Abstract
UV radiation induces systemic immunosuppression. Because nonsteroidal anti-inflammatory drugs suppress UV-induced immunosuppression, prostanoids have been suspected as a crucial mediator of this UV effect. However, the identity of the prostanoid involved and its mechanism of action remain unclear. Here, we addressed this issue by subjecting mice deficient in each prostanoid receptor individually or mice treated with a subtype-specific antagonist to UV irradiation. Mice treated with an antagonist for prostaglandin E receptor subtype 4 (EP4), but not those deficient in other prostanoid receptors, show impaired UV-induced immunosuppression, whereas administration of an EP4 agonist rescues the impairment of the UV-induced immunosuppression in indomethacin-treated mice. The EP4 antagonist treatment suppresses an increase in the number of CD4+/forkhead box P3-positive (Foxp3+) regulatory T cells (Treg cells) in the peripheral lymph nodes (LNs) and dendritic cells expressing DEC205 in the LNs and the skin after UV irradiation. Furthermore, the EP4 antagonist treatment down-regulates UV-induced expression of receptor activator of NF-κB ligand (RANKL) in skin keratinocytes. Finally, administration of anti-RANKL antibody abolishes the restoration of UV-induced immunosuppression by EP4 agonism in indomethacin-treated mice. Thus, prostaglandin E2 (PGE2)–EP4 signaling mediates UV-induced immunosuppression by elevating the number of Treg cells through regulation of RANKL expression in the epidermis.
It has been known for more than 3 decades that UV radiation in the UVB (280–320 nm) range induces immunosuppression in animals. Fisher and Kripke (1) first noticed that UV-irradiated mice were more susceptible to transplanted cancer than nonirradiated mice and that lymphoid cells from irradiated mice failed to eliminate cancer cells. Based on these findings, they suggested that UV radiation induces systemic immunosuppression in animals and that this immunosuppression contributes indirectly to causing skin cancer. Systemic immunosuppression induced by UV was confirmed later in several animal models including contact hypersensitivity (CHS) (2), delayed-type hypersensitivity (3), allergic asthma (4), and experimental autoimmune encephalomyelitis (EAE) (5). This immunosuppressive effect also is exploited clinically, and UV radiation is used to treat a variety of diseases, such as psoriasis, vitiligo, and atopic dermatitis (6).
Several immune modulatory factors and immune cells are implicated in UV-induced systemic immunosuppression, including TNF-α, IL-4, IL-10 (7), platelet-activating factor (8), histamine (9), cis-urocanic acid (10), and natural-killer T cells (NKT cells) (11). In the early 1980s, T lymphocytes were found to play an important role in UV-induced systemic immunosuppression (12). Quite recently, regulatory T cells (Treg cells) were implicated in UV-induced systemic immunosuppression (13, 14). Loser et al. (13) reported that epidermal receptor activator for NFκB ligand (RANKL) is associated with UV-induced Treg cells and immunosuppression. They suggested that UV exposure up-regulates RANKL expression in keratinocytes, leading to the induction of Treg cells through activating epidermal dendritic cells (DCs) expressing DEC205, which recently were confirmed to be specialized to induce forkhead box P3-positive (Foxp3+) Treg cells (15). However, definite proof of the involvement of RANKL and Treg cells in UV-induced systemic immunosuppression has yet to be obtained.
Among the factors involved in UV-induced immunosuppression are prostanoids. Prostanoids, comprising prostaglandin (PG) E2, PGD2, PGF2α, PGI2, and thromboxane (TX) A2, are oxygenated metabolites of arachidonic acid produced by sequential catalysis of cyclooxygenase (COX) and respective synthases. They are produced in large amounts in inflammatory sites in response to various stimuli, including UV, and exert a variety of physiological and pathophysiological actions by acting on G protein-coupled receptors that includes four subtypes of PGE receptor (EP1, EP2, EP3, and EP4), PGD receptors (DP1 and DP2), PGF receptor (FP), PGI receptor (IP) and TXA receptor (TP) (16). Implication of prostanoids in UV irradiation-induced immunosuppression has been indicated by many studies showing that nonsteroidal anti-inflammatory drugs (NSAIDs), including indomethacin, that exert their effect through COX inhibition can reverse the immunosuppressive effect of UV radiation (8, 9, 17). Combined with the fact that prostanoids such as PGE2 are produced abundantly by keratinocytes upon UV exposure (18, 19), this reversal strongly suggests that prostanoids are involved in UV-induced immunosuppression. However, the identity of the prostanoid involved and how it is related to other proposed mechanisms of UV-induced immunosuppression remain unknown. We combined genetic and pharmacological approaches and addressed this long-standing question on the role of prostanoids in UV-induced systemic immunosuppression.
