Abstract
Within the field of eukaryotic protein synthesis, one factor remained putative for decades: eukaryotic translation initiation factor (eIF) 5A. Because eIF5A is an essential protein required for cell proliferation, and one easily targeted by inhibitors, identifying its role in the cell remains important and urgent. Recent reports support early findings that eIF5A stimulates protein synthesis and newly assign the factor a role in elongation rather than initiation. Here we show that eIF5A directly stimulates protein synthesis on native mRNAs, that rapid depletion of eIF5A in vivo immediately leads to a 2-fold inhibition of protein synthesis, and that both the immediate and lasting effects of eIF5A depletion are a reduction in polysome size concomitant with eIF5A depletion. Addition of purified eIF5A to a depleted lysate results in a roughly 2-fold stimulation of protein synthesis in vitro, a result consistent with both older methionyl–puromycin synthesis data and more recently published findings. We find that although eIF5A is not required for protein synthesis, it stimulates the process by about 2- to 3-fold. Our data, along with other published results, reinforce the conclusion that eIF5A stimulates protein synthesis with one important difference: Polysome profiles observed immediately after eIF5A depletion are diagnostic for a role in initiation. This discrepancy is discussed.
Keywords: hypusine, ribosome
Eukaryotic translation initiation factor (eIF) 5A is a small (16–18 kDa), essential protein that is ubiquitous and highly conserved among eukaryotes and archaea (1, 2). Several crystal structures of archaeal aIF5A have been published within the last decade (2–4). The less-conserved C-terminal portion is classified as an oligonucleotide binding motif (4). The N-terminal portion is more highly conserved and bears the absolutely unique feature of a/eIF5A: a specific, posttranslationally modified lysine known as hypusine [Nϵ-(4-amino-2-hydroxybutyl)lysine] (5). This residue lies at the tip of an extended loop in the crystal structures, the sequence of which (STSKTG-hypusine-HGHAK) is universally conserved in eukaryotes (6). Remarkably, a crystal structure of the prokaryotic eIF5A homolog, called elongation factor P (EF-P), shows that both proteins structurally resemble tRNA (7).
The essential function of eIF5A remains obscure. The factor was originally isolated from ribosomal high-salt washes (8, 9), suggesting a role in translation. Purified eIF5A stimulates a model assay of translation initiation, methionyl–puromycin (met-puro) synthesis (9, 10). Although eIF5A does not affect any step prior to 80S initiation complex assembly (10), the factor increases met-puro formation by 2- to 3-fold (9, 10). Whether eIF5A stimulates translation in vivo was tested by depleting eIF5A in yeast (11). These depletion experiments used a “rapid-depletion” yeast strain, UBHY-R, engineered to express an unstable form of eIF5A (UBR5A). By rendering UBR5A expression dependent on the GAL1 promoter in a null genetic background, eIF5A is rapidly depleted by shifting UBHY-R cells into glucose-containing media. UBHY-R cells grown in glucose exhibited only a mild (30%) reduction in the rate of protein synthesis and appeared to have normal polysome profiles (11), suggesting that eIF5A is not required for general protein synthesis.
We chose to reexamine the possibility that eIF5A stimulates translation for several reasons. First, the degree to which eIF5A consistently stimulated met-puro formation did not substantially differ from what was observed when depleting UBR5A from yeast cells. Second, the primary physiological activity of eIF5A had remained elusive. Finally, we discovered potential artifacts in the eIF5A depletion experiments that were a basis for doubting that eIF5A plays a role in general translation initiation. By optimizing the depletion experiments we find that loss of eIF5A correlates with a substantial inhibition of protein synthesis in vivo, UBR5A depletion results in a pronounced effect on polysome distribution, and supplementing depleted lysates with eIF5A stimulates protein synthesis in vitro.
While this manuscript was being prepared, other laboratories reported quantitatively similar effects of eIF5A depletion on global translation (12, 13). However, although these reports conclude that eIF5A stimulates elongation, the polysome profiles we observe during and after eIF5A depletion are diagnostic for a role in initiation. Possible explanations for this discrepancy are discussed, as is a recently published structure suggesting that EF-P stimulates only the formation of the first peptide bond (14).
