Abstract
Hypothalamic amenorrhea (HA) is associated with dysfunction of the hypothalamic-pituitary-peripheral endocrine axes, leading to infertility and bone loss, and usually is caused by chronic energy deficiency secondary to strenuous exercise and/or decreased food intake. Energy deficiency also leads to hypoleptinemia, which has been proposed, on the basis of observational studies as well as an open-label study, to mediate the neuroendocrine abnormalities associated with this condition. To prove definitively a causal role of leptin in the pathogenesis of HA, we performed a randomized, double-blinded, placebo-controlled trial of human recombinant leptin (metreleptin) in replacement doses over 36 wk in women with HA. We assessed its effects on reproductive outcomes, neuroendocrine function, and bone metabolism. Leptin replacement resulted in recovery of menstruation and corrected the abnormalities in the gonadal, thyroid, growth hormone, and adrenal axes. We also demonstrated changes in markers of bone metabolism suggestive of bone formation, but no changes in bone mineral density were detected over the short duration of this study. If these data are confirmed, metreleptin administration in replacement doses to normalize circulating leptin levels may prove to be a safe and effective therapy for women with HA.
Hypothalamic amenorrhea (HA) is characterized by cessation of menstrual cycles because of dysfunction of the hypothalamic-pituitary-gonadal axis, abnormalities in gonadotropin pulsatility, and subsequent estrogen deficiency. This disorder is associated with chronic energy deficiency, usually caused by strenuous exercise, stress, and/or reduced food intake, and accounts for more than 30% of cases of amenorrhea in women of reproductive age (1). In addition to infertility, HA is associated with other neuroendocrine abnormalities, including dysfunction of the thyroid, growth hormone, and adrenal axes (2–7) as well as bone loss (8, 9) and propensity for fractures.
Circulating leptin levels reflect the amount of energy stores in fat as well as acute changes in energy intake (10). Hypoleptinemia, signaling a state of energy deficiency, may mediate the changes in the neuroendocrine axes observed in HA. We first showed that acutely depriving mice and then healthy men of energy by caloric restriction resulted in relative leptin deficiency and neuroendocrine abnormalities affecting the gonadal and thyroid axes, and these abnormalities were prevented with recombinant methionyl human leptin (metreleptin) replacement (11, 12). Women with HA are chronically energy deficient and, in observational studies, have low leptin levels and loss of diurnal leptin variation (13–16). In our proof-of-concept, open-label pilot study, we administered metreleptin s.c. for 3 mo to normalize leptin levels in women with HA and found that metreleptin treatment resulted in ovulatory menses and significant increases in levels of luteinizing hormone (LH), estradiol, insulin-like growth factor-1 (IGF1), thyroid hormones, and bone formation markers (17). Our results indicated that hypoleptinemia may be responsible for reproductive and neuroendocrine dysfunction in women with HA, but the open-label nature of the study could not prove this notion beyond any doubt because uncontrolled confounding factors could have accounted for these findings. Moreover, the adrenal axis and the full spectrum of bone metabolism were not studied fully in the earlier study (17), and the duration of the trial was not long enough to allow the study of long-term effects of metreleptin treatment.
We therefore performed a randomized, double-blinded, placebo-controlled trial of metreleptin treatment in women with HA. End points of the study were changes in reproductive and neuroendocrine functions, markers of bone metabolism, bone mineral density (BMD), and resting energy expenditure. Compared with our previous open-label pilot study, this study was randomized, placebo-controlled, and of substantially longer duration (36 wk), permitting the assessment of study outcomes against the background rate of developing spontaneous menstrual cycles and/or neuroendocrine changes over an extended period.
Results
Baseline Characteristics.
There were no significant baseline differences between the metreleptin- and placebo-treated groups in regards to age, weight, body mass index (BMI), body fat composition, duration of amenorrhea, leptin levels, LH, follicle-stimulating hormone (FSH), estradiol, and BMD (Table S1 and Fig. 1).
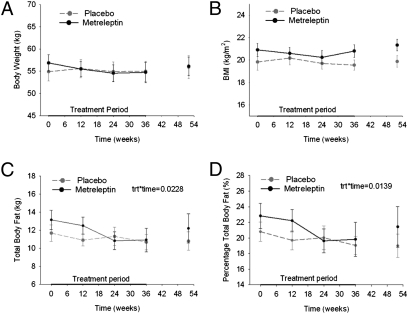
Fig. 1.
Body composition. Anthropometric changes over the 36 wk of treatment and at the follow-up visit at week 52 as assessed by body weight (A), body mass index (B), total body fat (C), and percentage total body fat (D). Solid black lines represent the metreleptin-treated group; gray dashed lines represent the placebo-treated group. Metreleptin treatment had a significant effect over time on total body fat (P = 0.02) and percentage total body fat (P = 0.01).
Subject Completion.
