Abstract
Predation is a fundamental process in the interaction between species, and exerts strong selection pressure. Hence, anti-predatory traits have been intensively studied. Although it has long been speculated that individuals of some species gain protection from predators by sometimes almost-uncanny resemblances to uninteresting objects in the local environment (such as twigs or stones), demonstration of antipredatory benefits to such “masquerade” have only very recently been demonstrated, and the fundamental workings of this defensive strategy remain unclear. Here we use laboratory experiments with avian predators and twig-mimicking caterpillars as masqueraders to investigate (i) the evolutionary dynamics of masquerade; and (ii) the behavioral adaptations associated with masquerade. We show that the benefit of masquerade declines as the local density of masqueraders relative to their models (twigs, in our system) increases. This occurs through two separate mechanisms: increasing model density both decreased predators’ motivation to search for masqueraders, and made masqueraders more difficult to detect. We further demonstrated that masquerading organisms have evolved complex microhabitat selection strategies that allow them to best exploit the density-dependent properties of masquerade. Our results strongly suggest the existence of opportunity costs associated with masquerade. Careful evaluation of such costs will be vital to the development of a fuller understanding of both the distribution of masquerade across taxa and ecosystems, and the evolution of the life history strategies of masquerading prey.
Keywords: camouflage, crypsis, habitat selection, misclassification, Selenia dentaria
Organisms are under strong selection to avoid predators and to capture prey, and understanding how animals’ visual appearances influence predation continues to be a stimulating challenge for evolutionary theory (1). Although the evolution of crypsis (avoiding detection; ref. 2), aposematism (warning coloration; ref. 3) and mimicry (resembling a defended organism; ref. 4) are intensively studied, one aspect of adaptive coloration has been almost completely ignored: masquerade (5). Masquerading organisms have evolved striking visual resemblances to inedible objects (termed “models”) found in the same locality. For example, the spider Ornithoscatoides decipiens looks like bird droppings, the leafy sea dragon Phyllopteryx eques may be misidentified as seaweed (6), and several species of caterpillar closely resemble twigs (7). It has long been assumed that individuals using masquerade avoid predation, or gain access to prey, by being misclassified as inedible objects by their predators or as innocuous objects by their prey (5). In short, whereas crypsis functions to prevent organisms being detected, masquerade is thought to function by ensuring that organisms are not correctly identified as predators/prey once they have been detected (fundamental differences between masquerade and both crypsis and Batesian mimicry have recently been reviewed from the theoretical perspective; ref. 5). However, this function has only recently been confirmed empirically (7), and neither the evolutionary dynamics of masquerade, nor the behavioral adaptations associated with it, have been extensively studied.
It has been suggested that the evolutionary dynamics of masquerade in some respects parallel those of Batesian mimicry (5). Palatable Batesian mimics gain greater protection from predators when they are rare in comparison with the defended species (or model) that they resemble (1). This is because when mimics are common relative to their models, predators learn of their presence and increase their attack rates on the model/mimic complex. As a result, natural selection is more likely to favor Batesian mimics of common, rather than rare, models. By analogy, the effectiveness of masquerade may also be determined by the relative abundance of masqueraders to their models: with the benefit of masquerade declining as the local density of masqueraders increases and/or the local density of models decreases. When masqueraders are common in comparison with their models, predators would be more likely to be rewarded with a masquerader when they attack an individual of the model–masquerader complex, and consequently they would find it more economic to spend time searching for masqueraders.
An entirely separate mechanism may also cause masquerade to increase in effectiveness as the local density of their model increases. Increasing model density may often lead to masqueraders being viewed against more complex visual backgrounds, which are known to complicate and prolong search times of predators, even if prey items are not cryptic (8, 9). For example, it may be more difficult to detect a twig-mimicking caterpillar when it is sat among 50 twigs than when it is sat among 5 twigs.
