (0.94–1.12) | 0.97 (0.94–1.00) | 0.95 (0.91–0.99)* | |
| 5 | 2,300 | 1.01 (0.92–1.11) | 0.96 (0.92–1.01) | 0.96 (0.91–1.00) | |
| PNC50–100 | Same day | 3,600 | 1.03 (0.99–1.07) | 1.01 (0.97–1.06) | 1.03 (0.99–1.07) |
| 1 | 3,600 | 1.03 (0.98–1.09) | 1.05 (1.01–1.08)* | 1.00 (0.96–1.04) | |
| 2 | 3,600 | 1.07 (1.00–1.15)* | 1.03 (1.00–1.06)* | 1.03 (0.98–1.07) | |
| 3 | 3,600 | 1.08 (1.00–1.17)* | 0.99 (0.97–1.02) | 1.00 (0.96–1.04) | |
| 4 | 3,600 | 1.05 (0.96–1.14) | 0.98 (0.95–1.01) | 0.96 (0.92–1.00)* | |
| 5 | 3,600 | 1.06 (0.97–1.17) | 1.01 (0.97–1.05) | 0.99 (0.95–1.03) | |
| PNC100–300 | Same day | 4,400 | 1.04 (1.00–1.08) | 1.04 (0.99–1.08) | 1.04 (1.00–1.08) |
| 1 | 4,400 | 1.05 (0.99–1.11) | 1.05 (1.02–1.08)* | 1.01 (0.97–1.06) | |
| 2 | 4,400 | 1.09 (1.02–1.16)* | 1.02 (0.99–1.04) | 1.03 (0.99–1.07) | |
| 3 | 4,400 | 1.08 (1.00–1.17)* | 0.99 (0.97–1.01) | 0.99 (0.96–1.03) | |
| 4 | 4,400 | 1.05 (0.96–1.14) | 0.98 (0.96–1.01) | 0.98 (0.94–1.01) | |
| 5 | 4,400 | 1.08 (0.99–1.18) | 1.04 (1.00–1.08)* | 1.02 (0.98–1.05) | |
| PNC300–1,000 | Same day | 830 | 1.04 (1.00–1.08) | 1.04 (0.99–1.08) | 1.04 (1.00–1.08) |
| 5 | 830 | 1.03 (0.96–1.10) | 1.04 (1.00–1.07)* | 1.02 (0.99–1.05) | |
| PSCtotal | Same day | 960 | 1.05 (1.00–1.09)* | 1.04 (1.00–1.08) | 1.05 (1.00–1.09)* |
| 1 | 960 | 1.04 (0.99–1.10) | 1.03 (1.00–1.05)* | 1.01 (0.98–1.05) | |
| 2 | 960 | 1.05 (0.99–1.11) | 1.00 (0.98–1.02) | 1.01 (0.98–1.05) | |
| 3 | 960 | 1.04 (0.98–1.11) | 0.98 (0.96–1.00) | 0.99 (0.96–1.03) | |
| 4 | 960 | 1.02 (0.95–1.09) | 0.99 (0.97–1.01) | 0.99 (0.96–1.02) | |
| 5 | 960 | 1.04 (0.97–1.12) | 1.04 (1.01–1.08)* | 1.02 (0.99–1.06) | |
| PSC50–100 | Same day | 60 | 1.03 (0.99–1.07) | 1.01 (0.97–1.06) | 1.03 (0.99–1.07) |
| 1 | 60 | 1.03 (0.98–1.09) | 1.05 (1.02–1.08)* | 1.00 (0.96–1.04) | |
| 2 | 60 | 1.07 (1.01–1.15)* | 1.03 (1.00–1.06)* | 1.03 (0.99–1.07) | |
| 3 | 60 | 1.09 (1.01–1.17)* | 1.00 (0.97–1.02) | 1.00 (0.96–1.04) | |
| 4 | 60 | 1.05 (0.96–1.14) | 0.98 (0.95–1.01) | 0.96 (0.93–1.00) | |
| 5 | 60 | 1.07 (0.97–1.17) | 1.01 (0.97–1.05) | 0.99 (0.95–1.03) | |
| PSC100–300 | Same day | 440 | 1.04 (1.00–1.09)* | 1.04 (1.00–1.09) | 1.04 (1.00–1.09)* |
| 1 | 440 | 1.05 (0.99–1.11) | 1.04 (1.01–1.07)* | 1.02 (0.98–1.06) | |
| 2 | 440 | 1.08 (1.01–1.15)* | 1.01 (0.99–1.04) | 1.02 (0.99–1.06) | |
| 3 | 440 | 1.07 (1.00–1.15) | 0.99 (0.96–1.01) | 0.99 (0.95–1.03) | |
| 4 | 440 | 1.04 (0.96–1.13) | 0.99 (0.96–1.01) | 0.98 (0.95–1.02) | |
| 5 | 440 | 1.07 (0.98–1.17) | 1.04 (1.00–1.08)* | 1.02 (0.98–1.06) | |
| PSC300–1,000 | Same day | 490 | 1.04 (1.00–1.08) | 1.04 (0.99–1.08) | 1.04 (1.00–1.08) |
| 1 | 490 | 1.03 (0.99–1.08) | 1.01 (0.99–1.04) | 1.01 (0.98–1.05) | |
| 5 | 490 | 1.02 (0.96–1.09) | 1.04 (1.01–1.07)* | 1.02 (0.99–1.06) | |
For a complete table, see Supplemental Material, Table 2 (doi:10.1289/ehp.1002203).
