Abstract
Adult neurogenesis is a process by which the brain produces new neurons once development has ceased. Adult hippocampal neurogenesis has been linked to the relational processing of spatial information, a role attributed to the contribution of newborn neurons to long-term potentiation (LTP). However, whether newborn neurons also influence long-term depression (LTD), and how synaptic transmission and plasticity are affected as they incorporate their network, remain to be determined. To address these issues, we took advantage of a genetic model in which a majority of adult-born neurons can be selectively ablated in the dentate gyrus (DG) and, most importantly, in which neurogenesis can be restored on demand. Using electrophysiological recordings, we show that selective reduction of adult-born neurons impairs synaptic transmission at medial perforant pathway synapses onto DG granule cells. Furthermore, LTP and LTD are largely compromised at these synapses, probably as a result of an increased induction threshold. Whereas the deficits in synaptic transmission and plasticity are completely rescued by restoring neurogenesis, these synapses regain their ability to express LTP much faster than their ability to express LTD. These results demonstrate that both LTP and LTD are influenced by adult neurogenesis. They also indicate that as newborn neurons integrate their network, the ability to express bidirectional synaptic plasticity is largely improved at these synapses. These findings establish that adult neurogenesis is an important process for synaptic transmission and bidirectional plasticity in the DG, accounting for its role in efficiently integrating novel incoming information and in forming new memories.
Keywords: memory, learning, glutamatergic transmission, synaptic strength
Adult neurogenesis is a process by which the brain produces new neurons once fetal and early postnatal development has ceased (1–3). The dentate gyrus (DG) of the hippocampus is one of the few regions of the adult mammalian brain in which thousands of newborn cells are generated daily. The functional role of newborn hippocampal neurons has been the subject of extensive research and debate. Based on several correlative pieces of evidence, it was initially hypothesized that these cells are involved in processing spatial memory (3–5). Recently, their participation in complex forms of hippocampal-mediated memory that require flexible, inferential memory expression has been demonstrated (6–8).
Because spatial learning has been associated with possible persistent changes in synaptic strength, studies have focused on the impact of adult neurogenesis on long-term potentiation (LTP) in the DG (9–11). The threshold for LTP induction is lower for glutamatergic synapses impinging on young granule cells than for those contacting mature neurons in the DG (10). Furthermore, LTP in newborn neurons is unaffected by GABAergic inhibition, whereas this manipulation greatly enhances potentiation in mature neurons (11). Our understanding of the contribution of adult neurogenesis to synaptic transmission and plasticity in the DG is far from complete, however. In particular, we still do not know whether long-term depression (LTD), the inverse correlate of LTP, is also influenced by newborn cells. Similarly, how the plastic properties of the DG network are modified as neo-neurons integrate their network is completely unknown.
To address these issues, we took advantage of an inducible transgenic mouse that allows the reduction of adult hippocampal neurogenesis (6, 12, 13). In this model, neural precursors (expressing nestin) can be selectively killed in double-transgenic mice (bigenic mice) through overexpression of the proapoptotic protein Bax after the oral administration of an exogenous tetracycline analog, doxycycline (Dox; SI Materials and Methods). We have previously shown that disruption of adult neurogenesis strongly affects the processing of spatial and emotional information (6, 12, 13).
Here, using this model, we report that adult neurogenesis influences not only LTP, but also LTD, in the DG. We further demonstrate that as newborn neurons are incorporated in the DG network, they improve the plastic properties of this structure by facilitating the expression of LTP and LTD in a sequential time-dependent manner.
Results
Reduction of Adult Neurogenesis Impairs Synaptic Transmission in the DG.
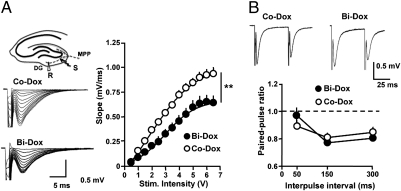
We first assessed whether excitatory transmission in the DG was modified as a result of reduced adult neurogenesis in bigenic mice treated with Dox (Bi-Dox). To this end, we recorded field excitatory postsynaptic potentials (fEPSPs) evoked in the DG in response to the stimulation of the medial perforant path (MPP), one of the main excitatory inputs to granule cells. The input-output (I/O) relationship was largely reduced in slices obtained from Bi-Dox mice compared with control mice (Nestin-rtTA, Tet-Bax, WT) treated with Dox (Co-Dox; Fig. 1A). This reduction did not result from a change in the likelihood of glutamate release at MPP terminals, because the paired-pulse ratio was not statistically different from that measured in the control animals (Fig. 1B). The most likely explanation for this change is that the reduction of adult-born neurons eliminated a significant number of the MPP synapses that were participating in the evoked response in control mice, resulting in fEPSPs of reduced amplitude.