If the effectiveness of masquerade is influenced by the local environment (e.g., model density in the situation considered here), then one might expect masqueraders to possess associated behavioral adaptations that allow them to best exploit the benefit of masquerade. Such behavioral adaptations have been demonstrated in cryptic prey (10–12), and there is some evidence that masquerading prey may select microhabitats in which they are most likely to be mistaken for their inedible model (13, 14). However, there is no evidence that selection to avoid predation (rather than to find an abundant food source) is driving microhabitat selection in masquerading prey. Given that the benefit of masquerade is likely to be density-dependent, we predict that masquerading individuals should select microhabitats where their models are common. Clearly, microhabitat selection will also be influenced by resource abundance, and in situations where masqueraders are forced to trade off food abundance with protection from predation, we would expect the outcome to be determined by the relative risks of starvation and being eaten.
We used domestic chicks, Gallus gallus domesticus, as predators and twig-mimicking caterpillars of the Early Thorn moth, Selenia dentaria, as masquerading prey to test whether predation on masquerading prey is influenced by the relative density of masqueraders and their models; and, if so, what the underlying mechanism for this effect is. By manipulating the relative density of models to masqueraders, without altering either birds’ experience with twig-mimicking caterpillars or the complexity of the visual task required to detect a caterpillar, we were able to ask whether the relative density of masquerading prey influenced birds’ motivation to attack them. By controlling birds’ previous experience of twigs and masqueraders, but altering the number of twigs present in a test trial, we were able to ask whether increased twig density made caterpillars more difficult to detect. Finally, by giving masquerading prey a series of trials in which they were allowed to choose between two different microhabitats, we were able to ask whether they possess behavioral strategies that reduce the costs associated with density-dependent predation and whether and how they trade off protection from predation and access to food when selecting microhabitats.
Results
Experiment 1: Does the Relative Density of Masquerading Prey Influence Predators’ Motivation to Attack Them?
Thirty-two domestic chicks were trained to forage on twig-mimicking caterpillars before being divided into four equal groups. Chicks in all experimental groups were habituated to the experimental arena. They were then given a series of 10 presentations designed to manipulate the number of twigs that chicks experienced without influencing their experience of caterpillars or the difficulty of finding caterpillars. All chicks received 4 presentations of a rewarding hawthorn branch containing 9 twigs and 1 caterpillar. Two groups also experienced a high exposure to unrewarding hawthorn branches with 10 twigs and no caterpillar (5 presentations) plus 1 null presentation of an empty arena (10 presentations in all); the other two groups experienced low exposure to unrewarding hawthorn branches containing 10 twigs and no caterpillar (1 presentation) and 5 null presentations (also 10 presentations in total). Thus, all groups had the same amount of experience with caterpillars, but differed in their experience of the relative commonness of caterpillars compared with twigs.
One group from each exposure treatment received unmanipulated branches, and the other received manipulated hawthorn branches that had been bound in purple cotton thread. The reason for training some groups on branches bound in thread was to test whether twig density influenced predatory behavior when caterpillars did not benefit from masquerade. This manipulation is known to remove resemblance between twigs and caterpillars (7) and allows us to remove the possibility that simply sampling lots of nonrewarding stimuli influenced predators' behavior.
All chicks were given a single test trial in which they were given a manipulated or unmanipulated branch (consistent with their initial experience) containing nine twigs and one caterpillar. The latency to attack the caterpillar was recorded. Note that the visual complexity of the search task was the same across treatments, the key differences between treatments were in the previous experience of predators and in whether caterpillars could benefit from masquerade or not.