Units for IQR: SO2, NO2, and PM10 (μg/m3); PNCx and UFP (1/cm3); PSCx (μm2/cm3).
Cumulative-effects models represent time-delayed effects with moving averages up to 6 days (mean of the same day and 5 previous days).
PDL models with the lag coefficients follow a third-degree polynomial of the lag number and a maximum lag of 5 days.
Single lag models represent time-delayed effects with lagged effect up to 5 days.
p < 0.05 (p-values for the null hypothesis that the corresponding parameter is zero).
Effect estimates for PNC, PSC, and NO2 were mostly higher in magnitude after adjustment for PM10 [Table 4; see also Supplemental Material, Table 3 (doi:10.1289/ehp.1002203)]. Associations with PNC and PSC were comparable in magnitude but less precise after adjustment for NO2, with borderline p-values (p < 0.09) only for PNC100–300 and PSC100–300 cumulative effects over 3 days (present and previous 2 days). In two-pollutant models, associations between respiratory ERV and NO2 were more consistent than those for other pollutants.
Table 4.
Overview of RRs (95% CIs) between respiratory ERV and an IQR increment of air pollutant while controlling for NO2 or PM10.a
| Pollutant | Time delay (days) | IQRb | While controlling for NO2 | While controlling for PM10 |
|---|---|---|---|---|
| NO2 | 3 | 40 | — | 1.07 (1.01–1.13)* |
| 4 | 40 | — | 1.07 (1.01–1.14)* | |
| 5 | 40 | — | 1.08 (1.01–1.15)* | |
| PNC50–100 | 2 | 3,600 | 1.06 (0.99–1.14) | 1.07 (1.00–1.15)* |
| 3 | 3,600 | 1.06 (0.98–1.16) | 1.08 (1.00–1.17)* | |
| PNC100–300 | 2 | 4,400 | 1.08 (1.00–1.17) | 1.10 (1.02–1.19)* |
| 3 | 4,400 | 1.06 (0.97–1.16) | 1.11 (1.02–1.21)* | |
| PSC50–100 | 2 | 60 | 1.06 (0.99–1.14) | 1.07 (1.01–1.15)* |
| 3 | 60 | 1.07 (0.98–1.16) | 1.09 (1.01–1.17)* | |
| PSC100–300 | 2 | 440 | 1.07 (0.99–1.16) | 1.10 (1.02–1.19)* |
| 3 | 440 | 1.05 (0.95–1.15) | 1.10 (1.01–1.20)* | |
For a complete table, see Supplemental Material, Table 3 (doi:10.1289/ehp.1002203). Estimates were calculated using cumulative effects models representing time-delayed effects with moving averages up to 6 days (mean of the same day and 5 previous days) and including both pollutants with the same lag, for example, same-day total particle number concentration and same-day NO2 concentration.
Units for IQR: NO2 (μg/m3); PNCx (1/cm3); PSCx (μm2/cm3).
p < 0.05 (p-values for the null hypothesis that the corresponding parameter is zero).
Associations with PNC100–300 were robust to variations in model parameters [see Supplemental Material, Table 4 (doi:10.1289/ehp.1002203)] in the sensitivity analyses.