Results
UV Irradiation Induces Systemic Immunosuppression and Increases the Number of Treg Cells Responsible for UV-Induced Systemic Immunosuppression.
UV-induced systemic immunosuppression usually is examined in mice by CHS response. We adopted a protocol of previous studies (7, 9) with some modifications (Fig. 1A). We shaved the back skin of C57BL/6 mice on day 0, irradiated them on the back with UV on day 1, sensitized them on the abdomen with 2,4-dinitrofluorobenzene (DNFB) on day 5, challenged them on the ear on day 10, and evaluated ear swelling on day 11. We first determined an optimal UV dose producing effective systemic immunosuppression by applying various doses of UV. UV doses more than 5 kJ/m2 showed effective immunosuppression as evidenced by significantly lower CHS responses in these groups of mice than in nonirradiated control mice (Fig. 1B). Although the skin was severely injured in mice irradiated with UV at more than 7 kJ/m2, irradiation at 5 kJ/m2 caused only slight erythema on the skin. We therefore used 5 kJ/m2 of UV in our subsequent study. The immunosuppression induced by this dose of UV lasted for at least 2 wk (Fig. S1). To confirm the immunosuppression at the cellular level, we collected regional lymph nodes (LNs) on day 10 and cultured LN cells in the presence of 2,4-dinitrobenzene sulfonic acid (DNBS), a water-soluble form of DNFB. Consistent with the CHS response being driven mainly by IFN-γ–producing T cells, the cells from the UV-irradiated mice showed significantly lower cell proliferation and significantly less IFN-γ production than cells from nonirradiated mice (Fig. 1 C and D). Because it has been suggested that Treg cells play a prominent role in the immunosuppressive effect of UV (14), we monitored the number of Treg cells in the LNs after UV irradiation. The number of CD4+Foxp3+ Treg cells (20) increased in a time-dependent manner and reached a significant increase 3 d after UV irradiation (Fig. 1E), a result that is in agreement with our finding in CHS (Fig. S1) and with a previous report that systemic immunosuppression in mice starts not earlier than 3 d after the irradiation (21). We then assessed the significance of Treg cells in UV-induced systemic immunosuppression by using Foxp3hCD2/hCD52 mice. This line of mice expresses human CD2 and human CD52 chimeric protein in Foxp3+ Treg cells (22). As reported, Treg cells in these mice, but not in wild-type mice, were markedly depleted using Mabcampath, an anti-human CD52 antibody (Fig. S2). Treg-depleted Foxp3hCD2/hCD52 mice irradiated with UV showed significantly higher CHS response than UV-irradiated wild-type mice (Fig. 1F). This result thus shows that Treg cells are required for UV-induced systemic immunosuppression.
Fig. 1.
UV irradiation suppresses CHS responses in mice and induces Treg cells in draining LNs. (A) The experimental protocol. Mouse back skin is shaved on day 0, subjected to UV or sham irradiation (No UV) on the back on day 1, sensitized with 0.5% DNFB applied to the shaved abdomen on day 5, and challenged by applying 0.2% DNFB on the ear. Ear swelling is evaluated 24 h after the challenge. (B) Dose-dependent suppressive effect of UV on CHS responses. Ear swelling was measured 24 h after challenge in sham-irradiated mice (No UV) and mice irradiated with indicated doses of UV (n = 4 mice per group). (C) Reduced DNBS-induced proliferation of LN cells from UV-irradiated mice. Cells were prepared from draining LNs of sham-irradiated mice (No UV) or mice irratiated with 5 kJ/m2 UV (UV) 5 d after sensitization and were cultured in the presence or absence of DNBS for 72 h. Cell proliferation was measured by [3H]thymidine incorporation (n = 3 mice per group). (D) Reduced DNBS-induced IFN-γ production in LNs cells from UV-irradiated mice. LN cells were prepared as above and were cultured with or without DNBS for 48 h. The amount of IFN-γ in the culture medium was measured by ELISA (n = 3 mice per group). (E) Increase in Treg cells in LNs after UV irradiation. LNs were collected from mice at indicated times after UV irradiation or from control mice without irradiation (No UV), and CD4+Foxp3+ Treg cells in LNs were quantified by flow cytometry (n = 3 mice per group). (F) Suppressed UV-induced immunosuppression in Treg-depleted Foxp3hCD2/hCD52 mice. Foxp3hCD2/hCD52 and wild-type mice were injected i.v. with 0.5 mg of Mabcampath 1 d before irradiation. Ear swelling was measured 24 h after challenge (n = 4 mice per group). Data are representative of at least three independent experiments with similar results and are shown as mean ± SEM. *P < 0.05; **P < 0.01.