Fig. 1.
Reduction of adult neurogenesis dramatically disrupts DG synaptic transmission. (A) (Left) Schematic representation of hippocampal circuits and position of the recording (R) and stimulating (S) electrodes and representative traces illustrating fEPSPs obtained in response to increasing stimulus intensity at MPP-DG synapses in both Co-Dox and Bi-Dox mice. (Right) I/O relationship for Co-Dox mice (open circles; n = 13) and Bi-Dox mice (solid circles; n = 14). **P < 0.01, two-way ANOVA. (B) (Upper) Representative traces illustrating PPR in both Co-Dox and Bi-Dox groups. (Lower) Summary plot of PPR measured at 50-ms, 150-ms, and 300-ms interpulse intervals for Co-Dox (open circles; n = 10) and Bi-Dox (solid circles; n = 8) littermates. P > 0.05, t test.
Reduction of Adult Neurogenesis Alters Synaptic Plasticity.
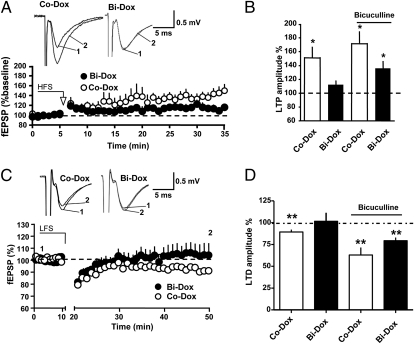
The DG was the first structure in which persistent activity-dependent changes in synaptic strength were documented (14). MPP synapses display different forms of synaptic plasticity, termed long-term potentiation (LTP) (15) and long-term depression (LTD) (16). We investigated the extent to which such forms of DG synaptic plasticity depend on adult-born neurons by monitoring LTP and LTD in both Bi-Dox and Co-Dox mice. LTP was induced by applying two trains of high-frequency stimuli (HFS; 500 ms, 100 Hz, 20 s apart). In slices obtained from Co-Dox animals, this protocol consistently yielded a slowly developing increase in synaptic strength that reached steady-state 15–20 min after induction (Fig. 2A). Interestingly, LTP could not be induced in Bi-Dox mice (Fig. 2A), but it was observed after GABAergic transmission was inhibited (Fig. 2B). These data indicate that reducing the number of adult-born neurons alters the ability to express LTP in the DG by increasing the threshold for LTP induction, confirming previous data obtained at the level of single cell recordings (9, 10).
Fig. 2.
Adult-born neurons are necessary for DG synaptic plasticity. (A and B) Neurogenesis blockade impairs LTP. (A) (Lower) Summary plot representing the time course of fEPSP in response to HFS for Co-Dox (open circles; n = 10) and Bi-Dox (solid circles; n = 8) mice. (Upper) Representative traces obtained from Co-Dox and Bi-Dox mice ore HFS (1) and 30 min after HFS (2). (B) Histogram summarizing LTP amplitude at 30 min after HFS in Co-Dox (open bar, 149.8 ± 14.6%; n = 10) and Bi-Dox (solid bar, 113.9 ± 6.3%; n = 8) mice under control conditions and in Co-Dox (open bar, 172.6 ± 17.3%; n = 4) and Bi-Dox (solid bar, 137.9 ± 11.0%; n = 7) mice in the presence of bicuculline. *P < 0.05, t test. (C and D) Impaired LTD in Bi-Dox mice. (C) (Upper) Representative superimposed traces obtaid before (1) and after (2) LTD induction. (Lower) Time course of fEPSPs after application of a LFS protocol to slices obtained from Co-Dox (open circles; n = 10) and Bi-Dox (solid circles; n = 10) mice. (D) Histogram summarizing LTD amplitude at 30–40 min after LFS in Co-Dox (open bar, 89.3 ± 2.7%; n = 10) and Bi-Dox (solid bar, 101.6 ± 9.8%; n = 10) mice under control conditions and in Co-Dox (open bar, 63.0 ± 8.5%; n = 9) and Bi-Dox (solid bar, 79.4 ± 3.5%; n = 9) mice in the presence of bicuculline. **P < 0.01, t test.