Discussion
We observed adverse associations between respiratory ERV and NO2 and particle number and surface area concentrations in several size ranges. Effects estimates for IQR increases in both particle number and surface area concentrations of 100–300 nm and 300–1,000 nm particles were comparable with estimated effects for IQR increases in NO2 concentrations, and effect estimates for NO2 were more consistent than those for other exposures in two-pollutant models. In most cases, particles < 50 nm were not positively associated with respiratory ERV, whereas particles in the size range of 50–100 nm were adversely associated with respiratory ERV, both being fractions of UFP.
Our findings are consistent with results from other studies reporting that levels of air pollution, often represented by PM10, PM2.5, NO2, SO2, O3, or UFP, are associated with short-term increases in ERV for respiratory complaints (Knol et al. 2009; Peel et al. 2005; Stieb et al. 2009; Tolbert et al. 2007).
Interestingly, we did not observe adverse associations of respiratory ERV with small particles (< 50 nm) but did observe positive associations with particle number concentration for particles > 100 nm. Andersen et al. (2008) investigated the association between short-term exposure to size-segregated particles and hospital admissions due to respiratory diseases in Copenhagen among the elderly (age > 65 years). They reported associations between total particle number concentrations and respiratory disease admissions (RR = 1.04; 95% CI, 1.00–1.07) comparable with the associations of PNC100–300 and PNC300–1,000 that we found.
Particle surface area concentrations also were adversely associated with total respiratory ERV, in agreement with Sager and Castranova (2009). This would be expected, as we derived particle surface area concentrations from particle number concentrations. Toxicologic studies report that surface area plays an important role in determining the biological activity of smaller particles, as they occupy less volume, resulting in a larger number of particles with a greater surface area per unit mass and an increased potential for biological interaction and absorption of chemical compounds (Oberdörster et al. 2005). However, it is not yet possible to measure particle surface area directly on a continuous scale.
Adverse associations between total respiratory ERV and both particle number and surface area concentrations remained after adjustment for particle mass (PM10). In two studies of respiratory health outcomes and air pollution, associations with particle number concentrations diminished after controlling for PM2.5 (Andersen et al. 2008; Halonen et al. 2008). Unfortunately, we did not have PM2.5 data available.
NO2 was associated with total respiratory ERV, in agreement with other studies (Peel et al. 2005; Stieb et al. 2009; Tolbert et al. 2007). NO2 itself has adverse health effects at high concentrations (> 200 μg/m3) (WHO 2005), but such levels are rare in Beijing. NO2 originates mainly from combustion processes and traffic, which are major sources of air pollution in Beijing (Sun et al. 2004), and NO2 is often correlated with other pollutants. Traffic-related air pollution has been associated with respiratory diseases in several studies (Brunekreef et al. 2009). In our two-pollutant models, associations with NO2 were most consistent. When controlling for NO2, associations with 100–300 nm stayed moderately significant (p < 0.09). A pollutant that exhibits a relatively strong association in a multipollutant model may be acting as a surrogate for an unmeasured or poorly measured pollutant (Peel et al. 2005). In addition, associations with pollutants based on single-pollutant models may be attributable to an association with another pollutant that is correlated with the measured pollutant. Typically, epidemiologic studies (including ours) cannot verify to what extent associations with NO2 are attributable to NO2 itself or to other pollutants correlated with NO2. Therefore, we cannot rule out the possibility that observed associations for NO2 were due to NO2-correlated air pollution.
We gathered daily concentrations of SO2, NO2, and PM10 from the Beijing Environmental Protection Bureau, but PM2.5 concentrations were not available. Because of size limitations, we did not investigate associations with disease-specific outcomes. Associations within population subgroups, such as the elderly or children, and disease specific analyses have suggested variation in effects and susceptibilities (Halonen et al. 2008). Associations between cardiovascular diseases and particle mass concentrations were not in the scope of our study, but are under investigation. Guo et al. (2009) reported that PM2.5, NO2, and SO2 concentrations were associated with hospital ERV for cardiovascular diseases in Beijing.