PGE2–EP4 Receptor Signaling Mediates UV-Induced Systemic Immunosuppression.
Using the system defined above, we next examined involvement of COX and PGs in UV-induced immunosuppression in our system. COX-2, but not COX-1, was strongly induced in skin keratinocytes of mice by UV irradiation (Fig. S3 A and B). Quantitative analysis of arachidonate metabolites revealed that, in comparison with other PGs, PGE2 was produced substantially in the skin 24 h after UV irradiation, and its production was suppressed significantly by indomethacin treatment (Fig. S3C). We then treated mice with 4 mg kg−1 d−1 indomethacin added in drinking water for 3 d beginning 24 h before UV irradiation. Treatment with indomethacin alone did not affect the CHS response. However, in agreement with previous studies (8, 9, 17), the indomethacin treatment reversed the UV-induced immunosuppression, as shown by significantly higher CHS response in the treated mice than in the control UV-irradiated mice (Fig. 2A). Similarly, treatment with a selective COX-2 inhibitor, SC-236, but not with a selective COX-1 inhibitor, SC-560, reversed the UV-induced immunosuppression (Fig. S4). Because these results suggest the involvement of PG in our experiment, we subjected mice deficient in each PG receptor individually (23) to our UV-induced systemic immunosuppression model. We excluded EP4-deficient mice from the experiment because they have a mixed genetic background of 129/Ola and C57BL/6 and show impaired CHS response (24). Instead we used an EP4 antagonist, ONO-AE-3–208 (24), and administered it to wild-type C57BL/6 mice to block the EP4 receptor pharmacologically. We also administered a TP/DP2 antagonist, ramatroban (25), to TP-deficient mice to examine involvement of DP2. We noted that UV irradiation induced immunosuppression in mice lacking DP1, EP1, EP2, EP3, FP, IP, or TP and in TP-deficient mice treated with ramatroban to a level similar to that found in controls (Fig. 2B), suggesting that these PG receptors do not play a crucial role in the induction of the systemic immunosuppression by UV exposure. To examine the involvement of EP4 receptor signaling, various doses of the EP4 antagonist were applied to wild-type mice in drinking water for the same period as described for indomethacin treatment. Notably, administration of the EP4 antagonist at 50 mg kg−1 d−1 and 100 mg kg−1 d−1 significantly prevented the UV-induced immunosuppression (Fig. 2C). The EP4 antagonist treatment restored the CHS response to a level found in mice treated with indomethacin or SC-236 (Fig. S4). Histological examination of the ear 24 h after the challenge showed that UV irradiation considerably decreased cell infiltration and edema in the dermis and that these changes were reversed by treatment with the EP4 antagonist (Fig. S5). Consistent with such changes, LN cells taken from EP4 antagonist-treated, UV-irradiated mice exhibited significantly increased cell proliferation and IFN-γ production in response to DNBS compared with LN cells from control irradiated mice (Fig. 2 D and E). These results suggest the importance of PGE2–EP4 signaling in UV-induced immunosuppression. To verify this hypothesis, we examined whether administration of an EP4 agonist (ONO-AE-1-329) (26) can restore immunosuppression in irradiated mice treated with indomethacin. Mice treated with 4 mg kg−1 d−1 indomethacin added to drinking water from day 0 to day 3 were subjected to UV irradiation on day 1 and were injected s.c. with different doses of the EP4 agonist immediately and 12 h after the UV irradiation (Fig. 3A). We confirmed that this injection of ONO-AE-1–329 can induce systemic EP4-blocking effects in mice injected with LPS (Fig. S6). Administration of ONO-AE-1–329 alone did not affect CHS responses (Fig. 3B). However, this compound dose-dependently restored the immunosuppression in the indomethacin-treated, UV-irradiated mice (Fig. 3B). This effect was mimicked by CAY10580, another EP4 agonist with a structure different from ONO-AE-1–329, but not by agonists for DP, EP2, or IP, which, like EP4, activate Gs protein (16) (Fig. S7). These findings affirm the action of PGE2–EP4 signaling in UV-induced systemic immunosuppression.
Fig. 2.