Results
Control Experiments Suggest That eIF5A Acts in Translation.
We first asked whether previously published data, obtained with strain UBHY-R, may have been subject to an artifact. Some degradation of UBR5A, the rapidly depleted protein expressed in strain UBHY-R, may have occurred during lysate preparation, inaccurately representing in vivo UBR5A levels. We found this effect to be negligible, although a striking media-dependent effect was observed, indicating that UBR5A depletion in SD glucose is impaired (Fig. S1). Depletion of UBR5A was previously demonstrated by blotting extracts of cells grown in rich media. If UBR5A depletion in minimal media is inefficient, then in vivo assays of [35S]methionine incorporation into protein by UBHY-R grown in minimal media could have been misleading. We also surmised that, because the TIF51A coding region was not completely deleted in UBHY-R, reversion to wild type at the TIF51A locus by homologous recombination with the plasmid-borne UBR5A construct was possible, absent selection. Reversion does occur during growth in rich media (Fig. S2). Growth in selective media prior to all experiments prevents contamination with revertants as determined by periodic selection tests and prolonged growth suppression in glucose-containing media. These results suggest that a role for eIF5A in translation may have been masked by UBHY-R-specific effects. Therefore we asked whether, as expected for a translation factor (9), eIF5A associates with polysomes. As since reported by others (15), we found that it does bind to ribosomes (Fig. S3), although not strongly. Finally, we reasoned that because eIF5A is not absolutely required for the formation of met-puro in vitro, a general reduction in translation activity (expected in cells grown in minimal media; see below) could mask a potential effect of UBR5A depletion on general translation. This possibility is addressed in the next section.
The Effect of eIF5A Depletion on Translation Is More Pronounced in Rich Media.
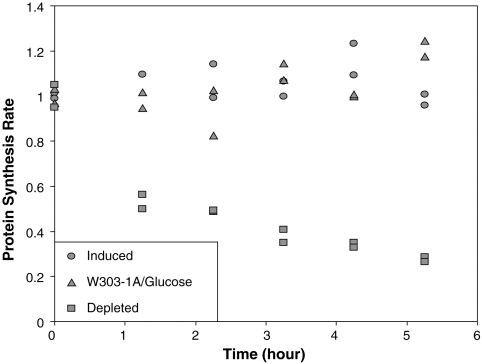
The growth medium used to propagate UBHY-R influences both the rate and extent of protein synthesis inhibition upon UBR5A depletion. By comparing the rate of [35S]methionine incorporation into total protein by UBHY-R cells before and during depletion, we observe a media-dependent effect on protein synthesis (Fig. 1 and Fig. S4). Extended depletion (for approximately 5 h) inhibits translation initiation when either rich [yeast extract peptone (YP)] or minimal [synthetic defined (SD)] media are employed, but the effect in rich media is more rapid and larger. Protein synthesis is inhibited by 2-fold after 1 h of UBR5A depletion in rich media (Fig. 1 and Fig. S4C), whereas a similar degree of inhibition is not observed until 2–3 h of incubation in minimal media (Fig. S4 A and B). Extended depletion for approximately 5 h results in a 4-fold inhibition of protein synthesis in rich media; in minimal media a lower inhibitory effect (between 2.5- and 3-fold) is observed after similar depletion times. In both minimal and rich media we observe a greater inhibition of protein synthesis than was initially reported in minimal media (about 1.4-fold; see ref. 11); based on our present findings we conclude that this difference arises from media effects and the artifacts described in the previous section. The effects of eIF5A depletion are seen as early as 1 h of depletion, when protein synthesis rates already have fallen by 2-fold in YP media. These differences are likely due to more efficient translation initiation by cells in YP media, although this is difficult to demonstrate by this method without quantifying intracellular methionine pools.
Fig. 1.
The effect of UBR5A depletion on protein synthesis rates is pronounced when cells are grown in rich media. W303-1A and UBHY-R cells were grown in YP galactose, then resuspended either in YP glucose (“Depleted” UBHY-R and “W303-1A/Glucose”) or galactose (“Induced” UBHY-R) as indicated. Duplicate samples were processed independently. [35S]methionine incorporation and protein concentrations were determined as described in Materials and Methods. Protein synthesis rates were measured as cpm incorporated per μg total protein per minute of labeling, then expressed as a percentage of the respective value at time zero.