Exposure assessment and misclassification of exposure is a well-recognized limitation of epidemiologic time-series studies. Measurements from only one station were used for the particle size distribution data, and we were not able to assess spatial variation in particle number concentrations. Our measurement site may be considered as an urban background station because of its location (20 m height and > 500 m from a major road). Average particle number size distributions at a PKU measurement site and another regional measurement site, located approximately 50 km south of the PKU, were similar (Yue et al. 2009), confirming that our measurement site may be considered as an urban background station. In Beijing, concentrations of fine PM (PM2.5 and PM10) are evenly distributed over the urban area, including the Haidian district (Cheng et al. 2007; Wang et al. 2009). In urban areas, differences of absolute particle number concentrations between different measurement sites can be great (Krudysz et al. 2009), with the largest variations in ultrafine (< 100 nm) and coarse (2,500–10,000 nm) particles and more homogenous spatial distributions of accumulation mode particles (100–2,000 nm) (Monn 2001). The low correlation between NO2—as an urban average, representing traffic exhaust levels and the main source of UFP—and UFP might suggest low correlation between UFP at the measurement site and UFP levels elsewhere in the city. On the other hand, moderate correlations between UFP and NO2 were observed for subgroups, for example, seasons or particle subfractions of UFP. Personal levels of UFP can differ substantially, such as in proximity to traffic (Kaminsky et al. 2009). However, Buzorius et al. (1999), Cyrys et al. (2008), and Puustinen et al. (2007) showed that daily temporal correlations of particle number concentration time series between different monitoring sites are high. The authors concluded that using one carefully chosen monitoring site is a reasonable approach to characterize exposure of particle number concentrations in epidemiologic time-series studies. When associations are estimated by Poisson regression model in time-series studies, Lipfert and Wyzga (1995) argued that exposure misclassification will reduce the precision of effect estimates, resulting in wider CIs, but will not bias estimates. On the other hand, bias away from the null can occur in time-series studies of multiple pollutants, such as this one, when measurement errors of different pollutants are correlated (Zeger et al. 2000).
The air pollution mixture in Beijing is different from that of Western cities. The main sources of particulate air pollution in Beijing are coal burning, traffic, and dust from long-range transport (Sun et al. 2004). The mean particle number concentrations in urban areas in Europe or North America are 60–80% of the Beijing values, and particulate mass concentrations in Beijing are about three times higher than in Europe (Wehner et al. 2008). Additionally, differences between populations with respect to age, diet, sex, ethnicity, and state of health have to be taken into account when comparing results from Beijing with other cities in China or abroad.
Our findings should be interpreted cautiously, because exposure to particle number concentrations was based on data from a single background-monitoring site. In addition, the possibility that some effects might have occurred by chance cannot be excluded. To verify our findings with respect to different pollutants and particle fractions, further studies should be conducted, including analyzing the spatial correlation of pollutant time series.
Conclusions
Present levels of air pollution were associated with respiratory ERV in Beijing, China. NO2 concentrations in Beijing appeared to be a better surrogate measure than PM10 or particle number concentrations for evaluating respiratory health effects of an air pollution mixture.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.1002203 via http://dx.doi.org/).
We thank all partners participating in this project, the Institute for Tropospheric Research for providing guidance of the particulate measurements, the Third Hospital for providing the emergency room cases, and weatherundergound.com for providing the meteorologic data.
This research was supported by the Deutsche Forschungsgemeinschaft (FR-1417/3-2) and the China National Natural Science Foundation (20420130348, 20637020).
References
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19((6)):716–723. [Google Scholar]
- Alfaro-Moreno E, Nawrot TS, Nemmar A, Nemery B. Particulate matter in the environment: pulmonary and cardiovascular effects. Curr Opin Pulm Med. 2007;13((2)):98–106. doi: 10.1097/MCP.0b013e328013f47e. [DOI] [PubMed] [Google Scholar]