Reversal of UV-induced immunosuppression by COX inhibition or EP4 receptor antagonism. (A) Effects of indomethacin. Mice were administered 4 mg kg−1 d−1 of indomethacin (Indo) or vehicle (Veh) in drinking water from day 0 to day 3, and the CHS response was measured 24 h after challenge. (B) Effects of PG receptor deficiency. Mice deficient in DP1, EP1, EP2, EP3, FP, IP, or TP (DP1KO, EP1KO, EP2KO, EP3KO FPKO, IPKO, and TPKO, respectively) and TP-deficient mice treated with 10 mg kg−1 d−1 of ramatroban (TPKO+Ram) were used with their wild-type counterparts as control. Ear swelling was measured 24 h after challenge (n = 4 mice per group). (C) Effects of ONO-AE3-208. Mice were administered the indicated doses of an EP4 antagonist, ONO-AE3-208 (EP4ant) or vehicle (Veh) dissolved in drinking water from day 0 to day 3. Ear swelling was measured 24 h after challenge (n = 4 mice per group). (D and E) Effects of the EP4 antagonist on LN cell proliferation and IFN-γ production. Mice administered 50 mg kg−1 d−1 ONO-AE3-208 or vehicle from day 0 to day 3 were subjected to UV or sham irradiation on day 1 and then were sensitized with 0.5% DNFB on day 5. LNs were excised on day 10, and LN cells were subjected to DNBS-induced cell proliferation (D) and IFN-γ production (E) (n = 3 mice per group). Data are representative of three experiments with similar results and are shown as mean ± SEM. **P < 0.01; N.S., not significant.
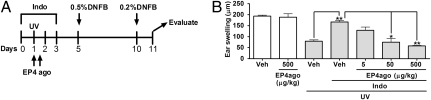
Fig. 3.
Restoration of UV-induced immunosuppression in indomethacin-treated mice by administration of an EP4 agonist. (A) The experimental protocol. Mice treated with indomethacin (Indo) or vehicle (Veh) as in Fig. 2A were irradiated with 5 kJ/m2 UV on day 1 and were injected s.c. with an EP4 agonist, ONO-AE1-329 (EP4ago), or vehicle (Veh) immediately and 12 h after the irradiation. The mice then were subjected to CHS induction as in Fig. 1A. (B) Effects of the EP4 agonist on indomethacin-induced impairment of UV-induced immunosuppression. Mice were treated with vehicle or the indicated doses of ONO-AE1-329, and the CHS response was measured (n = 4 mice per group). Data are representative of three experiments with similar results and are shown as mean ± SEM. *P < 0.05; **P < 0.01.
PGE2–EP4 Signaling Facilitates Increase of Treg Cells in Regional LNs After UV Irradiation.
To explore the mechanism of immunosuppressive action of PGE2–EP4 signaling, we collected axillary and inguinal LNs from UV-irradiated mice 4 d after irradiation and examined the effect of the EP4 antagonist on the number and composition of cells in these LNs. The UV irradiation significantly increased the total number of the LN cells, and the treatment of the EP4 antagonist did not affect this increase (Fig. 4A). The numbers of cells in the CD4 (CD4+CD8−), CD8 (CD8+CD4−), B (B220+), NK (DX5+Thy1.2−), and NKT (DX5+Thy1.2+) cell population also increased in the peripheral LNs after UV irradiation, but, again, there was no significant difference in the cell number of each of these populations between the mice treated with the EP4 antagonist and mice treated with the control vehicle (Fig. S8). However, the increase in Treg cells (defined as CD4+Foxp3+ cells) in the regional LNs after the irradiation was significantly lower both in number and percent in mice treated with either the EP4 antagonist or indomethacin than in control mice treated with vehicle (Fig. 4 B and C and Fig. S9). These results indicate the involvement of PGE2–EP4 signaling in increase in Treg cells after UV irradiation.
Fig. 4.
Selective suppression of the UV irradiation-induced increase in Treg cells in LNs by EP4 antagonism. Mice were administered 50 mg kg−1 d−1 of ONO-AE3-208 (EP4ant) or vehicle (Veh) from day 0 to day 3 and were subjected to UV irradiation and sensitization. Draining LNs were excised on day 5, and the number and composition of Treg cells were analyzed (n = 4 mice per group). (A) Total cell number of LNs. (B) The number and percent of CD4+Foxp3+ Treg cells in LNs. **P < 0.01. (C) Representative dot plot analysis of CD4+Foxp3+ cells in LNs from the vehicle-treated UV-irradiated mice and EP4 antagonist-treated UV-irradiated mice. Data are representative of three experiments with similar results and are shown as mean ± SEM.
PGE2–EP4 Signaling Mediates Epidermal RANKL Induction and Induces Epidermal DCs Expressing DEC205 in UV-Irradiated Mice.
