Abstract
Stable isotope and molecular data suggest that C4 grasses first appeared globally in the Oligocene. In East Africa, stable isotope data from pedogenic carbonate and fossil tooth enamel suggest a first appearance between 15–10 Ma and subsequent expansion during the Plio-Pleistocene. The fossil enamel record has the potential to provide detailed information about the rates of dietary adaptation to this new resource among different herbivore lineages. We present carbon isotope data from 452 fossil teeth that record differential rates of diet change from C3 to mixed C3/C4 or C4 diets among East African herbivore families at seven different time periods during the Late Miocene to the Pliocene (9.9–3.2 Ma). Significant amounts of C4 grasses were present in equid diets beginning at 9.9 Ma and in rhinocerotid diets by 9.6 Ma, although there is no isotopic evidence for expansive C4 grasslands in this part of the Late Miocene. Bovids and hippopotamids followed suit with individuals that had C4-dominated (>65%) diets by 7.4 Ma. Suids adopted C4-dominated diets between 6.5 and 4.2 Ma. Gomphotheriids and elephantids had mostly C3-dominated diets through 9.3 Ma, but became dedicated C4 grazers by 6.5 Ma. Deinotheriids and giraffids maintained a predominantly C3 diet throughout the record. The sequence of differential diet change among herbivore lineages provides ecological insight into a key period of hominid evolution and valuable information for future studies that focus on morphological changes associated with diet change.
Keywords: carbon isotopes, herbivore diet, bioapatite, paleodiet, mammal
Stable carbon isotope ratios from fossil tooth enamel, pedogenic carbonates, and terrestrial plant biomarkers are commonly used to determine the relative amounts of C3 and C4 vegetation in ancient habitats (1–4). These paleovegetation proxies integrate over different spatial and temporal scales, and each proxy has inherent strengths and weaknesses when used to reconstruct the relative amounts of C3 and C4 vegetation in ancient habitats (5, 6).
Carbon isotopes from fossil enamel of East African herbivores indicate C3 diets and presumably, C3 environments, in the records from Buluk, Fort Ternan, and the Tugen Hills that range in age from 17 to ∼9 Ma (7–9). An exception is carbon isotope data from four equid teeth from Chorora, Ethiopia, dated at 10.7–10.1 Ma, which indicate a mixed C3/C4 diet (10). Previously published isotope data on enamel from the Nakali, Namurungule, Nawata, and Nachukui formations (included and expanded significantly here) indicate a shift toward C4-dominated diets between 9.6 and 4.2 Ma (7). The previously published fossil enamel δ13C record from East Africa shows that herbivores incorporated C4 vegetation into their diets as early as ∼10 Ma and is generally consistent with the Late Miocene (8–6 Ma) global expansion of C4 vegetation documented in the Siwaliks, North America, and South America (1, 11–14).
Early to early Late Miocene pedogenic carbonate records from fossil sites in East Africa include Rusinga Island (15), the Lothidok Hills (16), Fort Ternan (17), the Tugen Hills (2), and Lothagam (7). All carbon isotope data from pedogenic carbonates older than 9 Ma indicate C3 environments, with the exception of the Tugen Hills (2). No pedogenic carbonates have been found in the Nakali Formation, and none have been sampled from the Namurungule Formation in the Samburu Hills. The earliest robust evidence for C4 vegetation from pedogenic carbonate is in the Lower Nawata Formation (7.4 Ma) at Lothagam, where the δ13C values (−9.0 to −2.2‰) indicate C3 to mixed C3/C4 environments that included significant amounts of C4 grass (7). Although isotope records from Late Miocene pedogenic carbonates are rare, abundant records show that C4 grasses became widespread during the Late Pliocene to Pleistocene throughout East Africa (ref. 5 and references therein).
Feakins et al. (4) analyzed the δ13C values of n-alkanoic acids in sediments from a Gulf of Aden core (Deep Sea Drilling Project Site 231) that coincide with two time periods represented in our record. The biomarker record shows input of predominantly C3-derived leaf waxes at 9.4 Ma and 5–15% C4 vegetation from 3.8 to 3.2 Ma (4). The data from the Gulf of Aden core record a noncontinuous, regional-scale signal that is well dated with high temporal resolution. Collectively, the fossil enamel, pedogenic carbonate, and biomarker records from East Africa indicate C3-dominated ecosystems throughout the Middle and Late Miocene and an expansion of C4 grasses in the Late Pliocene and Pleistocene. However, none of these previously published datasets provides a detailed record of the response of the fauna during this period.
