Abstract
A biomimetic hydrogel platform was designed to signal encapsulated cells using immobilized cell–cell communication cues, with a focus on enhancing the survival and function of encapsulated pancreatic β-cells to treat type 1 diabetes. When MIN6 cells, a pancreatic β-cell line, were encapsulated in poly(ethylene glycol) (PEG) hydrogels, their survival and glucose responsiveness to insulin were highly dependent on the cell-packing density. A minimum packing density of 107 cells/mL was necessary to maintain the survival of encapsulated β-cells without the addition of material functionalities (e.g., cell adhesion ligands). While single cell suspensions can improve diffusion-limited mass transfer, direct cell–cell interactions are limited. Thus, thiolated EphA5-Fc receptor and ephrinA5-Fc ligand were conjugated into PEG hydrogels via a thiol-acrylate photopolymerization to render an otherwise inert PEG hydrogel bioactive. The biomimetic hydrogels presented here can provide crucial cell–cell communication signals for dispersed β-cells and improve their survival and proliferation. Together with the cell-adhesive peptide RGDS, the immobilized fusion proteins (EphA5-Fc and ephrinA5-Fc) synergistically increased the survival of both MIN6 β-cells and dissociated islet cells, both at a very low cell-packing density (< 2 × 106 cells/mL). This unique gel platform demonstrates new strategies for tailoring biomimetic environments to enhance the encapsulation of cells that require cell–cell contact to survive and function.
Keywords: tissue engineering, cell encapsulation
Successfully designed biomaterials for cell delivery applications often integrate multiple factors, including cell–extracellular matrix (ECM) interactions, soluble biomolecules (e.g., growth factors, cytokines, etc.), and cell–cell interactions (1). Further, a material carrier should encourage the spatial and temporal presentation of these factors to the encapsulated cells. When engineering such synthetic microenvironments for cell delivery, poly(ethylene glycol) or PEG hydrogels are often considered advantageous, owing to their tunable and biocompatible material properties (2). The nonfouling properties of PEG materials provide a “blank slate” on which defined bioactive/functional motifs, such as peptides and proteins, can be easily incorporated without significantly affecting the bulk material properties. For example, cell-adhesive peptides (e.g., RGDS, IKVAV, etc.) have been routinely conjugated within synthetic PEG hydrogel networks to promote survival of anchorage-dependent cells (3, 4). Furthermore, hydrogels made from PEG macromers possess high permeability that permits the diffusion of small molecular weight nutrients and metabolic products that are critical in maintaining cell survival and function (2, 5).
While numerous hydrogel systems have been designed with the goal of promoting cell encapsulation (2, 6, 7), few strategies use material functionalities to mimic or recapitulate critical cell–cell interactions in a synthetic microenvironment. Cell–cell interactions are important for many cellular activities, ranging from directing differentiation during embryonic development to maintaining survival and function of certain cell types (e.g., pancreatic β-cells) (8, 9). In addition to direct cell–cell contact, cells also communicate with each other via paracrine signaling that can regulate cell fate in response to environmental stimuli (10, 11). In either case, a close proximity between cells is often the key to the survival, differentiation, and function of the individual cells.
Cell–cell interactions are mediated by cell surface receptors, and the direct effect of ligand-receptor binding is observed in intracellular signaling, which alters protein expression and subsequent cellular activities (12–15). Many cell surface receptors responsible for cell–cell interactions have been identified, including cadherins, connexins, gap junctions, Eph–ephrin, cell adhesion molecules (CAM), etc. Soluble formats of these receptors have been cloned and produced for studying mechanisms of intracellular signaling transduction. Some of these soluble receptors are in the form of fusion proteins, such as intercellular adhesion molecules 1 or ICAM-1 (16), E-cadherin (17), and ephrin–A1 (18) and have been used to create functional surfaces to enhance cell adhesion or to alter cell morphology.
Our group’s efforts have focused on creating functional PEG hydrogels to enhance the survival and function of encapsulated β-cells (3, 19) and to reduce proinflammatory cytokine-induced cellular damage (20). These gel formulations, however, lack the ability to recapitulate important cell–cell interactions, a critical characteristic required for maintaining β-cell survival and function in 3D. Gels functionalized with ECM components or peptide mimetics partially restore the viability of encapsulated β-cell (3, 21), but these gels cannot restore normal β-cell communication. In a recent publication, Konstantinova et al. showed that EphA–ephrinA binding regulates normal insulin secretion in pancreatic β-cells (22) through balancing EphA forward signaling and ephrinA reverse signaling. Furthermore, EphA–ephrinA signaling has been linked to various intracellular pathways related to cell survival [e.g., Phosphatidylinositol 3-kinases (PI-3K), focal adhesion kinases (FAK), or mitogen-activated protein kinases (MAPK) pathways] in many cell types (23–25), including neuronal cells and vascular endothelial cells. Similar signaling events, however, have yet to be demonstrated in pancreatic β-cells.
The central hypothesis for this study is that PEG hydrogels can be functionalized with molecules that mimic critical aspects of cell–cell communication and increase the survival and function of encapsulated β-cells. Due to its diverse role in many biological processes, we believe that EphA–ephrinA signaling not only regulates normal insulin secretion but also promotes β-cell communication and hence enhances their survival in PEG hydrogels. Thus, the aims of this study were to: (i) investigate the effects of β-cell-packing density in PEG hydrogels on their survival and insulin secretion following photo-encapsulation and in vitro culture; and (ii) fabricate biomimetic EphA–ephrinA dually functionalized PEG hydrogels via thiol-acrylate photopolymerization for enhancing β-cell encapsulation. EphA5 and ephrin–A5 fusion proteins were used in this study due to their known bidirectional signaling in β-cells (22).