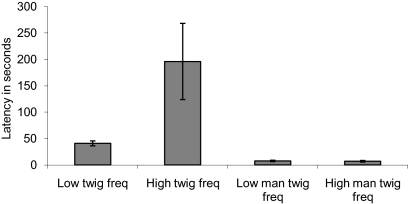
The time taken to attack the caterpillar differed among our experimental groups (Kruskal Wallis test: χ2 = 26.15, P < 0.001, df = 3; see Fig. 1). Chicks that had experienced unmanipulated branches took longer to attack caterpillars when they had higher levels of exposure to branches without caterpillars than when they had low levels of exposure (χ2 = 10.62, P = 0.001, df = 1). Thus, when prey could benefit from masquerade, predation rate (in a common prey-search task) was reduced when the predator's previous experience was that unrewarding models were more common. In contrast, when chicks were trained on manipulated branches, exposure level had no detectable effect on the time taken to attack the caterpillar (χ2 = 0.34, P < 0.56, df = 1). This result demonstrates that selection on nonmasquerading prey was not density dependent. Thus, it is not simply experience with nonrewarding stimuli that causes density-dependent predation, but experience with nonrewarding items that look like prey items. This finding demonstrates that increasing exposure to unrewarding branches decreased chicks’ motivation to search for masquerading caterpillars, but only when they resembled twigs, and thus the effectiveness of masquerade increases as caterpillar local density declines relative to twig local density.
Fig. 1.
The mean time in seconds (±SEM) taken to peck the caterpillar in the test trial in experiment 1.
Experiment 2: Does Increasing Twig Density Make it More Difficult for Predators to Detect Masquerading Prey?
Chicks from the two low-exposure groups in the previous experiment were given two trials to explore whether the number of twigs on the branch influenced the time taken to find the caterpillar (i.e., whether it was more difficult to find a caterpillar in more complex environments). In one trial, the chick was offered a branch with 5 twigs and 1 caterpillar; in the other trial, the branch had 15 twigs and 1 caterpillar (the order of these two trials was counterbalanced within groups). The latency to attack the caterpillar was recorded.
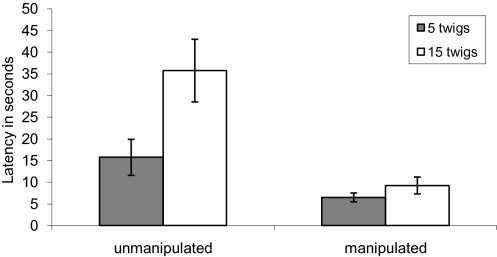
Chicks given unmanipulated branches took significantly longer to find masquerading prey at high local twig densities than at low twig densities (Sign test: P = 0.0078, n = 8; see Fig. 2), whereas chicks given manipulated branches took a similar amount of time to find masquerading prey irrespective of the number of twigs present (P = 0.726, n = 8). This result demonstrates that chicks found it more difficult to detect prey at high twig densities than at low twig densities, but only when prey benefitted from masquerade.
Fig. 2.
The mean time in seconds (±SEM) taken to peck the caterpillar in experiment 2. Filled bars represent latencies when 5 twigs were present, and open bars represent latencies when 15 twigs were present.
Experiment 3: Do Masquerading Prey Select Microhabitats that Minimize the Costs Associated with Density-Dependent Predation?
Caterpillars were placed singly into choice chambers with two different branches. They were left for 30 min, after which the branch on which the caterpillar was located was recorded. The primary difference between the two branches was in the number of twigs: one with four twigs (low twig density) and one with eight twigs (high twig density); twigs sometimes also differed in the availability of leaves used as food by the caterpillars. Trials were conducted both by day and by night. We predicted that daylight would increase the perceived risk of attack from visually hunting predators. In some trials, caterpillars were food deprived before the trial to enhance the value of leaves to them; in some trials, they were subjected to simulated handling by a predator before the trial, to enhance the perceived need for effective antipredator protection (see Table 1).
Table 1.
