Abstract
AKT activation requires phosphorylation of the activation loop (T308) by 3-phosphoinositide-dependent protein kinase 1 (PDK1) and the hydrophobic motif (S473) by the mammalian target of rapamycin complex 2 (mTORC2). We recently observed that phosphorylation of the AKT hydrophobic motif was dramatically elevated, rather than decreased, in mTOR knockout heart tissues, indicating the existence of other kinase(s) contributing to AKT phosphorylation. Here we show that the atypical IκB kinase ε and TANK-binding kinase 1 (IKKε/TBK1) phosphorylate AKT on both the hydrophobic motif and the activation loop in a manner dependent on PI3K signaling. This dual phosphorylation results in a robust AKT activation in vitro. Consistently, we found that growth factors can induce AKT (S473) phosphorylation in Rictor−/− cells, and this effect is insensitive to mTOR inhibitor Torin1. In IKKε/TBK1 double-knockout cells, AKT activation by growth factors is compromised. We also observed that TBK1 expression is elevated in the mTOR knockout heart tissues, and that TBK1 is required for Ras-induced mouse embryonic fibroblast transformation. Our observations suggest a physiological function of IKKε/TBK1 in AKT regulation and a possible mechanism of IKKε/TBK1 in oncogenesis by activating AKT.
Keywords: IKK-related protein kinase, protein kinase B, cancer
The AKT protein kinase is a major signaling hub and a key downstream effector of the PI3K pathway (1, 2). AKT plays a major role in cell growth, proliferation, and survival (3). Under physiological conditions, AKT activity is intricately controlled, whereas pathological AKT activation contributes to human cancers (4, 5). On PI3K activation, AKT is recruited to plasma membrane via its PH domain, which is critical for AKT phosphorylation (6, 7). Phosphorylation of the activation loop T308 by 3-phosphoinositide-dependent protein kinase 1 (PDK1) is essential for AKT activation (8). However, full AKT activation also requires phosphorylation of the regulatory hydrophobic motif S473 by mTORC2, which consists of mTOR, Rictor, Sin1, and mLST8 subunits (9–12). AKT (S473) phosphorylation is defective in Rictor or mSin1 knockout cells, demonstrating the importance of mTORC2 in AKT (S473) phosphorylation (10, 13, 14). DNA-PK also has been implicated in AKT hydrophobic motif phosphorylation in response to DNA double-strand breaks (15, 16).
Accumulating evidence indicates an mTOR-independent kinase responsible for AKT (S473) phosphorylation. Two studies using tissue-specific knockout mice indicated that mTOR is not required for AKT hydrophobic motif phosphorylation in skeletal and cardiac muscles (17, 18). We found that ablation of mTOR in heart cardiac muscles indeed decreased phosphorylation of the mTORC1 substrate S6K, but AKT (S473) phosphorylation was dramatically increased in these mice. Consistent with our observation, mTOR or Raptor/Rictor double-knockout in skeletal muscle also increases AKT (S473) phosphorylation (17–19). Collectively, these data provide compelling evidence for the existence of other AKT hydrophobic motif kinases besides mTORC2.
The noncanonical IκB kinase ε (IKKε) has been demonstrated to play an essential role in Ras-induced cell transformation, which requires activation of both the Raf-MEK-ERK and PI3K-AKT pathways (20). Of particular interest is the observation that IKKε can functionally replace the requirement of AKT to support cell transformation, which suggests that IKKε may act as an AKT regulator or effector. Consistently, IKKε was found to be amplified in several types of human cancer, including ovarian cancer and breast cancer (20, 21). TANK-binding kinase 1 (TBK1) is the closest IKKε homolog, and a systematic RNA interference screen has identified that TBK1 is required for Ras-induced oncogenesis. Down-regulation of TBK1 in active K-Ras–expressing cancer cells results in apoptosis and synthetic lethality (22, 23). These studies indicate that IKKε/TBK1 may function upstream or downstream of AKT to inhibit apoptosis and support oncogenic Ras-induced transformation.