Results
Effects of Cell-Packing Density on β-Cell Survival in Photopolymerized PEG Hydrogels.
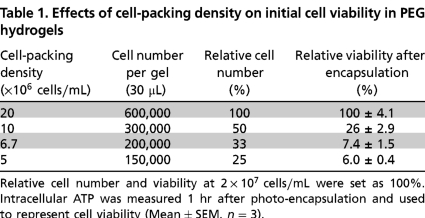
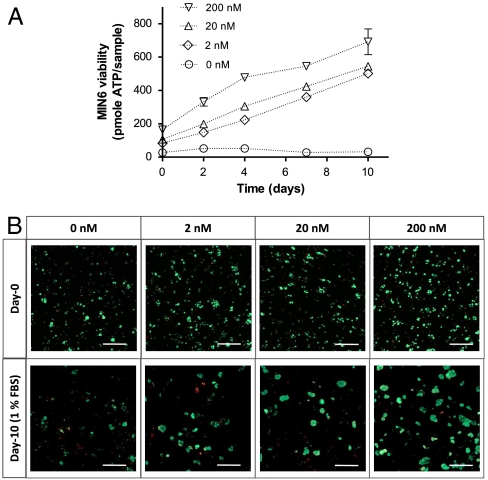
To reveal the importance of cell–cell interactions on maintaining β-cells survival in synthetic PEG hydrogels, we encapsulated MIN6 β-cells at various packing densities and monitored their survival. The latter was characterized via quantitative metabolic activity measurements, including intracellular ATP concentrations (Celltiter Glo® reagent) and qualitative live/dead staining and imaging. As shown in Table 1, the survival of MIN6 cells after photo-encapsulation depends largely on the initial cell-packing density. In Table 1, the relative cell number and viability (determined by ATP measurement) at a packing density of 2 × 107 cells/mL were set as 100%. When the cell-packing density was adjusted to 50% (1 × 107 cells/mL), 33% (6.7 × 106 cells/mL), or 25% (5 × 106 cells/mL), the corresponding cell survival decreased to 26%, 7.4%, or 6.0%, respectively. These results suggest a critical role of cell–cell contact and/or paracrine signaling for cell survival and function, which is severely limited at the lower cell-packing densities. Cells at relatively high densities (2 × 107 cells/mL) not only survived the photo-encapsulation process but also formed cell aggregates in the PEG gels, even at low serum conditions (1% FBS), for the duration of the experiment (Fig. 1A). Although cells encapsulated at 1 × 107 cells/mL appeared to have maintained intracellular ATP (Fig. 1A), it appeared to be a result of a few, sporadic, surviving cell clusters (Fig. 1B). Compared to culturing in low serum media, cell-laden hydrogels maintained in media containing 10% FBS showed even higher viability (Fig. S1). The differences in cell viability at various cell-packing densities were not likely due to the altered gel cross-linking densities. As shown in Table S1, the volume of cells at the highest density (2 × 107 cells/mL) tested was approximately 4% of the total gel volume. This also represents a 4% increase in the effective acrylate concentration, a factor that contributes to an increased polymerization rate that may affect cell viability during photo-encapsulation. To test this possibility, control experiments using hydrogels crosslinked from 9.6 wt%, 10 wt%, or 10.4 wt% PEGDA macromer revealed no statistical difference in cell viability following the photo-encapsulation procedure (Fig. S2). Overall, this study demonstrates a slight variation in gel cross-linking density does not significantly affect encapsulated cell viability in PEG hydrogels.
Table 1.
Effects of cell-packing density on initial cell viability in PEG hydrogels
Relative cell number and viability at 2 × 107 cells/mL were set as 100%. Intracellular ATP was measured 1 hr after photo-encapsulation and used to represent cell viability (Mean ± SEM, n = 3).
Fig. 1.
Effects of cell-packing density on the survival of MIN6 cells in PEG hydrogels. MIN6 cells were dispersed into single cells and encapsulated at different cell densities as indicated. Cell-laden hydrogels were maintained in RPMI-1640 medium containing 1% FBS. (A) Intracellular ATP of encapsulated MIN6 cells was measured at the indicated time after encapsulation and was used to represent cell metabolic activity and viability (Mean ± SEM, n = 3). (B) Representative confocal Z-stack (300 μm) images of encapsulated MIN6 cells stained with a live/dead viability staining kit at day-0 and day-10. Live cells were stained green while dead cells were stained red (scale: 200 μM).
Effects of Cell-Packing Density on Glucose-Stimulated Insulin Secretion.