We next assessed the contribution of newborn neurons to LTD. To this end, we stimulated the MPP with a low-frequency stimulation (LFS) protocol (1 Hz, 10 min). This induced a reliable and persistent decrease in synaptic strength in slices obtained from Co-Dox mice (Fig. 2 C and D). Interestingly, the same protocol failed to induce LTD in Bi-Dox mice. As for LTP, LTD could be induced when GABAergic transmission was inhibited (Fig. 2D), in agreement with an increased threshold for induction. Taken together, our data indicate that adult neurogenesis is an important contributor to the plastic properties of MPP synapses.
Different Time Courses for LTP and LTD Recovery After Adult Neurogenesis Rescue.
If the deficits that we observed in synaptic transmission and plasticity are the direct consequence of disrupting adult neurogenesis, then they should be abrogated in the Bi-Dox mice, in which neurogenesis returns to normal values after the cessation of Dox treatment. This approach also provides a unique glimpse of the impact of newborn neurons on the plastic properties of the DG as these cells become incorporated in the hippocampal network.
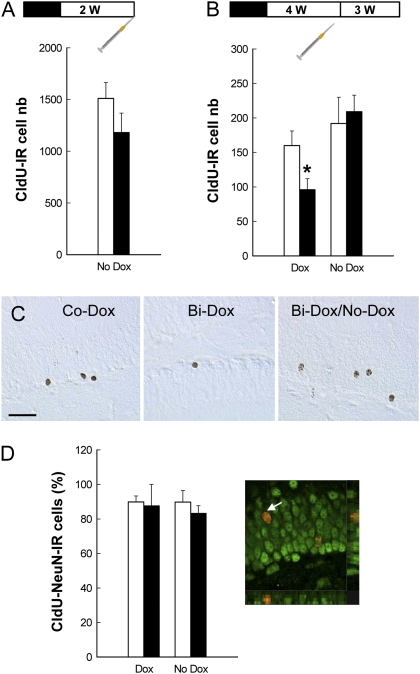
We first verified that cell genesis returned to normal values after the cessation of Dox treatment. After 4 wk of Dox impregnation, Bi-Dox and Co-Dox mice were treated with the sole vehicle for 2 wk. At this time point, the ability to generate newborn cells was completely recovered (Fig. 3A). Indeed, the number of newborn cells labeled with 5-chloro-2′-deoxychloridine (CldU) at 2 h before sacrifice was similar in Co-Dox/No-Dox and Bi-Dox/No-Dox mice.
Fig. 3.
Effect of Dox treatment and Dox removal on cell genesis, survival, and differentiation. All mice were first treated for 4 wk with Dox (black boxes in the experimental design). Then Dox was removed (white boxes), and the mice were treated with the sole vehicle for 2 wk (A) or 4 wk (B and D). The syringes represent CldU injections. (A) At 2 wk after Dox removal, cell genesis returned to control values (Co-Dox, n = 5; Bi-Dox, n = 6; t9 = 1.369; P = 0.20). (B) Dox was removed for one subgroup of animals from each genotype for 4 wk (Co-Dox, n = 10; Bi-Dox, n = 9). Dox treatment decreased the number of 3-wk-old CldU-IR cells in bigenic mice (t18 = 2.34; P = 0.03), an effect abolished when Dox was removed from the drinking solution (t17 = −0.35; P = 0.72). (C) Illustration of CldU-IR cells in a Co-Dox mouse, a Bi-Dox mouse, and a Bi-Dox/No-Dox mouse. (Scale bar: 50 μm.) (D) Cell differentiation measured by the percentage of CldU-IR cells expressing the mature neuronal marker NeuN was similar among the groups. (Right) A CldU-IR cell (red stain) expressing NeuN (green stain).
In a subsequent experiment, we examined cell survival. Dox was removed for one subgroup of mice from each genotype for 4 wk, after which the animals were injected with CldU and then killed 3 wk later (Fig. 3 A and B). Dox treatment decreased the number of 3-wk-old CldU-immunoreactive (IR) cells in bigenic mice, and this effect was abolished when Dox was removed from the drinking solution. To phenotype the newborn cells, we quantified the percentage of CldU-IR cells expressing the neuronal marker NeuN (Fig. 3D). Neuronal differentiation was similar among the groups, indicating that neurogenesis in Bi-Dox mice returned to normal values after the cessation of Dox treatment (Bi-Dox/No-Dox).
