Abstract
Plastids are DNA-containing organelles unique to plant cells. In Arabidopsis, one-third of the genes required for embryo development encode plastid-localized proteins. To help understand the role of plastids in embryogenesis and postembryonic development, we characterized proteins of the mitochondrial transcription termination factor (mTERF) family, which in animal models, comprises DNA-binding regulators of mitochondrial transcription. Of 35 Arabidopsis mTERF proteins, 11 are plastid-localized. Genetic complementation shows that at least one plastidic mTERF, BELAYA SMERT' (BSM), is required for embryogenesis. The main postembryonic phenotypes of genetic mosaics with the bsm mutation are severe abnormalities in leaf development. Mutant bsm cells are albino, are compromised in growth, and suffer defects in global plastidic gene expression. The bsm phenotype could be phenocopied by inhibition of plastid translation with spectinomycin. Plastid translation is essential for cell viability in dicotyledonous species such as tobacco but not in monocotyledonous maize. Here, genetic interactions between BSM and the gene encoding plastid homomeric acetyl-CoA carboxylase ACC2 suggest that there is a functional redundancy in malonyl-CoA biosynthesis that permits bsm cell survival in Arabidopsis. Overall, our results indicate that biosynthesis of malonyl-CoA and plastid-derived systemic growth-promoting compounds are the processes that link plant development and plastid gene expression.
Keywords: organellar gene expression, splicing, ClpPR protease, myrosinase, digitonin
Plastids are DNA-containing organelles of endosymbiotic origin that define plant cells (1). Plastid homeostasis is continually monitored, and gene expression is regulated by tetrapyrrole biosynthesis and the monitoring plastid gene expression machinery, the abundance of reactive oxygen species, plastidic redox status, and the ATP/ADP balance of organelles (2). Consequently, this so-called retrograde signaling also affects the progression of developmental programs (3). Among 339 nonredundant Arabidopsis genes required for proper embryo formation, 108 encode plastid-targeted proteins (4). To study the role of plastid gene expression in embryogenesis and postembryonic development, we have characterized proteins of the mitochondrial transcription termination factor (mTERF) family, a group of proteins named after the human mTERF1. These proteins have a modular architecture based on repetitions of a 30-aa mTERF motif (5). Studies of the crystal structure of mTERF1 have revealed that these mTERF motifs associate to create a helical structure apparently involved in nucleic acid binding (6). Vertebrates have four mTERF paralogs. In humans, mTERF1 is a sequence-specific DNA-binding protein responsible for mitochondrial transcription termination at a site adjacent to the mitochondrial rRNA genes (6). mTERF2 can bind to mitochondrial DNA (7) and, at least in mouse, seems to influence transcription (8). mTERF3 acts as a specific repressor of mammalian mtDNA transcription initiation in vivo (9).
Here, we show that 11 of 35 annotated Arabidopsis mTERFs are targeted to plastids. Genetic complementation indicated that early embryo arrest is a characteristic phenotype of mutation in mTERF/At4g02990, whereas in vitro cell culture and analysis of genetic mosaics revealed a postembryonic phenotype of a growth-compromised albino, which suggested the gene name BELAYA SMERT’ (BSM) or white death in Russian. Exogenously supplied phytohormones and plastidic homomeric acetyl-CoA carboxylase (ACC2) promoted the survival of bsm mutant cells, suggesting that biosynthesis of growth factors and malonyl-CoA underpin the nonautonomous and autonomous cell functions of plastids in Arabidopsis development.
Results
Arabidopsis Has at Least 11 Plastid-Localized mTERF Proteins.
Flowering plants have the highest number of mTERF genes among eukaryotes (Table S1). GFP fusions of all members of the Arabidopsis mTERF family showed that 11 mTERF proteins were targeted to chloroplasts and 17 were mitochondrial, whereas one fusion was distributed equally between the nuclei and the cytoplasm (Fig. S1). Six gene fusions showed no GFP expression. The detection of conserved protein motifs revealed the presence of two motifs specific for Arabidopsis mTERF proteins targeted to the mitochondria (Fig. S2 and Table S2). Integration of genome organization information (10) indicated species-specific gene family expansion through tandem duplication both in rice and Arabidopsis (with 45% and 25% of mTERF homologs in tandem, respectively) (Fig. S3). In contrast, plastid-targeted mTERFs belong to a single subtype, probably representing the ancestral family composition.
To determine the involvement of plastid mTERFs in embryo development, we surveyed transferred DNA (T-DNA) insertion mutant alleles. Plants with mutations in four genes developed seeds with arrested embryos (Table S3). The bsm mutant has been first identified and first characterized in detail.
bsm Cells Are Albino and Dependent on Exogenous Phytohormones.
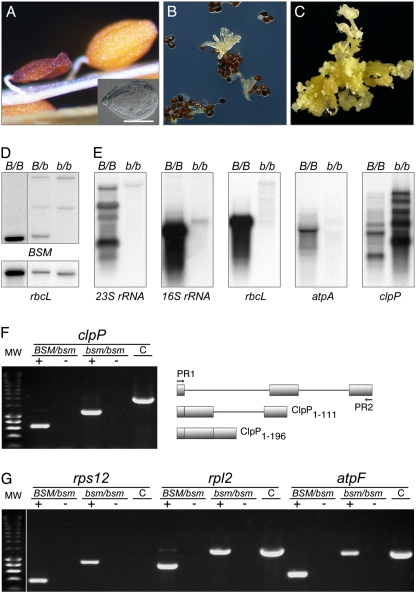
In a progeny of BSM/bsm plants, 25% of the seeds had embryos arrested at the late globular stage of development (Fig. 1A). Immature mutant seeds remained white, indicating a failure of the endosperm and embryo to differentiate chloroplasts. These developmental defects were complemented by the WT BSM protein as well as by its GFP and tandem affinity purification (TAP) tag fusions.
