The endocannabinoid system (ECS) includes cannabinoid (CB1 and CB2) receptors and their endogenous ligands (i.e., the endocannabinoids anandamide and 2-arachydonylglycerol) as well as proteins involved in endocannabinoids biosynthesis and degradation (1). The ECS is present in the liver and undergoes adaptive changes in response to noxious stimuli. Endocannabinoids as well as CB1 and CB2 receptors (which are, respectively, either faintly or not expressed in normal livers) are up-regulated in experimental liver injury and liver cirrhosis of various etiologies. In vivo, CB1 receptor activation promotes fat accumulation, triggers inflammation in nonalcoholic and alcoholic fatty liver diseases, contributes to the progression of chronic hepatitis to cirrhosis by stimulating fibrogenesis, and is also implicated in hemodynamic and neurological consequences associated with liver cirrhosis, including portal hypertension, encephalopathy, and cardiomyopathy (2–4). Conversely, activation of CB2 receptors exerts antifibrogenic and antiinflammatory effects in experimental models of liver disease (5). These pharmacological effects make CB2 agonists and CB1 antagonists promising candidates for the treatment of fibrosis in chronic liver pathologies. By disclosing a prominent CB1-mediated role of anandamide in the early phase of liver regeneration, Mukhopadhyay et al. (6) provide additional and significant support to the prominent role of the ECS in liver biology in an article in PNAS.
The liver is unique in that it has the unlimited capacity to regenerate in response to surgical removal as well as chemical or viral insults (7). This fascinating regenerative aspect invariably recalls the ancient legend of Prometheus, the Titan who stole the secret of fire from Zeus and passed it to humans. As a punishment, Prometheus was chained to a rock, and a giant bird ate his liver every day; every night, his liver regenerated. Liver regeneration depends on the concerted action of multiple growth factors, cytokines, and hormones that induce quiescent hepatocytes and other cells (biliary ductular, Kupffer, stellate, and endothelial cells) to replicate to restore liver mass (8). Mukhopadhyay et al. (6) studied the involvement of the ECS in liver regeneration by performing a mouse surgical procedure that removes two of three liver masses, a well-established technique developed in the rat in 1931 (9). In contrast to other experimental models involving necrosis of lobular zones induced by toxins, partial hepatectomy is not associated with massive necrosis, and thus, regeneration of the residual lobes is mediated by processes relevant only to liver tissues and not to necrosis or acute inflammation (8). In mice, the peak proliferative response, which can be evaluated by measuring the DNA synthesis, occurs between 36 and 42 h after partial hepatectomy. From 2 d after partial hepatectomy, liver regeneration can be quantified by measurement of liver mass. Seven days after partial hepatectomy, more than 75% of the original liver mass is restored (10).
The prominent role for hepatic CB1 receptor in liver regeneration was suggested by the delayed proliferative response (evaluated by quantifying the proliferating hepatocytes by bromodeoxyuridine staining at 40 h) in CB1 knockout mice, mice lacking the CB1 receptors in hepatocytes only, and WT mice treated with the CB1 antagonist rimonabant. The CB1 receptor was found to be involved in the early proliferative response only (i.e., 40 h after surgery), when the proliferative response is maximal. An increase of tissue weight was indeed observed at 40 h in WT but not CB1-deficient mice, whereas no weight differences between the two strains were observed 6 d after partial hepatectomy. These data suggests that, in the absence of CB1 receptor, hepatic regeneration was only delayed and not fully prevented. This is not surprising, because liver regeneration after partial hepatectomy is a very complex and well-orchestrated phenomenon, associated with signaling cascades involving a redundancy of hepatic mitogens (7). The transient—and not permanent—effect of CB1 activation on liver regeneration should be emphasized in the light of the possible use of CB1 antagonists in liver diseases. Indeed, the benefits of CB1 antagonists in slowing the progression of fibrosis to cirrhosis, attenuating the cardiovascular alteration associated with the advanced stage of the disease, and suppressing of the expression of cell cycle proteins implicated in hepatocellular carcinoma likely counterbalance the transient actions of CB1 antagonists at the early stages of regeneration.
