Abstract
Directed cell migration is a prerequisite not only for the development of the central nervous system, but also for topically restricted, appropriate immune responses. This is crucial for host defense and immune surveillance. Attracting environmental cues guiding leukocyte cell traffic are likely to be complemented by repulsive cues, which actively abolish cell migration. One such a paradigm exists in the developing nervous system, where neuronal migration and axonal path finding is balanced by chemoattractive and chemorepulsive cues, such as the neuronal repulsive guidance molecule-A (RGM-A). As expressed at the inflammatory site, the role of RGM-A within the immune response remains unclear. Here we report that RGM-A (i) is expressed by epithelium and leukocytes (granulocytes, monocytes, and T/B lymphocytes); (ii) inhibits leukocyte migration by contact repulsion and chemorepulsion, depending on dosage, through its receptor neogenin; and (iii) suppresses the inflammatory response in a model of zymosan-A–induced peritonitis. Systemic application of RGM-A attenuates the humoral proinflammatory response (TNF-α, IL-6, and macrophage inflammatory protein 1α), infiltration of inflammatory cell traffic, and edema formation. In contrast, the demonstrated anti-inflammatory effect of RGM-A is absent in mice homozygous for a gene trap mutation in the neo1 locus (encoding neogenin). Thus, our results suggest that RGM-A is a unique endogenous inhibitor of leukocyte chemotaxis that limits inflammatory leukocyte traffic and creates opportunities to better understand and treat pathologies caused by exacerbated or misdirected inflammatory responses.
Guiding migrating cells toward their destination is of prime importance in cell biology and physiology and is a prerequisite for tissue homeostasis, host defense, and immune surveillance (1, 2). Attracting cues, such as chemokines to guide leukocyte cell traffic, have been well characterized in recent years (3–5) and are likely to be complemented by repulsive cues, which actively abolish cell migration. One such a paradigm exists in the developing nervous system, where neuronal migration is regulated by chemoattractive and chemorepulsive cues, such as the neuronal repulsive guidance molecule A (RGM-A). The migration of leukocytes is a tightly orchestrated and controlled multiphasic biological cascade elicited by chemotactic signals during an acute inflammatory process (3, 4). Excessive immune cell migration into an inflammatory site might result in tissue destruction and organ dysfunction (functio laesa). Recently, neuronal guidance cues were identified that control and attenuate inappropriate migration and infiltration of leukocytes into acutely inflamed areas (6–12). Given that the neuronal repellent RGM-A is expressed at inflammatory sites but shares no sequence homology with any other known guidance cues, we explored whether it inhibits leukocyte migration in vitro and in vivo (7, 12, 13).
RGM was the first candidate for characterization as a topographic guidance cue and was originally described as a glycosylphosphatidylinositol-anchored proline- and cysteine-rich glycocprotein (13). Vertebrates comprise three homologs of RGM: RGM-A, RGM-B (DRAGON), and RGM-C (hemojuvelin). The human RGM-A isoform is located on chromosome 15q26.1 (14). RGM-A is thought to be shed from the cell membrane by cleavage at amino acid 412 or instability cleavage at the putative autoproteolytic cleavage site (amino acid 168), which is sensitive to pH drop-induced cleavage (14–17). During neuronal development, RGM-A is a chemorepulsive environmental cue that instructs navigating outgrowing axons (15–17) and also acts on neuronal migration and patterning, as demonstrated by neural tube closure defects in RGM-A knockout mice (17). In addition, RGM-A regulates neuronal survival and differentiation by binding to its dependence receptor neogenin (18–20). After development of the CNS is complete, RGM-A is present in the myelin sheath (21, 22). RGM-A reexpression is confined to the parenchymal lesion environment, specifically in areas of inflammation after rodent or human CNS injury (21, 22). Because axonal outgrowth and the transmigration of leukocytes into inflamed tissue share the conserved biological mechanisms of cytoskeleton remodeling (9), we hypothesized that RGM-A also repulses leukocyte migration in response to chemotactic factors.
