Abstract
Behavioral exposure therapy of anxiety disorders is believed to rely on fear extinction. Because preclinical studies have shown that glucocorticoids can promote extinction processes, we aimed at investigating whether the administration of these hormones might be useful in enhancing exposure therapy. In a randomized, double-blind, placebo-controlled study, 40 patients with specific phobia for heights were treated with three sessions of exposure therapy using virtual reality exposure to heights. Cortisol (20 mg) or placebo was administered orally 1 h before each of the treatment sessions. Subjects returned for a posttreatment assessment 3–5 d after the last treatment session and for a follow-up assessment after 1 mo. Adding cortisol to exposure therapy resulted in a significantly greater reduction in fear of heights as measured with the acrophobia questionnaire (AQ) both at posttreatment and at follow-up, compared with placebo. Furthermore, subjects receiving cortisol showed a significantly greater reduction in acute anxiety during virtual exposure to a phobic situation at posttreatment and a significantly smaller exposure-induced increase in skin conductance level at follow-up. The present findings indicate that the administration of cortisol can enhance extinction-based psychotherapy.
Keywords: memory, retrieval, consolidation
Phobic disorders can be characterized as disorders involving disturbed emotional learning and memory resulting in an enhanced fear response. A central mechanism in the pathogenesis of anxiety disorders is associative learning or conditioning that leads to formation of a fear memory (1–5). In phobic individuals, exposure to a phobic stimulus almost invariably provokes retrieval of stimulus-associated fear memory, which leads to the fear response (6–9). Exposure-based behavioral therapy of phobia is thought to rely on extinction of these fear responses (10–13). Extinction occurs when conditioned responding to a stimulus decreases when the reinforcer is omitted (12, 14). Accordingly, fear reduction induced by exposure therapy is the result of decrements in the conditioned response over successive extinction trials. Extinction leads to the formation of an alternative set of nonfearful memory associations (extinction memory) that competes with, but does not erase original fear memory associations (14, 15). Considering the importance of extinction learning for exposure therapy, pharmacological interventions aimed at enhancing extinction processes are promising approaches to enhance exposure therapy, as it has been demonstrated with d-cycloserine (16–18).
Glucocorticoids (cortisol in humans, corticosterone in rodents) are stress hormones released from the adrenal cortex and it has long been recognized that they readily enter the brain and affect learning and memory (19–24). Importantly, basic research studies in animals and humans have shown that the mnemonic effects of glucocorticoids can facilitate extinction processes (22, 25–29). Therefore, we aimed at investigating whether the administration of these hormones might be useful in enhancing exposure therapy.
Forty patients with a diagnosis of specific phobia for heights according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) were treated with three sessions of exposure therapy using virtual exposure to heights. Virtual reality (VR) exposure therapy has proven effective in the treatment of patients with acrophobia (30–34) and is ideal for clinical research, as it allows identical exposure of all patients and avoids unpredicted events that may occur in real environments (35). Cortisol (20 mg) or placebo was administered orally 1 h before each of the treatment sessions. Subjects returned for a posttreatment assessment 3–5 d after the last treatment session and for a follow-up assessment after 1 mo (28–35 d after last treatment session). Symptom severity was assessed with fear of heights questionnaires and subjective ratings of acute fear in height situations at pretreatment, posttreatment, and follow-up. Furthermore, we measured skin conductance response, which has been shown to be reduced after successful fear extinction (36).
Results
The cortisol group and the placebo group consisted each of 20 patients (11 males, 9 females). The groups did not differ significantly in demographic and clinical characteristics, or in any of the baseline measurements taken before treatment (Table 1). On all sessions with pharmacological treatment (i.e., treatment sessions 1–3), subjects who had received cortisol had significantly higher salivary cortisol concentrations before (P ≤ 0.001) and after (P ≤ 0.007) VR exposure than subjects who had received placebo (Table S1). No differences in baseline salivary cortisol concentrations were found between the treatment groups on any of the study days (P ≥ 0.332). None of the patients reported adverse effects due to substance administration.