In this study, we investigated the relationship between IKKε/TBK1 and AKT. We found that IKKε/TBK1 activates AKT by directly phosphorylating the activation loop and hydrophobic motif. Our observations suggest that pathological activation of IKKε or TBK1 promotes oncogenesis, at least in part, by activating AKT.
Results and Discussion
Overexpression of IKKε or TBK1 Activates AKT.
The observations that IKKε can substitute the requirement for active AKT to support in vitro transformation with active MEK and that TBK1 is required to suppress apoptosis in cells transformed by Ras suggest the possibility that IKKε/TBK1 may function to activate AKT (20, 22). To test this possibility, we examined whether ectopic expression of IKKε or TBK1 could activate AKT. Toward this end, WT and kinase dead mutants (KM) of IKKε or TBK1 were stably expressed in HeLa cells. We found that overexpression of WT TBK1, but not of KM TBK1, caused a dramatic increase in AKT phosphorylation on both S473 and T308 (Fig. 1A). In contrast, phosphorylation of the turn motif (T450) was not increased, although a mobility shift due to S473 and T308 phosphorylation was detected by this antibody. Similar to TBK1, overexpression of IKKε also induced a significant increase in AKT phosphorylation at both T308 and S473 (Fig. 1B). These results indicate that an elevated TBK1 or IKKε level, as may be found in cancer cells (20, 21), can induce AKT activation.
Fig. 1.
Overexpression of IKKε and TBK1 activates AKT. (A) Overexpression of TBK1 activates AKT. HeLa cells stably expressing WT TBK1, KM TBK1, or vector control (V) were tested for AKT signaling. Western blots with various antibodies are indicated. pi, phospho-antibody. (B) Overexpression of IKKε activates AKT. The experiments were similar to those shown in A.
FoxO1, TSC2, and GSK-3 are known physiological substrates of AKT involved in different downstream signaling (24–27). We observed that expression of either TBK1 or IKKε, but not their KMs, increased phosphorylation of FoxO1, TSC2, and GSK-3 (Fig. 1). Phosphorylation of FoxO1 by AKT activation should result in 14-3-3 binding and cytoplasmic localization (28). Indeed, in serum-starved control cells, little AKT (S473) phosphorylation was detected, and FoxO1 was nuclear-localized. However, IKKε overexpression resulted in a marked increase of AKT (S473) phosphorylation, and FoxO1 was detected mainly in cytoplasm (Fig. S1). These data demonstrate that expression of TBK1 or IKKε is sufficient to activate AKT signaling.
IKKε /TBK1 Are Important for Proper AKT Activation by Growth Factors.
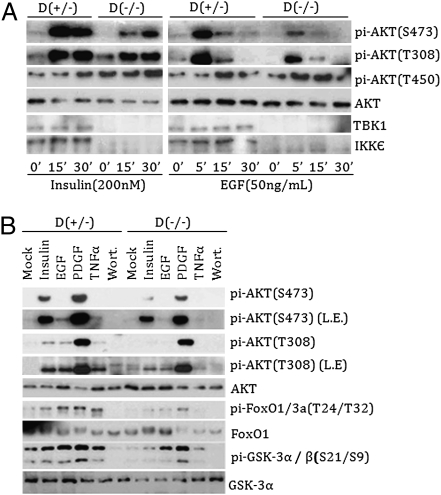
To determine a physiological role of IKKε/TBK1 in AKT activation, we investigated AKT activation in IKKε/TBK1 double-knockout mouse embryonic fibroblasts (MEFs) (29). Compared with IKKε/TBK1 double-heterozygous control cells, those double-knockout cells demonstrate normal Rictor or mTOR expression and normal PKCα (S657) or AKT (T450) phosphorylation (Fig. S2A). In IKKε and TBK1 heterozygous control cells, insulin induced a robust increase in AKT phosphorylation at both T308 and S473 sites (Fig. 2A); however, insulin-induced S473 phosphorylation was significantly reduced and delayed in the double-knockout MEFs. Similarly, EGF-stimulated phosphorylation of S473 was significantly diminished in the double-knockout cells. Phosphorylation of T308 also was reduced, but to a lesser extent. Consistent with decreased AKT activation, phosphorylation of FoxO1 and GSK3 in response to EGF, PDGF, and insulin also was compromised in the IKKε/TBK1 double-knockout cells (Fig. 2B). Reconstitution of TBK1 restored AKT phosphorylation in the double-knockout MEFs (Fig. S2B). Taken together, these data support a potential physiological role of IKKε and TBK1 in growth factor–induced AKT activation.