Fig. 1.
Mutant bsm phenotype and plastid gene expression. (A) Shrunken dry mutant seed, arrested embryo (Inset), and a WT seed. (B) Asynchronous germination of the seeds with arrested embryos. (C) Mixed callus/shoot bsm culture on a medium supplemented with phytohormones. (D) Albino cells are bsm/bsm homozygous. HindIII-digested genomic DNA from albino cells (b/b) was compared with DNA of green homo- (B/B) and heterozygous (B/b) plants by hybridization analysis with radioactive probes detecting nuclear BSM and plastid rbcL genes. (E) Analysis of plastid transcripts. RNA was extracted from green plants (B/B) or bsm albino shoot cultures (b/b). Analyzed plastid genes were rrn23S and rrn16S for ribosomal RNAs (23S rRNA and 16S rRNA), the large subunit of Rubisco (rbcL), the α-subunit of the plastidial ATPase (atpA), and the ClpP subunit of ClpPR protease (clpP). (F) bsm cells do not splice the second clpP group IIa intron. DNA products were amplified by PCR with specific primers (PR1 and PR2) for clpP/AtCg00670. The templates were first-strand cDNA (+), RNA (to control for DNA contamination; −), and genomic DNA (to provide a size marker for a PCR fragment representing fully nonspliced clpP transcript; C). The drawing illustrates the exon–intron gene structure, organization of processed transcripts, and lengths of encoded polypeptides. (G) atpF, rpl2, and rps12 intron splicing. The labeling is as in F. The first group IIb intron of rps12 is encoded by two different genes, which require a transsplicing event for processing, the reason why the PCR product is generated only with cDNA and not with DNA as a template.
Dry seeds with arrested embryos were cultured in vitro. Although WT seeds germinated within 2–3 d, the first germination events with bsm seeds were only observed after 3 wk and led to poorly growing, malformed albino seedlings (Fig. 1B). Therefore, to induce cell proliferation and de novo organogenesis, these bsm seedlings were transferred to medium supplemented with cytokinin and auxin, resulting in stable shoot cultures that maintained a slow growth over several years (Fig. 1C). DNA gel blot hybridization showed that the shoot cultures were homozygous for the mutant bsm allele and contained chloroplast DNA (Fig. 1D). We concluded that phytohormones promote the growth of albino bsm cells.
BSM Gene Deficiency Affects Processing and Steady-State Levels of Plastid Transcripts.
Plastids possess two types of DNA-dependent RNA polymerases, a nuclear-encoded bacteriophage-type RNA polymerase (NEP) and a bacterium-type multisubunit RNA polymerase (PEP). The PEP catalytic core requires products of the plastid genes rpoA, rpoB, rpoC1, and rpoC2 (11). Plastid-encoded class I genes such as rbcL are transcribed predominantly by PEP. Class II genes are transcribed by both PEP and NEP, whereas a few class III genes rpoB and accD, are thought to be transcribed exclusively by NEP (12). In Arabidopsis, rRNA genes belong to class II, but PEP is responsible for most transcriptional activity of rrn16S and rrn23S genes (12).
Mature 16S rRNA and 23S rRNA were not detectable in bsm cells (Fig. 1E). The PEP-dependent expression of the protein coding genes rbcL and atpA was similarly affected, whereas clpP transcripts levels increased (Fig. 1E). A similar response characterized albino tobacco plants mutated in PEP RNA polymerase (13).
To understand the changes in clpP transcript size, we analyzed its splicing by RT-PCR. In Arabidopsis, the coding region of clpP is disrupted by two introns that belong to group IIb and group IIa classes. In albino shoots, RT-PCR showed that the second clpP group IIa intron was not spliced (Fig. 1F). The last gene exon codes for the 85-aa residues that include two residues of a clpP catalytic triad, indicating that the mutant polypeptide is catalytically inactive (14). The plastid ClpPR protease complex is reported to be essential in both tobacco and Arabidopsis (15).
Protein-coding regions of plastid genes for the AtpF subunit of the ATP synthase and ribosomal proteins Rpl2 and Rps12 are interrupted by group IIa introns (16). The production of the rps12 ORF also requires a transsplicing event of a IIb intron. Results showed that rps12 transsplicing was normal but that atpF, rpl2, and rps12 group IIa introns were not spliced in bsm cells (Fig. 1G). These nonspliced transcripts encode mutant proteins, of which AtpF1–48 and Rpl21–130 are most probably nonfunctional. Arabidopsis plants with defective atpF splicing are albino (17).
RNA editing increases the complexity of the plastid transcriptome, can influence the protein activity, and be affected by pigment deficiency (18, 19). Sequencing of cloned cDNA-derived PCR fragments suggested that the clpP and accD transcripts were fully edited in the mutant.