Table 1.
Demographic and clinical characteristics and baseline measurements at pretreatment assessment
| Placebo group | Cortisol group | Significance, P | |
| Females/males | 9/11 | 9/11 | 1.0 |
| Age | 40.2 (2.6) | 42.8 (2.4) | 0.461 |
| BMI | 26.4 (1.1) | 25.6 (0.8) | 0.558 |
| Severity primary diagnosis | 5.4 (0.2) | 5.4 (0.2) | 0.874 |
| BDI total score | 5.7 (1.2) | 4.0 (1.0) | 0.280 |
| ASI total score | 17.9 (1.8) | 16.2 (1.8) | 0.498 |
| STAI trait total score | 39.2 (1.8) | 36.1 (1.4) | 0.185 |
| ITQ | 5.3 (1.2) | 4.2 (1.2) | 0.524 |
| AQ | 58.9 (4.4) | 58.4 (4.1) | 0.927 |
| ATHQ | 41.5 (2.7) | 39.3 (2.0) | 0.517 |
| DES | 18.3 (1.0) | 18.6 (0.9) | 0.847 |
| AES | 20.1 (1.3) | 20.0 (1.3) | 0.962 |
| Treatment credibility | 23.6 (0.8) | 22.8 (0.6) | 0.462 |
| SUD | 48.8 (5.3) | 49.4 (4.8) | 0.940 |
| BAT score | 4.3 (0.4) | 4.1 (0.4) | 0.792 |
Data presented as mean (SEM). AES, anxiety expectancy scale; AQ, acrophobia questionnaire; ASI, anxiety sensitivity index; ATHQ, attitude toward heights questionnaire; BAT, behavioral avoidance test; BDI, Beck depression inventory; BMI, body mass index; DES, danger expectancy scale; ITQ, immersive tendencies questionnaire; STAI, state-trait anxiety inventory; SUD, subjective units of discomfort.
Effects of VR Exposure.
We found a significant reduction of fear as measured with the acrophobia questionnaire (AQ) from pretreatment assessment (59.3 ± 2.8; mean ± SE) to posttreatment assessment (35.7 ± 2.6; F1,35 = 18.135; P < 0.001), and follow-up (30.1 ± 2.8; F1,35 = 18.636; P < 0.001). The controlled effect size from pretreatment to posttreatment assessment was d = 1.3 and from pretreatment to follow-up d = 1.6. Also in the danger expectancy scale (DES) fear symptoms decreased from pretreatment (18.1 ± 0.6) to posttreatment (14.8 ± 0.7; F1,34 = 17.828; P < 0.001) and follow-up (12.8 ± 0.6; F1,35 = 15.926; P < 0.001). No significant symptom decrease was observed with the anxiety expectancy scale (AES) and with the attitude toward heights questionnaire (ATHQ). No significant symptom change was measured from posttreatment to follow-up in any of the questionnaires (AQ, DES, ATHQ, and AES) (P ≥ 0.127). Performance in the behavioral avoidance test (BAT) was enhanced from pretreatment (4.1 ± 0.3) to posttreatment (5.5 ± 0.2, F1,35 = 6.088; P = 0.019) and follow-up (5.5 ± 0.2; F1,35 = 8.556; P = 0.006).
Effects of Cortisol.
Fear of heights.