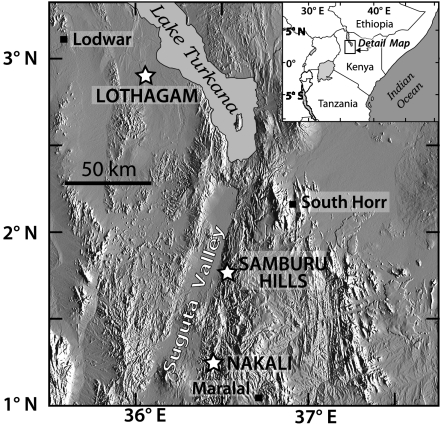
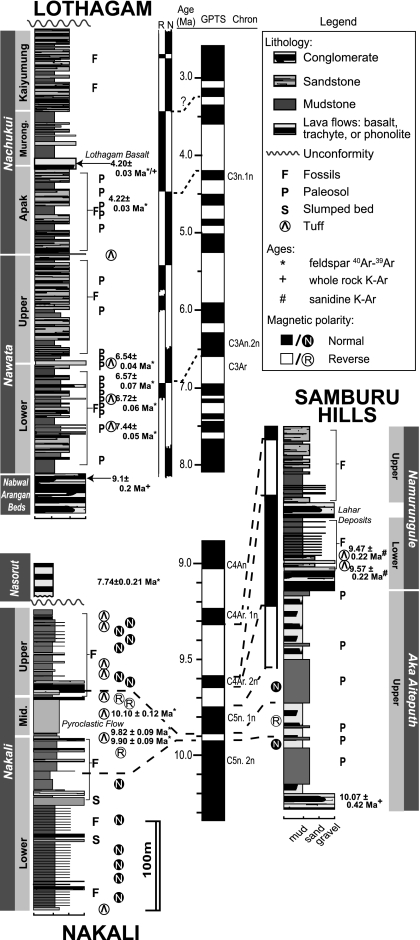
We use stable carbon isotope ratios from fossil tooth enamel identified from family to species level (SI Appendix, Table S1) to evaluate rates of change in herbivore diets from the Late Miocene to the Early Pliocene from three fossil localities in East Africa (Fig. 1). The sites range in age from 9.9 to 3.2 Ma; lithostratigraphy, radiometric ages, and stratigraphic intervals where samples were collected are shown in Fig. 2. The stable isotope data address two important issues in the East African fossil record. First, they illustrate the diverse paleoecological response of large herbivores to paleoenvironmental change associated with the appearance of C4 grasses during a key period of hominid evolution. Paleoenvironmental context is integral to understanding East African hominid emergence, radiation, and evolution from the Late Miocene onward. All sites in this study have yielded important hominid material. Nakali and the Samburu Hills, located in the Suguta Valley, Kenya, have yielded the Late Miocene hominids Nakalipithecus nakayami (18) and Samburupithecus kiptalami (19), respectively, which likely represent stem hominines (23). With Chororapithecus abyssinicus, these are the only known East African hominids between 11 and 9 Ma (24). The third site, Lothagam, is located west of Lake Turkana in northern Kenya (Fig. 1). The Nawata and Nachukui formations at Lothagam have yielded hominid material dated at ∼5 and 3.5 Ma. The older specimens are attributed to Hominidae indet., whereas the younger specimens are likely Kenyanthropus platyops or, possibly, Australopithecus cf Au. afarensis (25) (SI Appendix, Table S2). Second, the stable isotope record of diet change will allow paleontologists to compare the timing and rate of diet change to morphological changes associated with the dietary shift toward C4 grazing in herbivore lineages. Examples of craniodental and postcranial ecomorphic characteristics influenced by the transition from browsing to grazing include tooth morphology (i.e., hypsodonty, enamel thickness, and size), jaw structure, and forelimb bone length (26, 27).
Fig. 1.
Map of northern Kenya showing the three fossil localities in the Suguta Valley and the Turkana Basin.
Fig. 2.
Lithostratigraphy, magnetostratigraphy, and radiometric ages from Nakali, the Samburu Hills, and Lothagam. Geomagnetic Polarity Time Scale (GPTS) changes at the break between 9.0 and 8.0 Ma. The uppermost age from the Nakali Formation (10.10 ± 0.12 Ma) is from a pyroclastic flow and essentially synchronous with the two slightly younger ages below. Modified from refs. 18, 19, and 20–22.
Results and Discussion
Carbon Isotope Data by Age.
We use the terms “C3-dominated diet” for δ13C values < −8‰, “mixed C3/C4 diet” for −8‰ to −2‰, and “C4-dominated diet” for values > −2‰. These delineations are based on estimated paleoatmospheric δ13C values and an enrichment factor of 14.1‰ between diet and enamel (SI Appendix, Table S3) (28, 29). A Shapiro–Wilk test for normality demonstrates that the data are not distributed normally (SI Appendix, Table S4). Thus, the median and range of δ13C values are more appropriate than the mean and SD for describing central location and variance. Mann–Whitney U test results indicate significant shifts (P < 0.05) in the median δ13C value between successive age increments (i.e., 9.9–9.6 Ma) where there are more than five isotope analyses within a family (SI Appendix, Table S5). All isotopic results are reported in SI Appendix, Table S6; a summary is presented in Table 1.
Table 1.
Median, range, and number of δ13C values for herbivore families by age
| Formation or member (age) | Family | Median (‰) | Range (‰) | N |
| Kaiyumung (3.2 Ma) | All taxa | −1.8 | −11.1 to +0.8 | 13 |
| Rhinocerotidae | +0.5 | −0.6 to +0.8 | 3 | |
| Bovidae | — | −6.6 and −2.0 | 2 | |
| Suidae | −1.5 | −2.9 to −0.2 | 6 | |
| Giraffidae | −9.4 | — | 1 | |
| Deinotheriidae | −11.1 | — | 1 | |
| Apak (4.2 Ma) | All taxa | −2.4 | −12.5 to −0.2 | 53 |
| Equidae | −1.0 | −1.8 to −0.2 | 5 | |
| Rhinocerotidae | −2.4 | −11.2 to −2.0 | 11 | |
| Bovidae | +2.2 | −5.8 to −0.4 | 6 | |
| Hippopotamidae | −4.4 | −8.2 to −0.9 | 7 | |
| Suidae | −2.9 | −5.9 to −1.5 | 13 | |
| Gomphotheriidae | −0.9 | −2.2 to −0.7 | 4 | |
| Elephantidae | −0.8 | −1.1 to −0.2 | 5 | |
| Deinotheriidae | — | −12.5 and −12.0 | 2 | |
| Upper Nawata (6.5 Ma) | All taxa | −2.9 | −12.2 to +0.9 | 79 |
| Equidae | −0.2 | −0.5 to +0.5 | 6 | |
| Rhinocerotidae | −8.0 | −10.9 to −1.3 | 13 | |
| Bovidae | −3.3 | −9.0 to +0.2 | 7 | |
| Hippopotamidae | −2.7 | −7.6 to +0.9 | 28 | |
| Suidae | −5.8 | −9.1 to −2.0 | 12 | |
| Giraffidae | −12.2 | — | 1 | |
| Elephantidae | −1.1 | −2.1 to +0.3 | 11 | |