Fig. 2.
IKKε and TBK1 are important for proper AKT activation. (A) IKKε and TBK1 double-knockout MEFs are defective in AKT phosphorylation. Both heterozygous [D(+/−)] and homozygous knockout [D(−/−)] MEFs were serum-starved for 16 h and then stimulated with insulin (200 nM) or EGF (50 ng/mL) for the indicated times. Western blots with various antibodies are shown. (B) IKKε and TBK1 double-knockout MEFs are defective in AKT signaling. The experiments were similar to those shown in A, except that cells were treated with Wortmannin (100 nM), TNFα (50 ng/mL), EGF (50 ng/mL), PDGF (50 ng/mL), and insulin (200 nM) for 30 min. L.E., longer exposure.
IKKε and TBK1 Directly Phosphorylate and Activate AKT In Vitro.
Given the fact that AKT phosphorylation is constitutively elevated in IKKε- or TBK1- overexpressing cells (Fig. 1), we tested whether IKKε/TBK1 could directly phosphorylate AKT. Dual-epitope–tagged IKKε and TBK1 immunoprecipitated from transected HEK293 cells potently phosphorylated S473 of recombinant AKT in vitro, as detected by phospho S473 and T308 antibodies (Fig. 3A). We performed similar experiments with 32P-ATP and found that TBK1 efficiently phosphorylated the recombinant His-AKT (Fig. S3). At the highest TBK1 level tested, substantial phosphorylation (0.26 mol 32P-phosphate/mole protein) of AKT was achieved. Flag-mTOR coimmunoprecipitated with cotransfected HA-Rictor was included as a positive control. Because mTOR, IKKε, and TBK1 were tagged by the Flag epitope, the relative activity of these kinases could be compared. It appears that both IKKε and TBK1 displayed comparable kinase activity with mTOR toward AKT (S473) phosphorylation (Fig. 3A and Fig. S3). In contrast, IKK1 (a canonical IκB kinase) displayed no activity toward AKT, demonstrating the specificity of the kinase assay. Surprisingly, both IKKε and TBK1 also phosphorylated AKT activation loop T308, whereas mTOR did not (Fig. 3 A and B).
Fig. 3.
IKKε and TBK1 directly phosphorylate and activate AKT. (A) IKKε and TBK1 phosphorylate AKT on both S473 and T308 in vitro. IKKε, TBK1, IKK1, and Rictor plus mTOR were transfected into HEK293 cells and immunoprecipitated with HA antibody as indicated. The immunoprecipitates were used to phosphorylate the inactive 6XHis-AKT in vitro. Phosphorylation of AKT was determined by phospho antibodies. IB, immunoblotting; IP, immunoprecipitation. (B) Phosphorylation of AKT by endogenous TBK1. Endogenous IKK1, mTOR, PDK1, and TBK1 were immunoprecipitated from HeLa cells and then used for in vitro AKT phosphorylation as in A. (C) IKKε activates AKT in vitro. Tagged IKKε (WT and KM) were immunoprecipitated with anti-HA agarose-conjugated beads from transfected HEK293 cells and then incubated with inactive 6XHis-AKT to active the AKT kinase (IKKε assay). The IKKε immunoprecipitates were then removed by centrifugation. The activated AKT in the supernatant was subsequently assayed for its ability to phosphorylate TSC2 (AKT assay). (D) Activation of AKT by IKKε. The pi-TSC2(S939) and GST-TSC2 immunoblotting signals from two independent experiments were quantified using ImageJ. AKT activity was defined as the signal ratio between pi-TSC2(S939) and GST-TSC2.