Overview of experimental design for the caterpillar choice experiment
| Experimental group | Leaves on branch with low twig frequency? | Leaves on branch with high twig frequency? | Food deprivation? | Simulated predation? |
| Both leaves | Yes | Yes | No | No |
| Both no leaves | No | No | No | No |
| High leaves | No | Yes | No | No |
| Low leaves | Yes | No | No | No |
| Low leaves fasted | Yes | No | Yes | No |
| Low leaves fasted and predation | Yes | No | Yes | Yes |
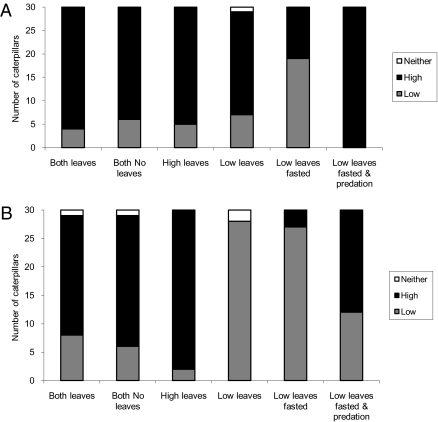
Caterpillars showed a preference for the branch with the higher number of twigs in trials when both twigs also had approximately equal numbers of leaves, when both twigs had no leaves, and when only the branch with a high number of twigs had leaves. This preference was shown both by day (Binomial test: P < 0.0001, n = 30; P = 0.0014, n = 30; and P = 0.0003, n = 30, respectively; see Fig. 3A), and by night (P = 0.0241, n = 29; P = 0.0023, n = 29; and P < 0.0001, n = 30 respectively; see Fig. 3B). This finding suggests that masquerading caterpillars chose branches that maximized both food abundance and protection from predators. It is unclear from these results whether food or predation were driving decisions (even when no food was present, high twig densities could still be a heuristic caterpillars use to find food). Therefore, we created situations in which one branch provided the best food source and the other provided the best protection from predators, and investigated what factors influenced the way caterpillars traded off the two benefits.
Fig. 3.
The number of caterpillars found on the low-twig-frequency branch (gray bars), on the high-twig-frequency branch (black bars), and on neither branch (open bars) in experiment 3. (A) Caterpillar choices made by day. (B) Caterpillar choices made by night.
Our remaining results refer to trials where only the branch with the low number of twigs had leaves on it; and so the branch that provided food for caterpillars provided poorer protection from predators through masquerade. Caterpillars tested in the day showed a significant preference for branches with a high twig number (Binomial test: P = 0.0081, n = 29) despite this branch offering them no food, whereas those tested at night showed a significant preference for branches with a low twig number (P < 0.0001, n = 28). This result shows that when food and maximizing antipredatory defense through masquerade conflict, caterpillars act to maximize effectiveness of masquerade by day (when visual predators are active) but change behavior to prioritize food at night (when the risk of predation from visually hunting predators is much lower, and so antipredatory masquerade is much less valuable).
During the day, caterpillars faced with this conflict between food and perceived predation risk were more likely to opt for the branch with food if they had previously been food deprived than if they had not (Fisher's test: P < 0.0038); when the value of food was increased, the benefit of obtaining food outweighed the costs associated with foraging in a microhabitat that posed a higher risk of predation. However, there was no effect of food deprivation when tested at night (P = 0.2377). At night, almost all caterpillars (in both groups) opted for the branch with leaves. This result shows that caterpillars are flexible in their behavior and modify habitat preference in response to variation in internal state (hunger in this case).
In both the day and the night, caterpillars that had been food deprived and subjected to simulated predation showed significantly stronger preferences for branches with a high twig number (and no leaves) than caterpillars that had only been food deprived (P < 0.0001 and P < 0.0001, respectively). This result again shows that caterpillars are flexible in their behavior, this time in response to variation in external environment (perceived predation risk). Specifically, when experiencing cues of high predation risk, caterpillars were more likely to forgo feeding opportunities to exploit an alternative microhabitat where the density-dependent antipredatory gains from masquerade were higher.
Discussion
We demonstrated that masquerade is less effective as an antipredatory defense when the density of masqueraders is increased relative to their models. This occurred through two separate mechanisms: first, predators were less motivated to search for masquerading caterpillars if their recent experience suggested a high, rather than low, local density of unrewarding twigs. Second, increasing the number of models made it more difficult for predators to detect masquerading prey.
The relative importance of these two processes is likely to differ between masquerading species. For example, the twig mimics may often be found in close proximity to their models (twigs), thus emphasizing the second mechanism. However, some caterpillars are considered to mimic bird droppings (15) and are often found on leaves that do not necessarily carry real droppings; this type of masquerade is still likely to show density dependence, but will do so more strongly through the first mechanism.