Splicing of the clpP Group IIa Intron Is BSM-Dependent.
Plastomes of most flowering plants encode a MatK maturase that is thought to be a transacting splicing factor for group IIa introns (20). Splicing of the clpP second intron is thought to be MatK-independent (16, 21). Inhibiting plastid translation in WT cells with the antibiotic spectinomycin (22) allowed us to replicate the growth-compromised albino phenotype of bsm (Fig. 2 A–C). Spectinomycin abolished splicing of atpF, rpl2, and rps12 group IIa introns but not of the clpP second intron (Fig. 2D). Thus, processing of the clpP transcript depends on BSM and probably does not require MatK, suggesting a more direct role for BSM in clpP second intron splicing.
Fig. 2.
Analysis of spectinomycin-treated plants of WT genome composition. (A) Effect of spectinomycin on WT plants. Seeds were placed on a medium without or with 500 mg/L spectinomycin; 7-d-old seedlings are shown. Treated seedlings are albino, because inhibition of plastid translation prevents biogenesis of chlorophyll-containing photosynthetic complexes. (B) Ninety-day-old plantlets developed in the presence of spectinomycin. Although by this time, green plants had completed their life cycle, albino seedlings were comparable with bsm seedlings of similar age (Fig. 1B). (C) Thirty-day-old shoot in vitro culture. Transfer of spectinomycin-induced albinos to a medium supplemented with phytohormones promoted the growth and established albino shoot cultures composed from undifferentiated calli and de novo developed shoots. (D) RT-PCR analysis of splicing. Splicing analysis of atpF, rpl2, rps12, and clpP introns is detailed in Fig. 1 F and G. RNA was extracted from albino (w) or green (g) shoots. Control PCR is on genomic DNA (C).
BSM Is an mTERF-Like Protein.
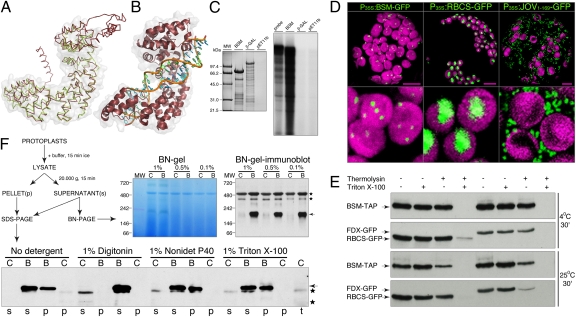
BSM protein sequence was aligned to create a structural model of the BSM protein with I-TASSER (23), taking advantage of the recently solved crystal structures of human mTERF1 (6). BSM is slightly larger than mTERF1, and the model suggests that BSM consists of a central core that is structurally homologous to the mammalian mTERFs flanked by N- and C-terminal extensions (Fig. 3A). The mTERF fold seems to have evolved to mediate protein–nucleic acid interactions. In this respect, the model predicts that BSM should be capable of binding dsDNA and suggests a similar mode of interaction to that observed in human mTERF1 (Fig. 3B). Nevertheless, conservation of the critical residues that enable the human mTERF1 to bind and recognize DNA (6) was not observed in BSM, probably reflecting a completely different nucleic acid binding specificity. Moreover, the N- and C-terminal extensions in BSM could conceivably confer additional functionalities to the protein, although it is also possible that they simply allow BSM to interact with a DNA sequence longer than the 21-bp bound by human mTERF1. To verify these conclusions, bacterially produced 6xHis-BSM was refolded on Ni+ beads and incubated with radioactively labeled, double-stranded restriction fragments of cloned Arabidopsis chloroplast DNA. Although 6xHis-BSM bound DNA, no preferential retention of cpDNA fragments was observed (Fig. 3C).
Fig. 3.
Structural homology modeling and biochemical properties of BSM. (A) Overlay between the BSM model and human mTERF1. The C-α trace of the BSM model constructed with I-TASSER is shown (red) in an overlay with human mTERF1 (green, PDB 3MVA). The central core of the BSM protein is predicted to fold very similarly to mTERF1. The root mean square deviation was of 2.28 Å for 280 C-α atoms. The molecular surface of human mTERF1 is transparent. (B) Predicted DNA binding mode for BSM. The central core of BSM is shown in ribbon representation (red) together with the DNA molecule from the human mTERF1 cocrystal (3MVA). The model indicates that the BSM-predicted fold is consistent with dsDNA binding. The molecular surface of the BSM core is transparent. The two DNA strands are in green or blue with an orange coil. (C) DNA binding. Six-histidine–tagged BSM (BSM) and bacterial β-galactosidase (β-GAL) were produced in Escherichia coli and purified on Ni+ resin. The empty pET11b plasmid was used as control (pET11b). Beads with absorbed proteins were incubated with 32P-labeled NotI-NcoI restriction fragments (probe) of cloned chloroplast DNA of Arabidopsis. Beads were washed, and bound DNA was purified and resolved by agarose gel electrophoresis. (D) BSM-GFP localization in chloroplasts. The control protein fusions RBCS-GFP and JOV1–169-GFP visualized chloroplast stroma and mitochondria. Shown are the entire protoplast and a close-up of chloroplasts from the same cell. Autofluorescence of chlorophyll visualizes chloroplasts, which are false-colored in magenta. Green is GFP fluorescence. (Scale bars, 100 μm.) (E) BSM-TAP is localized inside of chloroplasts. Percoll-purified chloroplasts of transgenic plants that coproduce BSM-TAP and a small subunit of Rubisco (RBCS-GFP) or ferredoxin (FDX-GFP) were incubated in an isotonic buffer at 4 °C or 25 °C for 30 min. Samples were differentially supplemented with a protease thermolysin and nonionic detergent Triton X-100. GFP fusions of proteins known to be localized in chloroplast stroma were used as controls and biological repeats. (F) BSM-TAP is involved in intermolecular interactions. Live protoplasts from leaves of WT plants (C) or genetically complemented plants (B) that produced the BSM-TAP were used as starting material. On the 3–12% BN gel and its blot with separated samples from protoplasts lysed in the presence of 1%, 0.5%, or 0.1% of digitonin, the abundant protein complex migrating at ∼500 kDa corresponds to a heteromeric Rubisco and provides a gel-loading control. Asterisks mark nonspecific signals also present in controls, and the arrow points to the BSM-TAP.