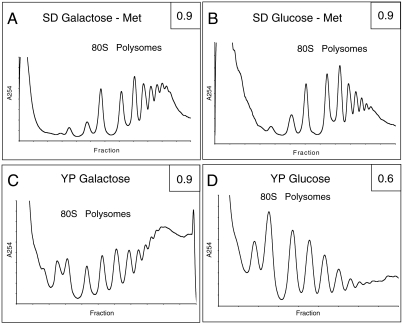
The effect of growth medium on protein synthesis rates is also readily seen when polysome profiles are determined. In the case of YP galactose (Fig. 2C), heavy polysomes are much more predominant compared to those from cells grown in SD galactose (Fig. 2A), indicative of more efficient translation initiation in rich media. Following depletion, polysomes are severely reduced in YP-glucose medium (Fig. 2D), whereas the effect in SD-glucose medium (Fig. 2B) is more modest, although polysome distribution changes similarly in both cases. These observations are consistent with the protein synthesis rate data (Fig. 1; ref. 11). The results obtained in YP media indicate that eIF5A stimulates the rate of protein synthesis by at least twofold. We conclude that the growth conditions used in earlier depletion studies masked important effects of eIF5A depletion on protein synthesis. In this experiment, UBHY-R cells were incubated in glucose-containing medium for 5–6 h in order to replicate conditions used previously (11).
Fig. 2.
The effect of UBR5A depletion on polysomes is more pronounced in rich media. UBHY-R cells were cultured in the indicated galactose-containing media (A and C) until reaching mid-log phase. Half of the culture was treated immediately with CHX and used to prepare a nondepleted extract. The other half was resuspended in the indicated media (B and D) with glucose and incubated at 30 °C for 5 (SD) or 6 (YP) h. After glucose incubation, extracts were prepared in the same manner. Approximately three OD units of each extract were loaded onto 10–60% (SD) or 10–50% (YP) sucrose gradients and centrifuged. Absorbance scans at 254 nm were obtained for each gradient. The ratio of polysomes to total ribosomes was calculated by numerical integration and is given for each profile (Upper Right). The profiles are representative of three independent analyses.
eIF5A Directly Stimulates Protein Synthesis in Vitro.
The demonstrations above that eIF5A depletion reduces global translation in yeast involve polysome profile analysis and in vivo protein synthesis assays. Both techniques show an effect of eIF5A depletion in intact cells, but the time required to reduce eIF5A levels means that we cannot rule out possible indirect effects of eIF5A depletion. The difference between the rates of UBR5A depletion (Fig. S1) and the reduction of [35S]methionine incorporation (Fig. 1) also suggests that secondary effects might contribute to translational repression. We therefore sought a more direct measure of eIF5A stimulation of protein synthesis and asked whether the addition of purified eIF5A to depleted lysates would stimulate protein synthesis in vitro.