Our results demonstrate that besides being expressed in the developing CNS and in lesions in the adult CNS, RGM-A is strongly expressed by epithelium, leukocytes [ie, polymorphonuclear leukocytes (PMNs), monocytes, and T/B lymphocytes], and tissues that are sensitive to infection. Both in vitro and in vivo, RGM-A inhibits PMN migration through its receptor neogenin and modifies the inflammatory milieu by attenuating the proinflammatory response. In addition, RGM-A is a potent inhibitor of leukocyte recruitment to sites of inflammation, mitigating hallmarks of inflammation, such as edema formation and proinflammatory cytokine expression, in a model of mouse peritonitis. RGM-A is essential to limiting the ability of leukocytes to transmigrate through epithelial cell layers, thereby attenuating the inflammatory milieu (“cytokine storm”), and also to reducing the number of leukocytes subsequently recruited. Thus, we have identified a previously uncharacterized function of the neuronal guidance cue RGM-A outside the CNS. Our findings identify RGM-A as a potent immunomodulatory protein in vitro and in vivo. Its anti-inflammatory functions through binding to its receptor neogenin. These results support a conserved molecular role for RGM-A as a guidance cue for neurons and leukocyte chemotaxis.
Results
RGM-A Is Expressed Outside the CNS.
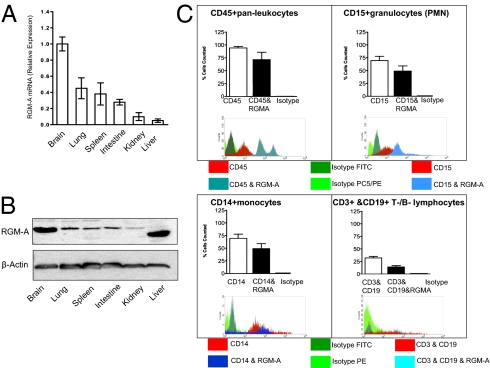
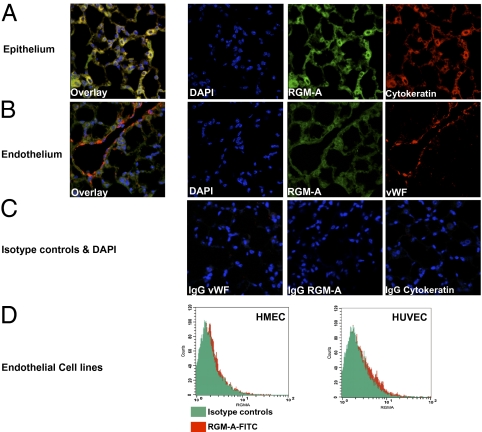
To perform an immunologic function, RGM-A must be expressed outside the CNS. We screened various murine organs for the presence of RGM-A mRNA and detected RGM-A expression in several organs, including some with a direct function during an immune response and some containing a localized immune compartment, including lung (alveolar macrophages), spleen, and intestine (Peyer's patches) (Fig. 1A). Elevated RGM-A RNA expression in the brain, lung, spleen, and intestine were verified to translate into protein synthesis by Western blot analysis (Fig. 1B). In addition, FACS analysis verified the expression of RGM-A by leukocyte subsets, including CD15+ granulocytes (PMNs), CD14+ monocytes, CD3+ T lymphocytes, and some CD19+ B lymphocytes (Fig. 1C). In response to perceived inflammatory stimulation, leukocytes down-regulate RGM-A expression contrasted by induced expression of the receptor neogenin by PMNs and monocytes (Fig. S1 A and B). Given the repulsive bioactivity of RGM-A, we investigated whether RGM-A is present in cells relevant to epithelial and endothelial barriers, both of which are essential to prevent the accumulation of inflammatory cells in mucosal organs (23). Based on the profound RGM-A expression on mRNA and protein levels, we investigated whether RGM-A is present on endothelium and/or epithelium by immunohistochemistry in the lung. Here RGM-A expression was confined to cytokeratin-positive epithelial cells (Fig. 2A). In contrast, RGM-A expression was absent on von Willebrand factor (vWF)-positive endothelial cells (Fig. 2B). Isotype controls did not exhibit any relevant signals (Fig. 2C). This finding was corroborated by the lack of RGM-A expression by two well-established endothelial cell lines, human dermal microvascular endothelial cell 1 (HMEC-1) and human umbilical vein endothelial cell (HUVEC) (Fig. 2D) (8).
Fig. 1.