- Andersen ZJ, Wahlin P, Raaschou-Nielsen O, Ketzel M, Scheike T, Loft S. Size distribution and total number concentration of ultrafine and accumulation mode particles and hospital admissions in children and the elderly in Copenhagen, Denmark. Occup Environ Med. 2008;65:458–466. doi: 10.1136/oem.2007.033290. [DOI] [PubMed] [Google Scholar]
- Birmili W, Stratmann F, Wiedensohler A. Design of a DMA-based size spectrometer for a large particle size range and stable operation. J Aerosol Sci. 1999;30((4)):549–554. [Google Scholar]
- Brunekreef B, Beelen R, Hoek G, Schouten L, Bausch-Goldbohm S, Fischer P, et al. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Res Rep Health Eff Inst. 2009;(139):5–89. [PubMed] [Google Scholar]
- Buzorius G, Hämeri K, Pekkanen J, Kulmala M. Spatial variation of aerosol number concentration in Helsinki city. Atmos Environ. 1999;33:553–565. [Google Scholar]
- Cheng S, Chen D, Li J, Wang H, Guo X. The assessment of emission-source contributions to air quality by using a coupled MM5-ARPS-CMAQ modeling system: a case study in the Beijing metropolitan region, China. Environ Modell Softw. 2007;22:1601–1616. [Google Scholar]
- Cyrys J, Pitz M, Heinrich J, Wichmann HE, Peters A. Spatial and temporal variation of particle number concentration in Augsburg, Germany. Sci Total Environ. 2008;401((1–3)):168–175. doi: 10.1016/j.scitotenv.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW. Systemic and cardiovascular effects of airway injury and inflammation: ultrafine particle exposure in humans. Environ Health Perspect. 2001;109((suppl 4)):529–532. doi: 10.1289/ehp.01109s4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Jia Y, Pan X-C, Liu L, Wichmann H-E. The association between fine particulate air pollution and hospital emergency room visits for cardiovascular diseases in Beijing, China. Sci Total Environ. 2009;407((17)):4826–4830. doi: 10.1016/j.scitotenv.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Halonen JI, Lanki T, Yli-Tuomi T, Kulmala M, Tiittanen P, Pekkanen J. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax. 2008;63:635–641. doi: 10.1136/thx.2007.091371. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Generalized additive models: some applications (with discussion) J Am Stat Assoc. 1987;82:371–386. [Google Scholar]
- Health Effects Institute. Revised Analyses of the National Morbidity, Mortality, and Air Pollution Study, Part II: Revised Analyses of Selected Time-Series Studies of Air Pollution and Health. Cambridge, MA: Health Effects Institute; 2003. [Google Scholar]
- Health Effects Institute. Special Report. Vol. 15. Cambridge, MA: Health Effects Institute, International Scientific Oversight Committee; 2004. Health Effects of Outdoor Air Pollution in Developing Countries of Asia: A Literature Review. [Google Scholar]
- Kan H, London SJ, Chen G, Zhang Y, Song G, Zhao N, et al. Differentiating the effects of fine and coarse particles on daily mortality in Shanghai, China. Environ Int. 2007;33((3)):376–384. doi: 10.1016/j.envint.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol AB, de Hartog JJ, Boogaard H, Slottje P, van der Sluijs JP, Lebret E, et al. Expert elicitation on ultrafine particles: likelihood of health effects and causal pathways. Part Fibre Toxicol. 2009;24(6):19. doi: 10.1186/1743-8977-6-19. [Online 24 July 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky JA, Gaskin EALM, Matsuda M, Miguel AH. In-cabin commuter exposure to ultrafine particles on commuter roads in and around Hong Kong’s Tseung Kwan O Tunnel. Aerosol Air Qual Res. 2009;9:353–357. [Google Scholar]
- Krudysz M, Moore K, Geller M, Sioutas C, Froines J. Intra-community spatial variability of particulate matter size distributions in Southern California/Los Angeles. Atmos Chem Phys. 2009;9:1061–1075. doi: 10.5194/acp-9-1061-2009. [Online 12 February 2009] [DOI] [Google Scholar]
- Lipfert FW, Wyzga RE. Air pollution and mortality: issues and uncertainties. J Air Waste Manag Assoc. 1995;45((12)):949–966. doi: 10.1080/10473289.1995.10467427. [DOI] [PubMed] [Google Scholar]
- Monn C. Exposure assessment of air pollutants: a review on spatial heterogeneity and indoor/outdoor/personal exposure to suspended particulate matter, nitrogen dioxide and ozone. Atmos Environ. 2001;35:1–32. [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [Online 6 October 2005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunsanya T, Prescott G, Seaton A. Acute respiratory effects of particles: mass or number? Occup Environ Med. 2001;58((3)):154–159. doi: 10.1136/oem.58.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16((2)):164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- Peng RD, Chang HH, Bell ML, McDermott A, Zeger SL, Samet JM, et al. Admissions for cardiovascular and respiratory coarse particulate matter air pollution and hospital diseases among Medicare patients. JAMA. 2008;299((18)):2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen P, Timonen KL, Tiittanen P, Mirme A, Ruuskanen J, Pekkanen J. Ultrafine particles in urban air and respiratory health among adult asthmatics. Eur Respir J. 2001;17:428–435. doi: 10.1183/09031936.01.17304280. [DOI] [PubMed] [Google Scholar]
- Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med. 1997;155:1376–1383. doi: 10.1164/ajrccm.155.4.9105082. [DOI] [PubMed] [Google Scholar]
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Puustinen A, Hämeri K, Pekkanen J, Kulmala M, de Hartog J, Meliefste K, et al. Spatial variation of particle number and mass over four European cities. Atmos Environ. 2007;41:6622–6636. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2003. [Google Scholar]
- Sager TM, Castranova V. Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: comparison to ultrafine titanium dioxide. Part Fibre Toxicol. 2009;6(15) doi: 10.1186/1743-8977-6-15. [Online 4 May 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen M, Nygaard UC, L⊘vik M. Particle size determines activation of the innate immune system in the lung. Scand J Immunol. 2009;69((5)):421–428. doi: 10.1111/j.1365-3083.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Szyszkowicz1 M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health. 2009;8:25. doi: 10.1186/1476-069X-8-25. [Online 10 June 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YL, Zhuang GS, Wang Y, Han LH, Guo JH, Dan M, et al. The air-borne particulate pollution in Beijing. Concentration, composition, distribution and sources. Atmos Environ. 2004;38((35)):5991–6004. [Google Scholar]
- Tolbert PE, Klein M, Peel JL, Sarnat SE, Sarnat JA. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol. 2007;17((suppl 2)):29–35. doi: 10.1038/sj.jes.7500625. [DOI] [PubMed] [Google Scholar]
- Touloumi G, Samoli E, Pipikou M, Le Tertre A, Atkinson R, Katsouyanni K. Seasonal confounding in air pollution and health time-series studies: effect on air pollution effect estimates. Stat Med. 2006;25((24)):4164–4178. doi: 10.1002/sim.2681. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Fiotakis K, Vlachogianni TJ. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol. 2008;26((4)):339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen L, Tao J, Zhang Y, Su L. Satellite-based estimation of regional particulate matter (PM) in Beijing using vertical-and-RH correcting method. Remote Sens Environ. 2009;114((1)):50–63. [Google Scholar]
- Weather Underground. Welcome to Weather Underground! 2009. [[accessed 10 October 2009]]. Available: http://www.wunderground.com.
- Wehner B, Birmili W, Ditas F, Wu Z, Hu M, Liu X, et al. Relationships between submicrometer particulate air pollution and air mass history in Beijing, China, 2004–2006. Atmos Chem Phys. 2008;8:6155–6168. [Google Scholar]
- Wehner B, Wiedensohler A, Tuch TM, Wu ZJ, Hu M, Slanina J, et al. Variability of the aerosol number size distribution in Beijing, China: new particle formation, dust storms, and high continental background. Geophys Res Lett. 2004:31. doi: 10.1029/2004GL021596. [Online 25 November 2004] [DOI] [Google Scholar]
- WHO (World Health Organization) International Classification of Diseases, 10th Revision. Geneva: WHO; 1993. [Google Scholar]
- WHO (World Health Organization) Air Quality Guidelines. Global Update 2005; Report on a working group meeting; Bonn, Germany. 18–20 October 2005; Copenhagen: WHO, Regional Office for Europe; 2005. EUR/05/5046029. [Google Scholar]
- Wichmann HE. Diesel exhaust particles. Inhal Toxicol. 2007;19((suppl 1)):241–244. doi: 10.1080/08958370701498075. [DOI] [PubMed] [Google Scholar]
- Wichmann HE, Spix C, Tuch T, Wölke G, Peters A, Heinrich J, et al. Daily mortality and fine and ultrafine particles in Erfurt, Germany, part I: role of particle number and particle mass. Res Rep Health Eff Inst. 2000;98:5–94. [PubMed] [Google Scholar]
- Willeke K, Baron PA. Aerosol Measurement, Principles, Techniques and Applications. 2nd ed. New York: Van Nostrand Reinhold; 1993. [Google Scholar]
- Wood SN. Generalized Additive Models: An Introduction with R. New York: Chapman and Hall/CRC; 2006. [Google Scholar]
- Yao X, Lau APS, Fang M, Chan CK, Hu M. Size distributions and formation of ionic species in atmospheric particulate pollutants in Beijing, China: 1—inorganic ions. Atmos Environ. 2003;37:2991–3000. [Google Scholar]
- Yue D, Hu M, Wu Z, Wang Z, Guo S, Wehner B, et al. Characteristics of aerosol size distributions and new particle formation in summer of mega-city Beijing, China. J Geophys Res. 2009:114. doi: 10.1029/2008JD010894. [Online 24 July 2009] [DOI] [Google Scholar]
- Zanobetti A, Schwartz J, Samoli E, Gryparis A, Touloumi G, Peacock J, et al. The temporal pattern of respiratory and heart disease mortality in response to air pollution. Environ Health Perspect. 2003;111:1188–1193. doi: 10.1289/ehp.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, Cohen A. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]