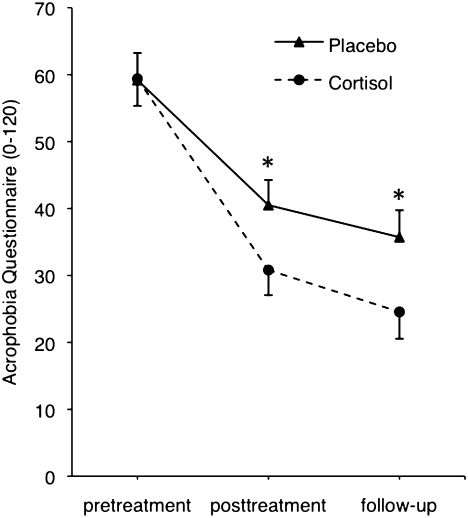
Compared with the placebo group, the cortisol group showed significantly less fear as measured with the AQ at posttreatment (cortisol, 30.4 ± 3.1; placebo, 40.2 ± 3.1; F1,34 = 5.090; P = 0.031) and at follow-up (cortisol, 24.2 ± 3.5; placebo, 35.4 ± 3.5; F1,34 = 5.053; P = 0.031) (Fig. 1). The controlled effect size for cortisol at posttreatment was d = 0.6 and at follow-up d = 0.6. Sex did not influence the cortisol effect (interaction sex X drug, P = 0.4). There was a significant difference for the DES at follow-up (cortisol, 11.5 ± 0.8; placebo, 14.2 ± 0.8; F1,34 = 5.322; P = 0.027). The controlled effect size for cortisol at follow-up was d = 0.6. Sex did not influence the cortisol effect on DES (interaction sex X drug, P = 0.2). We found a trend difference for the AES at follow-up (cortisol, 24.7 ± 1.4; placebo, 28.4 ± 1.4; F1,34 = 3.512; P = 0.07) and no significant difference for the ATHQ. There was a trend toward better performance of the cortisol group compared with the placebo group in the BAT at follow-up (cortisol, 5.9 ± 0.2; placebo, 5.2 ± 0.2; F1,34 = 3.767; P = 0.061) but not at posttreatment (cortisol, 5.8 ± 0.2; placebo, 5.2 ± 0.2; F1,34 = 2.435; P = 0.128). The BAT scale ranges from 0 to 6, where 6 means least fear. Compared with the placebo group, the cortisol group showed significantly lower anxiety (as measured in subjective units of discomfort (SUDs) while going up with the elevator during VR exposure) at posttreatment (cortisol, 15.9 ± 2.9; placebo, 29.4 ± 3.1; F1,30 = 9.803; P = 0.004), but not at follow-up (cortisol, 16.5 ± 4.3; placebo, 23.1 ± 4.5; F1,30 = 1.114; P = 0.3). The controlled effect size for cortisol at posttreatment was d = 1.00. Sex did not influence the cortisol effect on SUDs (interaction sex X drug, P = 0.6). In all treatment sessions, there were no significant treatment-related differences in subjects’ belief in having received active medication or placebo (P ≥ 0.248).
Fig. 1.
Adding cortisol to VR exposure resulted in reductions of self-reported fear of heights (measured with acrophobia questionnaire, range 0–120) at posttreatment and at follow-up. VR exposure took place in three treatment sessions between pretreatment and posttreatment assessment. Cortisol was administered 1 h before each VR exposure session. Values are depicted as mean and SEM. *P < 0.05 indicates significant difference between the placebo- and cortisol group at a certain time point (see text for details).
Skin conductance.