RGM-A is expressed outside the CNS postdevelopmentally. (A) RGM-A mRNA in murine tissues quantified by quantitative real-time RT-PCR compared with levels of RGM-A mRNA expression present in the murine brain. Along with lymphatic tissue such as spleen, pronounced RGM-A mRNA levels were detected in organs hosting an intrinsic immune compartment, including the brain (microglia), lungs (alveolar macrophages), and intestines (Peyer's patches). (B) Western blot analysis of RGM-A protein expression in pooled murine tissues compared with levels of expression in the brain. Values correspond to relative RGM-A mRNA levels. All data are mean ± SEM; n = 5. (C) FACS analysis verified the expression of RGM-A by leukocyte subsets including CD15+ granulocytes (PMNs), CD14+ monocytes, CD3+ T lymphocytes, and some CD19+ B lymphocytes (PMNs). Isotype controls demonstrated no relevant signal.
Fig. 2.
RGM-A is expressed at the epithelial barrier. Given the repulsive bioactivity of RGM-A, we investigated its presence in cells relevant for epithelial or endothelial barriers. (A) In the lung, RGM-A expression is confined to cytokeratin-positive epithelial cells. (B) In contrast, RGM-A expression is absent on vWF-positive endothelium. (C) Isotype controls demonstrated no relevant signal. (D) The absence of RGM-A in endothelial barriers was further corroborated by the lack of RGM-A expression by the major endothelial cell lines HMEC-1 and HUVEC, which serve as cell sources to investigate features of endothelial function (8).
RGM-A Reduces PMN Migration Through the Neogenin Receptor in Vitro.
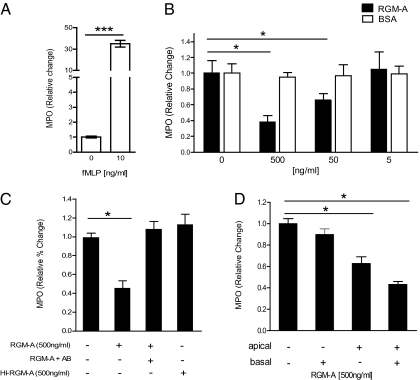
Appropriate adjusted leukocyte migration and recruitment to the inflammatory site is essential to control inflammation for effective host defense and also to avoid tissue damage due to an aberrant, exacerbated inflammatory response (3, 4). To test whether RGM-A is a chemorepulsive cue for PMN migration in vitro, we applied formyl-methionyl-leucyl-phenylalanine (fMLP)-induced chemotaxis using a paracellular flux assay (8). On stimulation by fMLP, granulocytes migrate across a semipermeable membrane seeded with epithelial CaCo cells, forming a cell monolayer (Fig. 3A). RGM-A reduced the fMLP-induced PMN migration in a dose-dependent manner, reaching minimum levels (32% ± 8%) at a concentration of 500 ng/mL (P < 0.05) compared with control application of a nonspecific control protein (BSA) in corresponding concentrations (Fig. 3B).
Fig. 3.
RGM-A inhibits active PMN migration in vitro. (A) fMLP (10 ng/mL) induced chemotaxis-dependent migration of PMNs through a CaCo epithelial cell monolayer. 1 × 106 granulocytes were placed in the apical compartment and transmigration of PMNs measured after 60 min. Measurement of MPO as representative marker was used to quantify basolateral PMN transmigration. (B) PMN transmigration in the presence of distinct concentrations of RGM-A or BSA, showing suppression of PMN migration by RGM-A in a dose-dependent fashion. (C) RGM-A–specific effects on PMN migration in the presence of RGM-A antibody (AB) and heat-inactivated RGM-A (HI-RGM-A). (D) PMN transmigration in the presence of distinct concentrations of RGM-A in the apical compartment, basolateral compartment, or both compartments of a transmigration chamber. All data are mean ± SEM; n = 6 per group. *P < 0.05; **P < 0.01; ***P < 0.001.
To further validate that the effect observed is specific to RGM-A, we exposed RGM-A to heat inactivation (95 °C for 45 min) or applied functional blocking RGM-A antibodies before the PMN migration assay. In both controls, chemotactic PMN migration was no longer attenuated (Fig. 3C). To further specify the profound effect of RGM-A on PMN migration, we added RGM-A on the apical side, basolateral side, or both sides of the semipermeable membrane. Apical and combined apical/basolateral exposure of PMNs to RGM-A suppressed chemotactic migration of PMNs to a significant degree, whereas isolated RGM-A application to the basolateral compartment did not (apical, 63% ± 6%, P < 0.05; apical/basolateral, 42% ± 6%, P < 0.05) (Fig. 3D).
