Abstract
Among the pathogenic processes contributing to dopaminergic neuron (DN) death in Parkinson disease (PD), evidence points to non–cell-autonomous mechanisms, particularly chronic inflammation mounted by activated microglia. Yet little is known about endogenous regulatory processes that determine microglial actions in pathological states. We examined the role of glucocorticoid receptors (GRs), activated by glucocorticoids released in response to stress and known to regulate inflammation, in DN survival. Overall GR level was decreased in substantia nigra of PD patients and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-intoxicated mice. GR changes, specifically in the microglia after MPTP treatment, revealed a rapid augmentation in the number of microglia displaying nuclear localization of GR. Mice with selective inactivation of the GR gene in macrophages/microglia (GRLysMCre) but not in DNs (GRDATCre) showed increased loss of DNs after MPTP intoxication. This DN loss in GRLysMCre mice was not prevented by corticosterone treatment, in contrast to the protection observed in control littermates. Moreover, absence of microglial GRs augmented microglial reactivity and led to their persistent activation. Analysis of inflammatory genes revealed an up-regulation of Toll-like receptors (TLRs) by MPTP treatment, particularly TLR9, the level of which was high in postmortem parkinsonian brains. The regulatory control of GR was reflected by higher expression of proinflammatory genes (e.g., TNF-α) with a concomitant decrease in anti-inflammatory genes (e.g., IL-1R2) in GRLysMCre mice. Indeed, in GRLysMCre mice, alterations in phosphorylated NF-κB levels indicated its protracted activation. Together, our data indicate that GR is important in curtailing microglial reactivity, and its deregulation in PD could lead to sustained inflammation-mediated DN injury.
A major hallmark of Parkinson disease (PD) is the loss of dopaminergic neurons (DNs) of the substantia nigra (SN), which results in severe depletion of striatal dopamine (DA) levels with ensuing cardinal motor symptoms, including resting tremor, rigidity, and bradykinesia (1). The etiology of the sporadic form of PD that accounts for the majority of cases remains unknown. Substantial evidence indicates that among the pathogenic mechanisms conducive to degeneration of DNs is an ongoing chronic inflammatory response mounted by activated microglia and astroglia as well as infiltrating peripheral T cells (2–4). Activated microglia can contribute to neuronal toxicity through secretion of proinflammatory mediators that can increase oxidative stress, directly trigger neuronal cell-death mechanisms, or act to amplify the inflammatory response. In PD, the experimental evidence for the role of microglia includes postmortem and PET studies that revealed the presence of reactive microglia both in the striatum and in the SN (5), an elevated expression of proinflammatory molecules such as TNF-α, IL-1β, and IFN-γ, inducible nitric oxide synthase (iNOS), and cyclooxygenase 2 (COX-2) in the affected regions. The deleterious role of these inflammatory mediators on DN survival has been demonstrated in various experimental animal and non-human primate models of PD as well as in vitro mesencephalic cell culture studies using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication that selectively destroys DNs (ref. 6 and refs. therein).
The mechanisms that trigger microglial activation and, more importantly, maintain it in a chronically activated state throughout disease progression in PD are poorly known. However, it can be postulated that the extent of DN injury inflicted by a chronic inflammatory reaction is likely to be determined by factors such as the intensity of the immune response as well as the activation of the mechanisms that can resolve it. One endogenous mechanism that is stimulated to restrict and terminate an inflammatory reaction is an activation of the hypothalamic–pituitary–adrenal (HPA) axis that results in a rise in the systemic level of glucocorticoids (GCs), which are produced and released by adrenal glands (7). As well as being released in response to an inflammatory reaction, GCs are released as a response to stress, and, in both cases, they exert their actions mostly through ubiquitously expressed type II glucocorticoid receptors (GRs) (8).
GC-GR responses to neuronal injury are complex, for instance, an excess of GCs (such as occurs in chronic stress) was found to exacerbate neuronal injury in experimental ischemia (9), while an important neuronal survival effect of GR was demonstrated in an acute lipopolysaccharide (LPS)-induced inflammatory lesion model (10). Several reports have suggested that the GC-GR responses might be crucially linked to PD pathogenesis. Thus, chronically high levels of GCs were shown to exacerbate motor deficits in 6-hydroxy-DA–treated rats (11). This experimental observation corroborates clinical data showing that stress can trigger or worsen motor symptoms in PD patients (12). The involvement of GC-GR in DN survival after MPTP intoxication was suggested previously in adrenalectomized mice (13) as well as in transgenic mice harboring antisense GR (14). In these experimental approaches, GR activity is compromised in most tissues and therefore do not allow a precise dissection of the molecular actions of GR in cell types involved in PD pathogenesis. Moreover, it remains to be established whether GC-GR activity is altered in PD patients.