The fact that higher local densities of unrewarding models made predators more reluctant to search for masquerading caterpillars can be understood in terms of foraging economics: if the predator's previous experience tells it that individuals of the masquerader–model complex are most likely to be unrewarding models, then predators will be less motivated to investigate such individuals in future, particularly when they can otherwise invest their time more profitably (16). Predators’ abilities to detect masquerading prey may also improve with recent experience of masqueraders. Such an effect would be akin to search-image formation (17), and would make investigating individuals of the model–mimic complex more profitable for predators with recent experience of masquerading prey. Furthermore, it is well known in the psychology of visual discrimination that performance is improved if the distracters are visually quite different from the targets (18).
Our results suggest that the evolutionary dynamics of masquerade may, in some respects, parallel those of Batesian mimicry. It is well established that the benefit of Batesian mimicry is density dependent, and density-dependent predation is considered to drive polymorphism within mimicking populations (1). Intriguingly, there may be analogous situations in masquerade, where twig-mimicking caterpillars sometimes display polyphenism, with individuals of a given local population differing in appearance so as to more closely resemble twigs of their current host plant (19). However, the evolutionary dynamics of masquerade are unlikely to exactly mirror those of Batesian mimicry. Batesian mimics cause predators to change their behavior toward the defended model in a way that influences its’ population or evolutionary dynamics; whereas, if masqueraders have any influence on the evolutionary dynamics of the model, they do so directly, either by damaging the models themselves (e.g., stick insect eating their host plants) or by removing animals that directly influence the reproductive success of the model (e.g., flower mantids eating seed-eating pests and/or pollinators; ref. 5).
We also demonstrate that caterpillars select microhabitats that take advantage of the density-dependent nature of masquerade. Caterpillars were able to select habitats that maximized both food abundance and protection from predation when the two were compatible; and to trade off the relative costs and benefits of protection from predation and feeding opportunities, in a state-dependent manner, when the two were incompatible. Although this behavior may appear complex, it need not imply that caterpillars use complex cognitive strategies. Rather, the behavior may be mediated by relatively simple unlearned rules, albeit ones that are responsive to both internal state and external conditions. For example, in the day, caterpillars may select sites with lots of twigs unless hunger levels exceed a certain level; at night, caterpillars could select habitats based on food abundance unless they have recently survived a predation event.
A clear consequence of our results is that the effectiveness of masquerade depends on the masquerader's microhabitat. Thus, selection for microhabitat choice that enhances the antipredatory effectiveness of masquerade may involve opportunity costs incurred through lost foraging opportunities. Such opportunity costs could potentially lead to changes in the ecology or life history strategies of animals that use masquerade, or could restrict the situations in which masquerade could evolve. For example, when foraging opportunities are limited in areas where animals gain protection from masquerade, animals may be under strong selection to feed at night, when visually hunting predators are less abundant. Furthermore, the strength of selection for night feeding may change over ontogeny. Several species of caterpillar cease feeding by day as they increase in size, and this is thought to be due to an increase in predation risk (20). Similar effects may be expected in masquerading prey because predators may be more motivated to search for larger individuals. As a result, the diurnal patterns of microhabitat preferences that we found in fourth instars of the Early Thorn moth may not be as pronounced in early instars. Careful evaluation of both the antipredatory effectiveness and the opportunity costs of these strategies will be required for a fuller understanding of the ecological situations where masquerade is likely to be selected.
Conclusions
This study investigates (i) the evolutionary dynamics of masquerade and (ii) the behavioral adaptations associated with masquerade. We provide compelling evidence that the benefit of masquerade declines as the local density of masqueraders relative to their models increases and that masquerading organisms have evolved complex microhabitat selection strategies that allow them to best exploit the density-dependent properties of masquerade. Our results strongly suggest the existence of opportunity costs associated with masquerade, and careful evaluation of such costs will be vital to the development of a fuller understanding of both the distribution of masquerade across taxa and ecosystems, and the evolution of the life history strategies of masquerading prey.
Materials and Methods
Caterpillars.