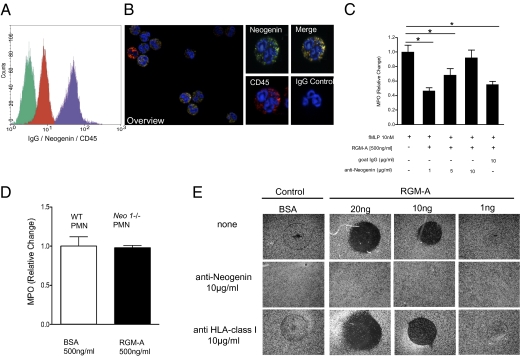
To determine in vitro whether RGM-A reduces PMN migration through a specific receptor-mediated mechanism, we first examined whether the RGM-A receptor neogenin is expressed by PMN (16, 18–20). We found robust expression of the RGM-A receptor neogenin on the PMN cell membrane/surface by FACS analysis and immunocytochemistry (Fig. 4 A and B). To delineate the ligand receptor dependency of the RGM-A–triggered effect, we preincubated neogenin-positive PMNs with increasing concentrations of neogenin-blocking antibodies. Here the application of neogenin antibodies abolished the previously observed inhibitory effect of RGM-A on PMN migration in a dose-dependent fashion, and the effect vanished completely at a dose of 10 μg/mL. In contrast, application of isotype control IgG antibodies did not attenuate the RGM-A–triggered effect (Fig. 4C). Furthermore, neo1-/- PMNs did not respond to RGM-A, demonstrating an intact regular migration response [myeloperoxidase (MPO)], comparable to that of WT- derived PMNs after apical BSA application (Fig. 4D).
Fig. 4.
RGM attenuates PMN migration through its receptor neogenin in vitro. (A) Flow cytometry (overlay histogram) of freshly isolated human PMNs stained with isotype-matched control antibody (green), anti-CD45 antibody (red), and anti-neogenin antibody (purple). (B) Immunohistochemical staining of freshly isolated human PMNs with anti-neogenin (FITC) or isotype control and anti-CD45 (Cy3) antibodies, with the DAPI label used for nuclear counterstaining. Isotype control antibodies demonstrated no detectable signal levels (Left, overlay; original magnification 200×). Demonstration of CD45+ PMNs to coexpress abundant neogenin (Right; original magnification 600×). (C) PMN transmigration in the presence of distinct concentrations of functionally blocking neogenin antibodies compared with isotype IgG controls. Data are mean ± SEM; n = 6 per group. *P < 0.05; **P < 0.01; ***P < 0.001. (D) Neo1−/− PMNs did not respond to RGM-A, revealing an intact, regular migration response (MPO) as demonstrated by WT-derived PMNs after apical BSA application. (E) To investigate a direct contact-repulsion property of RGM-A on PMNs, we analyzed PMN binding to differentially coated surfaces (± RGM-A). The contact-repulsive RGM-A effect on PMN blocked PMN surface binding and was neogenin-dependent. PMN binding was dose-dependently blocked by RGM-A coating (top row). This was completely reversible on preincubation of PMNs with the neogenin antibody (middle row), whereas anti-HLA does not interfere with RGM-A–mediated contact repulsion. One representative experiment out of three experiments conducted is shown.
After establishing the chemorepulsive properties of RGM-A, we sought to delineate putative contact-repulsive effects. We used a modified cell attachment assay to test the ability of seeded PMN to bind to an RGM-A–coated surface (24). In brief, RGM-A or BSA was immobilized on the well surface, and PMNs were allowed to adhere. Nonadherent cells were removed by gently rinsing the wells after 15 min. We observed that PMNs were repulsed, and in circumscribed areas with RGM-A protein coating up to 20 ng, PMNs were attached only sporadically. This effect was completely reversed when PMNs were preincubated with a neogenin-blocking antibody, which reduced viable binding sites for the ligand RGM-A. Here PMNs were rendered insensitive to RGM-A–coated surfaces. In contrast, coincubation using a nonspecific antibody directed against HLA-class I did not convert the contact-repulsive properties of RGM-A (Fig. 4E). Taken together, these findings indicate that RGM-A is a potent chemorepellent and chemorepulsive cue for leukocyte migration.
RGM-A Reduces Leukocyte Migration During Zymosan A-Induced Peritonitis.