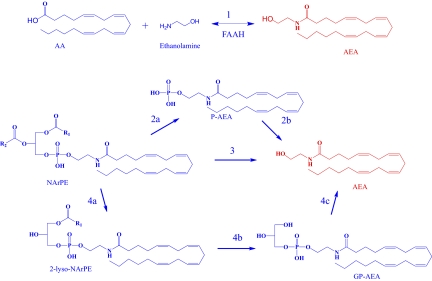
The biosynthesis of anandamide and its congeners occurs through a variety of enzymatic routes (pathways 1–4 in Fig. 1). Although questions remain regarding their relative significance, the one invoked to be operative in the early phase of liver regeneration (pathway 1) has always been thought of as the least likely to be of physiological significance (11). This is so because this reaction requires catalysis by the fatty acid amide hydrolase (FAAH) working in reverse. In vitro, this, in turn, requires very high concentrations of arachidonic acid and ethanolamine to drive the enzyme to anandamide production instead of hydrolysis (12–14). The more natural reaction catalyzed by FAAH (i.e., the hydrolysis of anandamide to yield arachidonoic acid and ethanolamine instead) occurs at more physiological concentrations of substrate and products. Normally, inhibitors of FAAH raise anandamide levels, and this is the basis of human clinical trials for treatment of pain and inflammation. However, after partial hepatectomy in mice, the in vivo levels of ethanolamine and arachidonic acid were increased dramatically, and this was suggested to drive the reaction to anandamide synthesis rather than hydrolysis (pathway 1). This view is bolstered by the finding of Mukhopadhyay et al. (6) that high arachidonic acid concentrations can reverse FAAH's amidase activity and drive its synthase activity in their in vitro assay. The same effect would also have been observed with high ethanolamaine if the radiolabel was in arachidonate rather than in the ethanolamine portion of anandamide. The nomenclature anandamide amidase and anandamide synthase was used historically before it was definitively known that both of these activities are catalyzed by the same enzyme (i.e., FAAH) (12). Consistent with the synthase hypothesis, FAAH null mice or mice treated with an FAAH inhibitor failed to increase hepatic anandamide levels.
Fig. 1.
Pathways for anandamide (AEA) biosynthesis. (i) FAAH catalyzes the condensation of arachidonic acid (AA) and ethanolamine to form anandamide (pathway 1). (ii) A type-C phospholipase hydrolyzes NArPE to phosphoanandamide (pAEA) (pathway 2a). PTPN22, Src homology 2 domain-containing inositol-5-phosphatase 1, or other uncharacterized phosphatases dephosphorylate pAEA to form AEA (pathway 2b). (iii) The metallo-β lactamase, N-arachidonoyl phosphatidylethanolamine, hydrolyzes NArPE to form AEA through a one-step reaction (pathway 3). (iv) The serine hydrolase, abhd4, sequentially removes acyl groups from NArPE to form lyso-NArPE and then, GP-AEA (pathway 4 a and b). The metal-dependant phosphodiesterase, GDE1, hydrolyzes GP-AEA to form AEA (pathway 4c). Details in Ueda and Tsuboi (11) and Simon and Cravatt (14). Anandamide is highlighted in red.
These results are very exciting in that they show that anandamide can be synthesized by FAAH in vivo when high concentrations of arachidonic acid and ethanolamine are present. Clearly, such a condition does not seem to occur very often, because it requires the concomitant overactivation of phospholipase A2 and phospholipase D enzymes, necessary to produce arachidonic acid and ethanolamine, respectively. Indeed, Mukhopadhyay et al. (6) show that phospholipase D activity is increased considerably by partial hepatectomy and that this effect might depend on CB1 activation. This raises the intriguing possibility that, through this FAAH-mediated pathway for anandamide biosynthesis, CB1 may increase its own activity in a positive feedback loop that is potentially useful when hyperactivation of the receptor is necessary, such as during liver regeneration.
What about the CB2 receptor? Experimental data suggest that the targeting of the CB2 receptor represents a promising strategy for circumventing the unwanted consequences of CB1 receptor activation (15). As already mentioned, CB2 receptors are undetectable in normal liver but can be induced in hepatic cells such as hepatocytes, cholangiocytes, myofibroblasts, and stellate cells under pathophysiological states (e.g., steatosis, cirrhosis, and nonalcoholic fatty liver disease) (2–4). The CB2 receptor has been identified as a possible target for the management of liver diseases based on the ability of CB2 agonists to reduce experimental fibrosis and exert curative effects in cirrhotic rats (5). More importantly, in the competing processes linked to liver regeneration, a recent study showed induction of CB2 mRNA 24–72 h after partial hepatectomy as well as a delayed regenerative response in CB2-deficient mice (16). Such data, together with the results by Mukhopadhyay et al. (6), suggest that anandamide—newly biosynthesised through an in vivo pathway involving conjugation of arachidonic acid and ethanolamine by FAAH —promotes liver regeneration after partial hepatectomy by activation both CB1 and CB2 receptors (Fig. 2).