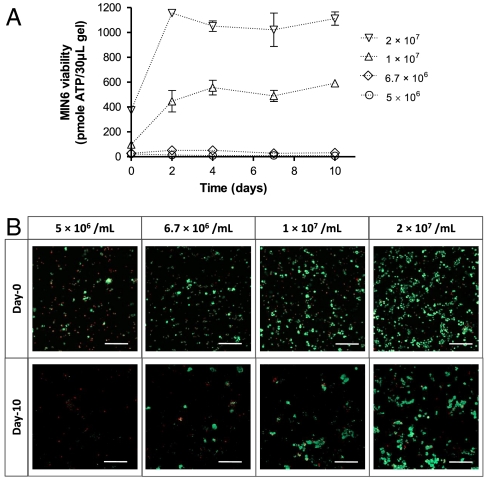
Fig. 2 reports insulin secretion from MIN6 cells encapsulated in PEG hydrogels at different packing densities. Insulin secreted from MIN6 cells encapsulated at 6.7 × 106 cells/mL appeared to respond to glucose concentration negatively, indicating that cell–cell contact is critical in maintaining normal glucose responsive insulin secretion. On the other hand, MIN6 cells encapsulated at higher cell-packing densities secreted higher amounts of insulin at higher glucose concentrations, as demonstrated by higher insulin secretion indexes.
Fig. 2.
Effects of MIN6 cell-packing density in PEG hydrogels on glucose responsive insulin secretion. MIN6 cells were dispersed into single cells and encapsulated at different cell densities as indicated. Cell-laden hydrogels were maintained in RPMI-1640 medium containing 10% FBS for 10 days before insulin secretion was tested in Krebs–Ringer buffer solution (KRB). The amount of insulin secreted from samples incubated in 25 mM glucose KRB was normalized to the amount of insulin secreted from the respective samples incubated in 2 mM glucose KRB and expressed as an insulin secretion index (Mean ± SEM, n = 4). All values of the insulin secretion index were significantly different from 1 (p < 0.05), with & indicating lower and * indicating higher than 1.
Protein-Functionalized Biomimetic PEG Hydrogels Enhance β-cell Survival.
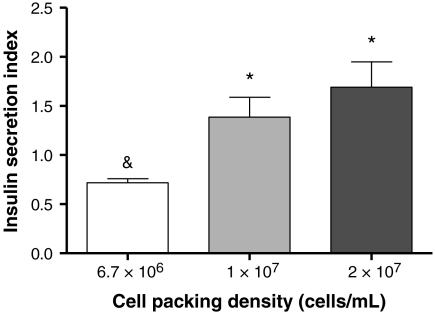
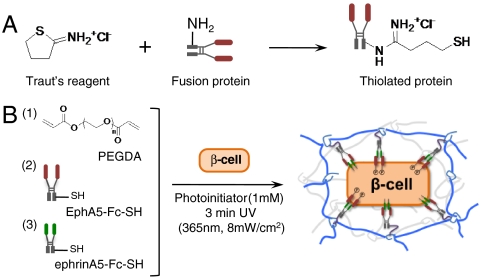
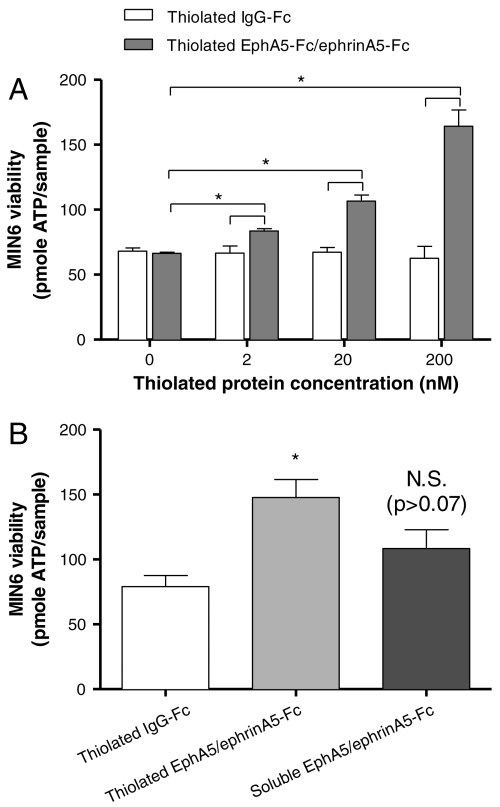
Soluble fusion proteins including IgG-Fc, EphA5-Fc, and ephrinA5-Fc were thiolated using Traut’s reagent (Scheme 1A). The degree of thiolation was quantified with Ellman’s reagent and was found to be approximately 2 thiols per fusion protein. Thiolated fusion proteins were conjugated in PEG hydrogels via a thiol-acrylate photopolymerization mechanism, following established protocols (Scheme 1B). MIN6 cells at 6.7 × 106 cells/mL were encapsulated in PEG hydrogels functionalized with fusion proteins, and their initial viability was quantified via ATP assay. Fig. 3A shows that the metabolic activity/viability of encapsulated MIN6 cells increased significantly with increasing concentrations of the functionally relevant thiolated proteins (EphA5–Fc and ephrinA5–Fc) in PEGDA hydrogels, while cell metabolic activity was not increased by the incorporation of a control thiolated fusion protein, IgG–Fc. These interesting results illustrate how materials-directed presentation of EphA5 receptor and ephrinA5 ligand can enhance β-cell survival in photopolymerized hydrogels, presumably by mimicking key cell–cell interactions. Additional control experiments using equivalent concentrations (200 nM total) of nonthiolated (soluble) EPhA5/ephrinA5–Fc did not enhance the viability of encapsulated MIN6 cells significantly, likely due to the diffusion of the untethered fusion proteins from the gels prior to quantification (Fig. 3B).