We next investigated whether endogenous TBK1, which has a higher expression level and wider tissue distribution than IKKε (30), also could phosphorylate AKT in vitro. We found that TBK1 immunoprecipitated from HeLa cells phosphorylated AKT on both T308 and S473, whereas the immunoprecipitated mTORC2 complex or PDK1 phosphorylated only S473 or T308, respectively (Fig. 3B). Phosphorylation of T308 and S473 would be sufficient to activate AKT. Indeed, WT IKKε, but not KM IKKε, activated AKT in vitro, as determined by the ability of the activated AKT to phosphorylate recombinant GST-TSC2 (Fig. 3 C and D). These data clearly demonstrate that IKKε and TBK1 can activate AKT by directly phosphorylating T308 and S473.
IKKε/TBK1 and mTORC2 Activate AKT Independently.
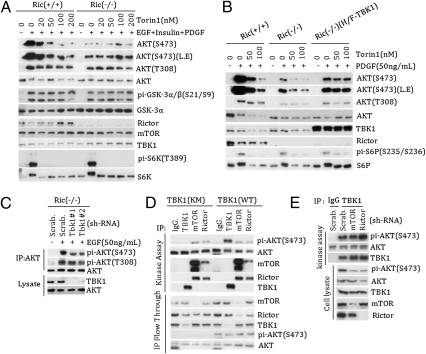
Although it has been well established that mTORC2 is the AKT (S473) kinase, our data indicate a role of IKKε/TBK1 in AKT activation. To clarify this issue, we tested several stimuli and found that PDGF could induce significant AKT (S473) phosphorylation in Rictor−/− MEFs (Fig. 4B and Fig. S4 A and B). Moreover, the combination of EGF, insulin, and PDGF induced even stronger AKT (S473) phosphorylation in the Rictor−/− cells (Fig. S4 A and B), establishing the existence of mTORC2-independent AKT (S473) kinase. Consistent with previous data, insulin-induced AKT (S473) phosphorylation was largely abolished in the Rictor−/− cells (Fig. S4A). It is worth noting that previous studies were aimed at emphasizing the importance of mTORC2, and no serious effort was made to achieve high AKT (S473) phosphorylation by examining different stimuli or combinations. A potent mTOR kinase inhibitor, Torin1, was used to further exclude the role of mTOR in AKT (S473) phosphorylation in Rictor−/− cells (31). We found that Torin1 had no effect on AKT (S473) phosphorylation in the Rictor−/− cells, but inhibited S6K phosphorylation (Fig. 4A). Consistent with this, phosphorylation of the AKT substrate GSK-3 and TSC2 was induced by growth factors (Fig. 4A and Fig. S4B).
Fig. 4.
TBK1 and mTORC2 act independently in AKT activation. (A) mTORC2-independent activation of AKT in Rictor−/− MEF. Rictor+/+ or Rictor−/− cells were pretreated with different doses of Torin1 for 30 min, then stimulated with or without the combination of EGF (50 ng/mL), insulin (200 nM), PDGF (50 ng/mL) (EGF + insulin + PDGF) for 20 min. Cell lysates were blotted with antibodies as indicated. (B) PDGF activates AKT in Rictor−/− cells in an mTOR-independent manner. Rictor+/+, Rictor−/−, and TBK1 stably expressed Rictor−/− cells were pretreated or not treated with mTOR inhibitor Torin1 at different concentrations for 30 min, then treated or not treated with PDGF for 20 min. Cell lysates were blotted with different antibodies as indicated. (C) TBK1 contributes to AKT (S473) phosphorylation in the Rictor−/− MEF cells. Control (Scrab.) and two different lentiviral-based shRNAs targeting Tbk1 (Tbk1#1 and Tbk1#2) were used to knock down TBK1 in the Rictor−/− MEF cells. To detect AKT (S473) phosphorylation, cells were treated with or without 50 ng/mL of EGF for 5 min, and AKT was immunoprecipitated from lysate of 10-cm dishes, AKT(S473) phosphorylation was detected in the AKT-concentrated immunoprecipitates, and Tbk1 levels were determined in the cell lysate. (D) TBK1 expression does not affect mTORC2 activity. TBK1, Rictor or mTOR was immunoprecipitated from TBK1-expressing WT or TBK1-expressing KM HeLa cells and assayed for AKT (S473) phosphorylation in vitro. The post-IP supernatants were also blotted with various antibodies, as indicated. (E) mTORC2 does not affect TBK1 activity. Rictor or mTOR was down-regulated by shRNA in HeLa cells. Endogenous TBK1 was immunoprecipitated, and activity was determined by in vitro AKT phosphorylation assays.