In this study, we examined the role of GR in PD both by analyzing the levels of GCs and GR in parkinsonian patients and by using MPTP treatment paradigms in mice that were generated for selective GR gene inactivation either in macrophages/microglia or in DNs by Cre/loxP technology. Our results show that GR is modulated in PD and highlight the crucial regulatory actions of GR in microglia for DN survival.
Results
GR and Cortisol Levels Are Modulated in PD and in MPTP-Intoxicated Mice.
The level of GR mRNA analyzed by quantitative PCR (qPCR) was lower in SN of parkinsonian patients compared with controls (P = 0.012) and, as expected, a reduction in tyrosine hydroxylase (TH) mRNA (P = 0.014) was also found (Fig. 1A). By contrast, an increase, not statistically significant (P = 0.14), in GR mRNA was observed in the putamen of parkinsonian patients (Fig. 1B). Because of the greater availability of striatal postmortem tissue, we were able to analyze GR protein levels in the putamen of parkinsonian patients and control subjects. An increase in GR protein level was found (P = 0.049) (Fig. 1C). To examine whether GC levels are modulated in PD, we measured plasma cortisol levels in nine healthy controls and 20 PD patients. Consistent with a previous report (15), our single–time point analysis revealed a twofold higher cortisol level in the PD group (Fig. 1D, P = 0.00016). These alterations in cortisol levels do not correlate with disease duration (P = 0.517, correlation coefficient r = −0.154) or the Unified Parkinson's Disease Rating Scale (UPDRS) score indicative of the severity of motor dysfunction (P = 0.652, r = −0.107; nonparametric Spearman test) (Table S1). Altogether, data from human patients suggest that GC-GR responses are most likely altered during PD.
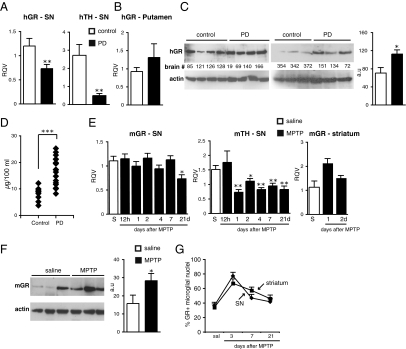
Fig. 1.
GR expression in SN and striatum in PD and in MPTP-treated mice. RT-qPCR of human (h) GR and TH mRNA from SN (A) and putamen (B) of control and PD (n = 5–6). (C) Western blot analysis of human striatal GR protein of two groups of PD and control subjects. The results were quantified with actin as loading control, a.u., arbitrary units. (D) Plasma cortisol levels of control subjects (n = 9) and PD patients (n = 20). (E) Time course of mouse (m) GR and TH mRNA levels in SN after saline (S) or acute MPTP treatment. GR mRNA levels in striatum after saline (S) or 1 or 2 d after MPTP treatment. (F) A representative experiment of Western blot analysis of striatal GR protein levels in mice injected with saline (S) or 7 d after MPTP. (G) Percentage of microglia showing nuclear GR localization quantified from confocal images of GR, Iba-1, and DAPI immunofluorescence in SN and striatum of saline control and MPTP-intoxicated mice killed at indicated days. (n = 3–5 for saline group, and n = 4–5 for each MPTP group.) *P < 0.05, **P ≤ 0.01, ***P < 0.001 (human control subjects vs. PD; saline vs. MPTP-injected mice, Mann-Whitney test).
To ascertain whether GR levels are similarly affected in the well-established MPTP mouse model of PD, RNAs extracted from SN of C57/BL6 mice killed at different time points (12 h to 21 d) after acute MPTP intoxication were analyzed. A decrease in GR mRNA (P = 0.027) was observed at day 21, whereas a diminution in TH mRNA (P = 0.014) was already evident after 1 d (Fig. 1E). Striatal GR mRNA levels showed a tendency to increase after MPTP treatment (P = 0.077) and, in line with striatal PD results, an increase (P = 0.048) in protein level was seen 7 d after MPTP intoxication (Fig. 1 E and F). We next took advantage of this experimental mouse model to examine whether there were intracellular changes occurring in GR, specifically in microglia after MPTP intoxication. Double immunofluorescence labeling of GR and microglial marker Iba-1 was performed on SN and striatal sections from mice either injected with saline or 3, 7, and 21 d after MPTP injections. Although GR labeling was not detected in the cytoplasm of microglia as previously reported (16), it was easily discernible in nuclei. Results of quantification of microglia with nuclear GR staining in SN and striatum revealed a sharp rise 3 d after MPTP treatment that subsequently declined at 7 and 21 d, raising the possibility that nuclear activity of GR is increased in microglia upon MPTP treatment (Fig. 1G). Collectively, our results indicate that MPTP-induced nigrostriatal pathway injury in mouse is associated with significant changes in the GC-GR system. To elucidate precisely the neuronal versus glial role of GR in DN injury, we produced mice by Cre/loxP technology that carried selective inactivation of GR gene either in macrophages (GRLysMCre mice) (17) or in DNs (GRDATCre mice) (18).