Next, we characterized BSM protein fusions produced in planta. BSM-GFP was targeted to chloroplasts (Fig. 3D). When purified chloroplasts were treated with protease, BSM-TAP proteolysis was facilitated by nonionic detergents, indicating that the protein is localized within plastids (Fig. 3E).
mTERFs are components of mitochondrial nucleoids in animals (7, 24). Chloroplast nucleoids are known to partition both into Triton-insoluble 20,000 × g chloroplast preparations (25) and soluble >3-MDa complexes of chloroplasts (26). In cell extracts prepared with nonionic detergents, Triton X-100 or Nonidet P-40 (Fig. 3F), one-half of the BSM-TAP was retained in a soluble complex that did not enter the Blue-Native (BN) polyacrylamide gels at a >2-MDa cutoff. The remainder of the BSM-TAP partitioned into Triton-insoluble 20,000 × g chloroplast preparations. This partitioning was disrupted by digitonin, and on BN gels, digitonin-extracted 70-kDa BSM-TAP comigrated with a discrete band of 200 kDa (Fig. 3F), representing either a heteromeric complex or a homomeric trimer. Digitonin sensitivity indicates that BSM could be a component of a peripheral membrane complex. Notably, chloroplast nucleoids are thought to be anchored to the membranes (27).
Previous studies have shown that mTERF pTAC15/At5g54180 copurifies with transcriptionally active plastid chromosomes (28). BSM was identified as a component of the soluble nucleoid fraction (26). Plants with a mutation in mTERF At2g03050 have reduced rRNA levels and protein synthesis rates in plastids (29). Taken together, these data suggest that BSM is likely to be implicated in organellar gene expression, possibly in a similar way to the mTERF proteins in metazoans.
Genetic Interactions Between BSM and ACC2.
Tobacco proteins encoded by the plastid genes ycf1, ycf2, clpP, accD, and matK and several components of the translational machinery have been shown to be essential for cell viability (30, 31). Our data suggest that a major deficiency of protein translation exists in bsm plastids. To understand why bsm cells are still viable, we applied genetic tests to investigate a possible functional redundancy in the accD gene function.
The plastid accD gene encodes the β-carboxyltransferase subunit of the heteromeric acetyl-CoA carboxylase (He-ACC), whereas the other three subunits are encoded by the nuclear CAC genes. He-ACC produces malonyl-CoA that is used for de novo biosynthesis of fatty acids, which in plant cells, occurs almost exclusively in plastids (32). However, because bsm cells are viable, mutant plastids most probably have sufficient malonyl-CoA for fatty acid synthesis. Indeed, analysis of fatty acid composition and content of albino bsm/bsm cells showed that there were no significant differences between mutant and WT cells, except for lower levels of polyunsaturated C18 fatty acid in the mutant and a correspondingly higher level of stearic acid (18:0) and oleic acid (18:1) (Fig. S4).
Malonyl-CoA in plastids can also be synthesized by the eukaryotic type, homomeric ACC (Ho-ACC) (32). Analysis of sequenced plant genomes indicates that three groups of plant species exist regarding the capacity for malonyl-CoA biosynthesis (Fig. 4A). Some species depend exclusively on an He-ACC (grapevine type), others rely only on an Ho-ACC (corn type), and some use both enzymes (canola type) (33). The Arabidopsis genome contains two genes for Ho-ACC that are positioned on chromosome 1 in a tandem duplication. The ACC1/GURKE/PASTICCINO3 is a cytosolic enzyme (34). Compared with ACC1, the ACC2 encoded by the gene At1g36180 is 107 aa residues longer at the N terminus. The current gene model (The Arabidopsis Information Resource) indicates that At1g36180 can produce two proteins (Fig. 4B), but only the 2,356-aa-long ACC2.1 is expected to be fully active. The functional significance of the alternatively spliced, C-terminally truncated ACC2.2 is unclear. We produced ACC21–204-GFP and ACC21–544-GFP in leaf protoplasts, and both protein fusions localized to the chloroplasts (Fig. 4C), suggesting that ACC2.1 is indeed a plastid protein.
Fig. 4.