Throughout both experiments, we used laboratory-reared, fourth instar larvae of the Early Thorn moth Selenia dentaria. This species is an inch-worm (family Geometridae) that it is polyphagous on a wide range of deciduous broadleaved shrubs and trees; benefits from masquerade (7); does not reflect light in the UV spectrum; and has no known color polymorphism (see SI Materials and Methods and Fig. S1 for further details).
Predation Experiment.
On day 2 of life, 32 chicks that had been acclimatized to the experimental arena (see SI Materials and Methods) were divided into two groups of 16. Individuals in each group where given two trials, in each of which they were allowed to find and consume one caterpillar from a branch that also contained nine twigs. The branch was manipulated for one group, but not manipulated for the other. Manipulated branches were bound in purple cotton thread to change their visual appearance without influencing their physical structure or odor (7). The purpose of these trials was to train chicks to forage for caterpillars on the branches.
On day 3, each group was split into two, giving four groups in all. Chicks in all groups experienced 10 presentations of an unmanipulated or manipulated branch (in line with their previous experience) in the arena. Each presentation lasted 90 s, and there was an interval of 90 s between presentations. The presentations could be of one of three types: a branch containing 9 twigs and a caterpillar, a branch containing 10 twigs and no caterpillar, or a null presentation where there was nothing in the arena. All groups experienced four presentations of a branch with nine twigs and a caterpillar. Two groups (one with manipulated branches and one without) experienced a high exposure level to unrewarding branches with 10 twigs and no caterpillar (five presentations) plus one null presentation; the other two groups experienced a low exposure level to unrewarding branches with 10 twigs and no caterpillar (one presentation) and five null presentations. The order of the 10 presentations was randomized for each individual. This method of presentation was chosen because it allowed twig number to be manipulated without changing either the number of caterpillars presented or the complexity of the visual task (chicks always had to find one caterpillar among nine twigs).
At the end of day 3, the chicks were given a single test trial. This consisted of a manipulated or unmanipulated (consistent with each chick's previous experience) branch containing nine twigs and one caterpillar. Latency to attack the caterpillar was recorded. In one case, the trial was stopped after 10 min because the chick showed no interest in the branch; this chick was awarded a latency of 601 s in the analysis.
On day 4, the chicks from the low-exposure groups were given two trials to explore whether the number of twigs on the branch affected the time taken to find the caterpillar (i.e., whether it was more difficult to find caterpillars in more complex environments). In one trial, the chick was offered a branch with 5 twigs and one caterpillar, in the other the branch had 15 twigs and one caterpillar. The order of trials was counterbalanced within groups. The trials were 90 s apart.
Caterpillar Choice Experiment.
Experiments were performed in the laboratory in which caterpillars were housed (see SI Materials and Methods for housing details), in July and August 2009. Daytime measurements were taken between 10:00 AM and 2:00 PM (illuminated by sunlight only), and nighttime measures were taken between 10:00 PM and 2:00 AM (illuminated by moonlight and starlight only). Caterpillars were placed into choice chambers, a clear plastic tank measuring 33 × 18 × 18 cm with a cling-film lid, individually using a paintbrush. White paper covered the floor of the tank and was changed every trial. A pencil mark indicated the center of the chamber, and a single branch was placed on either side of this line, 5 cm from the line at the closest point. Caterpillars were placed on the pencil line facing neither branch. They were left for 30 min, after which the branch on which the caterpillar was located was recorded: five caterpillars were not located on a branch so they were removed from the analysis. The primary difference between the two branches was in the number of twigs. Each branch was 25 cm in length, one with four twigs (low twig number) and one with eight twigs (high twig number). Twigs on both branches were ∼3.5 cm in length and 3 mm in diameter. The number of twigs on the branches was not manipulated in any way, and therefore represented natural variation. Experimental groups differed in which of the branches possessed leaves, their hunger levels, and the perceived predation risk: see Table 1 for details. When required, food deprivation was achieved by removing the leaves from the caterpillar's host plant 24 h before the start of the experiment. Predation was simulated by gently squeezing caterpillars with tweezers three times immediately before moving it to the experimental chamber (no caterpillar was visibly injured by this process). Each choice was investigated using 30 replicate caterpillars.