Selective Absence of GR in the Microglia of GRLysMCre Mice and Resultant GR Levels After Acute MPTP Treatment.
We first verified the efficiency of Cre recombination and microglial specificity of GR gene inactivation in GRLysMCre mice. Although 80–85% of microglial cells were positive for GR in primary cultures prepared from control GRloxP/loxP newborn pup brain cortices, only 15–20% of these cells expressed GR in cultures from GRLysMCre pups (Fig. S1A). This recombination efficiency is similar to that reported in macrophages (70%) (19). Additionally, Western blot analysis revealed an almost complete absence of GR protein in cultured mutant GRLysMCre microglia (Fig. S1B). Finally, although GR was expressed in both TH+ DNs and Iba1+ microglial cells in the SN of control GRloxP/loxP mice, it was almost completely absent in microglia from GRLysMCre mutants (Fig. S1C).
We examined whether the absence of microglial GR impacts the overall levels of GR after nigrostriatal pathway injury (SI Results) and found they were not overtly altered in GRLysMCre mice.
DN Loss Is Inhibited by Microglial GR and Not by GR in DA Neurons After Acute MPTP Treatment.
The number of TH-immunoreactive (IR) DNs in SN after saline injections in control GRloxP/loxP and GRLysMCre mice was similar. MPTP triggered a loss of TH-IR neurons in SN of GRloxP/loxP controls (P < 0.0001, saline vs. MPTP, post hoc Bonferroni/Dunn test); however, in the GRLysMCre mutants, a greater decrease was observed at all time points (P = 0.001, 3 d; P = 0.0007, 7 d; P = 0.001, 21 d; GRLysMCre mutants vs. controls, post hoc Bonferroni/Dunn test) (Fig. 2A). The analysis of DA nerve terminal parameters in GRLysMCre mutants and controls (SI Results and Fig. S2) showed similar neuropathological aggravation except for delayed diminution in [3H]DA uptake in mutants.
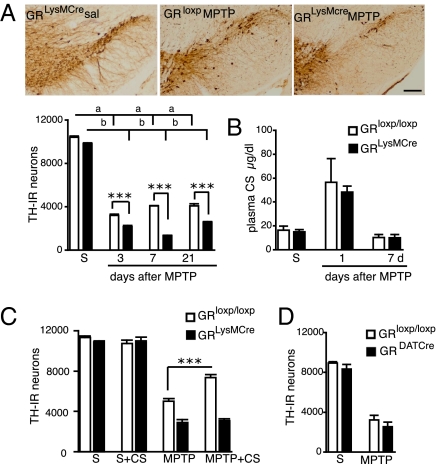
Fig. 2.
Microglial GR protects DNs after acute MPTP intoxication. (A) TH immunohistochemistry in SN of GRLysMCre and GRloxP/loxP mice 7 d after either saline (sal) or MPTP treatment. (Bar = 100 μm.) Quantification of the number of TH-IR neurons in SN shows a significant reduction in GRLysMCre mutants compared with GRloxP/loxP controls after MPTP injections. Two-way ANOVA analysis followed by post hoc Bonferroni/Dunn test showed statistical significance for genotype on MPTP treatment at all time points and significance of P = 0.01 with F = 9.3, degrees of freedom = 1 at 7 d for genotype × treatment. a, b = ***P < 0.001 saline vs. MPTP for GRloxP/loxP and GRLysMCre mice. ***P < 0.001, **P < 0.01 MPTP GRloxP/loxP vs. GRLysMCre MPTP mice. (B) Basal CS levels in saline-injected (S) and MPTP-treated control and mutant mice at times indicated. (C) GRloxP/loxP and GR LysMCre were treated with β-cyclodextrin or CS + β-cyclodextrin for 7 d after saline or acute MPTP intoxication. The results show number of TH-IR neurons in SN. ***P < 0.001. (D) TH-IR cells in SN in control GRloxP/loxP and GR DATCre mice after saline (S) or MPTP. (n = 5–7 mice per group and per time point.)
To rule out the possibility that the increased DN loss in mutants was not a result of an altered HPA axis and thus a difference in GC levels, basal corticosterone (CS) plasma levels were measured 1 and 7 d after MPTP treatment. A three- to fourfold rise in CS level after 1 d of MPTP treatment and a decline to pretreatment level at day 7 was of comparable magnitude in GRLysMCre mutant and GRloxP/loxP control mice (Fig. 2B). Also, differences in MPTP metabolism do not contribute to an increased susceptibility of mutant mice because striatal 1-methyl-4-phenylpyridinium (MPP+) levels were identical between mutants and control animals (Fig. S3A). Finally, the increased DN loss seen in GRLysMCre mutants is unlikely through a direct activating effect of MPP+ on mutant microglia because TNF-α mRNA expression was not induced by treatment of microglial cultures with MPP+, whereas significant induction was found with LPS (Fig. S3B).