Genetic interactions between BSM and ACC2. (A) Modes of malonyl-CoA biosynthesis in plastids of higher plants. ACC2, a eukaryote-type homomeric acetyl-CoA carboxylase encoded by genes in the nuclear genome. Prokaryote-type heteromeric acetyl-CoA carboxylase is composed of four subunits. CAC1, CAC2, and CAC3 are the nuclear genome-encoded polypeptides. AccD is a product of a plastid accD gene. (B) The ACC2 gene model in the genome annotation. Two alternative transcripts are encoded by the ACC2 Arabidopsis gene locus. Positions of the T-DNAs in the analyzed mutant alleles acc2-1 and acc2-2 are indicated. (C) Localization of ACC21–204-GFP and ACC21–544-GFP in chloroplasts of Arabidopsis. (Scale bar, 10 μm.) (D) Embryos of the indicated genotypes in almost mature seeds that are turning brown. Images were false-colored in accordance with the BSM gene state (magenta and green for the bsm mutant and the BSM WT embryos, respectively). (E) Seeds with arrested acc2-1/acc2-1;bsm/bsm and ACC2/ACC2;bsm/bsm embryos after 4 wk on a shoot-inducing medium. Arrested acc2-1/acc2-1;bsm/bsm embryos fail to develop shoots.
To assess whether BSM and ACC2 interact genetically, we confirmed two T-DNA insertion mutations in the ACC2 gene as annotated in the SALK Institute mutant collection (35). T-DNAs were located in the 21st and 27th exon of the mutant acc2-2 and acc2-1 gene alleles, respectively (Fig. 4B and Fig. S5). Homozygous acc2 plants had no obvious mutant phenotype compared with the WT, indicating that He-ACC is the major plastid acetyl-CoA carboxylase in Arabidopsis. Embryos from flowering acc2-1/acc2-1;bsm/BSM or acc2-2/acc2-2;bsm/BSM plants were compared with ACC2/ACC2;bsm/BSM plants that had been selected among the same F2 segregating populations. The acc2-1/bsm and acc2-2/bsm embryo arrested earlier than the ACC2/bsm embryos (Fig. 4D), and in addition, seeds with arrested acc2-1/bsm embryos did not germinate in vitro (Fig. 4E), indicating synthetic lethality between the bsm and acc2 mutations. Hence cytosolic ACC1 cannot compensate for a deficiency in plastid malonyl-CoA biosynthesis in bsm plastids and functional redundancy in malonyl-CoA biosynthesis in plastids of Arabidopsis promotes bsm cell survival.
BSM Mosaics Reveal a Noncell-Autonomous Role of Plastids in Development.
The bsm cell proliferation defects were partially alleviated by external application of cytokinin and auxin. Because phytohormones have a systemic action, we wondered whether our results were indicative of a noncell-autonomous function of plastids during development. Therefore, we characterized postembryonic mutant phenotypes in mosaic plants generated by heat-inducible CRE-loxP recombination (Fig. 5A).
Fig. 5.
Mosaic plants. (A) Design of the gene complementation constructs. Right (RB) and left (LB) borders delineate the T-DNA integrated into the plant nuclear genome. ORFs are BSM ORF (BSM1), TAP-tag (TAP), and CRE recombinase (CRE). There are three cis-elements: Pact2, promoter sequences of the Arabidopsis ACTIN2 (At3g18780); pA, the nopaline synthase polyadenylation signals; loxP, loxP recombination substrates. Heat stress-inducible expression was directed by the (Phsp) Arabidopsis HSP18.2 promoter. Heat stress (37 °C)-induced CRE excise ORF from the T-DNA in planta. Eventual decay of the BSM transcript and protein leads to the loss of the gene function. The BSM, BSM-TAP, and BSM-GFP ORFs were tested in this experimental design. For simplicity, only BSM-TAP ORF is drawn here. (B) Mosaic plants are in the two front rows (HS+), and nonstressed siblings used as controls (HS−) are in the back rows. (C) Close-up picture of a plant from B. Green leaves indicated with dots were harvested for the time-course analysis in G. (D and E) The same plant as in C 2 wk and 4 wk later, respectively. Abnormal leaf shapes and massive shoot proliferation are illustrated. (F) Mosaic developed from a heat-stressed plant that transitioned to flowering. (G) Protein gel-blot analysis of the BSM-TAP from green leaves of mosaic (HS+) and control (HS−) plants. The type of leaf material harvested for the time course is illustrated in C. To estimate the half-life time (Lower), protein extracts from plants 4 d after HS were serially diluted twofold with an extract from a WT plant.
When BSM gene loss was induced in 4-wk-old plants grown on soil, leaves preformed at the time of heat-shock application remained green (Fig. 5B). Young leaf primordia at the time of the heat shock, however, developed a proximo-distal gradient of pigment deficiency (Fig. 5C). The subsequent three to four leaf primordia developed serrated, small albino leaves, and the youngest emerging leaves were rod-like albino structures (Fig. 5D). Mosaics were photoautotrophic plants that often developed large masses of short albino shoots (Fig. 5E). Heat stress applied to 5- to 6-wk-old plants resulted in the development of mosaics with albino inflorescence shoots (Fig. 5F). Albino flowers on primary shoots had sepals, petals, anthers, and carpels, whereas in flowers of side shoots, only filamentous structures usually developed instead of the internal whorls of petals and anthers. No seeds were obtained after either self-pollination or cross-pollination with WT pollen, indicating major problems with gametophyte development.