| Deinotheriidae | −11.4 | — | 1 | |
| Lower Nawata (7.4 Ma) | All taxa | −5.1 | −11.4 to +2.2 | 91 |
| Equidae | −0.9 | −3.8 to +0.4 | 14 | |
| Rhinocerotidae | −9.1 | −11.0 to −4.0 | 15 | |
| Bovidae | −4.2 | −7.3 to +2.2 | 6 | |
| Hippopotamidae | −4.3 | −9.2 to −0.7 | 29 | |
| Suidae | −7.2 | −9.6 to −5.6 | 14 | |
| Giraffidae | −9.8 | −11.4 to −8.1 | 4 | |
| Gomphotheriidae | −2.1 | −3.9 to +0.7 | 4 | |
| Elephantidae | −3.2 | −6.2 to −1.0 | 4 | |
| Deinotheriidae | −9.7 | — | 1 | |
| Upper Namurungule (9.3 Ma) | All taxa | −5.0 | −10.7 to +0.2 | 34 |
| Equidae | −1.9 | −4.8 to +0.2 | 13 | |
| Rhinocerotidae | — | −9.7 and −4.9 | 2 | |
| Bovidae | — | −7.6 and −2.1 | 2 | |
| Hippopotamidae | −5.6 | −6.5 to −3.8 | 3 | |
| Suidae | −7.9 | −8.4 to −5.1 | 3 | |
| Giraffidae | −7.6 | −10.7 to −7.3 | 3 | |
| Gomphotheriidae | −8.2 | −9.1 to −4.6 | 8 | |
| Lower Namurungule (9.6 Ma) | All taxa | −5.6 | −10.4 to +0.7 | 69 |
| Equidae | −2.1 | −6.6 to +0.7 | 24 | |
| Rhinocerotidae | −4.4 | −9.6 to −1.0 | 12 | |
| Bovidae | −6.1 | −10.3 to −3.7 | 6 | |
| Hippopotamidae | −6.6 | −8.5 to −5.2 | 6 | |
| Suidae | −6.5 | −7.4 to −3.5 | 7 | |
| Giraffidae | −7.7 | −8.7 to −6.3 | 7 | |
| Gomphotheriidae | — | −10.3 and −6.3 | 2 | |
| Deinotheriidae | −9.5 | −9.8 to −8.9 | 5 | |
| Nakali (9.9 Ma) | All taxa | −9.4 | −12.3 to −0.9 | 113 |
| Equidae | −8.6 | −10.5 to −0.9 | 35 | |
| Rhinocerotidae | −9.3 | −11.6 to −6.5 | 11 | |
| Bovidae | −10.4 | −11.8 to −8.1 | 10 | |
| Hippopotamidae | −9.7 | −11.2 to −8.0 | 14 | |
| Suidae | −8.6 | −10.8 to −6.3 | 15 | |
| Giraffidae | −9.8 | −12.3 to −8.0 | 15 | |
| Gomphotheriidae | −7.7 | −9.6 to −6.9 | 7 | |
| Deinotheriidae | −10.4 | −11.7 to −9.5 | 6 |
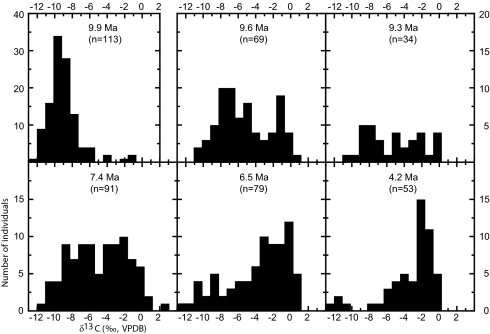
The δ13C values of 113 herbivore fossil teeth from the Nakali Formation (9.9 Ma) range from −12.3 to −0.9‰ and have a median value of −9.4‰. Of the 113 samples, 94 indicate C3-dominated diets (Fig. 3). Ten of 11 samples that have δ13C values > −7‰ come from the Upper Member. This includes three Upper Member equid teeth that range from −1.8 to −0.9‰, suggesting diets of ∼65% C4 grass. None of the samples with confirmed placement in the Lower Member (n = 28) have δ13C values > −8‰. Thus, C4 grasses were absent from the diets of all confirmed Lower Member taxa and were in high-enough abundance to comprise the majority of the diet of some Upper Member equids. Although C4 grass was available by 9.9 Ma at Nakali, most herbivores were exploiting C3 trees, shrubs, forbs, sedges, or grasses.
Fig. 3.
Histograms of δ13C values of fossil enamel from all herbivore taxa by age. Note the different vertical axis for the 9.9 Ma population.
In stark contrast to the Nakali (9.9 Ma) taxa, more than 80% (n = 103) of Namurungule taxa (9.6–9.3 Ma) consumed C4 grass (Fig. 3). The range of δ13C values in the Lower and Upper members of the Namurungule Formation are indistinguishable (Table 1), but the δ13C histogram for Lower Namurungule taxa is bimodal with peaks at −7 and −1‰. This is the earliest evidence for niche partitioning into C3 and C4 diets at the ecosystem scale in the East African fossil record. The Upper Namurungule histogram is not well defined (Fig. 3). The distribution of δ13C values from the Lower Nawata is also bimodal with peaks at −7‰ and −2‰. The Upper Nawata δ13C histogram is unimodal and skewed left. The Apak δ13C histogram is also unimodal and skewed left (Fig. 3). No histogram is plotted for the Kaiyumung because of the relatively small sample size (n = 13). The δ13C data record a dietary transition from nearly all C3 at 9.9 Ma to mixed C3/C4 from 9.6 to 7.4 Ma to predominantly mixed C3/C4 to C4-dominated diets from 6.5 Ma onward (Fig. 3). Advancing temporally from 10 to 3 Ma through the isotope record requires moving spatially within the Kenya Rift between the three sites (Fig. 1). Available data suggest differences in physiographic settings (e.g., paleoelevation and location in the Rift Valley) between the three sites that would have affected paleoclimatic variables such as temperature, precipitation, aridity, and seasonality (18, 20, 30, 31). None of the sites overlap in age; hence, it is not possible to discern the influence of local (i.e., site-specific) versus regional climate on vegetative structure at each site. Therefore, the temporal shift in δ13C toward more positive values may also be partly attributed to local physiographic influences on paleoclimate. The carbon isotope data are a single, albeit valuable, component for reconstructing the paleoecology of fossil sites. In the absence of additional geochemical, taxonomic, taphonomic, and lithofacies data from all sites, we refrain from a comprehensive reconstruction of paleoenvironment at each site and focus on diet change in herbivore lineages.