To show functionally that GR activation in microglia protects DNs against MPTP intoxication, GRloxP/loxP and GRLysMCre mice were given CS in drinking water starting immediately after the last saline or MPTP injection until their death 7 d later. Quantification of TH-IR neurons in SN showed that CS treatment has a significant protective effect on DNs in GRloxP/loxP but not GRLysMCre mice (Fig. 2C, P < 0.0001, GRloxP/loxP MPTP vs. GRloxP/loxP MPTP + CS mice, post hoc Bonferroni/Dunn test).
DN loss was also analyzed after acute MPTP treatment in mice selectively inactivated for the GR gene in DNs (GRDATCre). The cre recombination and GR inactivation in these mice has been previously reported (18, 20). No significant difference in the number of TH-IR neurons between GRloxP/loxP controls and GRDATCre mice was observed (Fig. 2D).
Overall, our data suggest that CS-stimulated GR nuclear localization and its activity in microglia are critical for maintaining the survival of DNs under PD-like neurodegeneration.
Microglial GR Gene Inactivation Exacerbates both Microglial and Astroglial Reactivity After Acute MPTP Treatment.
To examine whether microglial GRs have a role in determining the magnitude of glial activation after acute MPTP treatment, microglial and astroglial reactivity in striatum and in SN were analyzed by Iba-1 and GFAP staining, respectively. At day 3 after MPTP treatment, Iba-1 labeling revealed hypertrophied activated microglia in both SN and striatum of GRloxP/loxP controls and GRLysMCre mutants and, by day 7, this activation had declined (Fig. S4A). Quantification of hypertrophied microglia at day 3 after MPTP treatment revealed an increase in SN and striatum of GRLysMCre mutants compared with GRloxP/loxP controls (Fig. S4A, P = 0.04). Analysis of microglial soma size in the SN showed that 27.9 ± 5.0% of activated microglia in GRLysMCre mutants had a surface area >200 μm2 [i.e., a cell body width >16 μm, normally hypertrophied cell body being ≈16 μm (21)] compared with 12.9 ± 3.8% in GRloxP/loxP control mice (P = 0.04; one-way ANOVA). In addition, a significant difference was found in the number of GFAP-IR astrocytes between genotypes after MPTP treatment (Fig. S4B). Quantification of GFAP+ cells at day 7 after MPTP treatment showed a two- and eightfold increase in SN and striatum, respectively, in GRLysMCre mutants compared with GRloxP/loxP controls. This result is in concordance with increased GFAP protein levels at day 7 in the striatum, which remained high at day 21 in mutants (Fig. S4C).
Subchronic MPTP Treatment in GRLysMCre Mutants Leads to an Increased DN Vulnerability Associated with Sustained Activation of Microglia.
The substantial loss of nigral TH-IR neurons in the acute MPTP paradigm is known to be, at least in part, attributable to a strong activation of microglia (22). By contrast, the evidence for a similar microglial activation and its putative role in inducing DN death after moderate subchronic MPTP intoxication death is less well documented (19, 23). We therefore examined microglial GR activity in a subchronic MPTP paradigm. Analysis of nigral TH-IR neurons 7 d after the last MPTP injection showed a significant diminution in GRLysMCre mutants compared with GRloxP/loxP controls (P = 0.0019, post hoc Bonferroni/Dunn test). Moreover, this decrease in DN was sustained, as revealed by the analysis of mice 10 wk after the last MPTP injection (P = 0.0012, GRloxP/loxP MPTP vs. GRLysMCre MPTP mice) (Fig. 3A). Analysis of striatal levels of DA, its metabolites, and other monoamines 7 d after MPTP treatment showed a decrease in DA in GRLysMCre mutants compared with GRloxP/loxP controls (P = 0.049) (Fig. 3B) and a small increase in the dihydroxyphenylacetic acid (DOPAC)/DA ratio (0.366 in mutants vs. 0.255 in controls).
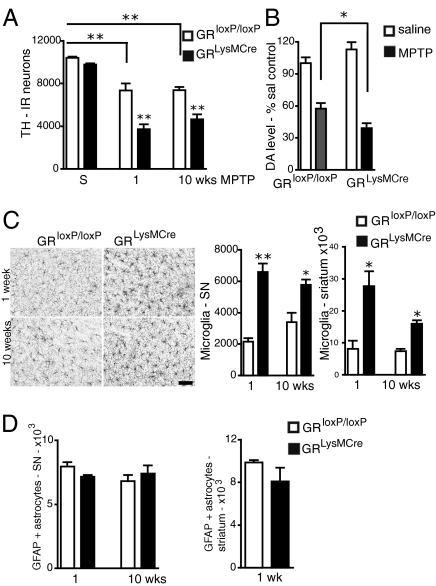
Fig. 3.