Loser et al. (13) previously reported that UV irradiation up-regulated RANKL expression in the epidermis and induced DCs expressing DEC205 there, both of which they suggested might be responsible for induction of Treg cells in the peripheral lymphoid organs. We therefore analyzed the effect of the EP4 antagonist on the expression of RANKL in the skin. Real-time RT-PCR analysis of the skin exhibited a marked increase in RANKL mRNA after UV irradiation, and this increase was significantly suppressed by treatment with the EP4 antagonist (Fig. 5A). We also immunostained for RANKL in the skin of control mice and mice treated with the EP4 antagonist. The UV irradiation markedly enhanced immunofluorescent signals for RANKL in keratinocytes that were costained with an antibody to cytokeratin, a marker for keratinocytes. Additionally, keratinocytes formed multiple layers after the irradiation, suggesting that they underwent activation. Treatment with the EP4 antagonist substantially suppressed RANKL expression in the epidermis to the level seen in nonirradiated control mice and prevented multilayer formation (Fig. 5B). We also found that treatment with indomethacin elicited the same result as treatment with the EP4 antagonist, and the addition of the EP4 agonist could restore keratinocyte activation and RANKL production after UV irradiation in mice treated with indomethacin (Fig. 5B). These results indicate that PGE2–EP4 signaling mediates RANKL expression induced by UV irradiation in keratinocytes. We next examined the number of DCs expressing DEC205 (CD11c+DEC205+) specialized to induce Foxp3+ Treg cells (15) in the LNs. We detected a markedly increased number of CD11c+DEC205+ cells in the peripheral LNs 2 d after UV irradiation, and this increase of CD11c+DEC205+ cells was suppressed significantly by treatment with the EP4 antagonist (Fig. 5C). Although these results indicate that fewer DEC205+ DCs are present in the LNs to induce Treg with the EP4 antagonism, we also noted that the total number of CD11c+ DCs in the LNs decreased with the EP4 antagonist treatment 2 d after UV irradiation (Fig. 5C). These results might reflect the interference of DC migration by the EP4 antagonist, as we previously reported (24), and raised a question whether the EP4 antagonism suppressed induction of CD11c+DEC205+ cells by UV irradiation in situ in the skin. We therefore isolated epidermal sheet 2 d after UV irradiation and examined the number of CD11c+DEC205+ cells in the epidermis. UV irradiation significantly increased the population of CD11c+DEC205+ cells in the epidermal cells, and the treatment with the EP4 antagonist suppressed this increase of CD11c+DEC205+ cells (Fig. 5D). These results clearly show that the lack of PGE2–EP4 signaling leads to reduced induction of CD11c+DEC205+ cells in the skin. To verify further that PGE2–EP4 signaling mediates UV-induced systemic immunosuppression by regulating epidermal RANKL, we treated mice with either anti-RANKL or the isotype control antibody 2 d before UV irradiation. Anti-RANKL treatment could diminish immunosuppression by UV irradiation to a degree similar to indomethacin treatment (Fig. 6). There was no additive effect of treatments with indomethacin and anti-RANKL antibody. Further, treatment with the EP4 agonist did not restore immunosuppression in indomethacin-treated mice cotreated with anti-RANKL. These results suggest that RANKL is indispensable for the PGE2–EP4 signaling to mediate UV-induced systemic immunosuppression. It also is noted that administration of either anti-RANKL antibody or indomethacin did not restore the immune response fully to the level observed in control non–UV-irradiated mice.
Fig. 5.
PGE2–EP4 signaling mediates the expression of RANKL in keratinocytes and induces DCs expressing DEC205 in UV irradiation. Mice were treated with 50 mg kg−1 d−1 ONO-AE3-208 (EP4ant) or vehicle (Veh) from day 0 and were subjected to UV or sham irradiation on the back on day 1. (A) Effects of EP4 antagonism on UV-induced expression of RANKL mRNA in the skin. The back skin was excised 1 d after irradiation, and Rankl mRNA expression in the skin was evaluated by quantitative real-time PCR analysis and is shown in arbitrary expression units (AU). Rankl expression is normalized to that of Gapdh (n = 3 mice per group). (B) Effect of EP4 antagonism and EP4 agonism on UV-induced increase in RANKL protein in keratinocytes. For EP4 agonist treatment, mice were treated with 4 mg kg−1 d−1 indomethacin (Indo) from day 0 on, were subjected to UV irradiation, and were injected s.c. with 500 μg/kg ONO-AE1-329 (EP4ago), or vehicle immediately and 12 h after the irradiation. The back skin was excised 2 d after irradiation and was stained for RANKL (red) and cytokeratin (green). Cell nuclei were stained with Hoechst-33342. (Scale bar, 50 μm.) (C) Effects of EP4 antagonism on the increase of DEC205+ DCs in LNs after UV irradiation. LNs were collected 2 d after UV irradiation, and the number of CD11c+ and CD11c+DEC205+ cells in LNs was analyzed by flow cytometry (n = 4 mice per group). (D) Effects of EP4 antagonism on the UV-induced increase of DEC205+ DCs in the skin. Epidermal cells were prepared from back skins excised 2 d after irradiation, and CD11c+ cells were isolated by magnetic-activated cell sorting (MACS). DEC205+ cells in isolated CD11c+ cells were analyzed by flow cytometry (n = 4 mice per group). Data are representative of three experiments with similar results and are shown as mean ± SEM. *P < 0.05; **P < 0.01.