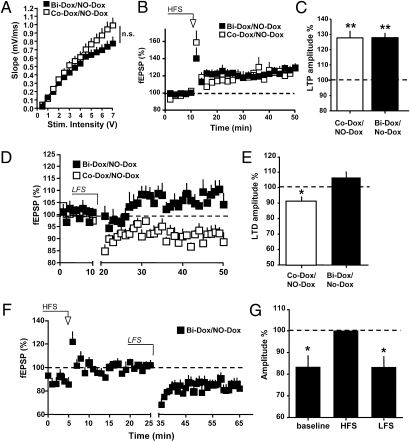
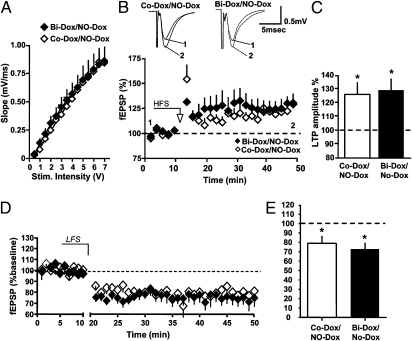
We next analyzed whether synaptic transmission and plasticity recover as neurogenesis returns to normal values. After 4 wk of Dox treatment, bigenic mice were treated with the sole vehicle for 2 or 4 wk (Bi-Dox/No-Dox mice) and compared with control animals (Co-Dox/No-Dox) that received the same protocol. At 2 wk after cessation of Dox, the I/O relationship at MPP synapses in Bi-Dox/No-Dox mice was no longer different from that measured in control animals (Co-Dox/No-Dox; Fig. 4A). Surprisingly, whereas LTP was perfectly normal in these animals (Fig. 4 B and C), LTD remained significantly impaired (Fig. 4 D and E). These animals’ ability to display LTD was not lost, as demonstrated by the findings that at the MPP synapses that were first potentiated, subsequent application of an LTD-induction protocol caused depotentiation (Fig. 4 F and G). These findings suggest that 2 wk after the cessation of Dox treatment, MPP synapses were maximally depressed, thereby preventing further LTD.
Fig. 4.
Two wk of restoration of neurogenesis is insufficient to fully rescue synaptic plasticity. (A) I/O relationship at MPP-DG synapses measured in Co-Dox/No-Dox (open squares; n = 7) and Bi-Dox/No-Dox (solid squares; n = 7) mice. P > 0.05, two-way ANOVA. (B) Time course of fEPSPs after HFS in Co-Dox/No-Dox (open squares; n = 8) and Bi-Dox/No-Dox (solid squares; n = 9) mice. (C) Histogram summarizing LTP amplitude measured at 30–40 min after HFS in Co-Dox/No-Dox (open bar; 127.9 ± 3.0%; n = 9) and Bi-Dox/No-Dox (solid bar; 127.7 ± 4.6%; n = 9) mice. **P < 0.01, t test. (D and E) Failure in the rescue of LTD at 2 wk after the cessation of Dox treatment. (D) Time course of fEPSPs after application of a LFS protocol to slices obtained from Co-Dox/No-Dox (open squares; n = 10) and Bi-Dox/No-Dox (solid squares; n = 10) mice. (E) Histogram summarizing LTD amplitude at 30–40 min after LFS in Co-Dox/No-Dox (open bar; 91.1 ± 3.0%; n = 9) and Bi-Dox/No-Dox (solid bar; 107.4 ± 4.1%; n = 6) mice. *P < 0.05, t test. (F and G) MPP synapses are depotentiated and show LTD only if they are first potentiated. (F) Time course of fEPSPs after application of a HFS and LFS protocols to slices obtained from Bi-Dox/No-Dox mice (solid squares; n = 8). (E) Histogram summarizing baseline, potentiation, and depotentation amplitudes in Bi-Dox/No-Dox mice (83.8 ± 5.4%, 100%, and 83.7 ± 5.2%, respectively; n = 8). *P < 0.05, t test.
At 4 wk after cessation of Dox treatment, the I/O relationship at MPP synapses in slices from Bi-Dox/No-Dox mice in which neurogenesis was restored (Fig. 3) no longer differed from that measured in Co-Dox/No-Dox animals (Fig. 5A). Similarly, both LTP (Fig. 5 B and C) and LTD (Fig. 5 D and E) were perfectly normal in these animals compared with control mice. Taken together, these findings confirm that the deficits in synaptic plasticity observed in Bi-Dox mice were caused by the disruption of adult neurogenesis (Fig. 6).