Absence of GR increases loss of TH-IR neurons in SN and chronically activates microglia in GRLysMCre mutant mice after subchronic MPTP treatment. (A) Quantification of TH-IR neurons in SN of saline (S) or subchronically MPTP-intoxicated control GRloxP/loxP and GRLysMCre mutant mice, 1 wk or 10 wk after last injection. Two-way ANOVA followed by post hoc Bonferroni/Dunn test showed genotype × MPTP treatment effect: P = 0.02, F = 0.47, degrees of freedom = 2 (n = 4–5). (B) DA levels 1 wk after MPTP or saline injections. The results are calculated as percentage change from values obtained in corresponding saline-injected mice. (C) Immunohistochemistry with anti–Iba-1 antibody in SN 1 and 10 wk after MPTP treatment shows strong microglial activation in the GR LysMCre mutants. (Bar = 50 μm.) The hypertrophied Iba1+ cells were quantified both in SN and striatum; the activation persists in GRLysMCre mutants at 10 wk. (D) Quantification of GFAP+ astrocytes in the SN and striatum show no difference between GRloxP/loxP control and GRLysMCre mutants. Note that GFAP+ cells are absent in striatum in controls and mutants 10 wk after MPTP treatment. *P < 0.05 (n = 5).
Strikingly, 7 d after the last MPTP injection, a threefold increase in the number of Iba-1–positive hypertrophied microglia was found in GRLysMCre mutants in both the striatum and SN. This activation was sustained in both regions even at 10 wk after MPTP treatment (Fig. 3C). In GRloxP/loxP controls, microglial activation in the SN and striatum was negligible at day 7 but slightly increased in the SN at 10 wk postintoxication. In contrast to the results showing a strong astroglial response in mutants after acute MPTP treatment, no difference in the number of GFAP+ astroglia was found either in SN or striatum between controls and GRLysMCre mutants 7 d after subchronic MPTP injections (Fig. 3D). At 10 wk, the number of GFAP+ astrocytes in the SN was also similar in controls and mutants; however, GFAP+ astrocytes were absent in the striatum.
Levels of Pro- and Anti-Inflammatory Genes and Upstream Activators of Innate Immunity Are Modulated by Microglial GR After MPTP-Induced DN Injury.
GR regulates the expression of a wide variety of inflammatory mediators and does so in part by restraining the transcriptional activation potential of NF-κB, activator protein 1 (AP-1), or IFN regulatory factor (IRF) (24). To gain insight into the identity of genes modulated by microglial GR in response to MPTP-triggered neurotoxicity, the nigral and striatal expressions of different classes of inflammatory genes were analyzed by RT-qPCR and compared between GRloxP/loxP control and GRLysMCre mutant mice (Fig. S6 A–C). Additionally, changes in the expression of these genes resulting from MPTP intoxication relative to saline treatment in GRloxP/loxP control mice were also examined (SI Results and Fig. S5). Overall, our data indicate that absence of microglial GR results in higher expression of proinflammatory factors [e.g., TNF-α and intracellular adhesion molecule 1 (ICAM-1)] concomitantly with lower expression of anti-inflammatory mediators [e.g., IL-1R2 and MAPK phosphatase 1 (MKP-1)] in the injured mesencephalon after MPTP exposure.
We observed an increased expression of procaspase-1 (×2) and procaspase-4 (×1.8) only in the SN in GRLysMCre mutants relative to GRloxP/loxP controls 48 h after MPTP intoxication, indicating that GR controls their expression (Fig. 4A). Toll-like receptors (TLRs) were the other major class of core innate-immunity components examined because GR is known to regulate the expression of some family members (25). MPTP treatment itself resulted in a strong induction of several TLRs (TLR3, TLR4, TLR7, and TLR9) and MyD88, a key adaptor in the TLR signaling pathway (Fig. S5). The levels of TLR3, TLR4, TLR9, and MyD88 in GRLysMCre mutant mice showed a further up-regulation, in the SN, 48 h after MPTP treatment (Fig. 4A). These results indicate that microglial GRs regulate important upstream activators of innate immunity during DN injury.
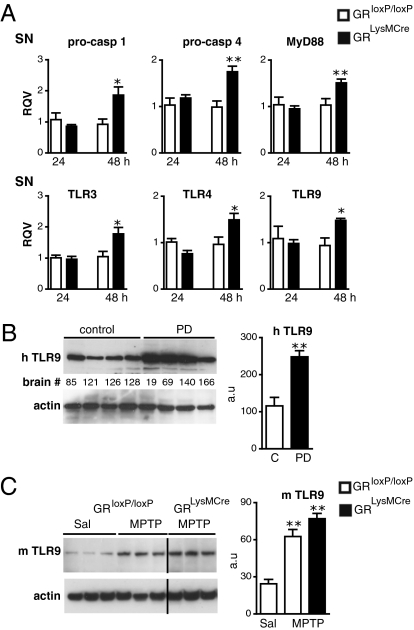
Fig. 4.