Supplementary Material
Acknowledgments
This work was supported by National Environment Research Council Grant NE/E016626/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014629108/-/DCSupplemental.
References
- 1.Ruxton GD, Sherratt TN, Speed MP. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals & Mimicry. New York: Oxford University Press; 2004. pp. 23–25. [Google Scholar]
- 2.Stevens M, Merilaita S. Animal camouflage: current issues and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2009;364:423–427. doi: 10.1098/rstb.2008.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mappes J, Marples N, Endler JA. The complex business of survival by aposematism. Trends Ecol Evol. 2005;20:598–603. doi: 10.1016/j.tree.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Speed MP. When is mimicry good for predators? Anim Behav. 1993;46:1246–1248. [Google Scholar]
- 5.Skelhorn J, Rowland HM, Ruxton GD. The evolution and ecology of masquerade. Biol J Linn Soc Lond. 2010;99:1–8. [Google Scholar]
- 6.Cott HB. Adaptive Colouration in Animals. London: Methuen; 1940. pp. 311–343. [Google Scholar]
- 7.Skelhorn J, Rowland HM, Speed MP, Ruxton GD. Masquerade: camouflage without crypsis. Science. 2010;327:51. doi: 10.1126/science.1181931. [DOI] [PubMed] [Google Scholar]
- 8.Merilaita S, Lyytinen A, Mappes J. Selection for cryptic coloration in a visually heterogeneous habitat. Proc Biol Sci. 2001;268:1925–1929. doi: 10.1098/rspb.2001.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimitrova M, Merilaita S. Prey concealment: visual background complexity and prey contrast distribution. Behav Ecol. 2010;21:176–181. [Google Scholar]
- 10.De Ruiter L. Countershading in caterpillars. An analysis of its adaptive significance. Arch Neerl Zool. 1956;11:285–341. [Google Scholar]
- 11.Edmunds M, Grayson J. Camouflage and selective predation in caterpillars of the poplar and eyed hawk moths (Laothoe populi and Smerinthus ocellata) Biol J Linn Soc Lond. 1991;42:467–480. [Google Scholar]
- 12.Stamp NE, Wilkens RT. In: Caterpillars: Ecological and Evolutionary Constraints on Foraging. Stamp NE, Casey TM, editors. New York: Chapman & Hall; 1993. pp. 283–330. [Google Scholar]
- 13.Herrebout WM, Kuyten PJ, de Ruiter L. Observations on colour patterns and behaviour of caterpillars feeding on Scots pine. Arch Neerl Zool. 1963;15:315–357. [Google Scholar]
- 14.Greene E. A diet-induced developmental polymorphism in a caterpillar. Science. 1989;243:643–646. doi: 10.1126/science.243.4891.643. [DOI] [PubMed] [Google Scholar]
- 15.Porter J. The Colour Identification Guide to Caterpillars of the British Isles (Macrolepidoptera) London: Viking; 1997. [Google Scholar]
- 16.Stephens DW, Krebs JR. Foraging Theory. Princeton: Princeton University Press; 1986. pp. 104–126. [Google Scholar]
- 17.Dukas R, Kamil AC. Limited attention: the constraint underlying search image. Behav Ecol. 2001;12:192–199. [Google Scholar]
- 18.Bauer B, Jolicoeur P, Cowan WB. Visual search for colour targets that are or are not linearly separable from distractors. Vision Res. 1996;36:1439–1465. doi: 10.1016/0042-6989(95)00207-3. [DOI] [PubMed] [Google Scholar]
- 19.Noor MAF, Parnell RS, Grant BS. A reversible color polyphenism in American peppered moth (Biston betularia cognataria) caterpillars. PLoS ONE. 2008;3:e3142. doi: 10.1371/journal.pone.0003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger D, Gotthard K. Time stress, predation risk and diurnal-nocturnal foraging trade-offs in larval prey. Behav Ecol Sociobiol. 2008;62:1655–1663. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.