Fig. 5.
Four wk of restoration of adult neurogenesis completely rescues DG synaptic transmission and plasticity. (A) I/O relationship at MPP-DG synapses measured in Co-Dox/No-Dox (open rhombs; n = 5) and Bi-Dox/No-Dox (solid rhombs; n = 8) mice. P > 0.05. two-way ANOVA. (B) (Lower) Time course of fEPSPs after HFS in Co-Dox/No-Dox (open rhombs; n = 5) and Bi-Dox/No-Dox (solid rhombs; n = 6) mice. (Upper) Superimposed representative traces before (1) and 40 min after HFS (2). (C) Histogram summarizing LTP amplitude measured at 30–40 min after HFS in Co-Dox/No-Dox (open bar; 126.1 ± 8.7%; n = 5) and Bi-Dox/No-Dox (solid bar; 129.2 ± 8.3%; n = 6) mice. *P < 0.05, t test. (D and E) Rescue of LTD at 4 wk after the cessation of Dox treatment. (D) Time course of fEPSPs after application of an LFS protocol to slices obtained from Co-Dox/No-Dox (open rhombs; n = 10) and Bi-Dox/No-Dox (solid rhombs; n = 10) mice. (E) Histogram summarizing LTD amplitude at 30–40 min after LFS in Co-Dox/No-Dox (open bar; 79.1 ± 8.0%; n = 6) and Bi-Dox/No-Dox (solid bar; 73.9 ± 7.2%; n = 9) mice. *P < 0.05, t test.
Fig. 6.
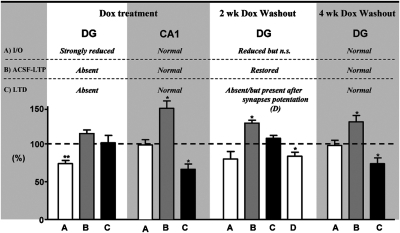
Graph summarizing the effect of blocking adult neurogenesis in bigenic mice on the I/O relationship, LTP, and LTD in the DG and CA1 areas (Left: Dox treatment). The same parameters are also plotted for experiments achieved in the DG in animals in which Dox treatment was stopped for 2 wk and 4 wk (Right: 2 wk and 4 wk Dox washout). Bars represent changes in percentage measured compared with control animals for I/O and compared with baseline for LTP and LTD. *P < 0.05.
Our conclusions are further supported by recordings obtained from the CA1 region of the hippocampus, a nonneurogenic area. We found that neither the I/O relationship nor LTP at Schaffer collateral synapses were affected in Bi-Dox mice, indicating that ablation of adult-born neurons in the DG did not affect synaptic transmission and synaptic plasticity in this area (Fig. 6 and Fig. S1).
Discussion
In this study, we tackle the importance of adult neurogenesis for hippocampal synaptic plasticity using an inducible reversible transgenic mouse model. This approach has several advantages, because it is not associated with any of the side effects that accompany the use of irradiation or the administration of pharmacologic compounds (17, 18). Moreover, it targets specifically hippocampal neurogenesis without affecting olfactory neurogenesis (6, 12, 13). More importantly, such a partial ablation of adult-born neurons is reversible, enabling validation of a direct role of adult neurogenesis in governing synaptic strength in the DG. Our data unambiguously demonstrate that reducing neurogenesis dramatically impairs synaptic transmission and both LTP and LTD at MPP synapses. Previous studies using x-irradiation demonstrated that ablation of adult-born hippocampal neurons decreases LTP (11, 19, 20). In those studies, however, the I/O relationship was not affected, suggesting that irradiation produced misleading results and/or that some compensatory mechanisms accounted for this apparent discrepancy in the findings. The simplest explanation for the diminished I/O relationship observed in bigenic mice is the reduction in the number of synapses recruited during MPP stimulation. As newborn neurons are lost, the synapses impinging on these cells can no longer participate in the evoked-synaptic responses.