GR regulation of upstream activators of innate immune response and relevance to PD. (A) qPCR results show up-regulation in the mRNA levels in SN of proinflammatory caspases and TLRs in GRLysMCre mutant mice compared with controls after MPTP. *P < 0.05, **P < 0.01, MPTP-treated GRloxP/loxP vs. GRLysMCre mice (n = 4). (B) TLR9 protein levels in striatum of human control subjects (C) and parkinsonian patients (PD). **P < 0.01, control subjects vs. PD (n = 5). (C) A representative Western blot experiment showing striatal protein levels of TLR9 in GRloxP/loxP control and GRLysMCre mutant mice 7 d after either saline (sal) or acute MPTP treatment. The signals were quantified in relation to actin. **P < 0.01, saline vs. MPTP treatment (n = 5).
To test the relevance of these findings to PD, we examined TLR9 expression in human striatal homogenates. Our results showed a dramatic two- to threefold increase in TLR9 level in striatal lysates from PD patients compared with control subjects (Fig. 4B). Moreover, a significant increase in TLR9 level in the striatum was also seen in MPTP-treated GRloxP/loxP and GRLysMCre mice (Fig. 4C), suggesting that glial cells trigger an innate immune response upon stimulation by endogenous signals produced by degenerating DA nerve terminals.
Absence of Microglial GR Alters NF-κB Activity.
To gain insights into the mechanistic actions of microglial GR, we examined its effect on NF-κB because GR negatively regulates NF-κB–induced expression of proinflammatory genes like TNF-α, iNOS, and ICAM, which are also up-regulated in PD. We observed that GR immunoprecipitated from nuclear extracts of microglia cells is associated with p65 NF-κB (Fig. S7A). To show that NF-κB transcriptional activity in microglia is regulated by GR activation, primary microglia prepared from control and GRLysMCre pups were transiently transfected with pGL4.3luc2p/NF-κB-RE vector comprising the luciferase reporter gene under the control of κB enhancer elements. Measurement of luciferase activity after LPS or LPS plus dexamethasone treatment in these cells showed that dexamethasone inhibits luciferase activity in control but not in mutant microglia (P = 0.033; Fig. S7B).
To test the possibility that microglial GR inactivation results in sustained NF-κB transcriptional function, we analyzed the phosphorylated NF-κB levels. p65 NF-κB phosphorylation at Ser276 is associated with induction of proinflammatory genes (26). The results showed that in both GRloxP/loxP and GRLysMCre microglial cell cultures, LPS treatment induces phosho-Ser276 p65 and phospho-Ser337 p105 NF-κB levels (Fig. S7C). However, in the dexamethasone pretreatment condition, the magnitude of reduction of these phosphorylated subunits was greater in GRloxP/loxP control compared with mutant cultures. (Fig. S7C). At 2 d after MPTP intoxication, phosho-Ser276 p65 and phospho-Ser337 p105 NF-κB levels were higher in SN and striatum of GRLysMCre mice than in controls (Fig. S7D). Next, we investigated the Ser536-phosphorylated p65 NF-κB. In macrophages, Ser536 phosphorylation by IKKα has been evoked in the clearance of p65 NF-κB from inflammatory target gene promoters (27). In vitro, we observed a strong induction of phospho-Ser536 p65 NF-κB upon LPS exposure of GRloxP/loxP but not GRLysMCre microglia (Fig. S7C). This induction was not observed in dexamethasone-pretreated GRloxP/loxP microglia cultures, indicating that it is sensitive to GR. Interestingly, the level of phosho-Ser536 was reduced in GRLysMCre compared with GRloxP/loxP mice after MPTP intoxication (Fig. S7D). Thus, mechanisms involved in the resolution of NF-κB activation at target genes are compromised in the absence of microglial GR.
Discussion
In this study, we appraised the role of GC-GR in DN degeneration in PD. Overall GR transcript level was lower in the SN of PD patients compared with control subjects. Our data obtained in humans revealed twofold higher cortisol levels in PD patients compared with control subjects. Although our study is limited because plasma cortisol levels were analyzed at one single time point, which obviously cannot reflect 24-h cortisol status, a study by Hartmann et al. (15) on the 24-h cortisol secretory pattern also showed a significantly high overall cortisol concentration in PD patients and, interestingly, a diminution of the normal diurnal changes. Because chronically high levels of GCs are known to compromise immune functions, in part by downregulating GR (28), we have studied the consequences of GR inactivation for the survival of DNs by using mouse models in which GR is specifically ablated in microglia or in DNs.