To characterize the timing of BSM decay after induction of gene deletion, the fate of BSM-TAP was analyzed. BSM protein had a half-life of 3 d in green leaves of mosaics (Fig. 5G) but was undetectable in albino tissues. Therefore, the relative stability of the BSM protein present at the time of heat-shock application maintains WT functionality in green tissues, suggesting that WT plastids in mosaic plants produce growth-promoting substances that had not been supplied to the in vitro grown bsm cells.
Characterization of foliar cell types showed that significantly less mesophyll cells were present in albino leaves (Fig. S6A), and whereas stomata complex cells and trichomes were recognizable, the epidermis pavement cells did not attain a WT interlocking jigsaw puzzle shape (Fig. S6B). In albino leaves, mutant bsm plastids differentiated as double membrane-bound, often doughnut-shaped small organelles without a developed membrane system (Fig. S6C). Albino leaves were also found to accumulate several abundant polypeptides (Fig. S6D), identified as thioglucoside glucohydrolases TGG1 and TGG2 or myrosinases. In Arabidopsis, myrosinases are specific markers of the myrosin cells of vascular bundles (36) and guard cells of the stomata complex (37). TGG1 is also required for key abscisic acid responses of guard cells (37). The observed proteome phenotype was not, however, caused by ectopic gene activation (Fig. S6 E and F).
Discussion
Plastids are the metabolic factories of plant cells (38). Disruption of amino acid, vitamin or nucleotide biosynthesis in plastids generally leads to arrested embryo development (39). We show that loss of the plastidic BSM protein results in an arrest of embryogenesis and abnormal postembryonic development. bsm cells are albino and defective in photosynthesis but can still be maintained in vitro on a minimal medium, indicating that they are not auxotrophic for basic metabolites.
Our results reveal that there is negligible protein translation in bsm plastids. Because seed abortion at the transition stage of embryogenesis in Arabidopsis is often caused by mutations in chloroplast ribosomal proteins (39) and aminoacyl-tRNA synthetases (40), defects in plastid gene expression could cause arrested bsm embryo development. Although plastid translation is essential for cell viability in tobacco (30), this is not the case in monocotyledonous grasses (1, 20). The dicot/monocot dichotomy is thought to depend on the plastome gene content (1), because grass genomes do not encode Ycf1, Ycf2, and AccD proteins. Indeed, it has been proposed that accD is the only truly essential gene of the tobacco plastome (31), implying that the loss of plastid translation results in auxotrophy for malonyl-CoA in dicots.
Genetic interactions between BSM and ACC2 genes suggest that a functional redundancy in plastid malonyl-CoA biosynthesis underlies survival of bsm cells. Thus, alternative modes of plastid malonyl-CoA biosynthesis (Fig. 4A) can explain the different sensitivities of dicot plant species to genetically or pharmacologically induced loss of plastid translation. Metazoans depend on Ho-ACC for their malonyl-CoA. Ho-ACC operates in the plant cell cytosol (34). The evolutionary forces that have led to the retention of either He-ACC and/or Ho-ACC in plastids are not known. However, biochemical enzyme properties (32) or protein neofunctionalization may have influenced the capacity for ecological adaptation. Interestingly, the seed oil-producing crops canola and soybean have retained genes for both the He-ACC and Ho-ACC plastid isoforms.
Materials and Methods
Plant Material and Growth Conditions.
The ecotypes and genetic markers were C24, C24 bsm/BSM (this study), Col acc2-1/acc2-1, and Col acc2-2/acc2-2 (SALK Institute Mutant Collection). Plants were grown as described (41). Mutant cell/shoot bsm/bsm lines were maintained on a shoot-inducing medium (MS salts, 20 g/L sucrose, 1 mg/L 6-benzyladenine, 0.1 mg/L α-naphthalene acetic acid). To induce mosaics, plants were heat-stressed at 37 °C.
Transient Assays and Microscopy Analysis.
GFP protein fusions were expressed transiently (http://genetics.mgh.harvard.edu/sheenweb/). Tissue sections were analyzed by light and transmission EM (41), embryos were analyzed by Nomarski optics (http://www.seedgenes.org/Tutorial.html), and protoplasts in isotonic solutions, epidermis peels, or hand sections of leaves in water were analyzed by laser scanning confocal microscopy (41). Surface morphology was examined with a tabletop electron microscope TM-1000 (Hitachi).
Other Methods.
Sequences were analyzed as described (10). GATEWAY recombinational cloning was used routinely (Invitrogen). mTERF primer sequences are in Table S4. The binary vector for mosaic analysis was pB6Act1 (42). Lipids were extracted from mutant and WT calli, and their fatty acid compositions and contents were quantified through their methyl esters as described (43). The NativePAGE Novex Bis-Tris Gel system (Invitrogen) was used for BN-PAGE.
TAP tags were visualized with the peroxidase-antiperoxidase soluble complex P1291 (Sigma-Aldrich) at a 1/1,000 dilution. The GFP antigen was detected with 1/2,000-diluted primary rabbit anti-GFP antibody AB3080 (Chemicon International) and 1/10,000-diluted secondary ECL anti-rabbit IgG horseradish peroxidase-linked whole antibody from donkey NA934V (GE Healthcare). Peroxidase activity was detected with the Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer) and high-performance chemiluminescent film Hyperfilm ECL (GE Healthcare). 32P-labeled DNA probes were prepared with the nucleic acid labeling Readiprime II random prime labeling system (GE Healthcare). Gene fragments for labeling were amplified by PCR with gene-specific primers (Table S4).