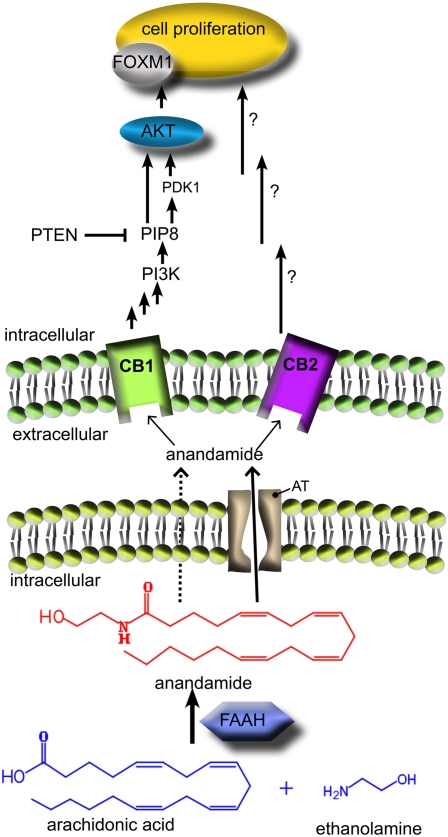
Fig. 2.
Partial hepatectomy stimulates anandamide production through a pathway involving conjugation of arachidonic acid and ethanolamine by FAAH. The generated anandamide, which may cross the cellular membrane either by passive diffusion (dotted arrow) or through a specific transporter [putative anandamide transporter (AT), solid arrow], promotes hepatocytes regeneration through activation of both CB1 (as shown by Mukhopadhyay et al.) (6) and CB2 (as shown by Teixeira-Clerc et al.) (16). After CB1 receptor activation, anandamide induces the forkhead-box M1 (FoxM1) transcriptor factor, which is involved in mitotic progression. The CB1-mediated induction of FoxM1 involves the Akt/PTEN (protein kinase B/phosphatase and tensin homolog deleted on chromosome 10) signaling pathway. The intracellular mechanisms associated with CB2-stimulated cell proliferation have been not studied to date. Note that anandamide could stimulate liver regeneration as a paracrine (as shown in this figure) and/or an autocrine lipid mediator. GP-AEA, glycerophospho-anandamide; NArPE, N-arachidonoyl phosphatidylethanolamine.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6323 in issue 15 of volume 108.
References
- 1.Di Marzo V. Targeting the endocannabinoid system: To enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 2.Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut. 2008;57:1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- 3.Caraceni P, Domenicali M, Giannone F, Bernardi M. The role of the endocannabinoid system in liver diseases. Best Pract Res Clin Endocrinol Metab. 2009;23:65–77. doi: 10.1016/j.beem.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Tam J, et al. Endocannabinoids in liver disease. Hepatology. 2011;53:346–355. doi: 10.1002/hep.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lotersztajn S, et al. CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol. 2008;153:286–289. doi: 10.1038/sj.bjp.0707511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay B, et al. Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc Natl Acad Sci USA. 2011;108:6323–6328. doi: 10.1073/pnas.1017689108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol (Chic) 1931;12:186–202. [Google Scholar]
- 10.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 11.Ueda N, Tsuboi K, Uyama T. Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim Biophys Acta. 2010;1801:1274–1285. doi: 10.1016/j.bbalip.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Arreaza G, et al. The cloned rat hydrolytic enzyme responsible for the breakdown of anandamide also catalyzes its formation via the condensation of arachidonic acid and ethanolamine. Neurosci Lett. 1997;234:59–62. doi: 10.1016/s0304-3940(97)00673-3. [DOI] [PubMed] [Google Scholar]
- 13.Katayama K, Ueda N, Katoh I, Yamamoto S. Equilibrium in the hydrolysis and synthesis of cannabimimetic anandamide demonstrated by a purified enzyme. Biochim Biophys Acta. 1999;1440:205–214. doi: 10.1016/s1388-1981(99)00124-9. [DOI] [PubMed] [Google Scholar]
- 14.Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teixeira-Clerc F, et al. Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology. 2010;52:1046–1059. doi: 10.1002/hep.23779. [DOI] [PMC free article] [PubMed] [Google Scholar]