In the absence of microglial GR (GRLysMCre mice), DN loss in the SN was significantly higher after both acute and subchronic MPTP intoxication compared with control mice. In contrast, MPTP-induced nigrostriatal pathway injury was not affected by GR ablation in DNs. The increased cell death of DNs observed in GRLysMCre mutants was also correlated with reductions in DA uptake and DA levels in the striatum. The exacerbation of neuropathological parameters in GRLysMCre mutants are not linked to altered MPTP metabolism, HPA axis, or receptiveness of mutant microglial cells to MPP+. Therefore, we suggest that microglial GR is a key determinant of DN survival. Additional evidence supporting this view is our finding that, although CS treatment immediately after acute MPTP intoxication significantly protected nigral DNs of GRloxP/loxP control mice, it was ineffective in the GRLysMCre mutants. Although we observed a significant increase in the number of microglia displaying nuclear localization of GR after MPTP, suggesting stimulation of transcriptional control of GR, the results of CS treatment underscore the point that endogenous CS rise after MPTP treatment is probably not of sufficient magnitude or rapid enough to enable GR to suppress microglial neurotoxicity.
GRs were found to regulate the magnitude of microglial activation. Thus, 3 d after acute MPTP intoxication, the number and size of hypertrophied microglia, particularly in the SN of GRLysMCre mutants, was augmented compared with controls. However, by day 7, this activation had declined (in mutants and controls), an observation in accordance with past studies (22). Our work on GR extends the findings reported by Sugama et al. (13) on the time course of exacerbation of microglial activation in adrenalectomized mice acutely intoxicated with MPTP. They also showed that CS treatment suppressed this activation and in parallel increased DN survival. Yet the short duration of activation by acute MPTP toxicity does not mirror the known chronic inflammatory response described in PD and in MPTP-intoxicated monkeys (6). Strikingly, however, our results obtained with the subchronic MPTP paradigm, in which overall MPTP toxicity is moderate, revealed significant microglial activation in the SN and striatum in GRLysMCre mice 7 d after treatment, with almost negligible activation in GRloxP/loxP controls. Importantly, this activation was still present at 10 wk, suggesting that it was chronic in nature. LPS injections in the nigral area have demonstrated that DN survival is particularly sensitive to the activation state of microglia. It is therefore conceivable that the actions of GR in regulating microglial activation in response to environmental changes around DNs are vital. The importance of controlling the magnitude of microglial activation during DN injury was also illustrated in mice lacking the myeloid-specific chemokine receptor CX3CR1 and work on nuclear hormone receptor Nurr1 (29, 30).
Molecular analysis of gene levels of potent proinflammatory mediators after acute MPTP treatment revealed a clear increase in TNF-α mRNA as well as comparatively smaller increases in iNOS and pro–IL-1β mRNA in the GRLysMCre mutants. Interestingly, significant up-regulation of IL-1R2 and MKP-1 expression was observed after MPTP treatment in control mice, indicating that there is a tight control of proinflammatory reaction. By contrast, their levels were lower in GRLysMCre mutants, suggesting that the gene induction action of GR is equally as important as its transrepressive action. MPTP treatment led to strong stimulation of genes coding for upstream core components of innate immunity, particularly TLR9, TLR3, TLR4, MyD88, and procaspase-1, indicating that they are involved in cross-talk between microglia and injured DA neurons. TLRs most likely play a role in PD because a strong up-regulation of TLR9 levels was observed in the striatum of PD patients. In MPTP-treated GRLysMCre mutant mice, there was further up-regulation in SN of procaspases-1 and -4, TLR3, TLR4, TLR9, and the adaptor protein MyD88, suggesting that they are targets of microglial GR and have a role in sustaining a positive feed-forward proinflammatory process that is likely to be deleterious for DNs or for crucial steps of phagocytic process of dying DNs.
Our results linking neuroinflammation in PD pathogenesis and the mechanistic actions of GR reveal that GR associates with the p65 subunit of NF-κB in microglia nuclei and regulates the transactivation potential of NF-κB. Selective inhibition of NF-κB activity was found to inhibit glial-associated neuroinflammation and DN loss in MPTP-treated mice (31); thus, NF-κB may be crucially involved in PD-associated neurodegeneration. Phosphorylation of Ser276 p65 NF-κB is essential for NF-κB oligomerization and DNA binding at promoter regions of inflammatory genes (26). Conversely, phosphorylation of Ser536 p65 NF-κB by IKKα was shown to be required for NF-κB turnover in macrophagic nuclei (27). Thus, our results in MPTP-treated mice showing increased phospho-Ser276 p65 NF-κB levels in the absence of microglial GR indicate prolongation of NF-κB transcriptional activity. Also, down-regulation of phospho-Ser536 p65 NF-κB in the absence of microglial GR suggests that termination of NF-κB activity is compromised in mutant microglial cells. Collectively, our data strongly suggest that GR-dependent regulation of NF-κB activity in microglial cells plays a key role in determining the intensity, pattern, and chronicity of inflammatory processes in the lesioned nigrostriatal pathway, which consequently influence the neurodegenerative outcome.