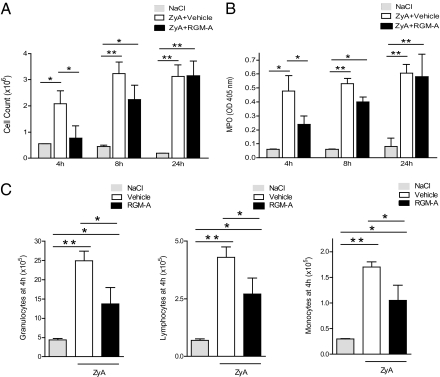
We next explored whether the observed chemorepulsive and contact-repulsive properties of RGM-A on PMN migration in vitro translate into an immunomodulatory role in vivo. Toward this end, we used a well-defined self-limiting model of zymosan A (ZyA)-induced peritonitis to evaluate the potential impact of RGM-A on acute inflammation. Epithelial cells are the main constituents of tissues lining mucosal surfaces and internal cavities (25), including specialized epithelial cells also known as mesothelial cells. Induction of peritonitis by i.p. injection of ZyA was followed by a single immediate i.v. injection of either vehicle (NaCl + BSA 0.2%) or 1 μg of RGM-A. Analysis of peritoneal exudates after 4 h, 8 h, and 24 h demonstrated a significant but transient reduction of infiltrating leukocytes. At 4 h, RGM-A–treated mice demonstrated significantly reduced recruitment of inflammatory cells (0.77 ± 0.47 × 106 cells) compared with vehicle controls (2.08 ± 0.5 × 106 cells; P < 0.05) (Fig. 5A). This reduction was observed up to 8 h postinjection but ceased by 24 h postinjection, as confirmed by significantly reduced MPO activity levels (Fig. 5B). RGM-A was seen to be a potent, broad inhibitor of leukocyte recruitment. Differential count analysis demonstrated reduced counts of all populations, including granulocytes, lymphocytes, and monocytes, in the inflammatory exudate (Fig. 5C). Concomitant RGM-A injection diminished histological signs of acute inflammation, including edema formation and leukocyte infiltration, whereas excessive inflammation occurred in vehicle controls. In summary, RGM-A was found to inhibit leukocyte recruitment to inflammatory sites, reducing acute inflammation.
Fig. 5.
RGM-A reduces leukocyte migration and dampens inflammation during ZyA-induced peritonitis. After WT mice were injected i.p. with 1 mg of ZyA (1 mg/mL), they were given recombinant murine RGM-A (i.v. 1 μg in 0.2% BSA) or vehicle. (A) Cell counts in peritoneal lavage fluid after 4 h, 8 h, and 24 h. (B) MPO activity in peritoneal lavage after 4 h, 8 h, and 24 h. (C) Cell counts of granulocytes, lymphocytes, and monocytes in peritoneal fluid after 4 h in mice with ZyA-induced peritonitis and vehicle or RGM-A injection. All data are mean ± SEM; n = 6 per group. *P < 0.05; **P < 0.01; ***P < 0.001.
RGM-A Fails to Attenuate ZyA-Induced Peritonitis in neo1−/− Mice.
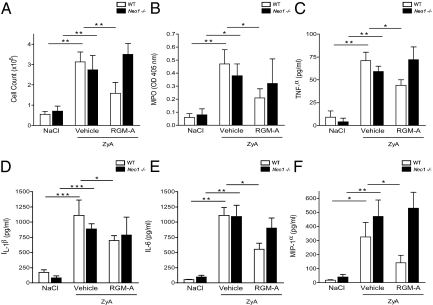
We investigated whether the immunemodulatory effect of RGM-A relies on successful binding to its receptor neogenin in vivo. We questioned whether the observed effect of RGM-A would also be present in mice with gene-targeted repression of the neogenin receptor (neo1−/− mice). We evaluated the extent of inflammation at 4 h after ZyA injection, when the maximum effect was reached (Fig. 5 A and B). Compared with WT controls, the RGM-A–induced reduction in leukocytes infiltrating the peritoneal cavity was not detectable in the neo1−/− mice (Fig. 6A). Correspondingly, the neo1−/− mice did not demonstrate reduced MPO activity levels after RGM-A injection compared with WT controls (Fig. 6B). To gather further evidence for the control of local inflammation through RGM-A, we evaluated the levels of proinflammatory cytokine production in the peritoneal lavage by ELISA analysis. RGM-A application suppressed the levels of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and macrophage inflammatory protein 1α, in the peritoneal cavity of the WT mice (Fig. 6 C–F). In contrast, neo1−/− mice did not demonstrate reduced cytokine levels in the inflammatory exudate (Fig. 6 C–F). Analysis of exudate, cytospins, and histological evaluation of peritoneal tissue revealed a dramatic reduction of recruited cells within the inflammatory exudates and attenuated histological inflammatory changes after RGM-A injection in WT mice compared with vehicle controls. In the neo1−/− mice, this attenuated acute inflammatory response was not detectable after RGM-A injection (Fig. S2). These results indicate that RGM-A does not reduce ZyA-induced peritonitis in neo1−/− mice.