Among the 20 participants who were enrolled in the study, 11 were assigned randomly to receive metreleptin, and nine received placebo. One participant in the metreleptin-treated group withdrew from the study because she developed injection-site reactions soon after the baseline visit. Thus, the analyses in Table 1 and Tables S1 and S2 include the results from 10 metreleptin-treated subjects. Seven of the 11 participants in the metreleptin-treated group and six of nine participants in the placebo-treated group completed the entire study (Table 2 and Table S3). Of the metreleptin-treated participants, one became pregnant at week 24, and one was discontinued from the study at week 28 because of persistent weight loss despite adjustments in study medication dose (SI Materials and Methods). One participant from the metreleptin-treated group at week 24 and three participants from the placebo-treated group at weeks 4, 16, and 24 decided not to continue the study because of traveling.
Table 1.
Changes in neuroendocrine axes and markers of bone turnover over time
| Analyte | Group | Week 0 (baseline) | Week 12 | Week 24 | Week 36 | Week 52 (follow-up) | P (trt)* | P (trt* time)† | P (follow-up)‡ |
| Hormones | |||||||||
| Leptin (ng/mL) | Metreleptin | 4.55 ± 0.64 | 44.51 ± 8.74 | 57.26 ± 11.36 | 59.33 ± 14.15 | 8.64 ± 3.92 | <0.0001 | <0.0001 | 0.05 |
| Placebo | 4.10 ± 0.64 | 3.65 ± 0.61 | 3.51 ± 0.52 | 3.09 ± 0.51 | 2.64 ± 0.57 | ||||
| Free leptin (ng/mL) | Metreleptin | 4.75 ± 1.24 | 23.87 ± 3.97 | 47.52 ± 11.98 | 49.04 ± 13.78 | 3.78 ± 1.00 | <0.0001 | <0.0001 | 0.18 |
| Placebo | 3.77 ± 0.62 | 3.35 ± 0.43 | 3.25 ± 0.41 | 2.75 ± 0.40 | 2.34 ± 0.30 | ||||
| Estradiol (pg/mL) | Metreleptin | 23.0 ± 9.0 | 19.3 ± 4.5 | 27.2 ± 8.0 | 25.4 ± 7.8 | 22.0 ± 7.8 | 0.01 | 0.52 | 0.22 |
| Placebo | 14.0 ± 1.7 | 13.9 ± 1.8 | 12.3 ± 1.4 | 11.8 ± 1.3 | 11.6 ± 1.8 | ||||
| Progesterone (ng/mL) | Metreleptin | 4.5 ± 0.4 | 4.9 ± 0.3 | 14.5 ± 5.6 | 7.3 ± 3.5 | 7.8 ± 3.9 | 0.03 | 0.33 | 0.30 |
| Placebo | 4.6 ± 0.4 | 4.4 ± 0.4 | 4.4 ± 0.3 | 5.2 ± 0.7 | 3.7 ± 0.2 | ||||
| Cortisol (μg/dL) | Metreleptin | 20.9 ± 1.1 | 18.2 ± 1.0 | 17.1 ± 1.1 | 12.8 ± 1.3 | 14.9 ± 1.6 | 0.02 | 0.24 | 0.17 |
| Placebo | 20.0 ± 1.3 | 19.6 ± 1.4 | 20.2 ± 1.0 | 19.7 ± 0.9 | 17.7 ± 1.1 | ||||
| IGF1 (ng/mL) | Metreleptin | 498.1 ± 66.7 | 462.2 ± 51.1 | 543.2 ± 49.7 | 491.0 ± 69.8 | 382.3 ± 71.7 | 0.23 | 0.08 | 0.58 |
| Placebo | 422.1 ± 41.9 | 434.3 ± 37.2 | 404.4 ± 33.1 | 388.4 ± 40.9 | 331.6 ± 50.6 | ||||
| IGF1:IGFBP-3 | Metreleptin | 5.35 ± 0.49 | 5.14 ± 0.37 | 6.09 ± 0.40 | 5.46 ± 0.61 | 4.58 ± 0.72 | 0.32 | 0.04 | 0.48 |
| Placebo | 5.05 ± 0.45 | 5.14 ± 0.38 | 4.82 ± 0.33 | 4.63 ± 0.42 | 3.91 ± 0.56 | ||||
| Bone markers | |||||||||
| BASP (U/L) | Metreleptin | 26.6 ± 5.7 | 28.2 ± 6.1 | 29.6 ± 7.3 | 30.7 ± 7.5 | 22.7 ± 4.5 | 0.09 | 0.29 | 0.20 |
| Placebo | 16.7 ± 4.4 | 17.2 ± 4.3 | 13.4 ± 2.7 | 13.5 ± 2.4 | 14.2 ± 4.2 | ||||
| Osteocalcin (ng/mL) | Metreleptin | 13.9 ± 2.6 | 20.9 ± 3.1 | 19.8 ± 3.1 | 20.9 ± 3.1 | 12.3 ± 2.1 | 0.0019 | 0.21 | 0.17 |
| Placebo | 9.3 ± 0.8 | 8.5 ± 1.0 | 8.1 ± 0.9 | 7.7 ± 0.7 | 8.7 ± 1.1 | ||||
| Urinary NTX:creatinine | Metreleptin | 49.4 ± 5.4 | 72.3 ± 9.0 | 56.1 ± 13.6 | 52.8 ± 13.4 | 45.9 ± 20.2 | 0.03 | 0.43 | 0.68 |
| Placebo | 30.8 ± 3.1 | 42.0 ± 10.0 | 56.9 ± 21.5 | 58.1 ± 12.0 | 38.2 ± 8.3 | ||||
All data are presented as mean± SE. For analysis, the last observation was carried forward to yield n = 10 for the metreleptin group and n = 9 for the placebo group, except BASP, which had n = 8 for the placebo group. On-treatment analysis yielded similar results. Log transformed data were used for leptin, free leptin, estradiol, and progesterone. Baseline level was adjusted for in the model for urinary NTX:creatinine. Overall P values were based on repeated measure ANOVA from baseline and every 4 wk through wk 36 for all variables except IGF1, IGFBP-3, and IGF1:IGFBP-3, which were analyzed using repeated measure ANOVA from baseline, week 12, week 24, and week 36.