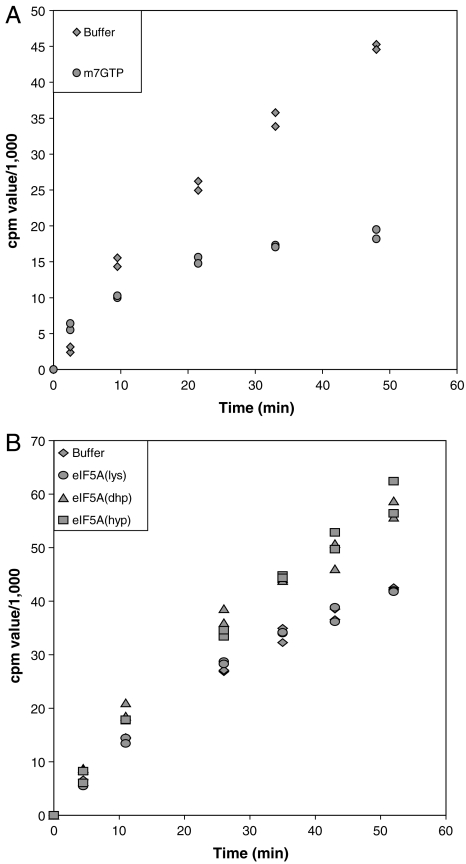
Toward this end, we prepared translationally active extracts from UBHY-R cells incubated in YP glucose for 2.5 h. The UBR5A-depleted extracts were then supplemented with purified eIF5A(lys or dhp) or eIF5A(hyp) purified from yeast. Protein synthesis on endogenous messages was measured by following the incorporation of [35S]methionine into acid-insoluble protein. It is important to recognize that such stimulation by eIF5A depends on having a sufficient level of methionine in the assay, and that the concentration of total methionine dramatically affects activity (Fig. S5). The use of recombinant eIF5A expressed in and prepared from bacteria, rather than eIF5A purified from yeast, eliminates possible contaminating yeast proteins that might affect protein synthesis. We also tested whether yeast-derived FLAG-tagged eIF5A(hyp) would differ in activity from the bacterially derived eIF5A preparations. Although eIF5A(hyp) shows greater stimulatory activity than eIF5A(dhp) during met-puro synthesis, both forms are active and specific; eIF5A(lys) or a homodeoxyhypusine-modified analog of eIF5A shows no stimulatory activity (16). We demonstrated that protein synthesis in depleted lysates depends on new initiation events by using a cap analog (m7GTP) to sequester eIF4E and block cap-dependent initiation. The result, shown in Fig. 3A, reveals an initial rapid incorporation of [35S]methionine resistant to inhibition, presumably due to elongation of nascent proteins on preexisting polysomes. After 10–20 min, protein synthesis in the presence of m7GTP virtually ceases, whereas uninhibited lysates show a near-linear increase in [35S]methionine incorporation. We interpret the latter to mean that [35S]methionine incorporation after 20 min depends on new initiation events. Thus the slope after 20 min is a measure of the rate of initiation. The two slopes in Fig. 3A differ by a factor of 10, indicating that m7GTP inhibits initiation by 90%. That protein synthesis and new initiation persist in severely depleted lysates is expected, given that polysomes also persist in depleted cells, albeit reduced in size (Fig. 2), and that methionyl-puromycin formation in vitro proceeds in the absence of added eIF5A (9, 10).
Fig. 3.
Although not required for initiation, eIF5A directly stimulates protein synthesis in vitro in a hypusine-dependent manner. Translation extracts depleted of UBR5A for 2.5 h were prepared and assayed in vitro for protein synthesis as described in Materials and Methods. To identical reaction aliquots was added m7GTP to inhibit initiation, or a buffer control (A). In B, extracts were supplemented with buffer alone, or at five molecules per 80S ribosome of eIF5A(lys), eIF5A(dhp), or FLAG-tagged eIF5A(hyp), the latter protein being purified from yeast. Ribosome concentration in the extract was determined by using the molar extinction coefficient of 80S as 5 × 107 M-1 cm-1 at 260 nm and by assuming that ribosomes are 80% of total RNA (25). Experiments shown are representative of multiple independent experiments.
The effect of adding various forms of eIF5A to depleted lysates is shown in Fig. 3B. Distinct stimulation of [35S]methionine incorporation is apparent and depends upon the presence of deoxyhypusine or hypusine (Fig. 3B and Fig. S6). This specificity was seen previously with the methionyl–puromycin assay, although unlike our current result, the quantitative activity of eIF5A(dhp) and eIF5A(hyp) then differed. This may reflect a disparity between mammalian and fungal translation (10). The average difference in slopes we observed, comparing added eIF5A(dhp) or eIF5A(hyp) with buffer alone at points after 20 min, was 1.8 ± 0.1 among nine experiments and multiple depleted lysates. Therefore exogenous eIF5A stimulates protein synthesis, likely at the initiation phase (see Discussion), by about 2-fold in this system. A slightly larger degree of stimulation of protein synthesis (2.5-fold) is seen if one simply subtracts the amount of [35S]methionine incorporated in the presence of m7GTP from each of the corresponding time points to give initiation-dependent protein synthesis. Also noteworthy is the fact that the rate of [35S]methionine incorporation during the first 10 min of incubation does not significantly differ between reactions stimulated and not stimulated by eIF5A (Fig. 3B), suggesting that eIF5A does not immediately affect the elongation rate (see Discussion). We conclude that eIF5A is not essential for global protein synthesis, but is required for optimal translation of a substantial number of endogenous mRNAs.