Fig. 6.
RGM-A fails to attenuate ZyA-induced peritonitis in neo1−/− mice. After neo1−/− mice were injected i.p. with 1 mg of ZyA (1 mg/mL), they received i.v. recombinant murine RGM-A (1 μg in 0.2% BSA) or vehicle. (A) Cell count in peritoneal lavage obtained after 4 h. (B) MPO activity in peritoneal lavage fluid obtained after 4 h. (C) Cytokine levels of TNF-α measured by ELISA in peritoneal fluid of WT neo1−/− mice 4 h after ZyA-induced peritonitis. (D) IL-1β, (E) IL-6, and (F) MIP-1α levels in peritoneal fluid WT compared with Neo−/− mice.
Because the entire effect elicited by RGM-A application is completely reversible when the high-affinity receptor neogenin is blocked in vitro and in vivo, the role of other RGM-A interaction partners (e.g., BMP2, BMP4) might be redundant or downstream (26, 27). Taken together, our findings provide evidence that the RGM-A is a highly conserved guidance cue for leukocyte migration mitigating inflammation.
Discussion
Here we identify a previously uncharacterized function for the repulsive neuronal guidance cue RGM-A outside the CNS as an inhibitor of leukocyte migration during acute inflammation. Outside the CNS, RGM-A is expressed by epithelium, leukocytes (i.e., PMNs, monocytes, and B/T lymphocytes) and identified in such organs as lung and kidneys, which are highly vascularized and thereby sensitive to infections. Because the RGM-A high-affinity receptor neogenin is strongly expressed by PMNs, its extracellular ligand RGM-A acts as a potent inhibitor of migration.
We demonstrate that epithelial RGM-A expression inhibits the transmigration of neogenin-positive PMNs, which is known to contribute to early epithelial injury by initiating an inflammatory lesion. In addition, RGM-A–driven autocrine functions might subsequently operate on leukocytes; either RGM-A–positive leukocytes may contact-repulse neogenin-positive PMNs, or membrane-cleaved extracellular RGM-A may chemorepulse neogenin-positive PMNs. Indeed, a pH drop in inflammatory lesion formation might cause autoproteolytic cleavage of cell membrane-bound RGM-A, resulting in an extracellular RGM-A gradient. The gradient might reach maximum levels in the inflammation core. This idea is supported by the detection of an extracellular RGM-A deposit only at the inflammatory lesion core (21). Thus, RGM-A might represent a barrier to aberrant leukocyte PMN migration. Epithelial barrier function maintained by RGM-A is essential for blocking the early onset and exacerbation of immunopathology affecting the gastrointestinal tract, eyes, urinary tract, and lungs. Besides its specific, primary action on migrating neogenin-positive PMNs, RGM-A also attenuates the subsequent development of an inflammatory milieu enriched with chemokines and proteases. Although not expressed by endothelial cells, RGM-A thus may have an indirect and secondary effect on endothelial barrier function by attenuating the emerging proinflammatory milieu as RGM-A reduces all leukocyte populations in a rather broad, symmetrical way.
Evidence of a role of RGM-A in regulating inflammation under physiological conditions was demonstrated by the recent identification of an RGM-A polymorphism as a genetic predisposition to develop misdirected inflammatory responses leading to exacerbated inflammation in animal models of multiple sclerosis and in human multiple scelerosis (up to an odds ratio of 1.33) (28). Thus, under physiological conditions, RGM-A might be an essential element of appropriate immune surveillance to prevent misdirected immune responses leading to exacerbation or autoimmunity. In the light of our results, circulating constitutively neogenin-positive PMNs are repulsed by RGM-A–positive epithelial barriers. The RGM-A–neogenin mediated inhibition of migration is overcome by pathogenic lesions (inflammatory, ischemic, or traumatic). Here lesional RGM-A expression can be considered an integral part of the endogenous anti-inflammatory program essential for the return to homeostasis (29, 30). Thus, like other leukocyte-derived anti-inflammatory cues, such as cytokines (e.g., IL-10), mediators (e.g., heme oxygenase 1), and immunoregulatory growth factors (e.g., TGF-β), RGM-A might be essential for the return to homeostasis by orchestrating appropriate inflammatory responses during postlesional tissue remodeling (29, 30).