*Effect of metreleptin and placebo treatment.
†Metreleptin and placebo treatment over time interaction.
‡One-way ANOVA was used to compare the difference between metreleptin and placebo treatment groups at week 52 follow-up.
Table 2.
Individual data on presence of menstruation, metreleptin dose, and weight: Treatment group
| Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | Week 28 | Week 32 | Week 36 | Week 52 | ||
| A | Menstruation | − | − | − | − | − | − | − | − | + | + | − |
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | |||
| Weight (kg) | 56.35 | 56.95 | 58.20 | 56.60 | 56.80 | 55.95 | 57.00 | 56.30 | 55.40 | 55.50 | 55.50 | |
| B | Menstruation | − | − | + | + | − | + | + | + | + | + | + |
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | |||
| Weight (kg) | 63.00 | 62.40 | 61.80 | 60.35 | 60.55 | 60.40 | 60.20 | 59.30 | 59.70 | 59.00 | 59.55 | |
| C | Menstruation | − | + | + | − | + | + | + | + | + | − | + |
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | |||
| Weight (kg) | 54.40 | 53.60 | 52.90 | 53.50 | 52.75 | 52.95 | 53.50 | 54.30 | 53.30 | 53.05 | 53.30 | |
| D | Menstruation | − | − | − | − | − | − | − | Withdrew from study. | |||
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.12 | 0.12 | 0.12 | 0.12 | |||||
| Weight (kg) | 57.80 | 59.15 | 58.80 | 57.95 | 57.15 | 56.90 | 57.85 | |||||
| E | Menstruation | − | − | + | − | + | + | + | Withdrawn from study because of pregnancy. | |||
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | |||||
| Weight (kg) | 56.95 | 54.75 | 54.20 | 54.95 | 55.45 | 55.25 | 53.20 | |||||
| F | Menstruation | − | − | − | − | − | − | − | − | − | − | − |
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.12 | 0.12 | 0.12 | 0.12 | 0.08 | 0.08 | |||
| Weight (kg) | 49.60 | 49.70 | 49.05 | 48.70 | 48.05 | 48.10 | 47.40 | 48.60 | 47.30 | 46.25 | 50.95 | |
| G | Menstruation | Withdrawn from study because of injection-site reactions. Subject was not included in the analyses. | ||||||||||
| Dose (mg/kg) | ||||||||||||
| Weight (kg) | ||||||||||||
| H | Menstruation | − | − | − | − | − | + | − | + | Withdrawn from study because of persistent weight loss. | ||
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.08 | 0.04 | 0.04 | 0.04 | |||||
| Weight (kg) | 52.50 | 51.38 | 50.45 | 47.75 | 46.85 | 47.05 | 46.00 | 47.40 | ||||
| I | Menstruation | − | − | − | − | − | − | − | − | − | − | − |
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.12 | 0.12 | 0.12 | 0.08 | 0.08 | 0.08 | |||
| Weight (kg) | 52.60 | 53.40 | 55.10 | 53.75 | 52.30 | 52.70 | 50.55 | 51.55 | 53.30 | 50.80 | 49.80 | |
| J | Menstruation | − | − | − | + | − | + | + | + | − | + | − |
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | |||
| Weight (kg) | 55.80 | 55.50 | 55.70 | 55.45 | 55.20 | 54.65 | 54.30 | 53.90 | 53.15 | 53.60 | 57.90 | |
| K | Menstruation | − | − | + | + | + | − | − | − | + | + | + |
| Dose (mg/kg) | 0.08 | 0.08 | 0.08 | 0.08 | 0.04 | 0.04 | 0.04 | 0.04 | 0.08 | |||
| Weight (kg) | 69.65 | 68.60 | 67.60 | 65.95 | 65.20 | 65.80 | 65.60 | 64.80 | 66.45 | 65.10 | 65.80 | |
Weight, Body Composition, and Metabolic Rate.