Supplementary Material
Acknowledgments
We thank Hans-Peter Braun and Mark Davey for critical reading of the manuscript; Mansour Karimi and Bernd Reiss for plasmids; Duncan Rix and Dow AgroSciences for chemicals; John Ohlrogge, Christian Schmitz-Linneweber, Ian Small, and Thomas Börner for discussions; and Martine De Cock and Karel Spruyt for help in preparing the manuscript. This work was supported by the VIB Institutional Valorization Funds, National Institutes of Health Grant R00 ES015421 (to M.G.-D.), a grant from the Deutsche Forschungsgemeinschaft (to M.F.), and European Union Project QLG2-CT-1999-00846 (to S.K.). K.V. is a postdoctoral fellow of the Research Foundation-Flanders.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103442108/-/DCSupplemental.
References
- 1.Stern DB, Hanson MR, Barkan A. Genetics and genomics of chloroplast biogenesis: Maize as a model system. Trends Plant Sci. 2004;9:293–301. doi: 10.1016/j.tplants.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Woodson JD, Chory J. Coordination of gene expression between organellar and nuclear genomes. Nat Rev Genet. 2008;9:383–395. doi: 10.1038/nrg2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Juez E. Steering the solar panel: Plastids influence development. New Phytol. 2009;182:287–290. doi: 10.1111/j.1469-8137.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- 4.Hsu S-C, Belmonte MF, Harada JJ, Inoue K. Indispensable roles of plastids in Arabidopsis thaliana embryogenesis. Curr Genomics. 2010;11:338–349. doi: 10.2174/138920210791616716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberti M, et al. The MTERF family proteins: Mitochondrial transcription regulators and beyond. Biochim Biophys Acta. 2009;1787:303–311. doi: 10.1016/j.bbabio.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Yakubovskaya E, Mejia E, Byrnes J, Hambardjieva E, Garcia-Diaz M. Helix unwinding and base flipping enable human MTERF1 to terminate mitochondrial transcription. Cell. 2010;141:982–993. doi: 10.1016/j.cell.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellegrini M, et al. MTERF2 is a nucleoid component in mammalian mitochondria. Biochim Biophys Acta. 2009;1787:296–302. doi: 10.1016/j.bbabio.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Wenz T, Luca C, Torraco A, Moraes CT. mTERF2 regulates oxidative phosphorylation by modulating mtDNA transcription. Cell Metab. 2009;9:499–511. doi: 10.1016/j.cmet.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Park CB, et al. MTERF3 is a negative regulator of mammalian mtDNA transcription. Cell. 2007;130:273–285. doi: 10.1016/j.cell.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Proost S, et al. PLAZA: A comparative genomics resource to study gene and genome evolution in plants. Plant Cell. 2009;21:3718–3731. doi: 10.1105/tpc.109.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki JY, et al. Affinity purification of the tobacco plastid RNA polymerase and in vitro reconstitution of the holoenzyme. Plant J. 2004;40:164–172. doi: 10.1111/j.1365-313X.2004.02195.x. [DOI] [PubMed] [Google Scholar]
- 12.Ishizaki Y, et al. A nuclear-encoded sigma factor, Arabidopsis SIG6, recognizes sigma-70 type chloroplast promoters and regulates early chloroplast development in cotyledons. Plant J. 2005;42:133–144. doi: 10.1111/j.1365-313X.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 13.Krause K, Maier RM, Kofer W, Krupinska K, Herrmann RG. Disruption of plastid-encoded RNA polymerase genes in tobacco: Expression of only a distinct set of genes is not based on selective transcription of the plastid chromosome. Mol Gen Genet. 2000;263:1022–1030. doi: 10.1007/pl00008690. [DOI] [PubMed] [Google Scholar]
- 14.Peltier J-B, Ytterberg J, Liberles DA, Roepstorff P, van Wijk KJ. Identification of a 350-kDa ClpP protease complex with 10 different Clp isoforms in chloroplasts of Arabidopsis thaliana. J Biol Chem. 2001;276:16318–16327. doi: 10.1074/jbc.M010503200. [DOI] [PubMed] [Google Scholar]
- 15.Zybailov BG, et al. Large scale comparative proteomics of a chloroplast Clp protease mutant reveals folding stress, altered protein homeostasis, and feedback regulation of metabolism. Mol Cell Proteomics. 2009;8:1789–1810. doi: 10.1074/mcp.M900104-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tillich M, Krause K. The ins and outs of editing and splicing of plastid RNAs: Lessons from parasitic plants. New Biotechnol. 2010;27:256–266. doi: 10.1016/j.nbt.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Asakura Y, Barkan A. Arabidopsis orthologs of maize chloroplast splicing factors promote splicing of orthologous and species-specific group II introns. Plant Physiol. 2006;142:1656–1663. doi: 10.1104/pp.106.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng C-C, et al. Editing of accD and ndhF chloroplast transcripts is partially affected in the Arabidopsis vanilla cream1 mutant. Plant Mol Biol. 2010;73:309–323. doi: 10.1007/s11103-010-9616-5. [DOI] [PubMed] [Google Scholar]