Scheme 1.
(A) Conjugation schemes to synthesize thiolated EphA5-Fc and ehprinA5-Fc. (B) Thiol-acrylate photopolymerization used to fabricate cell-laden biomimetic hydrogels.
Fig. 3.
(A) Effects of EphA5–Fc and ephrinA5–Fc immobilization on viability of MIN6 cells in PEG hydrogels. MIN6 cells were encapsulated at 6.7 × 106/mL. (A) Intracellular ATP of encapsulated MIN6 cells was quantified 1 hr after encapsulation. Hydrogels immobilized with Fc protein were used as controls (Mean ± SEM, n = 3 or 4). (B) Initial viability of MIN6 cells encapsulated (6.7 × 106 cells/mL) in PEG hydrogels functionalized with 200 nM Fc (white bar), 200 nM total of thiolated (light gray bar), or nonthiolated EphA5–Fc and ephrin–A5–Fc (dark gray bar). Asterisks indicate statistical significance between assigned groups (* p < 0.05) (Mean ± SEM, n = 3).
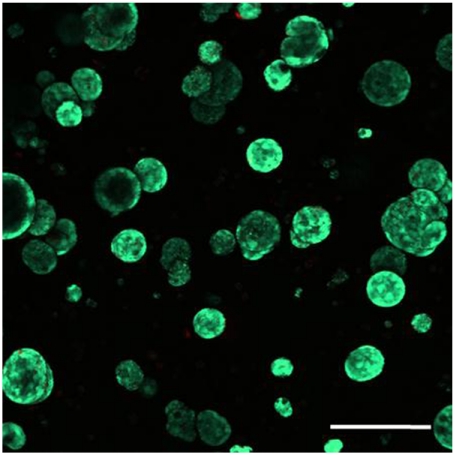
Confocal stacking images of cell-laden hydrogels dually stained with calcein AM (live cells) and ethidium bromide (dead cells) revealed a higher percentage of live cells in the biomimetic hydrogels immobilized with high EphA5/ephrinA5 hydrogels (Fig. 4B) compared to controls. Hydrogels dually functionalized with EphA5–Fc and ephrinA5–Fc also support the proliferation of encapsulated MIN6 cells under low serum (1% FBS) condition (Fig. 4). Fig. 5 shows the formation of spherical MIN6 β-cell aggregates in EphA5/ephrinA5–Fc (100 nM each) dually functionalized PEG hydrogels after 21 d of in vitro culture (MIN6 cells were encapsulated at an initial density of 6.7 × 106 cells/mL).
Fig. 4.
(A) Intracellular ATP of encapsulated MIN6 cells at the indicated time after encapsulation as a measure of cell viability (Mean ± SEM, n = 3). (B) Representative confocal Z-stack (300 μm) images of encapsulated MIN6 cells stained with live/dead viability staining kit in EphA5/ephrinA5 dual immobilized hydrogels with indicated concentrations. Live cells stained green while dead cells stained red (scale: 200 μM).
Fig. 5.
Representative confocal Z-stack (300 μm) image of encapsulated MIN6 cells stained with live/dead viability staining kit 21 days after encapsulation. Thiolated EphA5/ephrinA5–Fc: 200 nM (1∶1). Initial MIN6 cell-packing density: 6.7 × 106 cells/mL. Live cells were stained green while dead cells were stained red (scale: 200 μM).
Protein-Functionalized Biomimetic PEG Hydrogels Enhance Insulin Secretion.
Fig. S3 summarizes results from the glucose-stimulated insulin secretion from MIN6 cells encapsulated in control (thiolated IgG–Fc) or biomimetic (Thiolated EphA5/ephrinA5–Fc) gels. A higher insulin secretion index was measured from cells encapsulated in biomimetic gels, indicating that protein-presenting gels not only promote the survival of the dispersed MIN6 cells but also enhance their glucose-dependent insulin secretion. It is worth noting that the enhancement did not reach the same level as in the case where cells formed natural aggregates at higher packing density (Fig. 2). We believe that the intricate and complex cell–cell interactions that regulate insulin secretion from β-cells cannot be completely reestablished using EphA–ephrinA engineered hydrogels. Our approach, however, provides a foundation on which the survival and β-cells can be enhanced.
Synergistic Effects of Cell–ECM and Cell–Cell Interactions in Promoting β-cell Survival.
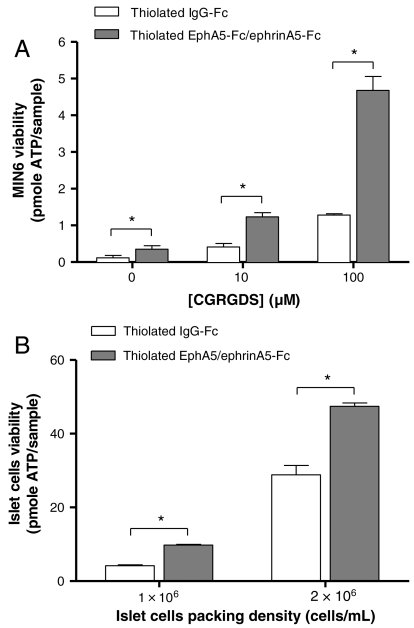
Hydrogels functionalized with defined compositions of both cell-adhesive peptides (CGRGDS) and thiolated fusion proteins (EphA5–Fc and ephrinA5–Fc) were used to encapsulate dispersed MIN6 β-cells or digested islet cells. To isolate the prosurvival effects of cell–ECM interactions from actual cell–cell contact, a very low cell encapsulation density (2 × 106 cells/mL) was used. As shown in Fig. 6A, the incorporation of the cell-adhesive peptide, CGRGDS, within PEG hydrogels significantly enhanced the survival of MIN6 β-cells in a dose-dependent manner (white bars). Further enhancement in MIN6 cell viability at all RGD concentrations was observed when 100 nM EphA5–Fc and 100 nM ephrinA5–Fc were incorporated (gray bars). Note that the concentrations of RGD used in the hydrogels were 3 orders of magnitude higher than the EphA5 and ephrinA5 proteins. Control experiments using immobilized RDG peptide show no enhancement in cell viability as determined by the Alamarblue cell metabolic activity assay (Fig. S4).