Except for one participant who was removed from the study because of weight loss at week 28, all participants in both the metreleptin- and placebo-treated groups maintained stable weights during the study with dose adjustments (Fig. 1A and Table 2 and Table S3). Four participants taking metreleptin required decreased doses because of weight loss. The BMI did not change significantly in the metreleptin group (20.8 ± 0.6 kg/m2 at week 36 compared with 21.1 ± 0.6 kg/m2 at baseline) compared with the control group (19.6 ± 0.4 kg/m2 at week 36 compared with 19.8 ± 0.7 kg/m2 at baseline) (P = 0.23) (Fig. 1B). However, over the 36-wk study period the metreleptin-treated group had progressive loss of total body fat mass and percentage measured by dual-energy X-ray absorptiometry (DEXA) scan, with a mean loss of 2.02 kg of fat, compared with the placebo-treated group (P = 0.023 for total body fat mass, P = 0.014 for total body fat percentage) (Fig. 1 C and D). The fat loss occurred in both peripheral and central fat compartments. This difference occurred during the first 24 wk of the study and was minimized with appropriate adjustments of metreleptin dosing as per protocol. After metreleptin withdrawal of 16 wk, the metreleptin-treated women regained fat mass to the levels of their baseline total body fat mass at week 52 (P = 0.63). Similar results were obtained with the use of bioelectrical impedance. There were no significant differences between the two treatment groups in resting energy expenditure throughout the study (P = 0.78). Respiratory quotient increased in both groups but increased significantly more in the metreleptin group (P = 0.047), perhaps suggesting greater carbohydrate oxidation in the metreleptin group. Every 3 mo participants recorded food intake on two weekdays and one weekend day. There were no significant differences between the two treatment groups in the reported total caloric intake at baseline (P = 0.84) or throughout the study (P = 0.91). At the end of 36 wk, there were no significant differences between the two groups in fat (P = 0.13), protein (P = 0.73), or carbohydrate intake (P = 0.43).
Leptin, Free Leptin, and Antileptin Antibody Levels.
In the metreleptin-treated group, the serum total leptin level increased significantly after 4 wk of metreleptin administration (25.99 ± 5.43 ng/mL compared with 3.54 ± 0.59 ng/mL for placebo; P < 0.0001) and continued to rise throughout the study (Fig. 2A). At 12 wk, the treatment dose of four participants in the metreleptin-treated group was increased to 0.12 mg/kg of metreleptin because of lack of menstruation; two of these participants (one at 24 wk, one at 28 wk) required readjustment of the doses back down to 0.08 mg/kg because of weight loss (Table 2). Two additional participants required decreases in metreleptin dosing to 0.04 mg/kg because of weight loss at 16 wk; one of these participants had her dose increased back to 0.08 mg/kg at week 32. Of the six participants in the metreleptin group who completed the study at a dose of 0.08 mg/kg, the mean leptin level was 69.67 ± 20.45 ng/mL at week 36. One participant completed the study at a dose of 0.12 mg/kg with a leptin level of 58.38 ng/mL. The mean leptin level in the metreleptin-treated group decreased 16 wk after discontinuation of metreleptin to 8.64 ± 3.92 ng/mL at week 52, a level that was not significantly different from the placebo-treated group (P = 0.054). The control group, as expected, did not have any significant changes in leptin levels throughout the study duration (4.10 ± 0.64 ng/mL at baseline compared with 3.09 ± 0.51 ng/mL at week 36; P = 0.54). Similarly, free leptin levels increased significantly over the study duration in the metreleptin-treated group, starting at 4.75 ± 1.24 ng/mL and reaching a peak of 49.04 ± 13.78 ng/mL at week 36, whereas the free leptin levels remained stable in the control group, starting at 3.77 ± 0.62 ng/mL and ending at 2.75 ± 0.40 ng/mL at week 36 (P < 0.0001) (Fig. 2B). Antileptin antibody levels were assessed in eight of 10 metreleptin-treated subjects and seven of nine placebo-treated subjects because of serum availability. Seven of eight metreleptin-treated subjects developed antileptin antibodies starting at the first check point at week 12. The antibody levels decreased slightly or were maintained at similar levels until the completion of treatment. None of the placebo-treated subjects developed antileptin antibodies.
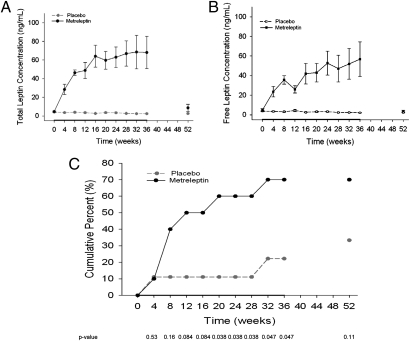
Fig. 2.
Total and free leptin levels and restoration of menses. (A) Total leptin levels during the 36 wk of treatment and at the week 52 follow-up visit. The solid black line represents mean total leptin levels in the metreleptin-treated group; the gray dashed line represents mean total leptin levels in the placebo-treated group. The serum total leptin level increased significantly in the metreleptin-treated group, compared with the placebo-treated group (P < 0.0001). The leptin level decreased quickly with discontinuation of metreleptin and was not significantly different from that in the placebo-treated group at the week 52 follow-up visit (P = 0.0536). The control group, as expected, did not have any significant changes in leptin levels throughout the study duration (P = 0.5395). (B) Free leptin levels during the 36 wk of treatment and at the week 52 follow-up visit. The solid black line represents the mean free leptin levels in the metreleptin-treated group; the black dashed line represents the mean free leptin levels in the placebo-treated group. Similar to total leptin levels, free leptin levels increased significantly over the study duration in the metreleptin-treated group but remained stable in the placebo-treated group (P < 0.0001). (C) The cumulative percentage of women in which menstruation occurred. The black solid line represents the metreleptin-treated group; the gray dashed line represents the placebo-treated group. Over time, significantly more participants in the metreleptin group than in the placebo group regained menses (P = 0.0046). The table reports P values at every time point using Fisher's exact test.