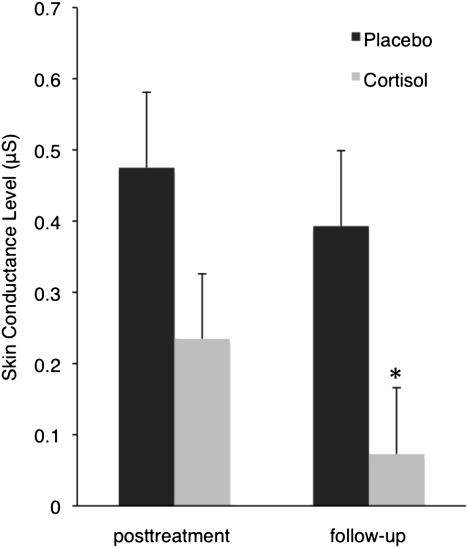
Due to technical reasons, skin conductance level (SCL) was only available from 25 subjects at posttreatment and from 20 subjects at follow-up. The treatment groups did not differ significantly in demographic and clinical characteristics or in any of the baseline measurements taken before treatment (Tables S2 and S3). Due to the smaller sample sizes, we analyzed the data for possible outliers and found that all values lay within 2.3 times the SD from the mean (data were normally distributed; Kolmogorov–Smirnov test, P ≥ 0.36). At follow-up, there was a significantly smaller SCL increase from the 60-s period on the floor to the 60-s period on the roof after going up with the elevator during VR (difference score SCL, in microsiemens) in the cortisol group compared with the placebo group (cortisol, 0.073 ± 0.093; placebo, 0.393 ± 0.106; F1,14 = 5.024; P = 0.042) (Fig. 2). Sex did not significantly influence the cortisol effect (interaction sex X drug, P = 0.2). The analysis at posttreatment showed a trend toward a smaller SCL difference score in the cortisol group (cortisol, 0.235 ± 0.091; placebo, 0.475 ± 0.106; F1,19 = 2.906; P = 0.105). SCL during the 60-s period on the floor did not differ significantly between treatment groups at posttreatment and follow-up (P ≥ 0.185; Tables S4 and S5 show SCL data during the 60-s periods on the floor and roof).
Fig. 2.
Skin Conductance Level (SCL) response to VR exposure to heights at posttreatment and follow-up. Values depicted are estimated means and SEM of SCL difference scores (mean SCL during the 60-s period on the floor subtracted from mean SCL during the 60-s period on the roof after going up with the elevator). *P < 0.05 indicates significant difference between the placebo and cortisol group at follow-up.
Discussion
The results of the present study indicate that cortisol facilitates the effects of VR exposure therapy. As measured with the acrophobia questionnaire, a standard questionnaire to reliably assess fear of heights (37), patients who received cortisol together with the three VR exposure sessions, showed significantly greater reductions in phobic fear both at posttreatment and at 1-mo follow-up (Fig. 1), compared with patients who received placebo. The controlled (for placebo treatment) effect size of the cortisol treatment was d = 0.6 at follow-up, which corresponds to a medium effect size. Acute anxiety as measured in SUDs while going up with the elevator during VR exposure was significantly more reduced in patients receiving cortisol than in patients receiving placebo at posttreatment, but not at follow-up. In the BAT, which consists of a real-life heights situation, there was only little room for drug-related improvements, as the mean score in the placebo group was 5.2 at follow-up (maximal score 6). In the cortisol group the mean score at follow-up was 5.9, resulting in a trend in treatment effect. Further studies are needed to investigate the cortisol effects in more challenging real-life situations and using reinstatement and renewal tests. Finally, we found evidence that cortisol reduced VR exposure-induced elevation of SCL at follow-up (Fig. 2). Taken together, the present findings indicate that the administration of cortisol can enhance exposure therapy of a specific phobia.
Basic research studies in animals and healthy humans have shown that glucocorticoids can enhance memory consolidation of new information (21, 38–41), but impair memory retrieval of already stored information (22–24, 42, 43). Moreover, recent evidence indicates that emotionally arousing information, including traumatic memory, is especially sensitive to the retrieval-reducing effects of glucocorticoids (22, 44–46). In a study in patients with social phobias, we recently reported that a single administration of cortisone (25 mg) reduces fear in a social stress situation (29). Moreover, in patients with spider phobia, we found that repeated oral administration of cortisol (10 mg) before presenting a spider photograph induces a progressive reduction of stimulus-induced fear (29). This effect was maintained when subjects were presented the stimulus again 2 d after the last cortisol administration, suggesting that cortisol facilitates the extinction of phobic fear (29). On the basis of the known effects on memory processes, glucocorticoids may facilitate the extinction of a fear memory trace in two ways: (i) because of the glucocorticoid-induced reduction of memory retrieval, an aversive cue is no longer followed by the usual, full-blown retrieval of fear memory and related clinical symptoms but, instead, becomes associated with a less aversive experience, which is stored as extinction memory and (ii) because glucocorticoids are known to enhance memory consolidation of new information, it is possible that glucocorticoids also enhance the storage of corrective experiences (extinction memory). This notion is supported by recent animal studies showing that postretrieval administration of glucocorticoids is able to enhance the consolidation of extinction memory (27, 47). In conclusion, the above-mentioned mnemonic effects of glucocorticoids are likely to contribute to their capability of facilitating memory extinction processes (22, 25–29).