We show that LTP and LTD are compromised under conditions in which GABAergic transmission is left intact, whereas they can be induced in the presence of GABA-A receptor antagonists. These results are in agreement with previous findings reporting a lower threshold for LTP induction at MPP synapses impinging on newborn neurons compared with those contacting mature DG neurons (9, 10). These findings support the involvement of adult-born neurons in LTD. These results are not surprising when one considers the similarity of LTP and LTD. Both depend on Ca2+ influx through NMDA receptors and lead either to an insertion or an endocytosis of AMPA receptors (21). Thus, a very likely explanation for the deficiency of LTP and LTD in bigenic mice is a positive shift of the threshold for inducing synaptic plasticity.
Thanks to the use of our bigenic model, we were able to rescue adult neurogenesis by stopping Dox treatment and monitoring synaptic transmission and plasticity at different time windows. Surprisingly, we found that the I/O relationship and LTP, but not LTD, were completely restored 2 wk after the rescue of neurogenic functions. Because we were able to depotentiate MPP synapses that were first potentiated, it appears that the absence of LTD under these conditions is due to a floor effect in which synapses are maximally depressed and can no longer respond to an LTD-induction protocol. These findings suggest that newborn neurons that are incorporated into the DG network are first contacted by synapses that can be strengthened only through LTP. This situation changes later as those synapses acquire the ability to display bidirectional plasticity, as indicated by the data obtained at 4 wk after cessation of Dox treatment.
Previous work established that synapses on newborn neurons have an increased capacity for potentiation compared with synapses impinging on mature neurons (10). This is in agreement with our present results showing that as newborn cells are incorporated, the plastic properties of the network improve dramatically, as demonstrated by the ability of MPP synapses to first express LTP before being able to display both LTP and LTD. It has been proposed that these unique characteristics may make new neurons within this critical period more sensitive to life experience. In particular, it has been shown that learning or experiencing an enriched environment determines the survival, dendritic development, and population response of the new neurons (22–26). This exceptional plasticity of the young neurons may be particularly useful for information processing (27, 28).
In conclusion, adult hippocampal neurogenesis is a key process influencing the plastic properties of the DG by facilitating the expression of LTP and LTD in a sequential time-dependent manner as newborn neurons are incorporated into the hippocampal network. This structural and functional plasticity may be an important contributor to the ability to form new memories.
Materials and Methods
Subjects and in Vivo Dox Treatment.
Adult male transgenic mice were obtained from the breeding of Tet-Bax with Nestin-rtTA founder mice, as described previously (6, 12, 13). In brief, in this model, Dox administration to bigenic mice activates the nestin-controlled transactivator (rtTA), which drives the transcription of the proapoptotic Bax gene in the adult brain and not in peripheral tissues. It specifically induces death of nestin-expressing cells in the subgranular zone of the DG, leading to reduced neurogenesis.
Eight-wk-old male nestin-rtTA/Tet-Bax bigenic mice and their control littermates (nestin-rtTA, Tet-Bax, and WT) were housed individually from the beginning of the Dox treatment through the end of the experiments. Dox was added to the drinking solution for 4 wk (2 mg/mL, 2.5% sucrose) starting when the mice were 8 wk of age. For the “reversal” experiments, Dox was removed from the drinking solution for 2 wk (first batch) or for 4 wk (second batch). In this latter experiment, Dox treatment started when the mice were 16 wk of age. All experiments were conducted in strict compliance with European Convention and institutional regulations.
CldU Injection.
Newborn cells were labeled by the incorporation of CldU (one daily i.p. injection of 42.7 mg/kg—the molecular equivalent of 50 mg/kg of BrdU—for 4 d). Animals were injected at 2 wk (first batch) or 4 wk (second batch) after the cessation of Dox treatment and were killed at 2 h (first batch) or 3 wk (second batch) after injection to study cell proliferation (first batch) or cell survival/phenotype (second batch).
Electrophysiology.