Menstruation and Fertility.
Seven of 10 subjects receiving metreleptin therapy developed menstruation during the course of the study, and two of nine subjects on placebo developed menstruation (P = 0.0046) (Fig. 2C and Table 2 and Table S3). Menstruation appeared at various stages of metreleptin therapy, ranging from 4 to 32 wk after initiation of treatment, and on average occurred earlier in subjects treated with metreleptin. All subjects who menstruated had irregular but sustained cycles, missing up to a total of three cycles after resumption and no more than 37.5% of the total duration of menstruation. Menstruating subjects had progesterone levels measured at day 21 of their cycles. Four of the menstruating subjects in the metreleptin arm were determined to be ovulatory, as defined by serum progesterone >10 ng/mL at the midluteal phase (18). One subject who resumed menses after 8 wk of metreleptin therapy and continued to have regular menstrual cycles became pregnant at week 24. Of the five metreleptin-treated subjects who regained menses and completed the study, three continued to have menses until the week 52 follow-up visit. Among the two participants who developed menses on placebo therapy, one had menses once during the first 4 wk of treatment, and the other had regular menses starting at week 32. One additional placebo-treated subject had menses at the week 52 follow-up visit.
Hormone Levels.
Estradiol and progesterone levels increased significantly in the participants treated with metreleptin as compared with the participants treated with placebo (P = 0.0137 and P = 0.0342, respectively, by treatment effect) (Table 1). The LH and FSH levels did not differ between the two groups (P = 0.40 and P = 0.70, respectively) (Table S2). There was no significant difference in inhibin B levels between the two groups (P = 0.27) (Table S2). Finally, testosterone levels did not differ between the metreleptin- and placebo-treated groups (P = 0.88) (Table S2). Thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), and free thyroxine (fT4) levels were normal at baseline and did not differ between the metreleptin- and placebo-treated groups (P = 0.71, P = 0.57, and P = 0.14, respectively). As compared with the placebo-treated group, fT3 increased significantly in the metreleptin-treated group over time (P = 0.02) (Table S2). There were no significant changes in fT4 or TSH throughout the study duration between the groups (P = 0.95 and P = 0.70, respectively) (Table S2). There was no significant difference between the metreleptin- and placebo-treated groups in cortisol levels at baseline (P = 0.61). The placebo-treated subjects had stable cortisol levels during the study compared with the metreleptin-treated subjects, who had a significant decline in cortisol levels (P = 0.019 by treatment effect) (Table 1). The mean cortisol level of the metreleptin-treated group increased slightly at the week 52 follow-up visit (16 wk after discontinuation of metreleptin); this level was not significantly different from the mean cortisol level of the placebo group at week 52 (P = 0.17). Spot free urinary cortisol:creatinine ratios were not significantly different between the placebo- and metreleptin- treated groups (P = 0.95) (Table S2). The ratio of IGF1:IGF binding protein 3 (IGF1:IGFBP-3) was significantly higher in the treatment group than in the placebo group over the study duration (P = 0.035), but there was only a borderline difference in IGF1 levels and no difference in IGFBP-3 levels (P = 0.08 and P = 0.81, respectively) (Table 1 and Table S2). There was no significant difference between the two groups in prolactin levels over the 36-wk period (P = 0.56) (Table S2).
Bone Metabolism.
There was no significant difference between the treatment groups in BMD at the lumbar spine (P = 0.97), hip (P = 0.51), radius (P = 0.76), or in total (P = 0.34) over 36 wk. The changes in biochemical markers for bone metabolism are summarized in Table 1 and Table S2. Levels of osteocalcin, a marker of bone formation, increased quickly with metreleptin treatment by week 4 and remained significantly elevated compared with the placebo treatment during the 36-wk study (P = 0.0019 by treatment effect). Levels of osteocalcin returned to baseline at the 52-wk follow-up visit. There were no significant differences between the two groups in levels of bone-specific alkaline phosphatase (BSAP), another marker of bone formation, or osteoprotegerin, an osteoclastogenesis inhibitory factor, over the study duration (P = 0.29 and P = 0.17, respectively). With respect to the markers of bone resorption, there was a higher increase in the urinary N-telopeptides of type 1 collagen (NTX):creatinine ratio in the placebo group than in the metreleptin group (P = 0.0282 by treatment effect). There was no significant difference between the two groups in C-telopeptides of type 1 collagen (CTX) levels over the study duration (P = 0.39).
Safety of Metreleptin Therapy.
One subject developed local injection-site reactions with erythematous rashes within a few weeks after starting metreleptin. She withdrew from the study, and the symptoms resolved spontaneously within 1 wk. Another subject receiving metreleptin therapy had persistent weight loss (more than 8% from her baseline weight) and withdrew from the study. Antileptin antibodies were determined to be nonneutralizing antibodies. No other clinically significant adverse effects related to the study medication or procedures were observed.