Exposure-based psychotherapy of phobia is thought to rely on extinction of fear responses (10–12). Therefore, the present results indicating that glucocorticoids enhance exposure therapy are consistent with the notion that glucocorticoids facilitate extinction. The results add further evidence to the relevance of restructuring memory traces in successful exposure therapy and to the amenability of these processes to pharmacological interventions. Our study is part of recent attempts to use pharmacological approaches or behavioral interventions to facilitate extinction or block reconsolidation processes (17, 48, 49). Such studies may not only help us to better understand the role of these memory processes in fear reduction, but they may also contribute to the development of novel therapeutic strategies to treat anxiety disorders.
Materials and Methods
Subjects.
Subjects aged 18–60 with fear of heights (acrophobia) were recruited via newspaper advertisements and flyers posted at the University of Basel and health institutions. A total of 260 subjects responded and, after a telephone screening, 75 subjects were invited for a diagnostic assessment with the diagnostic interview for mental disorders (DIPS) (50). A total of 42 patients (23 males and 19 females) who fulfilled criteria for specific phobia environmental-type based on the DSM-IV (51) were included in the study. Exclusion criteria were: a recent history of systemic or oral glucocorticoid therapy, another axis I disorder that was considered to be more impairing and distressing than the acrophobia, severe acute or chronic disease, pregnancy and lactating, current pharmacological treatment, or behavioral therapy. Pregnancy was determined by means of a urine pregnancy test that was performed for all female participants before administration of study medication. Two participants were excluded after allocation to study groups. One participant refrained from study participation between treatment sessions 1 and 2; another participant was excluded because of dizziness during VR therapy. Both subjects were allocated to the cortisol group. The remaining 40 patients completed the study and entered the analyses. The ethics committee of the University of Basel and the Swiss agency for the authorization and supervision of therapeutic products (Swissmedic) approved the study. After describing the study to the patients, written informed consent was obtained. Patients were quasi-randomly assigned to a double-blind, placebo-controlled design (matched for age, body mass index (BMI), severity of acrophobia, measured by the anxiety subscales of the acrophobia questionnaire (AQ) (37) and capability to immerse into virtual reality, measured by five questions taken from the immersive tendencies questionnaire (ITQ) (52). The blind was maintained throughout the study. All subjects received 100 Swiss francs as compensation for their participation.
Procedure and Measurements.
Treatment.
The study took place at the laboratories of the Department of Clinical Psychology and Psychotherapy of the University of Basel. Participation included six appointments: an initial screening session to clarify study eligibility and to assess symptoms before treatment (pretreatment assessment), three treatment sessions (treatment sessions 1–3) within 1 week (Monday, Wednesday, and Friday), an assessment 3–5 d after the last treatment session (posttreatment assessment), and a follow-up assessment 28–35 d after the last treatment session (follow-up assessment). During each treatment session, either cortisol (20 mg, 2 tablets of 10 mg each of hydrocortisone; Galepharm, Küsnacht, Switzerland) or placebo (2 similarly looking tablets) was administered 1 h before each of the three VR sessions. The initial 1-h resting period allowed the absorption of the medication before starting the VR session. On the pretreatment assessment, posttreatment assessment, and on the follow-up assessment, no medication was administered. During these assessments, participants had limited and structured exposure to the VR heights environment (using an elevator in the same VR environment as used for the treatment sessions) during a behavioral test in VR. During this behavioral test, participants were asked about their fear while going up with the elevator by means of SUDs on a scale ranging from 0 to 100 (100 being the most intense fear). To prepare the patients for the exposure session, the patients received some psychoeducative material about exposure therapy and instructions on how to cope with former avoidance strategies during pretreatment assessment. No other cognitive-behavioral techniques, such as breathing or relaxation techniques, were used.