In conclusion, our results show that the GC-GR system is modulated in PD and that this may adversely affect DN survival. GR dysfunction in PD may result in a chronic inflammatory reaction, and further in-depth work on its glial actions might open innovative therapeutic perspectives.
Materials and Methods
All methods used in this article are routinely used in our laboratories except for the production of the mice, which are referenced (8, 24) and described in detail in SI Materials and Methods.
GRLysMCre and GRDATCre Mice and Genotyping.
The GRLysMCre mouse line was produced by crossing Nr3c1loxP/loxP (designated GRloxP/loxP) mice with LysMCre mice (17, 32). Mice were backcrossed to 10 generations on C57/BL6 background at the start of experiments. The 2- to 4-mo-old male GRLysMCre and GRloxP/loxP mice used were generated by crossing male GRLysMCre mice with female GRloxP/loxP. Generation of DATcre (Tg BAC-DATiCrefto) and GRDATCre mice is described in Turiault et al. (20) and Ambroggi et al. (18). The animals were genotyped for the presence of Cre transgene either by dot blot or PCR analysis.
Supplementary Material
Acknowledgments
We are grateful to C. Lobsiger, S. O'Regan, and S. Rivaud for helpful comments. This work was supported by Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Université Pierre et Marie Curie, Direction de l'Hospitalisation et de l'Organisation des Soins (J.-C.C.), European Economic Community Grant LSHM-CT-2006-037378 (to F.T.), Association France Parkinson (S.V.), and Fondation de France (S.V.). F.R.B. had a short-term European Molecular Biology Organization fellowship, a studentship from the Spanish Ministry of Science and Innovation (BECA FPI BES-2005-8437), and Fundación Séneca (PI-05662). G.H. and D.A.-F. acknowledge support from the German Ministry of Education and Research (NGFNplus 01GS08136-4).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017820108/-/DCSupplemental.
References
- 1.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988;24:574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- 3.Kurkowska-Jastrzebska I, Wrońska A, Kohutnicka M, Członkowski A, Członkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, \6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- 4.Benner EJ, et al. Nitrated α-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouchi Y, et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: A target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 7.Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol. 2007;275:79–97. doi: 10.1016/j.mce.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 8.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 9.Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: When glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23:5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith LK, Jadavji NM, Colwell KL, Katrina Perehudoff S, Metz GA. Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson's disease. Eur J Neurosci. 2008;27:2133–2146. doi: 10.1111/j.1460-9568.2008.06177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AD, Castro SL, Zigmond MJ. Stress-induced Parkinson's disease: A working hypothesis. Physiol Behav. 2002;77:527–531. doi: 10.1016/s0031-9384(02)00939-3. [DOI] [PubMed] [Google Scholar]
- 13.Sugama S, Takenouchi T, Kitani H, Fujita M, Hashimoto M. Microglial activation is inhibited by corticosterone in dopaminergic neurodegeneration. J Neuroimmunol. 2009;208:104–114. doi: 10.1016/j.jneuroim.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Morale MC, et al. Glucocorticoid receptor deficiency increases vulnerability of the nigrostriatal dopaminergic system: Critical role of glial nitric oxide. FASEB J. 2004;18:164–166. doi: 10.1096/fj.03-0501fje. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer's and Parkinson's disease compared to normal controls: Ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18:285–289. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 16.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- 17.Tuckermann JP, et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest. 2007;117:1381–1390. doi: 10.1172/JCI28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambroggi F, et al. Stress and addiction: Glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- 19.Mount MP, et al. Involvement of interferon-γ in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007;27:3328–3337. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turiault M, et al. Analysis of dopamine transporter gene expression pattern—Generation of DAT-iCre transgenic mice. FEBS J. 2007;274:3568–3577. doi: 10.1111/j.1742-4658.2007.05886.x. [DOI] [PubMed] [Google Scholar]
- 21.Glenn JA, Ward SA, Stone CR, Booth PL, Thomas WE. Characterisation of ramified microglial cells: Detailed morphology, morphological plasticity and proliferative capability. J Anat. 1992;180:109–118. [PMC free article] [PubMed] [Google Scholar]
- 22.Liberatore GT, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Fischer D, et al. Modelling Parkinson-like neurodegeneration via osmotic minipump delivery of MPTP and probenecid. J Neurochem. 2008;107:701–711. doi: 10.1111/j.1471-4159.2008.05651.x. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa S, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinenov Y, Rogatsky I. Glucocorticoids and the innate immune system: Crosstalk with the toll-like receptor signaling network. Mol Cell Endocrinol. 2007;275:30–42. doi: 10.1016/j.mce.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 28.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardona AE, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 30.Saijo K, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh A, et al. Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci USA. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.