Along with cell–cell receptor–ligand interactions, RGM-A might bind to neogenin on the same cell. The receptor and ligand likely are expressed on the same cell, as was shown for the ephrin guidance molecule family (31). Repulsive proteins may be expressed by the same cell due to modulation of their sensitivity to respond to repulsive cues. This effect has been demonstrated in several elegant experiments by the ephrins (31). By cis interaction, ephrin-A5 was hypothesized to bind to its receptor on the axonal surface, thereby minimizing receptors available for sensing its environment. Consequently, a reduced number of axonal surface receptors available led to a reduced sensitivity of these axons (31).
This finding is in agreement with the fact that the broad inhibitory effect is not due to a direct interaction of RGM-A with the chemoattractant, because pretreatment of cells with RGM-A renders them refractory to subsequent stimulation. In analogy, the guidance cue slit was found to repulse leukocyte traffic (6), which was able to prevent exuberant cytokine release by the host in sepsis and influenza models (7). The identification of endogenous pathways that down-regulate infiltrating cell traffic and dampen inflammation might provide important targets for novel interventional approaches.
Materials and Methods
Cell Culture.
CaCo, HMEC-1, and HUVEC cells were used as described previously (8).
Transcriptional, Western Blot, and ELISA Analyses.
RT-PCR was used to determine RGM-A mRNA expression according to standard protocols (8). Western blot was applied to SDS gels using monoclonal RGM-A antibodies (R&D Systems) or rabbit anti–β-actin (Santa Cruz Biotechnology). Binding to species-matched secondary antibodies was visualized by chemiluminescence (Amersham). Cytokine levels (TNF-α, IL-1β, IL-6, and macrophage inflammatory protein 1α) were measured in the peritoneal lavage fluid by standard ELISA (R&D Systems).
Immunohistochemistry and Histopathology.
Slides prepared from fresh frozen cryopreserved mice lungs were labeled with monoclonal RGM-A antibodies (R&D Systems). Polyclonal RGM-A and vWF (Abcam) and monoclonal cytokeratin (Santa Cruz Biotechnology) antibody binding was visualized with Alexa Fluor–conjugated anti-rabbit or anti-mouse antibodies (Invitrogen) and compared with mouse and rabbit IgG isotype controls (Santa Cruz Biotechnology). Following peritonitis, paraffin-embedded tissue was stained with H&E.
Cytology and Flow Cytometry.
Blood was collected, pooled, and incubated with erythrocyte lysing solution for 5 min at room temperature. The following antibodies were used: anti–RGM-A (R&D Systems), anti-neogenin (SCBT), anti-CD45 (Immunotech), anti-CD15 (Immunotech), anti-CD14 (Serotec), anti-CD3 (Caltag), and anti-CD19 (BD Bioscience). FITC and Cy3 fluorescence of species-matched secondary donkey antibodies (Santa Cruz Biotechnology) was evaluated with a BD FACS Canto II unit using BD FACS Diva Software.
PMN Isolation and Transmigration Assays.
In short, PMNs were freshly isolated from whole blood anticoagulated with acid citrate/dextrose. The resulting cell population was >97% PMNs as assessed by microscopic evaluation. PMNs were studied within 2 h of their isolation. In subset of experiments, PMNs were isolated from whole blood obtained from neo1−/− mice.
Peritonitis.
All animal experiments were performed in accordance the regulations of the Regierungspräsidium Tübingen. Eight- to 12-wk-old C57BL/6 mice were injected i.p with 1 mL of ZyA (1 mg/mL; Sigma-Aldrich), as described previously (32).
Cell Adhesion Assay.
Cell adhesion assays were carried out as described previously (24). RGM-A or BSA was immobilized onto plastic dishes in decreasing concentrations, and the cells were allowed to attach for 15 min.
Splenocte Stimulation.
Splenocyte cell suspensions were prepared following standard procedures. Splenocytes were cultured in the presence or absence of 10 μg/mL ZyA (disolved 0.1% ethanol) at 37 °C for 24 h and then analyzed by FACS.