Discussion
In this randomized, double-blinded, placebo-controlled, 36-wk treatment study, administration of metreleptin to correct leptin deficiency in women with HA resulted in restoration of menses (>50% ovulatory); increases in estradiol, progesterone, and fT3 levels; decrease in cortisol level; increases in IGF1:IGFBP-3 ratio and osteocalcin level; and stabilization of urinary NTX:creatinine ratio. These results suggest that hypoleptinemia contributes significantly to the reproductive, neuroendocrine, and bone abnormalities associated with HA.
Although leptin first was discovered as an antiobesity hormone (19, 20), soon thereafter it was recognized as a hormonal mediator of adaptation to energy deprivation. Studies have shown that short-term starvation of mice (11) and humans (12, 21, 22) results in hypoleptinemia and alterations in the reproductive, thyroid, and growth hormone axes, which are normalized with exogenous administration of leptin. Women with HA are chronically energy-deprived and have both hypoleptinemia and similar neuroendocrine abnormalities. It thus would be reasonable to hypothesize that these abnormalities can be treated with metreleptin administration in replacement doses. Herein, we expand and extend our pilot data from our 3-mo, open-label, interventional study on leptin replacement in women with HA (17) and demonstrate metreleptin's ability to correct these abnormalities. In this study, daily s.c. injection of replacement doses of metreleptin resulted in significantly elevated levels of leptin within the first month of treatment. Furthermore, we show that, despite the development of antileptin antibodies, free leptin levels increased and were maintained throughout the study duration. The transient presence of antileptin antibodies also has been noted in the past in treatment of children with congenital leptin deficiency (23, 24) and adults with obesity (25).
In our study, metreleptin administration over 36 wk resulted in significantly more participants recovering menstruation compared with placebo. Study participants had had amenorrhea for a mean duration of 4–5 y. As might be expected on the basis of the known recovery rate of women with HA, two placebo-treated subjects had menses during the 36-wk study period, only one of which was regular. This response rate in the placebo group and the time frame over which it occurred provide context for interpreting the response rate in the metreleptin group. More than 50% of the seven metreleptin-treated subjects who resumed menses were found to have ovulatory cycles through determination of progesterone levels on day 21 of the menstrual cycle. Notably, one metreleptin-treated subject became pregnant despite required and reported use of barrier contraception. All subjects were instructed to use barrier methods and/or abstain from sexual intercourse to avoid pregnancy during treatment with the investigational medication. Five of the seven metreleptin-treated subjects who developed menses did so within 12 wk of beginning treatment. This finding is consistent with our previous pilot study in which ovulatory cycles and/or dominant follicles with withdrawal bleeding occurred in the majority of treated subjects over a similar time frame. Notably, two subjects developed menses later in the course of metreleptin treatment, suggesting that a lag in response may occur in some subjects. Alternatively, these later events (especially the menses occurring at 32 wk) could represent a lack of response to metreleptin and the natural history of the condition with spontaneous menstrual cycles. Subjects were instructed to maintain their exercise patterns and eating habits as stably as possible over the course of the study. The possibility remains that lifestyle changes could have contributed to restoration of menstrual cycles in some subjects, but we controlled for these potential confounders to the best of our abilities in this randomized and blinded study.
In addition, we demonstrated that there have been significant increases in estradiol and progesterone levels with metreleptin replacement. No changes in LH levels were found in this study, contrary to the previous one, which assessed LH pulsatility based on frequent overnight blood sampling. The blood specimens collected in the present study were not timed with respect to menstrual cycles, and, given the pulsatile nature of LH and FSH secretion, the measured levels may not reflect accurately changes in mean levels and/or pulsatility. Treatment with metreleptin also has improved reproductive function in other leptin-deficient conditions, including congenital leptin deficiency (23) and lipodystrophy (26), in uncontrolled pilot studies.
Chronic energy deficiency in women with HA also is associated with other neuroendocrine abnormalities, including decreased thyroid hormone, increased growth hormone, decreased IGF1, and increased cortisol levels (2–7). A significant increase in fT3, although not fT4, levels, as well as in the IGF1:IGFBP-3 ratio in the metreleptin-treated group as compared with the placebo group was observed in this study. Finally, we show that, compared with placebo, metreleptin administration results in significantly lower levels of cortisol in women with HA. Given the pulsatile nature of cortisol levels, this result will need to be assessed further with measures of adrenocorticotropic hormone (ACTH) pulsatility and free cortisol by 24-h urine collection in future studies. In addition to confirming an increase in osteocalcin, a marker of bone formation, with metreleptin treatment as seen in our previous pilot study (17), we observed a stabilization in urinary NTX:creatinine ratio, a measure of bone resorption. These findings suggest that continued use of metreleptin over a longer time frame may have beneficial effects on bone metabolism. Markers of bone formation are significantly reduced in mature adolescents with anorexia nervosa (27, 28), and high baseline osteocalcin has been shown to predict subsequent increases in lumbar bone mineral content of bone area (29). Although no significant changes in BMD were seen in our study, a 36-wk period probably is not long enough for a potential change to be observed. An ongoing, open-label extension of this study will assess the effect of up to 2 y of metreleptin administration on BMD.