Carbon Isotope Data by Family.
Equidae.
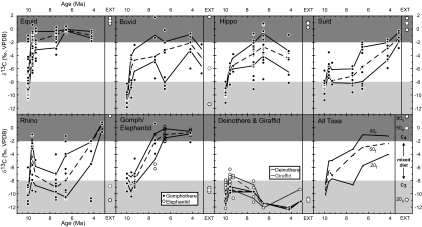
The majority of Nakali Formation equids have C3-dominated diets. Nine have intermediate δ13C values (−7.8 to −4.1‰) indicative of a mixed C3/C4 diet, and three specimens collected within the upper 30 m of the Nakali Formation have values that range from −1.8 to −0.9‰ (Fig. 4). These three specimens comprise the earliest C4-dominated diets from the East African fossil record. Lower Namurungule equids display a greater range in δ13C values than equids in the Upper Namurungule. The ranges of δ13C values in both the Lower and Upper Nawata are small, 4.2‰ and 1.0‰, respectively. Equids from the Apak have a median δ13C value of −1.0‰ (n = 5) and a range of 1.6‰. No equids were sampled from the Kaiyumung.
Fig. 4.
The δ13C value vs. age (Ma) for nine herbivore families and for all taxa. Data points represent individual samples. Lines are the 20th, 50th (dashed), and 80th percentile δ13C values for fossil (f) populations. White circles to the right of the fossil data for each family indicate the 20th, 50th (dashed), and 80th percentile δ13C values for extant (x and EXT) herbivores from Northern Kenya (32). The δ13C values from extant herbivores are corrected by +1.5‰ for recent changes in atmospheric δ13C values.
The equids were the first to incorporate significant amounts of C4 grass into their diet at 9.9 Ma. Beginning at 9.6 Ma, the isotope record shows a rapid transition toward dedicated C4 grazing characterized by an increasing median and a decreasing range of δ13C values through time (Fig. 4). Nearly three-fourths of the Nakali equids have C3-dominated diets, but not a single equid from the Lower Namurungule has a C3 diet. Between 9.6 and 9.3 Ma, the minimum δ13C value increases from −6.6 to −4.8‰, indicating a shift away from C3 vegetation in diet. By 6.5 Ma, equids were dedicated C4 grazers, as are extant equids.
Isotopic data are supported by mesowear analysis of fossil teeth, which is a fast, qualitative method for determining diet preference (browse vs. graze) using two morphological criteria. Cusp shape describes the buccal apices of molar cusps as sharp, round, or blunt, and occlusal relief describes the height of the valley between them (33). Sharp-to-round cusp shape and high occlusal relief, akin to serrations on a knife, are common in extant browsers. Round or blunt cusp shape and low occlusal relief are common in extant grazers. Nakaya et al. (34) found that 85% (n = 68) of upper molars from Nakali equids have high occlusal relief and that 92% (n = 60) have a sharp or round cusp shape, suggesting a diet of C3 browse, not C3 graze. In contrast, in the Namurungule Formation, 52% (n = 54) of upper molars have low occlusal relief and 97% (n = 36) have a round or blunt cusp shape, suggesting grazing diets. The isotope and mesowear results suggest that the East African equid diet transitioned from C3 browsing (with some minor amounts of C4 grazing) at 9.9 Ma to a C4-dominated grazing diet by 7.4 Ma. However, in the absence of mesowear (or microwear) data for other lineages that eventually include C4 grass in their diets, prior or continued use of C3 grass cannot be ruled out.
Rhinocerotidae.
All but one of the Nakali rhinocerotids had C3-dominated diets. The δ13C values of Lower Namurungule rhinocerotids (−9.6 to −1.0‰) indicate a range of C3- to C4-dominated diets. The range in δ13C values of rhinocerotids increases from the Lower Nawata to the Upper Nawata and decreases slightly in the Apak. In the Kaiyumung, three rhinocerotid samples have a median δ13C value of +0.5‰.
Some rhinocerotids adopt a C4-dominated diet by 9.6 Ma. However, unlike the equids, the rhinocerotids maintain a diverse diet ranging from C3- to C4-dominated diets through 4.2 Ma (Fig. 4). In the Lower Namurungule, half of the population (potentially Ceratotherium sp.) has a mixed C3/C4 to C4-dominated diet (−3.2 to −1.0‰), and the other half has a mixed C3/C4 to C3-dominated diet (−9.6 to −5.7‰), suggesting niche partitioning in the rhinocerotid diet as early as 9.6 Ma. By the Apak, mixed diets become less common in the record than C3- or C4-dominated diets, a profile that more closely resembles the dietary niche partitioning among the extant African rhinoceros where the white, Ceratotherium, is a grazer, and the black, Diceros, is a browser. Only the grazing rhinocerotid, Ceratotherium sp., is present at 3.2 Ma in the Kaiyumung.
Bovidae.
The median δ13C value of Nakali bovids (−10.4‰; n = 10) is, along with the deinotheriids, the lowest among all East African lineages at 9.9 Ma. The median δ13C value increases to −6.1‰ (n = 6) in Lower Namurungule bovids and appears to increase further in the two bovids analyzed from the Upper Namurungule (δ13C values = −7.6‰ and −2.1‰). This trend continues through bovids from the Lower Nawata, Upper Nawata, and Apak. Two bovids analyzed from the Kaiyumung have δ13C values of −6.6‰ and −2.1‰.