We next tested the function of TBK1. Knockdown of TBK1 in Rictor−/− MEFs significantly reduced the residual AKT (S473) phosphorylation stimulated by EGF (Fig. 4C), suggesting a role of endogenous TBK1 in AKT (S473) phosphorylation. When TBK1 was ectopically expressed, phosphorylation of AKT (S473) was increased in the Rictor−/− cells (Fig. 4B). Notably, Torin1 did not inhibit the TBK1-induced AKT (S473) phosphorylation, indicating that mTOR is not required for TBK1 to activate AKT. Similarly, IKKε expression-induced AKT (S473) phosphorylation was not inhibited by Torin1 (Fig. S4C).
We explored the relationship between IKKε/TBK1 and mTORC2 further by knocking down Rictor or mTOR in the TBK1-overexpressing cells (Fig. S5 A and B). As expected, knockdown of either Rictor or mTOR strongly decreased AKT (S473) phosphorylation in the TBK1 KM-expressing cells, indicating that AKT phosphorylation depends on mTORC2 in these cells. In contrast, knockdown of Rictor or mTOR only partially decreased the elevated AKT phosphorylation in the TBK1 WT-expressing HeLa cells. The interference of mTOR function by knockdown is evident in the reduced S6K phosphorylation (Fig. S5A). These results indicate that TBK1 activates AKT in a manner independent of mTORC2. Consistently, mTORC2 immunoprecipitated with Rictor or mTOR antibody from either TBK1 WT- or KM-expressing cells displayed similar activity to phosphorylated AKT (S473) (Fig. 4D), indicating that mTORC2 was not affected by TBK1 expression. Similarly, IKKε overexpression did not affect mTORC2 activity (Fig. S5C). To investigate whether mTORC2 plays a role in TBK1 activation, Rictor or mTOR was down-regulated in HeLa cells and endogenous TBK1 was immunoprecipitated for determining its kinase activity toward AKT. Knockdown of Rictor or mTOR had no effect on TBK1 kinase activity for AKT (S473) phosphorylation in vitro (Fig. 4E). These observations indicate that mTORC2 and IKKε/TBK1 indeed act independently to activate AKT.
PI3K Signaling Is Required for IKKε/TBK1 to Activate AKT.
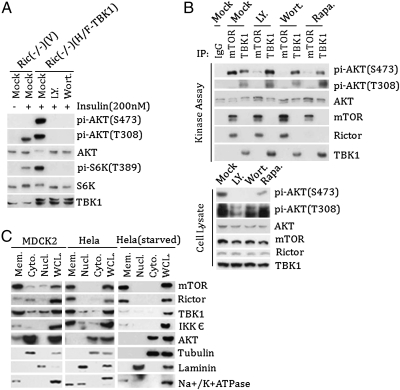
We next investigated the relationship between PI3K and IKKε/TBK1. TBK1-overexpressing Rictor−/− MEF cells were treated with PI3K inhibitor LY249002 or Wortmannin. Inhibition of PI3K potently reduced AKT (S473) phosphorylation in the TBK1-expressing Rictor−/− MEFs (Fig. 5A), indicating that PI3K signaling is required for TBK1 to phosphorylate AKT. Consistently, LY294002 and Wortmannin, but not Torin1, inhibited AKT phosphorylation in IKKε-expressing HeLa cells (Figs. S4C and S6A). To further test the effect of PI3K on TBK1, endogenous TBK1 was immunoprecipitated from cells treated with the PI3K inhibitors, and its kinase activity was measured. We found that treatment of PI3K inhibitors or prolonged rapamycin treatment had no effect on TBK1 kinase activity, but inhibited mTORC2 activity (Fig. 5B). Moreover, neither LY249002 nor Wortmannin inhibited TBK1 kinase activity when added in vitro (Fig. S6B).