- 19.Robbins JC, Heller WP, Hanson MR. A comparative genomics approach identifies a PPR-DYW protein that is essential for C-to-U editing of the Arabidopsis chloroplast accD transcript. RNA. 2009;15:1142–1153. doi: 10.1261/rna.1533909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel J, Börner T, Hess WR. Comparative analysis of splicing of the complete set of chloroplast group II introns in three higher plant mutants. Nucleic Acids Res. 1999;27:3866–3874. doi: 10.1093/nar/27.19.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoschke R, et al. An organellar maturase associates with multiple group II introns. Proc Natl Acad Sci USA. 2010;107:3245–3250. doi: 10.1073/pnas.0909400107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zubko MK, Day A. Stable albinism induced without mutagenesis: A model for ribosome-free plastid inheritance. Plant J. 1998;15:265–271. doi: 10.1046/j.1365-313x.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- 23.Roy A, Kucukural A, Zhang Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogenhagen DF, Rousseau D, Burke S. The layered structure of human mitochondrial DNA nucleoids. J Biol Chem. 2008;283:3665–3675. doi: 10.1074/jbc.M708444200. [DOI] [PubMed] [Google Scholar]
- 25.Phinney BS, Thelen JJ. Proteomic characterization of a Triton-insoluble fraction from chloroplasts defines a novel group of proteins associated with macromolecular structures. J Proteome Res. 2005;4:497–506. doi: 10.1021/pr049791k. [DOI] [PubMed] [Google Scholar]
- 26.Olinares PDB, Ponnala L, van Wijk KJ. Megadalton complexes in the chloroplast stroma of Arabidopsis thaliana characterized by size exclusion chromatography, mass spectrometry, and hierarchical clustering. Mol Cell Proteomics. 2010;9:1594–1615. doi: 10.1074/mcp.M000038-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato N, Albrieux C, Joyard J, Douce R, Kuroiwa T. Detection and characterization of a plastid envelope DNA-binding protein which may anchor plastid nucleoids. EMBO J. 1993;12:555–561. doi: 10.1002/j.1460-2075.1993.tb05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfalz J, Liere K, Kandlbinder A, Dietz K-J, Oelmüller R. pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell. 2006;18:176–197. doi: 10.1105/tpc.105.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meskauskiene R, et al. A mutation in the Arabidopsis mTERF-related plastid protein SOLDAT10 activates retrograde signaling and suppresses 1O2-induced cell death. Plant J. 2009;60:399–410. doi: 10.1111/j.1365-313X.2009.03965.x. [DOI] [PubMed] [Google Scholar]
- 30.Rogalski M, Ruf S, Bock R. Tobacco plastid ribosomal protein S18 is essential for cell survival. Nucleic Acids Res. 2006;34:4537–4545. doi: 10.1093/nar/gkl634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kode V, Mudd EA, Iamtham S, Day A. The tobacco plastid accD gene is essential and is required for leaf development. Plant J. 2005;44:237–244. doi: 10.1111/j.1365-313X.2005.02533.x. [DOI] [PubMed] [Google Scholar]
- 32.Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulte W, Töpfer R, Stracke R, Schell J, Martini N. Multi-functional acetyl-CoA carboxylase from Brassica napus is encoded by a multi-gene family: Indication for plastidic localization of at least one isoform. Proc Natl Acad Sci USA. 1997;94:3465–3470. doi: 10.1073/pnas.94.7.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baud S, et al. gurke and pasticcino3 mutants affected in embryo development are impaired in acetyl-CoA carboxylase. EMBO Rep. 2004;5:515–520. doi: 10.1038/sj.embor.7400124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 36.Ueda H, et al. AtVAM3 is required for normal specification of idioblasts, myrosin cells. Plant Cell Physiol. 2006;47:164–175. doi: 10.1093/pcp/pci232. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Zhang W, Stanley BA, Assmann SM. Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell. 2008;20:3210–3226. doi: 10.1105/tpc.108.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuhaus HE, Emes MJ. Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:111–140. doi: 10.1146/annurev.arplant.51.1.111. [DOI] [PubMed] [Google Scholar]
- 39.Bryant N, Lloyd J, Sweeney C, Myouga F, Meinke D. Identification of nuclear genes encoding chloroplast-localized proteins required for embryo development in Arabidopsis thaliana. Plant Physiol. 2011 doi: 10.1104/pp.110.168120. 10.1104/pp.110.168120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg M, Rogers R, Muralla R, Meinke D. Requirement of aminoacyl-tRNA synthetases for gametogenesis and embryo development in Arabidopsis. Plant J. 2005;44:866–878. doi: 10.1111/j.1365-313X.2005.02580.x. [DOI] [PubMed] [Google Scholar]
- 41.Kushnir S, et al. A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell. 2001;13:89–100. doi: 10.1105/tpc.13.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babiychuk E, et al. Allelic mutant series reveal distinct functions for Arabidopsis cycloartenol synthase 1 in cell viability and chloroplast differentiation. Proc Natl Acad Sci USA. 2008;105:3163–3168. doi: 10.1073/pnas.0712190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babiychuk E, et al. Arabidopsis phosphatidylglycerophosphate synthase 1 is essential for chloroplast differentiation, but is dispensable for mitochondrial function. Plant J. 2003;33:899–909. doi: 10.1046/j.1365-313x.2003.01680.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.