Even in the Late Miocene and Early Pliocene, bovids were taxonomically diverse (SI Appendix and references therein), which is reflected isotopically in the range of diets after 9.9 Ma. The highest δ13C value from each age population increases incrementally from the Lower Namurungule to the Lower Nawata (Fig. 4). As with the rhinocerotids, the bovids show a diverse diet throughout most of their record, despite being represented by a relatively limited sample size (n = 39). Although bovid fossils are abundant, especially at Lothagam, many are horn cores or postcranial elements, whereas teeth suitable for isotope analysis are rare. Future stable isotope work between and within the bovid tribes has great potential for elucidating their dietary preferences and radiation.
Hippopotamidae.
Nakali hippopotamids have a median δ13C value of −9.7‰ whereas in Lower Namurungule hippopotamids the median δ13C value is −6.6‰. Three hippopotamids from the Upper Namurungule have a median δ13C value of −5.6‰. By the Lower Nawata, the median δ13C value is −3.8‰ (n = 29) and in the Upper Nawata the median increases to −2.7‰ (n = 28). In the Apak, the median δ13C value is −4.4‰ (n = 7) and the range is from −8.2 to −0.9‰ (Fig. 4). No hippopotamids were analyzed from the Kaiyumung.
Kenyapotamus coryndoni had a C3 diet at 9.9 Ma, but by 9.6 Ma it was a mixed C3/C4 feeder and only one individual had a C3 diet. Hippopotamids from 9.3 Ma incorporated even more C4 grass into their diet than those from 9.6 Ma. By 7.4 Ma, Kenyapotamus is replaced by Archaeopotamus species that exploit nearly the entire δ13C range of C3 to C4 vegetation (Fig. 4). Hippopotamid diet remains diverse through the remainder of the record at Lothagam. The common extant hippopotamus in East Africa is primarily a grazer, but individuals display a diverse diet ranging from −13.7 to +1.5‰, with a mean value of −3.5 ± 1.7‰ (35).
Suidae.
Nakali Formation suids have a median δ13C value of −8.6‰ (n = 15), a value that increases to −6.5‰ (n = 6) in Lower Namurungule suids. The three suids from the Upper Namurungule have a median δ13C value of −7.9‰. At Lothagam, suid δ13C values increase from the Lower Nawata to the Upper Nawata and still further in the Apak and the Kaiyumung.
Between 9.9 and 3.2 Ma, suids make a slow transition from a C3- to a C4-dominated diet (Fig. 4). Only 2 of 15 nyanzachoeres from 9.9 Ma have minor amounts of C4 grass in their diet, but by 9.6 Ma all have some C4 grass in their diet. Suid diet remains fairly static through the Lower Nawata and is even slightly more reliant on C3 vegetation at 9.3 and 7.4 Ma than at 9.6 Ma. In the Upper Nawata, Nyanzochoerus syrticus has a wide dietary range, but that of N. australis is C4-dominated. By 4.2 Ma, the suids have mixed C3/C4 to C4-dominated diets. Kaiyumung nyanzachoeres and notochoeres have C4-dominated diets with a relatively restricted range of δ13C values. Extant East African suids occupy a wide range of ecosystems from closed canopy forests to mosaic grassland/woodlands, and their diets reflect the wide range of habitats that they occupy. Extant suids from the Turkana Basin have C4-dominated diets (36).
Giraffidae.
Giraffids have a C3-dominated diet from 9.9 to 3.2 Ma with the exception of six values from 9.6 and 9.3 Ma in the Namurungule Formation, where the maximum value is −6.3‰. No giraffids were sampled from the Apak, and the sole giraffid value from the Kaiyumung is −9.4‰. Giraffa camelopordalis, the extant giraffe, has been extirpated from the Turkana Basin, but elsewhere in Africa it and the Okapi have C3-dominated diets.
Gomphotheriidae and Elephantidae.
Results for these two probscidean lineages are discussed together and shown on the same plot (Fig. 4) because of general morphological similarities and because neither family is present throughout the entire record. In general, gomphotheriid δ13C values indicate C3-dominated diets from 9.9 to 9.3 Ma. By 7.4 Ma, no gomphotheriids or elephantids have C3 diets, and they occupy nearly the entire δ13C range of mixed C3/C4 to C4 diet (Fig. 4). Between 7.4 and 6.5 Ma, they become dedicated grazers. The Apak exhibits the highest taxonomic diversity of gomphotheriids and elephantids, but the lowest range of δ13C values, suggesting that, shortly before their extinction, gomphotheriids and elephantids were competing directly with each other for food. In complete contrast to their Plio-Pleistocene relatives, the extant elephantids, Loxodonta africana and Elephas maximus, are primarily browsers (37, 38).
Deinotheriidae.
Deinotheriids are extinct proboscideans that had two downward-curving tusks in the lower jaw, and they are relatively rare throughout the record. From the δ13C values available (Table 1), it appears that they maintain a C3 diet throughout the record, in agreement with previous interpretations of diet based on carbon isotope data and low crowned tooth morphology (37, 39). The δ13C values from this taxon, like those of Giraffa, are useful for defining the C3 end member at a given fossil locality.
Patterns of Differential Diet Change.