Fig. 5.
PI3K signaling is required for AKT activation by TBK1. (A) Inhibition of PI3K abolishes TBK1-dependent AKT phosphorylation. TBK1-expressing Rictor−/− MEF cells were treated with LY429002 (25 μM) or Wortmannin (100 nM) as indicated. Phosphorylation of AKT was determined by Western blot analysis. (B) TBK1 activity is insensitive to PI3K inhibitor treatment. HeLa cells were treated with LY249002 (25 μM) or Wortmannin (100 nM) for 30 min or rapamycin (50 nM) for 16 h as indicated. TBK1 and mTOR were immunoprecipitated, and kinase activity was determined using AKT as a substrate. (Lower) Phosphorylation of AKT in cell lysate was determined as well. (C) Both IKKε and TBK1 are membrane-associated. HeLa cells (serum-starved for 16 h or not) and MDCK2 cells were fractionated into nuclear, cytosolic, and membrane fractions. The distributions of IKKε and TBK1 in different subcellular fractions were determined by Western blot analysis along with fractional markers.
If PI3K signaling does not directly affect TBK1 activity, then how can TBK1-induced AKT activation be inhibited by LY249002 or Wortmannin in cells? One possibility is that if IKKε/TBK1 were membrane-localized, then PI3K-dependent AKT membrane recruitment would be required for its phosphorylation by IKKε/TBK1. Subcellular fractionation revealed that most of the IKKε/TBK1 was constitutively localized in the membrane fraction of either HeLa or MDCK2 cells (Fig. 5C). As expected, both mTOR and Rictor were also membrane-associated. These data support a model in which the PI3K-dependent membrane association of AKT is required for the phosphorylation by membrane-associated IKKε/TBK1, although IKKε and TBK1 activity is not directly affected by PI3K signaling.
TBK1 Is Required for Ras-Induced Transformation and Is Elevated in mTOR Knockout Cardiac Muscles.
We found that conditional mTOR knockout in mouse heart resulted in a dramatic increase of AKT phosphorylation on both S473 and T308 (Fig. 6A). We next examined the expression of both IKKε and TBK1. Interestingly, TBK1 protein levels were significantly increased in the knockout heart tissues, whereas IKKε expression was unchanged (Fig. 6B). TBK1 phosphorylation was elevated at Ser172, which correlates with TBK1 activation. Furthermore, phosphorylation of IRF3, a physiological TBK1 substrate, was elevated in the mTOR knockout heart tissues, indicating increased TBK1 activity. These data suggest that elevated TBK1 activity may contribute to the AKT hyperphosphorylation in mTOR knockout cardiac muscle. Combinatory knockout of mTOR together with TBK1 and/or IKKε would be required to further demonstrate that IKKε/TBK1 are the kinases responsible for AKT phosphorylation in the absence of mTOR.
Fig. 6.
TBK1 is activated in mTOR knockout heart tissue and is required for Ras-induced MEF transformation. (A) AKT phosphorylation is elevated in cardiac-specific mTOR knockout myocardium. Heart homogenate from control mice (WT-Cre) and mTOR cardiac knockout (cKO) mice at 1 mo after tamoxifen injection were prepared and probed for AKT phosphorylation and other antibodies as indicated. Two representative animals of each genotype are shown. (B) TBK1 expression and signaling are elevated in mTOR (cKO) myocardium. The same samples as in A were probed for TBK1 protein levels, Ser172 phosphorylation, and IRF3 phosphorylation. (C) TBK1 is required for K-Ras (V12)-induced focus formation in MEFs. IKKε/TBK1 double-knockout MEFs with TBK1 re-expression or vector control (the same cells as in Fig. S2B) were infected with K-Ras (V12). Cells were cultured for 4 wk, and cell foci were stained by crystal violet.