Fig. 6.
(A) Initial viability of MIN6 cells encapsulated (2 × 106 cells/mL) in PEG hydrogels functionalized with different concentrations of CGRGDS and either 200 nM Fc (white bars) or 100 nM EphA5–Fc and 100 nM ephrin–A5–Fc (gray bars). Asterisks indicate statistical significance between assigned groups (* p < 0.05) (Mean ± SEM, n = 3). (B) Initial viability of dissociated islet cells encapsulated in PEG hydrogels functionalized with 100 μM CGRGDS and 200 nM thiolated IgG–Fc or thiolated EphA5–Fc and ephrin–A5–Fc (1∶1). Asterisks indicate statistically significance between assigned groups (* p < 0.05) (Mean ± SEM, n = 3).
EphA5/ephrinA5 Promotes the Survival of Dissociated Islet Cells.
Fig. 6B shows the synergistic effects of cell–ECM and cell–cell interactions on dissociated islet cells survival. All hydrogels were functionalized with 100 μM CGRGDS, as well as 200 nM thiolated IgG-Fc or EphA5/ephrinA5–Fc (1∶1). To fabricate sufficient numbers of gels for analysis and to exclude the effects of actual cell–cell contact, low islet cell densities (1 × 106 or 2 × 106 cells/mL) were used. Similar to Fig. 6A, a synergistic effect between cell–ECM and biomimetic cell–cell interactions on promoting dissociated islet cell survival was observed. Qualitative live/dead staining and imaging results also support the enhanced survival of dissociated islet cells (Fig. S5).
Discussion
The survival and function of pancreatic β-cells rely heavily on cell–cell and/or cell–ECM interactions (9, 26). While previous studies using β-cells cultured on tissue culture plates have revealed the importance of cell–cell contact in maintaining normal β-cells phenotype, these studies do not recapitulate critical aspects of the three-dimensional organization of β-cell aggregates. Studies using “pseudoislets” produced by controlled β-cell aggregation have also demonstrated important aspects of microenvironmental effects on β-cell survival and function (27, 28). The understanding of critical cell–ECM and cell–cell interactions is the key to the engineering of improved biomimetic hydrogel carriers suitable for encapsulation of anchorage-dependent cells requiring cell–cell contact, including pancreatic β-cells.
Previously, Weber et al. revealed the importance of cell–ECM interactions on promoting the survival of pancreatic β-cells in synthetic PEG hydrogels (3, 21, 29). The “blank slate” nature of PEG hydrogels allows researchers to selectively and systematically decorate an inert and synthetic gel environment with defined ECM molecules from which to understand the distinct role of selected biomolecules on cell survival and function. Previous studies have concluded that without cell–ECM interactions, dispersed β-cells do not survive in synthetic hydrogels (3, 19, 30). While current results partly agree with this finding, we also found that MIN6 β-cells survived and proliferated when encapsulated in PEG hydrogels at sufficiently high cell-packing density (Table 1 and Fig. 1). Surprisingly, previous studies on β-cell encapsulations have been conducted primarily at very low cell-packing densities (< 106 cells/mL) and focus on the effects of other parameters/functionalities on β-cell survival and function (3, 19, 30, 31). A study from the Desai’s group did explore the effect of β-cell density on insulin secretion in a macroencapsulation device consisting of collagen gel enclosed by a membrane (32). However, because collagen is inherently bioactive due to the presence of cell binding motifs, it was difficult to isolate the specific effect of cell–cell interactions from cell–matrix contact. This study also did not quantitatively examine the effect of cell-packing density on cell survival. Our studies suggest that decreased cell survival at low cell-packing densities may be attributed to: (i) loss of actual cell–cell contact, (ii) increased diffusion distance for paracrine signaling, and (iii) photopolymerization induced cell damage. While in this study we did not explicitly delineate one from the others, increasing cell-packing density indeed improved the survival of encapsulated β-cells. We confirmed the enhanced survival quantitatively and qualitatively (Celltiter Glo assay and live/dead staining). Although MIN6 cells used in this study, regardless of cell-packing density, were dissociated into single cell suspension prior to encapsulation, increasing the initial cell-packing density at the time of encapsulation increases the probability of cell–cell contact and improves cell survival. Increasing cell-packing density also decreases the distance between cells and hence increases paracrine signaling, another mechanism proven to be important in β-cell physiology (33, 34). Nonetheless, current results reemphasize the importance of cell–cell interactions on maintaining β-cell survival in 3D hydrogels.