Fig. 6.
RANKL is indispensable for EP4-mediated, UV-induced systemic immunosuppression. Mice were injected s.c. with 100 μg of either anti-RANKL or isotype control antibody (Control) 2 d before UV irradiation. Indomethacin (Indo), 4 mg kg−1 d−1, and ONO-AE1-329 (EP4ago), 500 μg/kg, were administered as in Fig. 3A. The CHS response was measured (n = 4 mice per group). Data are representative of two experiments with similar results and are shown as mean ± SEM. *P < 0.05; **P < 0.01.
Discussion
UV radiation is not only carcinogenic but also suppresses immunity. Here, we answered a long-standing question regarding the role of prostanoids in UV-induced systemic immunosuppression by showing that PGE2–EP4 signaling mediates UV-induced systemic immunosuppression. We showed impairment of the immunosuppressive effect of UV by EP4 antagonism and reversal of the indomethacin-induced impairment of immunosuppression by EP4 agonism. Notably, treatment with the EP4 agonist alone, without UV irradiation, did not result in immunosuppression (Fig. 3B). Because several immune-modulatory mediators have been reported to play roles in the UV-induced systemic immunosuppression (7–10), it is likely that PGE2 acts in collaboration with other mediators to induce the immunosuppression after UV irradiation. Indeed, we previously showed that PGs such as PGE2 and PGI2 collaborate with IL-1β and enhance induction of various cytokines and remodeling factors, including RANKL, in a model of collagen-induced arthritis (27). Which mediator collaborates with PGE2 to induce immunosuppression remains to be determined. By examining the cell populations of the peripheral LNs after UV irradiation, we found that only an increase in the Treg cell population after UV irradiation was significantly suppressed by treatment with the EP4 antagonist, whereas the EP4 antagonist did not affect increases in numbers of other LN cell populations, including NKT cells, which previously were reported to mediate systemic immunosuppression after UV irradiation (11). Using Foxp3hCD2/hCD52 mice, we further showed that Treg cells play an indispensable role in UV-induced systemic immunosuppression. We also used anti-RANKL antibody and showed that RANKL makes a major contribution to this process. Our results, however, do not exclude an involvement of signaling molecules other than PGE2 in immunosuppression in a process independent of PGE2–EP4–RANKL signaling, because the EP4 antagonism did not completely suppress the increment of Treg cells after UV irradiation, and anti-RANKL antibody did not restore ear swelling to the level found in control mice.
Here we identified PGE2–EP4 signaling as an initiating factor for RANKL expression in keratinocytes. Classically, PGE2 was known as an osteolytic factor that functions in osteoblasts downstream of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α in induction of osteoclasts from bone marrow precursor cells. We previously identified the receptor mediating this action as EP4 (28). The molecule induced by PGE2 treatment and responsible for osteoclastogenesis was identified as RANKL (29). Thus, our present study has revealed that the identical PGE2–EP4–RANKL signaling operates in different types of cells with different consequences, one for osteolysis and the other for immunomodulation. It also is noted that PGE2–EP4 signaling can exhibit apparently opposite immunomodulatory actions in different situations. We recently used the EAE mouse model and showed that PGE2–EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion (30), whereas our current study showed that the identical signaling promotes immunosuppression. How such context-dependent differences arise should be defined clearly so that the EP4 antagonist can be used properly and safely in therapeutics in the future. In addition, UV-induced immunosuppression has been implicated in nonmelanoma skin cancers caused by UV radiation (1, 12, 31), and the incidence of nonmelanoma skin cancers was lower in subjects receiving a selective COX-2 inhibitor, celecoxib, than in subjects receiving placebo (32). We hope that the action of PGE2–EP4 signaling we have described here is exploited in various clinical settings, including this malignancy.