Leptin may improve BMD through several mechanisms, either indirectly via effects on neuroendocrine axes (e.g., increases in estradiol and IGF1 and decrease in cortisol, as shown herein) and/or via direct effects on bone. Recently, Lawson et al. (30) showed that women with HA had hypercortisolemia, and cortisol levels were negatively associated with BMD at the anterior–posterior spine, lateral spine, and hip. Alterations in the growth hormone axis, particularly low IGF1 concentrations, also have been proposed to contribute to bone loss associated with anorexia nervosa (27, 28, 31, 32). Dysfunction of the cortisol and growth hormone axes in addition to the gonadal axis may explain the inconsistent efficacy of estrogen replacement in restoring BMD in women with HA (33).
Leptin also has several direct effects on bone metabolism. Circulating leptin acts on bone marrow stromal cells, directing them toward an osteogenic rather than adipogenic pathway in mice (34–36). Studies have revealed that leptin treatment directly increases osteoblast proliferation and decreases osteoclastogenesis both in vitro and in vivo (37, 38). The extent to which changes in hormone levels and/or the direct effects of leptin contribute to the increase in markers of bone formation remain to be determined. Moreover, it is reasonable to assume that chronic metreleptin treatment over longer periods of time may improve BMD, given metreleptin's beneficial effects in restoring all these neuroendocrine axes. This effect remains to be demonstrated.
Although weight and BMI were controlled for by adjusting the treatment doses, the metreleptin-treated group had a significant loss of body fat in the beginning of the study, before treatment dose adjustments. This finding also has been observed in studies assessing the effect of metreleptin on metabolic parameters in humans with severe lipodystrophy and other conditions associated with relative leptin deficiency (39–41). We questioned whether the loss of body fat was caused by increased energy expenditure or decreased fat intake. Given a mean loss of 2.02 kg of fat over 36 wk, we would expect either an increase in energy expenditure or a decrease in fat intake of 72 calories per day. It also is possible that there is a direct lipolytic effect of metreleptin on adipose tissue, and this possibility needs to be explored in the future further. There was no change in resting energy expenditure in the metreleptin-treated group compared with the placebo-treated group over the course of the study. However, this study measured only resting rather than total energy expenditure, which would have provided a more thorough assessment of energy expenditure. Likewise, 3-d food diaries may not be sensitive enough to distinguish reliably changes in food intake on this order of magnitude.
Thus, the measures of energy expenditure and food intake used in this study proved to be limited, but the required level of sensitivity was not known beforehand. A 24-h measure of energy expenditure by direct calorimetry would provide a more accurate representation, but this method would not be easy to implement in a long-term, randomized study. Another theoretical limitation of this study is the withdrawal rate. An early withdrawal rate of 10% by week 20 and 35% by week 36 might have been expected, based on the intensity of the intervention and the level of commitment required to complete the study. Also, weight loss presents a potential concern in a lean to normal-weight population that already may have less body fat than controls of similar body weight (42); however, this weight loss appears to be a dose-related phenomenon that can be limited, given appropriate monitoring and dose adjusting. Determining an effective dose that has beneficial effects on reproductive parameters without inducing excessive weight loss is warranted, and the results herein provide evidence that such dosing is feasible and likely needs to be individualized.
In summary, this randomized, double-blinded, placebo-controlled study demonstrates that hypoleptinemia underlies the dysfunction of neuroendocrine axes and bone metabolism associated with HA. Treatment with metreleptin in physiologic doses may be a safe, effective therapeutic option. Aside from attempting to decrease exercise and/or increase food intake and body weight, the standard of treatment for HA is estrogen, which does not adequately address infertility, other associated neuroendocrine abnormalities, or bone loss. The neuroendocrine normalization observed in the metreleptin-treated group compared with placebo suggests a therapeutic role of this hormone with an onset of action much earlier than could be achieved with lifestyle changes alone. Longer studies are in process to determine the effect of metreleptin therapy on BMD, and larger studies are needed to determine the safety and efficacy of metreleptin as a treatment for this condition.
Materials and Methods
Subjects, study design, biochemical analysis, and other methods are described in SI Materials and Methods. In brief, eligible subjects were women between 18 and 35 y old with secondary HA for at least 6 mo coincident with a period of strenuous exercise and/or low body weight. The participants were assigned randomly in a 1:1 ratio to receive either metreleptin or placebo.
Supplementary Material
Acknowledgments
Amylin Pharmaceuticals, Inc., supplied metreleptin for this study. We thank Dr. K. Aronis for contributions in the laboratory. We also thank Dr. J. Chan for her role in the initial phases of this trial. This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK58785, DK79929, DK81913, and AG032030. The C.S.M. laboratory also is supported by a discretionary grant from Beth Israel Deaconess Medical Center and a small grant from Amylin Pharmaceuticals, Inc., administered through Beth Israel Deaconess Medical Center. Support also was received from Grants UL1 RR025758 and M01-RR-01032 (to Harvard Clinical and Translational Science Center) from the National Center for Research Resources. G.M. was supported by grants from the European Union Ideas Programme, European Research Council Starting Independent Grant LeptinMS 202579, and Telethon-Juvenile Diabetes Research Foundation Grant GJT08004.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015674108/-/DCSupplemental.