Mice were anesthetized with isoflurane and killed by decapitation. Brains were rapidly removed and chilled in an ice-cold, carbogenated (i.e., bubbled with 95% O2-5% CO2) artificial cerebrospinal fluid (ACSF) containing the following: 125 mM NaCl, 1.25 mM NaH2PO4, 25 mM glucose, 2.5 mM KCl, 2.5 mM CaCl2, 2 mM MgCl2, and 25 mM NaHCO3. Transverse entorhinal/hippocampal slices (300 μm thick) were cut using a Leica VT1200S vibratome and incubated with cutting ACSF for 30 min at room temperature. The slices were subsequently transferred to a holding chamber in bathing ACSF, where they were maintained for 30 min at room temperature until experiments began. Slices were individually transferred to a submerged chamber for recording and continuously perfused with oxygenated (95% O2-5% CO2) bathing medium (3–5 mL/min). All experiments were performed at room temperature. Extracellular fEPSPs were recorded using glass micropipettes (2–4 mOhm) filled with normal ACSF bathing medium. Slices from the middle hippocampus were used preferentially. Responses were evoked by stimulation (0.1 ms duration, 0–10 V amplitude) delivered to the middle molecular layer to stimulate the MPP using the same glass electrodes used for the recordings. The placement of electrodes in the MPP was corroborated by observing paired-pulse depression at 150-ms interpulse intervals. Recordings were obtained using an Axon Multiclamp 700B amplifier (Molecular Devices). Signals were filtered at 2 kHz, digitized, sampled, and analyzed using Axon Clampfit software (Molecular Devices).
In preliminary experiments, nestin-rtTA, Tet-Bax, and WT littermates treated with Dox and tested for I/O relationship, paired-pulse, LTP, and LTD, demonstrated no significant differences. We used them in equal proportions in these experiments, in which they collectively served as the control group (Co-Dox).
Immunohistochemistry and Stereological Analysis.
Mice were perfused transcardially with a phosphate-buffered solution of 4% paraformaldehyde. One in 10 free-floating sections were processed in a standard immunohistochemical procedure to visualize CldU using rat anti-BrdU (1/4,000; Accurate). Immunoreactivity was visualized by the biotin-streptavidin technique (ABC Kit; DAKO) using 3,3′-diaminobenzidine as a chromogen. The number of IR cells was counted under a 100× microscope objective throughout the entire septotemporal axis of the granule and subgranular layers of the DG as described previously (6). The total number of cells was estimated using the optical fractionator method.
Analysis of Cellular Phenotypes.
To examine the phenotype of CldU-IR cells, we incubated one in 10 with a BrdU antibody (1/1,000; Accurate), which was revealed using a CY3− anti-rat antibody (1/1,000; Jackson Laboratory). Then sections were incubated with a mouse monoclonal anti-NeuN antibody (1/1,000), which was visualized with Alexa Fluor 488 anti-mouse IgG (1/1000; Jackson Laboratory). The percentage of BrdU-labeled cells expressing NeuN was determined throughout the DG using a confocal microscope with HeNe and Argon lasers (Leica DMR TCSSP2AOBS).
Supplementary Material
Acknowledgments
We thank D. Gonzales and M. Manse for the mouse genotyping (genotyping platform of INSERM U862), C. Dupuy for the excellent care of the mice, and J. M. Israel and G. Marsicano for a critical reading of the manuscript. This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Université de Bordeaux, Agence Nationale pour la Recherche (to D.N.A. and S.H.R.O.), Fondation pour la Recherche Médicale (Equipe FRM, to S.H.R.O.), and Conseil Régional d'Aquitaine (to D.N.A. and S.H.R.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016928108/-/DCSupplemental.
References
- 1.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: From precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 2.Gross CG. Neurogenesis in the adult brain: Death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 3.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrous DN, Wojtowicz JM. Neurogenesis and Hippocampal Memory System. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2008. pp. 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 6.Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 11.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 12.Revest JM, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 13.Tronel S, et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2010 doi: 10.1002/hipo.20895. in press. [DOI] [PubMed] [Google Scholar]
- 14.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bramham CR. Control of synaptic consolidation in the dentate gyrus: Mechanisms, functions, and therapeutic implications. Prog Brain Res. 2007;163:453–471. doi: 10.1016/S0079-6123(07)63025-8. [DOI] [PubMed] [Google Scholar]
- 16.Bear MF. Homosynaptic long-term depression: A mechanism for memory? Proc Natl Acad Sci USA. 1999;96:9457–9458. doi: 10.1073/pnas.96.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupret D, et al. Methylazoxymethanol acetate does not fully block cell genesis in the young and aged dentate gyrus. Eur J Neurosci. 2005;22:778–783. doi: 10.1111/j.1460-9568.2005.04262.x. [DOI] [PubMed] [Google Scholar]
- 18.Wojtowicz JM. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus. 2006;16:261–266. doi: 10.1002/hipo.20158. [DOI] [PubMed] [Google Scholar]
- 19.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- 21.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Dupret D, et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 24.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 25.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tronel S, et al. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci USA. 2010;107:7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman T, et al. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171:769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 28.Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci USA. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.