Providing β-cells with sufficient cell–cell interactions (i.e., high cell-packing density) is not only critical for maintaining their survival in 3D but also enhances their responsiveness to glucose, as demonstrated by an increased insulin secretion index (Fig. 2). When encapsulated at lower cell-packing densities (< 1 × 107 cells/mL), MIN6 β-cells failed to secrete higher amounts of insulin in high glucose buffer solutions. Previous studies have shown that when cultured on tissue culture plates at low seeding density, β-cells lose normal glucose and insulin homeostasis (22). Without normal and sufficient cell–cell communication, β-cells secrete more insulin at low glucose concentrations, and vice versa. The low insulin secretion index (< 1) obtaining from MIN6 cells encapsulated in PEG hydrogels at a low cell-packing density (6.7 × 106 cells/mL) agrees with prior 2D studies.
Toward creating a functional hydrogel system that supports the survival and function of pancreatic β-cells, we have designed several hydrogel platforms, including the immobilization of glucagon-like peptide 1 (GLP-1) to enhance β-cell encapsulation (19), and the conjugation of affinity peptides capable of sequestering cytokine (20) or chemokines (31) to improve immunoisolation of hydrogels. Here, we further expand the functionalities of synthetic hydrogels by including critical cell–cell interaction signals—namely, EphA–ephrinA binding in photopolymerized PEG hydrogels. The signaling of EphA receptor and ephrinA ligand (both are cell surface-bound proteins) binding has been studied mostly in neuronal, vascular, and tumorous cells (23). Recent reports illustrate that EphA–ephrinA bidirectional binding also regulates normal insulin secretion from pancreatic β-cells (22). Furthermore, binding of Eph receptors and ephrin ligands has been shown to activate integrin signaling and cell survival pathways (e.g., MAPK, FAK, PI3K, etc.) (23–25). For example, ephrinA signaling activates integrin pathway, hence increasing cell adhesion or changing their morphology and motility (35–37). EphA–ephrinA binding also induces integrin clustering for cell segregation during development (38). We reasoned that Eph–ephrin binding might enhance integrin signaling induced by cell-adhesive ligands (e.g., RGDS), thus increasing β-cell survival in synthetic hydrogels. Indeed, enhanced survival of MIN6 β-cells in EphA5/ephrinA5 dually functionalized PEG hydrogels was observed immediately following photo-encapsulation (Figs. 3 and 4). At 6.7 × 106 cells/mL (cell-packing density), MIN6 cells did not survive in nonfunctionalized PEG hydrogels over the course of 10 d in vitro culture, a result consistent with previous studies conducted at low cell-packing density. In the presence of EPhA5/ephrinA5–Fc, however, encapsulated cells at the same packing density not only survived the photo-encapsulation procedure but also proliferated under both normal (10%) and low (1%) serum conditions. The immobilization of EphA5/ephrinA5 in the hydrogel network is critical in maintaining sustained signals to the encapsulated β-cells, because soluble EphA5–ephrinA5–Fc fails to elicit same level of enhanced survival (Fig. 3B). Although the PEG hydrogels used in this study did not contain immediately degradable linkers (for the duration of the experiments), MIN6 β-cells proliferate and form spherical cell aggregates (Figs. 1B, 4B, and 5). We believe that the additional space for β-cells growth was created as a result of: (i) high degree of gel swelling, and (ii) proliferating cells “push” away the elastic PEG polymer chains surrounding the cell clusters, as has been observed with neurosphere cultures in degradable PEG hydrogels (39). Potentially, this culture platform can also be utilized to expand β-cell mass when alternative cell sources for β-cell transplantation become available (40).
The major benefit of using PEG hydrogels to study cell–ECM and cell–cell interactions is that specific and defined components of cell-signaling molecules can be conjugated. We found that at concentrations below 10 μM, RGDS has no effect in enhancing MIN6 cell survival. In contrast, EphA5/ephirn–A5 significantly increased the survival of MIN6 cells at nano-molar concentrations, suggesting that cell–cell interactions may be more potent than cell-ECM interactions in influencing pancreatic β-cells. Furthermore, RGDS (at micro-molar concentrations) and EphA5/ephrinA5–Fc (at nano-molar concentrations) synergistically increased β-cell survival. These results are valid not only for immortalized MIN6 β-cells (Fig. 6A) but also for dissociated primary islet cells (Fig. 6B) encapsulated at a very low cell density where no direct cell–cell contact is present in the PEG hydrogels.
While islets in the pancreas contain extensive capillaries that support cell survival, these capillaries are lost following islet isolation. Islet cells located at the interior of larger clusters (diameter > 150 μm) often suffer from hypoxia and lack of nutrient supply and become apoptotic or necrotic during in vitro culture and following transplantation (41, 42). To overcome this limitation, methodologies for islet digestion and reaggregation have been utilized to form smaller “pseudoislets” (43). Our biomimetic hydrogel platform may provide suitable 3D scaffolding materials for directing cell expansion and reaggregation due to the ability to conjugate defined signaling cues critical for cell–ECM and cell–cell interactions. While the biomimetic strategy presented herein is not likely to compensate completely for the benefits of actual cell–cell contact, we believe this is a striking example of using materials design to recapitulate critical aspects of cell–cell signaling. Furthermore, this strategy may be useful in creating an artificial “niche” to regulate stem/progenitor cell fate in 3D. For example, stem/progenitor cells can be encapsulated as dispersed cells while still receiving synergistic signals from ECM and cell–cell contact mimetic motifs conjugated in hydrogels. This approach may provide a more uniform exposure to culture conditions (e.g., soluble growth factor) to the encapsulated stem/progenitor cells for the purpose of cell expansion and/or differentiation.