The carbon isotope data illustrate patterns of diet change in nine herbivore lineages, but do not reveal underlying drivers of the observed changes. We compiled isotope data from fossil and extant herbivore teeth in northern Kenya and summarized diet change patterns in herbivore lineages over the last 10 million years in East Africa (Fig. 4). The perissodactyls rapidly shift to C4-dominated diets. The equids’ shift begins around 9.9 Ma, and the rhinocerotids’ by 9.6 Ma. However, the rhinocerotids and equids differ in that rhinocerotids have C3-, mixed C3/C4, and C4-dominated diets from 9.6 until 4.2 Ma, when mixed C3/C4 diets disappear from the record, whereas the equids become dedicated C4 grazers sometime between 9.3 and 7.4 Ma (Fig. 4). Two proboscidean families, the gomphotheriids and elephantids, make a similar shift to C4 grazing that begins later than that of the equids (Fig. 4). At some time in the last million years or so, elephantids return to C3-dominated diets (37). The deinotheriids, a third proboscidean family, maintain a C3-dominated diet up to their demise in the Pleistocene. Among the artiodactyls, bovids shift to a mixed C3/C4 diet by 9.6 Ma and to a wide range of diets by 7.4 Ma, including individuals with C4-dominated diets. This diversity in diet may have contributed to their successful radiation in the Pliocene and Pleistocene in East Africa. Likewise, hippopotamids transition from a C3 diet to a mixed C3/C4 diet between 9.9 and 9.3 Ma. By 7.4 Ma, their diets had become quite diverse and remain so today (35). The suids incorporate small amounts of C4 grass into their diet by 9.6 Ma, but do not begin their shift toward a C4-dominated diet until 6.5 Ma (Fig. 4).
The underlying driver of the diverse dietary response to C4 grass among lineages can broadly be attributed to paleoenvironmental change. Paleoenvironmental records in East Africa from 10 to ∼5.5 Ma are essentially limited to sites in this study and in the Tugen Hills. At present, the discontinuous and incomplete paleoenvironmental record renders linkages to diet change weak. This is in contrast to the continuous, multiproxy paleoenvironmental records from 8 to 5 Ma in the Siwalik Hills of India and Pakistan, where a spatiotemporally continuous isotopic record of soil carbonates and a rich, coeval faunal collection, which includes isotope data from enamel, show that long-term climate change resulted in major faunal turnover (12). Building a multiproxy data set from existing East African sites during this time period has the potential of elucidating the primary drivers of herbivore diet change related to changes in paleoenvironment and paleoclimate.
Conclusions
From the Late Miocene through the Pliocene, East African herbivore families exhibit differential rates of diet change from C3-dominated to mixed C3/C4 or C4-dominated diets. C4 grasses were available by 9.9 Ma at Nakali, but of the population sampled (n = 113), only a dozen equids, a single rhinocerotid, and two suids and gomphotheriids, had mixed C3/C4 or C4-dominated diets. By 9.6 Ma, 80% of the sampled fauna, which includes the equids, rhinocerotids, bovids, hippopotamids, gomphotheriids, and suids, were incorporating C4 grass into their diets. The paleosol record indicates mosaic environments from 7.4 to 4.2 Ma, but there is no evidence for long-lived, ecosystem-scale C4 grasslands. With the exception of deinotheriids and giraffids, all sampled lineages incorporate significant amounts (>50%) of C4 grasses into their diet by 6.5 Ma. The change from C3 to mixed C3/C4 or C4-dominated diets between 9.9 and 9.3 Ma took place within a single species in both equids and hippopotamids. High rates of dietary change later in the record (9.3–3.2 Ma) likely occurred in conjunction with major habitat change that, in turn, produced faunal change, including the appearance of stem hominins in the fossil record. The timing and rates of morphological change (e.g., craniodental and postcranial) can be evaluated with respect to the ∼7-million-year isotope record of diet change presented here.
Materials and Methods
Stable isotope ratios are reported as δ values relative to the Pee Dee Belemnite (PDB) standard using permil (‰) notation where δ13C = (Rsample/Rstandard − 1) × 1,000, and Rsample and Rstandard are the 13C/12C ratios in the sample and in the standard, respectively, and the δ13C value of PDB is defined as 0‰. The δ13C isotope ratios of enamel were measured at the Stable Isotope Ratio Facility for Ecological Research at the University of Utah on a Finnigan 252 isotope ratio mass spectrometer. The SD of an internal carbonate standard (Carrara marble) analyzed with these samples was 0.2‰ (n = 63). Additional details of the sampling and analytical methods are provided in SI Appendix. Taxonomic identification of mammalian fauna from the Nakali, Namurungule, Nawata, and (Lothagam) Nachukui formations is presented in SI Appendix, Table S2, and reviewed in SI Appendix (18, 30, 40–51). We analyzed fossil enamel from large herbivores listed in SI Appendix, Table S1. Many are identified only by family because permission to sample specimens for stable isotope analysis is often restricted to fragmentary material that precludes identification at the genus or species level. A Simpson Index shows that the fauna from Nakali and Namurungule formations are similar at the family, genus, and species levels at 82, 78, and 50%, respectively (30, 52). Significant faunal turnover occurs between 9.3 and 7.4 Ma.
Supplementary Material
Acknowledgments
We thank the Government of Kenya and the National Museums of Kenya (KNM) for permission to sample fossil material from Nakali, the Samburu Hills, and Lothagam. This work would not be possible without the contributions of the KNM and Koobi Fora Research Project field crews and the KNM preparation and curatorial staff, especially Dr. E. Mbua and M. Muungu. K.T.U. thanks F. H. Brown, T. Sakai, and N. E. Levin for assistance in the field and insightful discussion. We thank A. K. Behrensmeyer and an anonymous reviewer whose critical comments significantly improved the manuscript. This work was supported by National Science Foundation Grant BCS-0621542 and Japan Society for the Promotion of Science Grants 19107007 and 22255006.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018435108/-/DCSupplemental.