Discussion
Our findings lead to three important conclusions. First, eIF5A stimulates protein synthesis directly and globally, presumably on all or most yeast mRNAs, although further study must determine its precise mechanism of action. Second, assay optimization is required when characterizing eIF activities that have only a modest effect on translation rates. Third, and related, suboptimal experimental conditions contributed to the erroneous and widely cited conclusion that eIF5A does not act in general translation based on work employing strain UBHY-R. These conclusions are addressed in detail below.
Our data indicate that eIF5A stimulates translation directly. Depletion of eIF5A from yeast cells results in a rapid decrease in ribosomal loading on messages (Fig. 2) that is concomitant with the factor’s disappearance (Fig. S1). Under optimal growth conditions, the immediate effect of depleting eIF5A is a twofold inhibition of protein synthesis (Fig. 1 and Fig. S4C). Although translation initiation proceeds in the absence of eIF5A (Fig. 1 and Fig. S1), supplementing the factor in vitro (Fig. 3B and Fig. S6) directly restores translation activity on endogenous messages to a degree that is surprisingly consistent with multiple met-puro synthesis assay results (stimulation factor: 2.8 ± 0.6) (9, 10). The parity between our in vivo and in vitro data is particularly important. When cells are depleted of eIF5A for 2.5 h, protein synthesis rates fall by a factor that is essentially identical to the degree of stimulation observed when supplementing extracts prepared from these cells. This result suggests that any immediate secondary effects of depleting eIF5A activity are nominal, although such secondary effects may play a role during later phases of depletion.
The question of whether eIF5A plays a role in protein synthesis has been analyzed in detail by other groups recently (12, 13, 17). Their results, and our data, demonstrate that eIF5A plays a direct role in translation. A recent analysis of mutant forms of eIF5A also supports this conclusion (18). Therefore, the numerous pleiotropic phenotypes seen with mutant forms of eIF5A may be due to changes in the translational efficiencies of specific mRNAs.
That eIF5A acts in translation is now well-supported. However, our data must be reconciled with other reports that indicate distinctly different roles of eIF5A in protein synthesis. While this work was in preparation, two groups reported data supporting a role for eIF5A in translational elongation (12, 13); a third group reported a crystal structure, EF-P bound to 70S, that suggests a role for the eIF5A homolog in the formation of the first peptide bond only (14). Our data agree with the interpretation that eIF5A acts as an elongation factor, but only with respect to formation of the first peptide bond. The polysome profiles we observe upon eIF5A depletion indicate that eIF5A is required for efficient initiation; we see no other interpretation that explains a decrease in efficiently loaded mRNAs after depletion of eIF5A. However, the polysome profiles obtained by Dever and coworkers (13) lead to the conclusion that eIF5A promotes translation elongation. It is unclear how to reconcile our polysome profiles with theirs. However, their other data suggesting a role in elongation are more difficult to interpret. In particular, some of the results were obtained after a number of hours of eIF5A depletion, when secondary effects of eIF5A depletion likely occur. The ribosome transit time experiments were not done under steady-state conditions; total and released incorporation data do not generate parallel lines, suggesting that elongation rates decreased throughout their experiment. Finally, the dipeptide release experiment required isolation of the substrate, during which time the tRNA in the ribosomal E site may have been lost. EF-P binding to the E site, and by homology eIF5A binding, is incompatible with simultaneous occupancy by stripped tRNA at the same site (14). Because E-site occupancy by 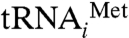 is observed or expected following formation of the first peptide bond and translocation (and by other tRNAs in all subsequent elongation steps), it is difficult to rationalize a general elongation activity for eIF5A after formation of the first peptide bond.
is observed or expected following formation of the first peptide bond and translocation (and by other tRNAs in all subsequent elongation steps), it is difficult to rationalize a general elongation activity for eIF5A after formation of the first peptide bond.