Materials and Methods
Animals.
Mice lacking each type or subtype of PG receptor individually were generated and backcrossed more than 10 times onto C57BL/6 background as described previously (23). Foxp3hCD2/hCD52 mice with C57BL/6 background were generated as described (22). Female mice of each genotype were used at age 8–10 wk. Wild-type C57BL/6CrSlc mice (Japan SLC) were used as controls. All mice were maintained on a 12-h/12-h light/dark cycle under specific pathogen-free conditions. All experimental procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Committee on Animal Research of Kyoto University Faculty of Medicine.
UVB Irradiation and CHS.
A bank of sunlamps emitting 280–360 nm with a peak emission at 313 nm (FL 20SE; Toshiba) arranged in parallel was used as a source of UVB. The irradiance, measured by an UVR-305/365D radiometer (Tokyo Kogaku), was 5 J/m2 s−1 at a distance of 40 cm. On day 0, back fur was shaved with electric clippers. On day 1, mice were exposed to 5 kJ/m2 of UVB on the shaved back with their ears and eyes protected. They were sensitized by applying 25 μL of 0.5% (wt/vol) DNFB (Sigma) in acetone/olive oil (4/1, vol/vol) on the shaved abdomen on day 5. Then, on day 10, the mice were challenged by application of 20 μL of 0.2% DNFB to the dorsal and ventral surfaces of both ears. The ear thickness of each mouse was measured before and 24 h after elicitation at a predetermined site with a micrometer, and the difference was expressed as ear swelling.
For drug treatment, 4 mg kg−1 d−1 of indomethacin (Nacalai) or 10–100 mg kg−1 d−1 of ONO-AE-3–208 in drinking water was given ad libitum from day 0 to day 3; 5 mg kg−1 of Ramatroban (Tocris) was injected s.c. every 12 h to TP-deficient mice from day 0 to day 3; and 5–500 μg/kg of ONO-AE-1–329 in 0.9% saline was injected s.c. immediately and 12 h after UV irradiation.
For anti-RANKL treatment, mice were injected s.c. with 100 μg of anti-mouse RANKL (Oriental Yeast) 2 d before UV irradiation. The same amount of rat IgG2a antibody (eBioscience) was used as a control.
Lymphocyte Culture.
Single-cell suspensions were prepared from inguinal and axillary LNs of mice 5 d after sensitization with 0.5% DNFB. The cells were cultured at 5 × 105 cells per well in a 96-well plate in 200 μL of RPMI-1640 containing 10% FBS with or without 100 μg/mL DNBS (Sigma) for 3 d and were pulsed with 0.5 μCi [3H]thymidine for the last 24 h of culture. The acid-insoluble radioactivity was determined by liquid scintillation counting. For measurement of cytokine production, the cells were cultured for 48 h, and the culture supernatants were collected. IFN-γ in the supernatants was measured using an IFN-γ ELISA kit (Endogen).
Immunofluorescence.
Immunostaining was performed on 10-μm cryostat sections of mouse back skin embedded in optimal cutting temperature (OCT) compound. Tissue sections were fixed in precooled acetone for 10 min. Anti-RANKL and anti-cytokeratin antibodies were used as primary antibodies. Biotinylated anti-rat IgG (Vector) and Alexa Fluor 488 anti-mouse IgG (Invitrogen) were used as secondary antibodies. The RANKL signal was amplified using the tyramide signal amplification (TSA) Plus Cyanine-3 System (Perkin-Elmer) according to the manufacturer's protocol. Cell nuclei were stained using Hoechst-33342 (Invitrogen) according to the manufacturer's instruction.
Statistical Analysis.
Data were analyzed by Student's t test, and differences were considered significant at P < 0.05. All bar graphs represent the mean ± SEM.
Supplementary Material
Acknowledgments
We thank S. Kongpatanakul, A. Wongkajornsilp, and P. Akarasereenont for encouragement to K.S. We also thank L. Loser for advice on UV irradiation, R. Hanada and C. Miyaura for advice on RANKL, and M. Mizutani and T. Arai for assistance. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and in part by a collaborative grant to Kyoto University from Ono Pharmaceuticals, which partially supported the mouse colonies and supplied ONO-AE-3-208 and ONO-AE-1-329 used in this study. K.S. was a recipient of a Novartis Fellowship in 2008.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018625108/-/DCSupplemental.