References
- 1.Reindollar RH, Novak M, Tho SP, McDonough PG. Adult-onset amenorrhea: A study of 262 patients. Am J Obstet Gynecol. 1986;155:531–543. doi: 10.1016/0002-9378(86)90274-7. [DOI] [PubMed] [Google Scholar]
- 2.Yen SS. Female hypogonadotropic hypogonadism. Hypothalamic amenorrhea syndrome. Endocrinol Metab Clin North Am. 1993;22:29–58. [PubMed] [Google Scholar]
- 3.Warren MP, et al. Functional hypothalamic amenorrhea: Hypoleptinemia and disordered eating. J Clin Endocrinol Metab. 1999;84:873–877. doi: 10.1210/jcem.84.3.5551. [DOI] [PubMed] [Google Scholar]
- 4.Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83:25–32. doi: 10.1210/jcem.83.1.4502. [DOI] [PubMed] [Google Scholar]
- 5.Misra M, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–5623. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- 6.Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril. 1997;67:1024–1030. doi: 10.1016/s0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- 7.Genazzani AD, et al. Increased adrenal steroid secretion in response to CRF in women with hypothalamic amenorrhea. J Steroid Biochem Mol Biol. 2001;78:247–252. doi: 10.1016/s0960-0760(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 8.Grinspoon S, et al. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab. 1999;84:2049–2055. doi: 10.1210/jcem.84.6.5792. [DOI] [PubMed] [Google Scholar]
- 9.Biller BM, et al. Osteopenia in women with hypothalamic amenorrhea: A prospective study. Obstet Gynecol. 1991;78:996–1001. [PubMed] [Google Scholar]
- 10.Mantzoros CS. Role of leptin in reproduction. Ann N Y Acad Sci. 2000;900:174–183. doi: 10.1111/j.1749-6632.2000.tb06228.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 12.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audi L, et al. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry. 1998;3:544–547. doi: 10.1038/sj.mp.4000418. [DOI] [PubMed] [Google Scholar]
- 14.Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased serum leptin in bulimia nervosa. J Clin Endocrinol Metab. 2000;85:4511–4514. doi: 10.1210/jcem.85.12.7051. [DOI] [PubMed] [Google Scholar]
- 15.Mantzoros C, Flier JS, Lesem MD, Brewerton TD, Jimerson DC. Cerebrospinal fluid leptin in anorexia nervosa: Correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab. 1997;82:1845–1851. doi: 10.1210/jcem.82.6.4006. [DOI] [PubMed] [Google Scholar]
- 16.Miller KK, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: The effects of body composition and nutritional intake. J Clin Endocrinol Metab. 1998;83:2309–2312. doi: 10.1210/jcem.83.7.4975. [DOI] [PubMed] [Google Scholar]
- 17.Welt CK, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 18.Hull MG, Savage PE, Bromham DR, Ismail AA, Morris AF. The value of a single serum progesterone measurement in the midluteal phase as a criterion of a potentially fertile cycle (“ovulation”) derived form treated and untreated conception cycles. Fertil Steril. 1982;37:355–360. doi: 10.1016/s0015-0282(16)46095-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 20.Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 21.Chan JL, et al. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-I in leptin deficiency states. J Clin Endocrinol Metab. 2008;93:2819–2827. doi: 10.1210/jc.2008-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schurgin S, Canavan B, Koutkia P, Depaoli AM, Grinspoon S. Endocrine and metabolic effects of physiologic r-metHuLeptin administration during acute caloric deprivation in normal-weight women. J Clin Endocrinol Metab. 2004;89:5402–5409. doi: 10.1210/jc.2004-1102. [DOI] [PubMed] [Google Scholar]
- 23.Farooqi IS, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 24.Farooqi IS, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heymsfield SB, et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 26.Musso C, et al. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism. 2005;54:255–263. doi: 10.1016/j.metabol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–4496. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 28.Grinspoon S, et al. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3864–3870. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- 29.Misra M, et al. Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. J Clin Endocrinol Metab. 2008;93:1292–1297. doi: 10.1210/jc.2007-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson EA, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–4716. doi: 10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB., Jr The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75:762–767. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- 32.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- 33.Vescovi JD, Jamal SA, De Souza MJ. Strategies to reverse bone loss in women with functional hypothalamic amenorrhea: A systematic review of the literature. Osteoporos Int. 2008;19:465–478. doi: 10.1007/s00198-007-0518-6. [DOI] [PubMed] [Google Scholar]
- 34.Thomas T, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 35.Hamrick MW, et al. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 36.Hamrick MW, et al. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res. 2007;327:133–141. doi: 10.1007/s00441-006-0312-3. [DOI] [PubMed] [Google Scholar]
- 37.Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 38.Holloway WR, et al. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 39.Mulligan K, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94:1137–1144. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91:2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 41.Javor ED, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 42.Frisch RE, et al. Magnetic resonance imaging of overall and regional body fat, estrogen metabolism, and ovulation of athletes compared to controls. J Clin Endocrinol Metab. 1993;77:471–477. doi: 10.1210/jcem.77.2.8345054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.