Finally, we tested whether IKKε/TBK1 is required for Ras transformation in MEFs. Active K-Ras was expressed in IKKε/TBK1 double-knockout cells that were reconstituted with TBK1 or vector. The IKKε/TBK1 double-knockout MEFs could not be transformed by active K-Ras (V12). In contrast, re-expression of TBK1, which restored AKT activation (Fig. S2B) but was insufficient to transform MEFs alone, supported a robust transformation by K-Ras (V12), as indicated by focus formation (Fig. 6C). These observations indicate an obligatory role of IKKε/TBK1 in Ras-induced oncogenic transformation of MEF cells. Future studies are needed to determine whether AKT activation is the major downstream effector of TBK1 supporting Ras-induced transformation.
As a major signaling hub, AKT regulates a wide range of cellular processes from cell growth, proliferation, to apoptosis (2). Pathological AKT activation is observed in many cancers and clearly plays a major role in tumorigenesis (1). It has been well established that PDK1 and mTORC2 are responsible for AKT activation in many cell types examined (9–11, 14, 32). Based on our present findings, we propose that IKKε/TBK1 also contributes to AKT activation. Uniquely, IKKε/TBK1 can phosphorylate both the activation loop and the hydrophobic motif to a sufficient degree to activate AKT. Under normal physiological conditions, PDK1 and mTORC2 may play major roles in most cell types; however, even MEFs have mTORC2-independent kinase that can be activated by growth factors and contributes to AKT (S473) phosphorylation. TBK1 appears to play such a role in AKT activation in the Rictor−/− cells. Moreover, IKKε/TBK1 might contribute to AKT activation in some tissues, such as skeletal and cardiac muscle tissues. As an oncogene, IKKε is amplified and overexpressed in >30% of breast carcinomas and cell lines (33, 34). Activation of NF-κB signaling by IKKε/TBK1 may contribute to oncogenesis (20, 30). Activation of AKT by IKKε/TBK1 also may contribute to tumorigenesis or innate immune response.
We have shown that PI3K signaling is required for IKKε/TBK1 to phosphorylate AKT in vivo, although clearly the kinase activity is not dependent on PI3K. We speculate that the membrane associated IKKε/TBK1 is unable to phosphorylate the cytosolic AKT in the absence of PI3K signaling. When PI3K is activated, AKT is recruited to membrane where it is phosphorylated and activated by the membrane-residing IKKε/TBK1. Activation of AKT by IKKε/TBK1 not only may play a role in physiological AKT activation in certain cell types, but also contributes to tumorigenesis, especially when IKKε/TBK1 is dysregulated. Future studies using combinatory tissue-specific knockout mouse models are needed to demonstrate the specificity and the relative contributions of IKKε/TBK1, PDK1, and mTORC2 in AKT activation.
Materials and Methods
Antibody, Plasmids, and Chemicals.
Antibodies were obtained from Santa Cruz Biotechnology (anti-mTOR, rabbit IgG,), Covance (anti-HA), Sigma-Aldrich (anti-IKKε), Bethyl Laboratories (anti-TBK1, anti-PDK1, anti-IKK1, and anti-Rictor), and Cell Signaling (all others). Anti-HA agarose-conjugated beads were obtained from Sigma-Aldrich. Torin1 (31), lentiviral shRNAs targeting human mTOR and Rictor were obtained from Sabatini Lab (11, 25), and shRNAs targeting mouse Tbk1 were obtained from Sigma-Aldrich (MISSION shRNA clones TRCN0000026954 and TRCN0000027015). IKK1, IKKε, and TBK1 plasmids from M. Karin's lab were subcloned into a HA/Flag-tagged retroviral vector. Other plasmids were from laboratory stock. Recombinant 6XHis-AKT was obtained from Upstate Biotechnology. Insulin, PDGF, EGF, TNFα, rapamycin, LY294002, and Wortmannin were obtained from Cell Signaling.
Cell Lines, Transfections, Virus Infection, and Mouse Tissues.