References
- 1.Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci USA. 1977;74:1688–1692. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noonan FP, De Fabo EC, Kripke ML. Suppression of contact hypersensitivity by UV radiation and its relationship to UV-induced suppression of tumor immunity. Photochem Photobiol. 1981;34:683–689. [PubMed] [Google Scholar]
- 3.Molendijk A, van Gurp RJ, Donselaar IG, Benner R. Suppression of delayed-type hypersensitivity to histocompatibility antigens by ultraviolet radiation. Immunology. 1987;62:299–305. [PMC free article] [PubMed] [Google Scholar]
- 4.Van Loveren H, et al. UV exposure alters respiratory allergic responses in mice. Photochem Photobiol. 2000;72:253–259. doi: 10.1562/0031-8655(2000)072<0253:uearar>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Becklund BR, Severson KS, Vang SV, DeLuca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci USA. 2010;107:6418–6423. doi: 10.1073/pnas.1001119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanolli M. The modern paradigm of phototherapy. Clin Dermatol. 2003;21:398–406. doi: 10.1016/j.clindermatol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Rivas M, Ullrich SE. The role of lL-4, IL-10, and TNF-α induced by ultraviolet radiation in the immune suppression. J Leukoc Biol. 1994;56:769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 8.Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart PH, Townley SL, Grimbaldeston MA, Khalil Z, Finlay-Jones JJ. Mast cells, neuropeptides, histamine, and prostaglandins in UV-induced systemic immunosuppression. Methods. 2002;28:79–89. doi: 10.1016/s1046-2023(02)00201-3. [DOI] [PubMed] [Google Scholar]
- 10.Walterscheid JP, et al. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc Natl Acad Sci USA. 2006;103:17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: Regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 12.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 13.Loser K, et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 14.Gorman S, Tan JW-Y, Yerkovich ST, Finlay-Jones JJ, Hart PH. CD4+ T cells in lymph nodes of UVB-irradiated mice suppress immune responses to new antigens both in vitro and in vivo. J Invest Dermatol. 2007;127:915–924. doi: 10.1038/sj.jid.5700600. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki S, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodward DF, Jones RL, Narumiya S. International Union of Basic and Clinical Pharmacology: Classification of prostanoid receptors, updating 15 years of progress. Pharmacol Rev. 2011 doi: 10.1124/pr.110.003517. in press. [DOI] [PubMed] [Google Scholar]
- 17.Chung HT, Burnham DK, Robertson B, Roberts LK, Daynes RA. Involvement of prostaglandins in the immune alterations caused by the exposure of mice to ultraviolet radiation. J Immunol. 1986;137:2478–2484. [PubMed] [Google Scholar]
- 18.Ruzicka T, Walter JF, Printz MP. Changes in arachidonic acid metabolism in UV-irradiated hairless mouse skin. J Invest Dermatol. 1983;81:300–303. doi: 10.1111/1523-1747.ep12519287. [DOI] [PubMed] [Google Scholar]
- 19.Kuwamoto K, et al. Possible involvement of enhanced prostaglandin E2 production in the photosensitivity in xeroderma pigmentosum group A model mice. J Invest Dermatol. 2000;114:241–246. doi: 10.1046/j.1523-1747.2000.0883x.x. [DOI] [PubMed] [Google Scholar]
- 20.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 21.Noonan FP, De Fabo EC. Ultraviolet-B dose-response curves for local and systemic immunosuppression are identical. Photochem Photobiol. 1990;52:801–810. doi: 10.1111/j.1751-1097.1990.tb08685.x. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu N, et al. Heterogeneity of natural Foxp3+ T cells: A committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabashima K, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabashima K, et al. Prostaglandin E2-EP4 signaling initiates skin immune responses by promoting migration and maturation of Langerhans cells. Nat Med. 2003;9:744–749. doi: 10.1038/nm872. [DOI] [PubMed] [Google Scholar]
- 25.Satoh T, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177:2621–2629. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- 26.Esaki Y, et al. Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:12233–12238. doi: 10.1073/pnas.0915112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda T, Segi-Nishida E, Miyachi Y, Narumiya S. Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J Exp Med. 2006;203:325–335. doi: 10.1084/jem.20051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyaura C, et al. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J Biol Chem. 2000;275:19819–19823. doi: 10.1074/jbc.M002079200. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao C, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 31.Welsh MM, et al. A role for ultraviolet radiation immunosuppression in non-melanoma skin cancer as evidenced by gene-environment interactions. Carcinogenesis. 2008;29:1950–1954. doi: 10.1093/carcin/bgn160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elmets CA, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: A randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.