It is important to note that robust quantification of eIF5A activity in translation was observed only after optimizing experimental conditions in vivo and in vitro. First, artifacts unique to strain UBHY-R were determined (Figs. S1 and S2). Second, we observed a strong effect of UBR5A depletion only after maximizing in vivo protein synthesis (Ref. 11, Figs. 1 and 2 and Fig. S4). Third, efficient protein synthesis on endogenous mRNAs in vitro depends on raising the molar concentration of radioactive methionine by cold methionine supplementation (Fig. S5). Although we have not determined the step(s) inhibited by low methionine concentrations in vitro, relieving such inhibition is a sensible and likely required step to take when examining the function of weakly stimulating factors such as eIF5A (9, 10).
We favor a model in which the primary physiological activity of eIF5A is to stimulate formation of the first peptide bond. In support of this idea we note the following: (i) eIF5A was initially found not to stimulate elongation on polyU in vitro in the presence of saturating levels of eEF1A and eEF2 (8); (ii) eIF5A was more recently found to stimulate only the formation of the first two peptide bonds on polyU, but not other templates, in the presence of eEF activity (13); (iii) most compelling, the eIF5A homolog EF-P makes contacts with L1 in the large ribosomal subunit that are incompatible with simultaneous stripped tRNA E-site occupancy (14). Considering our experiments showing diminished polysome profiles upon depletion, a failure to stimulate elongation on preexisting mRNAs in vitro, and the above considerations, we conclude that eIF5A stimulates the formation of the first peptide bond. However, we cannot rule out the possibility that differences in genetic backgrounds, effects of prolonged eIF5A inactivation, or experimental procedures may account for other interpretations of eIF5A activity.
Materials and Methods
Plasmids and Proteins.
Plasmids pYAH-F5A and pYAH-F5A(K51R) were constructed by PCR (19) from pBM-TIF51A (20) and YRp7-5A(K51R) (21). The amplified fragment was inserted into the BamH1 and EcoR1 sites of p414Gal1 (22) and used to express FLAG-tagged eIF5A and its K51R mutant form in strains YAH2 and YAH60. His6-eIF5A(lys) was expressed in strain BAH6 [BL21(DE3) carrying the eIF5A cDNA in pET28c]. To generate eIF5A(lys), His6-eIF5A(lys) was purified from strain BAH6 and treated with thrombin to remove the tag. Similarly, eIF5A(dhp) was obtained from BAH6 by treating purified eIF5A(lys) with His6-yDHS (23, 24). Native FLAG-tagged eIF5A [F5A(hyp)] was purified from yeast strain YAH2 with an anti-FLAG resin. Details of these procedures are provided in SI Materials and Methods.
Extract Preparation and Western Blotting.
Cells were propagated at 30 °C in YP medium containing either 2% glucose or 2% galactose. For depletion studies, cells were washed once with sterile room-temperature water containing 2% glucose, then resuspended in either YP or SD glucose or galactose as indicated. Two lysis methods were employed. The nondenaturing method involved glass bead vortexing and was described previously (11). For the denaturing method, a similar glass bead procedure was used, except that the buffer was 40 mM Tris-HCl, pH 6.8, 4% SDS, 4% glycerol, and 0.1 M DTT, and samples were boiled for 10 min. Lysate proteins were resolved and blotted with rabbit anti-yeast eIF5A (11). UBR5A bands were quantified with TotalLab TL100 (Nonlinear Dynamics, version 2006b).
In Vivo Protein Synthesis Measurements.
W303-1A and UBHY-R cells (11) were pulse-labeled with [35S]methionine at various times following the shift from YP galactose to YP glucose. About three OD595 units of cells (processed independently in duplicate) were washed in water/carbon source, resuspended in 300 μL YP (glucose or galactose), and incubated with 100 μCi Trans-35S-Label (MP Biomedicals) at 30 °C for 15 min with shaking. Labeling was terminated by adding 1-mL ice-cold stop solution [1.2 mg/mL methionine, 0.1 mg/mL cycloheximide (CHX)] and incubating on ice briefly. Cells were collected and snap-frozen in liquid nitrogen, then resuspended in 15% trichloroacetic acid (TCA) (1 mL), incubated on ice for 15 min, and boiled at 95 °C for 15 min to deacylate tRNAs. Denatured protein was pelleted, suspended in 1 mL acetone at −20 °C, stored 30 min at -20 °C, and centrifuged again. Precipitated proteins were dissolved in 400 μL 1% SDS, boiled for 10 min, and clarified by centrifugation. The protein content in 50-μL aliquots was determined with a BCA kit (Pierce) against a bovine serum albumin control. To measure radiolabel incorporation, 25-μL aliquots were counted in a Beckman LS 6000 IC scintillation counter. The rate of protein synthesis was calculated for each sample as cpm/μg protein/ min.