HeLa, HEK293, 293P, and MEF cell lines were cultured in DMEM supplemented with 10% FBS and penicillin (100 U/mL) and streptomycin (100 μg/mL). Transfections were performed with polyethylenimine. Lentiviral and retroviral infections were performed as described previously (11, 25). Cardiac-specific mTOR-inducible knockout mice were generated and tissue samples were prepared as described previously (17).
Immunoblotting, Quantification, Immunoprecipitation, and Kinase Assay.
Immunoblotting and immunoprecipitation was performed as described previously (10). The Western blot signals on scanned films were quantified using ImageJ software. For the kinase assay, the immunoprecipitates were washed with kinase buffer and then incubated for 20 min at 37 °C in 50 μL of kinase reaction buffer containing 100 ng of recombinant 6XHis-AKT, as described previously (11). Phosphorylation of AKT was determined by Western blot analysis. To measure AKT kinase activity, the immunoprecipitated IKKε/TBK1 was removed from the kinase reaction by centrifugation, and AKT activity was measured using purified GST-TSC2 fragments as a substrate (25). Phosphorylation of TSC2 was determined by Western blot analysis.
Cell Fractionation.
Cells were harvested in low-salt buffer [10 mM Hepes (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 50 mM NaF, 1.5 mM Na3VO4, protease inhibitor mixture (Roche), 1 mM DTT, 1 mM PMSF] and passed through a 25G needle 15 times. Nuclear fractions were collected by centrifugation at 1,000 × g for 5 min at 4 °C. Mitochondrial fractions were collected by further centrifugation of the supernatants at 10,000 × g for 5 min at 4 °C. The supernatants were further fractionated by centrifugation at 100,000 × g for 30 min at 4 °C to collect membrane and cytosolic fractions.
Foci Formation Assay.
The K-Ras (V12) virus-infected MEF cells were plated onto 10-cm dishes. After confluency was reached, the medium was changed every 2–3 d for 4 wk. The cells were then fixed with 4% paraformaldehyde and stained with 0.5% crystal violet in 25% methanol. Dried plates were photographed.
Supplementary Material
Acknowledgments
We thank Dr. Osamu Takeuchi and Dr. Shizuo Akira for the IKKε/TBK1 knockout MEFs; Dr. M. Karin for the IKK1, IKKε, and TBK1 plasmids; and Dr. David M. Sabatini for the mTOR and Rictor shRNA. This work was supported by National Institutes of Health Grants GM51586, GM62694, and CA113793 (to K.-L.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016132108/-/DCSupplemental.
References
- 1.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–107. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 3.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Manning BD, Cantley LC. Targeting the PI3K–Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 5.Vogt PK, Gymnopoulos M, Hart JR. PI 3-kinase and cancer: Changing accents. Curr Opin Genet Dev. 2009;19:12–17. doi: 10.1016/j.gde.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andjelković M, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 7.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The akt kinase: Molecular determinants of oncogenicity. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alessi DR, et al. Characterization of a 3-phosphoinositide–dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 9.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 12.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Jacinto E, et al. SIN1/MIP1 maintains rictor–mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Guertin DA, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Risson V, et al. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol. 2009;187:859–874. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentzinger CF, et al. Skeletal muscle–specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Boehm JS, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Guo JP, et al. Deregulation of IKBKE is associated with tumor progression, poor prognosis, and cisplatin resistance in ovarian cancer. Am J Pathol. 2009;175:324–333. doi: 10.2353/ajpath.2009.080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbie DA, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien Y, et al. RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–170. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 24.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 25.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 26.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor-suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 27.Tang ED, Nuñez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 28.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi H, et al. The roles of two IκB kinase–related kinases in lipopolysaccharide and double-stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:1–13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 31.Thoreen CC, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarbassov DD, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Adli M, Baldwin AS. IKK-i/IKKε controls constitutive, cancer cell–associated NF-κB activity via regulation of Ser-536 p65/RelA phosphorylation. J Biol Chem. 2006;281:26976–26984. doi: 10.1074/jbc.M603133200. [DOI] [PubMed] [Google Scholar]
- 34.Eddy SF, et al. Inducible IκB kinase/IκB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-κB activation in breast cancer cells. Cancer Res. 2005;65:11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.