Materials and Methods
Materials.
Fusion proteins including IgG–Fc, EphA5–Fc, and ephrinA5–Fc were obtained from R&D Systems. 2-Iminothiolane-HCl (Traut’s reagent) was purchased from Thermo Fisher Scientific. Cell culture media and reagents were obtained from Invitrogen unless otherwise noted. Celltiter Glo® viability kit was obtained from Promega. LIVE/DEAD® viability/cytotoxicity kit and Almarblue® reagent were obtained from Invitrogen. The mouse insulin ELISA kit was supplied by Mercodia Inc. All other chemicals were purchased from Sigma-Aldrich unless noted otherwise.
Thiolation of Receptors and Synthesis of Biomimetic PEG Hydrogel.
Free thiol groups were introduced onto fusion proteins (IgG–Fc, EphA5–Fc, and ephrinA5–Fc) using 2-Iminothiolane-HCl (Traut’s reagent). Briefly, fusion proteins were dissolved in PBS containing 2 mM EDTA. Traut’s reagent (fourfold molar excess) was added to the fusion protein solution and incubated for 1 h at room temperature. Excess Traut’s reagent was separated from the thiolated proteins using a desalting column (PIERCE). The degree of sulfation (sulfhydryl groups per protein) was determined using Ellman’s assay according to the manufacturer’s protocol.
Poly(ethylene glycol) diacrylate (PEGDA: M.W. 10 kDa) macromer (44, 45), as well as LAP photoinitiator (lithium phenyl-2,4,6-trimethylbenzoylphosphinate or lithium acylphosphinate) (31, 46) were synthesized according to published protocols. Biomimetic PEGDA hydrogels immobilized with thiolated receptors were synthesized using a mixed mode thiol-acrylate photopolymerization as described previously (19, 47).
Cell Culture and Photo-Encapsulation.
MIN6 cells, a mouse insulinoma cell line, were maintained in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 0.2% Fungizone. Medium was changed every 2–3 d. For photo-encapsulation into PEGDA hydrogels, MIN6 cells were trypsinized from tissue culture flasks and resuspended in Hank’s Balanced Salt Solution (HBSS) at a predetermined cell density. Prepolymer solutions containing PEGDA macromer, LAP photoinitiator, and thiolated proteins were prepared in HBSS in which the resuspended cells were added. Cell encapsulation was performed, under 3 min UV exposure (365 nm, 10 mW/cm2), in 1 mL sterile syringes with blunted tips to allow for easy solution loading and gel retrieval. Following photo-encapsulation, cell-laden gels were washed in fresh media for 1 h at 37 °C to remove any extractables (e.g., the sol fraction) and then maintained in culture media containing 1% FBS.
Cell Viability Assays, Confocal Microscopy, Insulin Secretion, Islet Cell Dissociation, and Statistics.
These methods can be found in SI Text.
Conclusion
Using PEG hydrogels as a β-cell culture platform, we show that cell–cell interactions are critical in maintaining β-cells survival and normal insulin secretion. Our results also demonstrate that through proper material design, it is possible to incorporate not only ECM components but also cell–cell communication signals to synergistically increase the survival and function of encapsulated anchorage-dependent cells. This previously undescribed biomaterial platform should be readily adapted to include other important cell-signaling proteins, as well as for encapsulation of other anchorage-dependent cell types.
Supplementary Material
Acknowledgments.
The authors thank Mr. Philip Pratt (Barbara Davis Center for Childhood Diabetes) for isolating mouse islets, as well as Dr. Alex Aimetti for assistance in peptide synthesis. This work was supported by the Howard Hughes Medical Institute, the National Institutes of Health (R01DK076084), the Colorado Clinical and Translational Sciences Institute (CO-Pilot award to C.L.), and a Research Support Funds Grant from Indiana University–Purdue University at Indianapolis.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014026108/-/DCSupplemental.