Neo1−/− Mice.
Neo1 gene trap (encoding neogenin) mice were kindly provided by Dr. Marc Tessier-Lavigne (The Rockefeller University) (33).
Supplementary Material
Acknowledgments
We thank Dr. Marc Tessier-Lavigne and Genentech for providing the neo1 gene trap mice. We thank Marion Faigle, Alice Mager, Stefanie Zug, and Sabine Conrad for excellent technical assistance. This work was supported by a Tübingen University IZKF Promotionskolleg Grant (to S.B.), Tübingen University Fortune Grant IZKF 1639-0 (to V.M.), Deutsche Forschungsgemeinschaft Grant DFG-RO 3671/5-1 (to P.R.), DFG-Graduate School 1258 “Impact of Inflammation on Nervous System Function” (to B.B.), Berlin Center for Regenerative Therapies Grant FKZ 313911, International Foundation for Research in Paraplegia Grant P102, and a Wings for Life Spinal Cord Research Foundation Travel Grant (to J.M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015605108/-/DCSupplemental.
References
- 1.Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 5.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 6.Wu JY, et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London NR, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberger P, et al. Hypoxia-inducible factor–dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 9.Rao Y, Wong K, Ward M, Jurgensen C, Wu JY. Neuronal migration and molecular conservation with leukocyte chemotaxis. Genes Dev. 2002;16:2973–2984. doi: 10.1101/gad.1005802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki K, et al. Semaphorin 7A initiates T-cell–mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 11.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 12.Ly NP, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:14729–14734. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stahl B, Müller B, von Boxberg Y, Cox EC, Bonhoeffer F. Biochemical characterization of a putative axonal guidance molecule of the chick visual system. Neuron. 1990;5:735–743. doi: 10.1016/0896-6273(90)90227-7. [DOI] [PubMed] [Google Scholar]
- 14.Mueller BK, Yamashita T, Schaffar G, Mueller R. The role of repulsive guidance molecules in the embryonic and adult vertebrate central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1513–1529. doi: 10.1098/rstb.2006.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monnier PP, et al. RGM is a repulsive guidance molecule for retinal axons. Nature. 2002;419:392–395. doi: 10.1038/nature01041. [DOI] [PubMed] [Google Scholar]
- 16.Rajagopalan S, et al. Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol. 2004;6:756–762. doi: 10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- 17.Niederkofler V, Salie R, Sigrist M, Arber S. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J Neurosci. 2004;24:808–818. doi: 10.1523/JNEUROSCI.4610-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsunaga E, et al. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004;6:749–755. doi: 10.1038/ncb1157. [DOI] [PubMed] [Google Scholar]
- 19.Matsunaga E, Nakamura H, Chédotal A. Repulsive guidance molecule plays multiple roles in neuronal differentiation and axon guidance. J Neurosci. 2006;26:6082–6088. doi: 10.1523/JNEUROSCI.4556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsunaga E, Chédotal A. Repulsive guidance molecule/neogenin: A novel ligand-receptor system playing multiple roles in neural development. Dev Growth Differ. 2004;46:481–486. doi: 10.1111/j.1440-169x.2004.00768.x. [DOI] [PubMed] [Google Scholar]
- 21.Schwab JM, et al. Central nervous system injury–induced repulsive guidance molecule expression in the adult human brain. Arch Neurol. 2005;62:1561–1568. doi: 10.1001/archneur.62.10.1561. [DOI] [PubMed] [Google Scholar]
- 22.Hata K, et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: Implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- 24.Klein G, Müller CA, Tillet E, Chu ML, Timpl R. Collagen type VI in the human bone marrow microenvironment: A strong cytoadhesive component. Blood. 1995;86:1740–1748. [PubMed] [Google Scholar]
- 25.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: In search of the “epimmunome.”. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babitt JL, et al. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:29820–29827. doi: 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 27.Samad TA, et al. DRAGON, a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:14122–14129. doi: 10.1074/jbc.M410034200. [DOI] [PubMed] [Google Scholar]
- 28.Nohra R, et al. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Genes Immun. 2010;11:279–293. doi: 10.1038/gene.2009.111. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 30.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 31.Hornberger MR, et al. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron. 1999;22:731–742. doi: 10.1016/s0896-6273(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 32.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leighton PA, et al. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.