Polysome Profile Analysis.
Cells were grown as above, brought to 0.1 mg/mL CHX and rapidly cooled on ice. Pellets were washed, resuspended in polysome lysis buffer [20 mM Hepes, pH 7.5, 140 mM KCl, 5 mM Mg(OAc)2, 0.5 mM DTT, 1% Triton X-100, 1 mg/mL heparin, 100 U/mL RNAprime (Eppendorf), 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF) and complete protease inhibitors (Roche)] at approximately 1-mL buffer per gram of cells and lysed by vortexing with glass beads. Lysates were centrifugation briefly at 1,000 × g, and three A260 units were loaded onto sucrose gradients and centrifuged at 54,000 rpm in a Beckman TL100 centrifuge in a TLS55 rotor for approximately 1 h. Polysome profiles were generated by following absorbance at 254 nm and fractions collected using an ISCO fractionator.
In Vitro Translation Assays.
Cells were grown in galactose media, shifted to glucose to deplete eIF5A, and lysed as described in detail in SI Materials and Methods. Translation assay reactions contained 60 ± 10% extract and eIF5A proteins as indicated, with final reaction conditions of 30 mM Hepes, pH 7.5, 3.8 ± 0.1 mM total MgCl2, 2 mM ATP, 0.5 mM GTP, 0.2 mg/mL creatine kinase, 12 mM creatine phosphate, 165 ± 10 mM KOAc/KCl, 100 μM amino acids (lacking methionine), 30–40 μM unlabeled methionine, 0.17 μM translation grade [35S]methionine (1175 Ci/mmol; MP Biomedicals), 1 mM AEBSF (added fresh), and 0.3 units/μL RNAprime (added fresh). Reactions were initiated by adding extract and were performed at 20 ± 2 °C. At the indicated times, 5-μL aliquots were withdrawn, spotted on Whatman #1 filters, soaked in fixative (15% TCA, 20 mM cold methionine), swirled, and stored at 4 °C until assay completion. The fixative was decanted and replaced, boiled briefly, and allowed to cool to room temperature. Filters were washed twice with methanol, air-dried, and then counted in a Beckman LS 6000 IC scintillation counter.
Supplementary Material
Acknowledgments.
We thank Hans Johansson, Myung Hee Park, and Martin Hoyt for helpful discussions. This work was supported by National Institutes of Health (NIH) Grants GM22135 and GM073723 from the US Public Health Service. C.A.H. was supported in part by NIH training Grant GM07377.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008150108/-/DCSupplemental.