References
- 1.Quade J, et al. A 16-Ma record of paleodiet using carbon and oxygen isotopes in fossil teeth from Pakistan. Chem Geol Isot Geosci Sect. 1992;94:183–192. [Google Scholar]
- 2.Kingston JD, Hill A, Marino BD. Isotopic evidence for neogene hominid paleoenvironments in the Kenya rift valley. Science. 1994;264:955–959. doi: 10.1126/science.264.5161.955. [DOI] [PubMed] [Google Scholar]
- 3.Cerling TE, Quade J, Wang Y, Bowman JR. Carbon isotopes in soils and palaeosols as ecology and palaeoecology indicators. Nature. 1989;341:138–139. [Google Scholar]
- 4.Feakins SJ, deMenocal PB, Eglinton TI. Biomarker records of late Neogene changes in northeast African vegetation. Geology. 2005;33:977–980. [Google Scholar]
- 5.Ségalen L, Lee-Thorp JA, Cerling T. Timing of C4 grass expansion across sub-Saharan Africa. J Hum Evol. 2007;53:549–559. doi: 10.1016/j.jhevol.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Kingston JD. Shifting adaptive landscapes: Progress and challenges in reconstructing early hominid environments. Am J Phys Anthropol. 2007;134(Suppl 45):20–58. doi: 10.1002/ajpa.20733. [DOI] [PubMed] [Google Scholar]
- 7.Cerling T, Harris J, Leakey M. Isotope paleoecology of the Nawata and Nachukui Formations at Lothagam, Turkana Basin, Kenya. In: Leakey MG, Harris JM, editors. Lothagam: The Dawn of Humanity in Eastern Africa. New York: Columbia University Press; 2003. pp. 605–623. [Google Scholar]
- 8.Cerling TE, Harris JM, Ambrose SH, Leakey MG, Solounias N. Dietary and environmental reconstruction with stable isotope analyses of herbivore tooth enamel from the Miocene locality of Fort Ternan, Kenya. J Hum Evol. 1997;33:635–650. doi: 10.1006/jhev.1997.0151. [DOI] [PubMed] [Google Scholar]
- 9.Morgan M, Kingston J. Carbon isotope evidence for the emergence of C4 plants in the Neogene from Pakistan and Kenya. Nature. 1994;367:162–165. [Google Scholar]
- 10.Bernor RL, Kaiser TM, Nelson SV. The oldest Ethiopian Hipparion (Equinae, Perissodactyla) from Chorora: Systematics, paleodiet and paleoclimate. Courier Forschungsinstitut Senckenberg. 2004;246:213–226. [Google Scholar]
- 11.Cerling TE, et al. Global vegetation change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. [Google Scholar]
- 12.Badgley C, et al. Ecological changes in Miocene mammalian record show impact of prolonged climatic forcing. Proc Natl Acad Sci USA. 2008;105:12145–12149. doi: 10.1073/pnas.0805592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Cerling TE, MacFadden BJ. Fossil horses and carbon isotopes: New evidence for Cenozoic dietary, habitat, and ecosystem changes in North America. Palaeogeogr Palaeoclimatol Palaeoecol. 1994;107:269–279. [Google Scholar]
- 14.Latorre C, Quade J, McIntosh WC. The expansion of C4 grasses and global change in the late Miocene: Stable isotope evidence from the Americas. Earth Planet Sci Lett. 1997;146:83–96. [Google Scholar]
- 15.Bestland EA, Krull ES. Palaeoenvironments of Early Miocene Kisingiri volcano Proconsul sites: Evidence from carbon isotopes, palaeosols and hydromagmatic deposits. J Geol Soc London. 1999;156:965–976. [Google Scholar]
- 16.Cerling TE. Development of grasslands and savannas in East Africa during the Neogene. Global Planet Change. 1992;5:241–247. [Google Scholar]
- 17.Cerling TE, Quade J, Ambrose SH, Sikes NE. Fossil soils, grasses, and carbon isotopes from Fort Ternan, Kenya: Grassland or woodland? J Hum Evol. 1991;21:295–306. [Google Scholar]
- 18.Kunimatsu Y, et al. A new Late Miocene great ape from Kenya and its implications for the origins of African great apes and humans. Proc Natl Acad Sci USA. 2007;104:19220–19225. doi: 10.1073/pnas.0706190104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawada Y, et al. K-Ar ages of Miocene Hominoidea (Kenyapithecus and Samburupithecus) from Samburu Hills, northern Kenya. Comptes Rendus de l'Academie des Sciences, Serie II. Sciences de la Terre et des Planetes. 1998;326:445–451. [Google Scholar]
- 20.Sakai T, et al. Climate shift recorded at around 10 Ma in Miocene succession of Samburu Hills, northern Kenya Rift, and its significance. Geological Society, London, Special Publications. 2010;342(1):109–127. [Google Scholar]
- 21.McDougall I, Feibel CS. Numerical age control for the Miocene-Pliocene succession at Lothagam, a hominoid-bearing sequence in the northern Kenya Rift. J Geol Soc London. 1999;156:731–745. [Google Scholar]
- 22.Feibel C. Stratigraphy and depositional history of the Lothagam sequence. In: Leakey MG, Harris JM, editors. Lothagam: The Dawn of Humanity in Eastern Africa. New York: Columbia University Press; 2003. pp. 17–29. [Google Scholar]
- 23.Harrison T. Dendropithecoidea, Proconsuloidea, and Hominoidea. In: Werdelin L, Sanders WJ, editors. Cenozoic Mammals of Africa. Berkeley, CA: University of California Press; 2010. pp. 429–469. [Google Scholar]
- 24.Suwa G, Kono RT, Katoh S, Asfaw B, Beyene Y. A new species of great ape from the late Miocene epoch in Ethiopia. Nature. 2007;448:921–924. doi: 10.1038/nature06113. [DOI] [PubMed] [Google Scholar]
- 25.Leakey M, Walker A. The Lothagam hominids. In: Leakey MG, Harris JM, editors. Lothagam: The Dawn of Humanity in Africa. New York: Columbia University Press; 2003. pp. 249–257. [Google Scholar]