References
- 1.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 2.Lin C-C, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber LM, et al. The effects of cell–matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004–3011. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 5.Lin C-C, Metters AT. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv Drug Deliver Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 7.Shoichet MS. Polymer scaffolds for biomaterials applications. Macromolecules. 2010;43:581–591. [Google Scholar]
- 8.Kohen E, Kohen C, Rabinovitch A. Cell-to-cell communication in rat pancreatic-islet monolayer-cultures is modulated by agents affecting islet-cell secretory activity. Diabetes. 1983;32:95–98. doi: 10.2337/diab.32.1.95. [DOI] [PubMed] [Google Scholar]
- 9.Luther MJ, et al. Cell-to-cell contact influences proliferative marker expression and apoptosis in MIN6 cells grown in islet-like structures. Am J Physiol-Endoc M. 2005;288:E502–E509. doi: 10.1152/ajpendo.00424.2004. [DOI] [PubMed] [Google Scholar]
- 10.Beck L, Damore PA. Vascular development: Cellular and molecular regulation. Faseb J. 1997;11:365–373. [PubMed] [Google Scholar]
- 11.Brogi E, et al. Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J Clin Invest. 1996;97:469–476. doi: 10.1172/JCI118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatia SN, et al. Effect of cell–cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. Faseb J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 13.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell B. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Bi. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 15.Rogers GJ, Hodgkin MN, Squires PE. E-cadherin and cell adhesion: A role in architecture and function in the pancreatic islet. Cell Physiol Biochem. 2007;20:987–994. doi: 10.1159/000110459. [DOI] [PubMed] [Google Scholar]
- 16.Hume PS, Anseth KS. Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations. Biomaterials. 2010;31:3166–3174. doi: 10.1016/j.biomaterials.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue XS, et al. A fusion protein N-cadherin-Fc as an artificial extracellular matrix surface for maintenance of stem cell features. Biomaterials. 2010;31:5287–5296. doi: 10.1016/j.biomaterials.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Moon JJ, Lee SH, West JL. Synthetic biomimetic hydrogels incorporated with Ephrin-A1 for therapeutic angiogenesis. Biomacromolecules. 2007;8:42–49. doi: 10.1021/bm060452p. [DOI] [PubMed] [Google Scholar]
- 19.Lin C-C, Anseth KS. Glucagon-like peptide-1functionalized PEG hydrogels promote survival and function of encapsulated pancreatic beta-cells. Biomacromolecules. 2009;10:2460–2467. doi: 10.1021/bm900420f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C-C, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNF alpha. Biomaterials. 2009;30:4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber LM, Hayda KN, Anseth KS. Cell–matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Pt A. 2008;14:1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konstantinova I, et al. EphA-ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129:359–370. doi: 10.1016/j.cell.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Murai KK, Pasquale EB. ‘Eph’ective signaling: Forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 24.Aoki M, Yamashita T, Tohyama M. EphA receptors direct the differentiation of mammalian neural precursor cells through a mitogen-activated protein kinase-dependent pathway. J Biol Chem. 2004;279:32643–32650. doi: 10.1074/jbc.M313247200. [DOI] [PubMed] [Google Scholar]
- 25.Holen HL, et al. Signaling through ephrin-A ligand leads to activation of Src-family kinases, Akt phosphorylation, and inhibition of antigen receptor-induced apoptosis. J Leukocyte Biol. 2008;84:1183–1191. doi: 10.1189/jlb.1207829. [DOI] [PubMed] [Google Scholar]
- 26.Bosco D, et al. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro—role of alpha 6 beta 1 integrin. Diabetes. 2000;49:233–243. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- 27.Hauge-Evans AC, et al. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli—enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes. 1999;48:1402–1408. doi: 10.2337/diabetes.48.7.1402. [DOI] [PubMed] [Google Scholar]
- 28.Luther MJ, et al. MIN6 beta-cell-beta-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem Bioph Res Co. 2006;343:99–104. doi: 10.1016/j.bbrc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Weber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667–673. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su J, et al. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31:308–314. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin C-C, et al. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. J Control Release. 2010;142:384–391. doi: 10.1016/j.jconrel.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.La Flamme KE, et al. The effects of cell density and device arrangement on the behavior of macroencapsulated beta-cells. Cell Transplant. 2007;16:765–774. doi: 10.3727/000000007783465262. [DOI] [PubMed] [Google Scholar]
- 33.Ilieva A, et al. Pancreatic islet cell survival following islet isolation: The role of cellular interactions in the pancreas. J Endocrinol. 1999;161:357–364. doi: 10.1677/joe.0.1610357. [DOI] [PubMed] [Google Scholar]
- 34.Hardikar AA, et al. Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc Natl Acad Sci USA. 2003;100:7117–7122. doi: 10.1073/pnas.1232230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huai JS, Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J Biol Chem. 2001;276:6689–6694. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki T, et al. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J Cell Sci. 2009;122:243–255. doi: 10.1242/jcs.036467. [DOI] [PubMed] [Google Scholar]
- 37.Sharfe N, et al. EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol Immunol. 2008;45:1208–1220. doi: 10.1016/j.molimm.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Julich D, et al. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- 39.Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27:2265–2274. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Narushima M, et al. A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol. 2005;23:1274–1282. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann R, et al. Superiority of small islets in human islet transplantation. Diabetes. 2007;56:594–603. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 42.MacGregor RR, et al. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol-Endoc M. 2006;290:E771–E779. doi: 10.1152/ajpendo.00097.2005. [DOI] [PubMed] [Google Scholar]
- 43.Cavallari G, et al. Assembly of standardised pancreatic islets by the “hanging drop”-technology: Improved secretory function of small pseudoislets. Diabetologia. 2007;50:S200–S200. [Google Scholar]
- 44.Lin C-C, Metters AT. Metal-chelating affinity hydrogels for sustained protein release. J Biomed Mater Res A. 2007;83A:954–964. doi: 10.1002/jbm.a.31282. [DOI] [PubMed] [Google Scholar]
- 45.Lin C-C, Anseth KS. Controlling affinity binding with peptide-functionalized poly(ethylene glycol) hydrogels. Adv Funct Mater. 2009;19:2325–2331. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fairbanks BD, et al. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30:6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salinas CN, Anseth KS. Mixed mode thiol-acrylate photopolymerizations for the synthesis of PEG-peptide hydrogels. Macromolecules. 2008;41:6019–6026. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.