References
- 1.Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JWB. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3105–3114. doi: 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KK, Hung LW, Yokota H, Kim R, Kim SH. Crystal structures of eukaryotic translation initiation factor 5A from Methanococcus jannaschii at 1.8 Å resolution. Proc Natl Acad Sci USA. 1998;95:10419–10424. doi: 10.1073/pnas.95.18.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peat TS, Newman J, Waldo GS, Berendzen J, Terwilliger TC. Structure of translation initiation factor 5A from Pyrobaculum aerophilum at 1.75 Å resolution. Structure. 1998;6:1207–1214. doi: 10.1016/s0969-2126(98)00120-8. [DOI] [PubMed] [Google Scholar]
- 4.Yao M, Ohsawa A, Kikukawa S, Tanaka I, and Kimura M. Crystal structure of hyperthermophilic archaeal initiation factor 5A: a homologue of eukaryotic initiation factor 5A (eIF-5A) J Biochem-Tokyo. 2003;133:75–81. doi: 10.1093/jb/mvg011. [DOI] [PubMed] [Google Scholar]
- 5.Cooper HL, Park MH, Folk JE, Safer B, Braverman R. Identification of the hypusine-containing protein hy+ as translation initiation factor eIF-4D. Proc Natl Acad Sci USA. 1983;80:1854–1857. doi: 10.1073/pnas.80.7.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Facchiano AM, et al. Homology modelling of the human eukaryotic initiation factor 5A (eIF-5A) Protein Eng. 2001;14:881–890. doi: 10.1093/protein/14.11.881. [DOI] [PubMed] [Google Scholar]
- 7.Hanawa-Suetsugu K, et al. Crystal structure of elongation factor P from Thermus thermophilus HB8. Proc Natl Acad Sci USA. 2004;101:9595–9600. doi: 10.1073/pnas.0308667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemper WM, Berry IW, and Merrick WC. Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Ba and M2Bß. J Biol Chem. 1976;251:5551–5557. [PubMed] [Google Scholar]
- 9.Benne R, Brown-Luedi ML, Hershey JWB. Purification and characterization of protein synthesis initiation factors eIF-1, eIF-4C, eIF-4D, and eIF-5 from rabbit reticulocytes. J Biol Chem. 1978;253:3070–3077. [PubMed] [Google Scholar]
- 10.Benne R, Hershey JWB. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978;253:3078–3087. [PubMed] [Google Scholar]
- 11.Kang HA, Hershey JWB. Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J Biol Chem. 1994;269:3934–3940. [PubMed] [Google Scholar]
- 12.Gregio APB, Cano VPS, Avaca JS, Valentini SR, Zanelli CF. eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun. 2009;380:785–790. doi: 10.1016/j.bbrc.2009.01.148. [DOI] [PubMed] [Google Scholar]
- 13.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaha G, Stanley RE, Steitz TA. Formation of the first peptide bond: The structure of EF-P bound to the 70S ribosome. Science. 2009;325:966–970. doi: 10.1126/science.1175800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jao DL, Chen KY. Tandem affinity purification revealed the hypusine-dependent binding of eukaryotic initiation factor 5A to the translating 80S ribosomal complex. J Cell Biochem. 2006;97:583–598. doi: 10.1002/jcb.20658. [DOI] [PubMed] [Google Scholar]
- 16.Park MH, Wolff EC, SmitMcBride Z, Hershey JWB, Folk JE. Comparison of the activities of variant forms of eIF-4D. The requirement for hypusine or deoxyhypusine. J Biol Chem. 1991;266:7988–7994. [PubMed] [Google Scholar]
- 17.Zanelli CF, Valentini SR. Is there a role for eIF5A in translation? Amino Acids. 2006;33:351–358. doi: 10.1007/s00726-007-0533-0. [DOI] [PubMed] [Google Scholar]
- 18.Dias CAO, et al. Structural modeling and mutational analysis of yeast eukaryotic translation initiation factor 5A reveal new critical residues and reinforce its involvement in protein synthesis. FEBS J. 2008;275:1874–1888. doi: 10.1111/j.1742-4658.2008.06345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Vol. 3. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2001. [Google Scholar]
- 20.Schwelberger HG, Kang HA, Hershey JWB. Translation initiation factor eIF-5A expressed from either of two yeast genes or from human cDNA: Functional identity under aerobic and anaerobic conditions. J Biol Chem. 1993;268:14018–14025. [PubMed] [Google Scholar]
- 21.Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3105–3114. doi: 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mumberg D, Muller R, Funk M. Regulatable promoters of Saccharomyces cerevisiae: Comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang KR, Wolff EC, Park MH, Folk JE, Chung SI. Identification of YHRO68w in Saccharomyces cerevisiae chromosome VIII as a gene for deoxyhypusine synthase. J Biol Chem. 1995;270:18408–18412. doi: 10.1074/jbc.270.31.18408. [DOI] [PubMed] [Google Scholar]
- 24.Lipowsky G, et al. Exportin 4: A mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J. 2000;19:4362–4371. doi: 10.1093/emboj/19.16.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorsch J, Herschlag D. Kinetic dissection of fundamental processes of eukaryotic translation initiation in vitro. EMBO J. 1999;18:6705–6717. doi: 10.1093/emboj/18.23.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.