- 26.Stromberg CAE. Evolution of hypsodonty in equids: Testing a hypothesis of adaptation. Paleobiology. 2006;32:236–258. [Google Scholar]
- 27.Cerling T, Harris J, Leakey M. In: Environmentally Driven Dietary Adaptations in African Mammals. A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems, Ecological Studies. Baldwin IT, et al., editors. Vol. 177. Berlin: Springer; 2005. pp. 258–272. [Google Scholar]
- 28.Cerling T, Harris J. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia. 1999;120:347–363. doi: 10.1007/s004420050868. [DOI] [PubMed] [Google Scholar]
- 29.Tipple B, Meyers S, Pagani M. Carbon isotope ratio of Cenozoic CO2: A comparative evaluation of available geochemical proxies. Paleoceanography. 2010;25 [Google Scholar]
- 30.Tsujikawa H. The palaeoenvironment of Samburupithecus kiptalami based on its associated fauna. Afr Stud Monogr. 2005;(Suppl 32):51–62. [Google Scholar]
- 31.Leakey M, Harris J. Lothagam: The Dawn of Humanity in Eastern Africa. New York: Columbia University Press; 2003. [Google Scholar]
- 32.Cerling T, Harris J, Leakey M, Mudidia N. Stable isotope ecology of Northern Kenya, with emphasis on the Turkana Basin. In: Leakey MG, Harris JM, editors. Lothagam: The Dawn of Humanity in Eastern Africa. New York: Columbia University Press; 2003. pp. 583–603. [Google Scholar]
- 33.Fortelius M, Solounias N. Functional characterization of ungulate molars using the abrasion-attrition wear gradient: A new method for reconstructing paleodiets. Am Mus Novit. 2000;3301:1–36. [Google Scholar]
- 34.Nakaya H, et al. Late Miocene paleoenvironmental change of hominoid sites in Kenya: Mesowear analysis of Hipparion cheek teeth, paper presented at the 33rd. International Geological Congress at Oslo 2008. 2008 [Google Scholar]
- 35.Cerling TE, et al. Stable isotope ecology of the common hippopotamus. J Zool. 2008;276:204–212. [Google Scholar]
- 36.Harris J, Cerling T. Dietary adaptations of extant and Neogene African suids. J Zool. 2002;256:45–54. [Google Scholar]
- 37.Cerling T, Harris J, Leakey M. Browsing and grazing in elephants: The isotope record of modern and fossil proboscideans. Oecologia. 1999;120:364–374. doi: 10.1007/s004420050869. [DOI] [PubMed] [Google Scholar]
- 38.Sukumar R, Ramesh R. Elephant foraging: Is browse or grass more important? In: Daniel JC, Datye H, editors. A Week with Elephants. New Delhi: Bombay Natural History Society, Bombay and Oxford University Press; 1995. pp. 368–374. [Google Scholar]
- 39.Sanders W, Gheerbrant E, Harris J, Saegusa H, Delmer C. Proboscidea. In: Werdelin L, Sanders WJ, editors. Cenozoic Mammals of Africa. Berkeley, CA: University of California Press; 2010. pp. 161–251. [Google Scholar]
- 40.Aguirre E, Alberdi MT. Hipparion remains from the northern part of the Rift Valley; Kenya. Proc K Ned Akad Wet, B Palaeontol Geol Phys Chem. 1974;77:146–157. [Google Scholar]
- 41.Aguirre E, Leakey P. Nakali; nueva fauna de Hipparion del Rift Valley de Kenya. Estudios Geologicos (Madrid) 1974;30:219–227. [Google Scholar]
- 42.Aguirre E, Guerin C. First discovery of a Iranotheriinae (Mammalia, Perissodactyla, Rhinocerotidae) in Africa: Kenyatherium bishopi Nov. gen. Nov. sp. Training Vallesian (Upper Miocene) of Nakala (Kenya) (Translated from French) Estudios Geologicos (Madrid) 1974;30:229–233. [Google Scholar]
- 43.Flynn LJ, Sabatier M. A muroid rodent of Asian affinity from the Miocene of Kenya. J Vertebr Paleontol. 1984;3:160–165. [Google Scholar]
- 44.Benefit B, Pickford M. Miocene fossil cercopithecoids from Kenya. Am J Phys Anthropol. 1986;69:441–464. [Google Scholar]
- 45.Morales J, Pickford M. A large Percrocutid carnivore from the Late Miocene (ca. 10–9 Ma) of Nakali, Kenya. Annales de Paleontologie. 2006;92(4):359–366. [Google Scholar]
- 46.Nakaya H, Pickford M, Nakano Y, Ishida H. The late Miocene large mammal fauna from the Namurungule Formation, Samburu Hills, northern Kenya. Afr Stud Monogr. 1984;(Suppl 2):87–131. [Google Scholar]
- 47.Nakaya H, Pickford M, Yasui K, Nakano Y. Additional large mammalian fauna from the Namurungule Formation, Samburu Hills, northern Kenya. Afri Stud Monogr. 1987;(Suppl 5):79–130. [Google Scholar]
- 48.Nakaya H, Watade M. Hipparion from the upper Miocene Namurungule Formation, Samburu Hills, Kenya: Phylogenetic significance of newly discovered skull. Geobios. 1990;23:195–219. [Google Scholar]
- 49.Nakaya H. Faunal change of late Miocene Africa and Eurasia: Mammalian fauna from the Namurungule Formation, Samburu Hills, northern Kenya. Afri Stud Monogr. 1994;(Suppl 20):1–103. [Google Scholar]
- 50.Tsujikawa H. The updated late Miocene large mammal fauna from Samburu Hills, northern Kenya. Afr Stud Monogr. 2005;(Suppl 32):1–50. [Google Scholar]
- 51.Werdelin L, Sanders W. Cenozoic Mammals of Africa. University of California Press, Berkeley, CA; 2010. [Google Scholar]
- 52.Nakaya H, Tsujikawa H. Late Cenozoic mammalian biostratigraphy and faunal change. In: Ishida H, Tuttle R, Pickford M, Ogihara N, Nakatsukasa M, editors. Human Origins and Environmental Backgrounds. Berlin: Springer; 2006. pp. 59–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.