VR treatment.
The exposure treatment took place in a temperature-controlled (temperature was between 19.7 °C and 27 °C; mean 22.3 °C) and sound-attenuated experimental room connected to an adjoining control room. During the exposure session the room was darkened. The participant stood on a wooden platform (1.5 × 2.1 feet high; height above floor about 6 inches). Before the exposure session started, physiological sensors and a headset with integrated video display glasses (head-mounted display) and headphones were attached. Via these glasses, patients were exposed to a VR height environment (Virtual Reality Medical Center, San Diego, CA) that was simulated by a computer program. A sensor registered head movements and altered the display to reproduce a change in gaze direction.
The therapist controlled the treatment via a personal computer keyboard located in the control room and adapted the standardized therapy protocol depending on the strength of reported fear of the patient. The subjects were instructed to move their heads and look around. Patients were guided through a VR height environment with different platforms connected by bridges and elevators. All patients were systematically guided through the environment and had to pass different predefined stations with increasing difficulty. Patients started the treatment session with using a lift at a rather low building and finished it with crossing a long and small bridge connecting two very high platforms. The following predefined schema for the treatment session in VR was used: patients had to stay at a particular station for at least 60 s. During this period they had to give two SUDs, the first one after 30 s and the second one after another 30 s. The second SUD at a particular station was used as “reference anxiety” for how long a patient had to stay at a particular station. If the second SUD was 30 or below, the patient was guided to the next predefined station. If the second SUD was above 30, the patient had to stay at this station until the anxiety had decreased at least 20%. After a decrease of at least 20%, the therapist asked for one further SUD. If anxiety did not further decrease, the patients were guided to the next station. If anxiety decreased, the patient stayed at this station until no further decrease occurred or until seven SUDs were taken, which was predefined as a maximum for each station. SUDs were asked every 30 s, therefore a patient never stayed longer than 210 s at each station. This procedure was applied at each station. A maximum of 10 stations was available. Every patient stayed for 20 min in the VR. The time spent at each station and the number of stations varied between patients and between sessions. All participants were instructed to avoid cognitive avoidance strategies and the cognitive avoidance was measured with several questions afterward.
Saliva measurements.
Saliva was collected with Salivette (Sarstedt). Baseline saliva samples were taken pretreatment, posttreatment, and on the follow-up assessment immediately before the BAT and on treatment sessions 1–3 immediately before the administration of the study medication. In treatment sessions 1–3, two additional samples were taken, one immediately before the VR exposure (1 h after the administration of the study drugs) and one immediately after the VR exposure. The saliva samples were stored at −20 °C until biochemical analysis. After thawing, salivettes were centrifuged at 3,000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Free cortisol in saliva was analyzed by using commercially available chemiluminescence immunoassay with high sensitivity (IBL International). The inter- and intra-assay coefficients of variation were <10%. To reduce error variance caused by imprecision of the intra-assay, all samples of one subject were analyzed in the same run.
Self-Report Measures.
Fear of heights questionnaires.
The following measures were applied at pretreatment, posttreatment, and follow-up assessments to measure fear of heights: (i) The German translation of the anxiety subscale of the AQ (37). This questionnaire describes 20 situations that can cause fear of heights (e.g., driving over a bridge, walking over a sidewalk grating, or sitting on an airplane) and asks for anxiety ratings on 7-point (0–6) Likert-type scales (0 = not at all afraid to 6 = very afraid, range 0–120, α = 0.80). (ii) The ATHQ, German version (53) and (iii) the DES, German version (54) to assess dysfunctional cognitions. The ATHQ consists of six questions assessing participants’ attitudes toward height situations (e.g., “I think heights are … good/bad, secure/insecure”). Patients evaluate their attitudes toward heights by 12 adjectives on an 11-point scale ranging from a positive (0) to a negative (10) adjective (range 0–60; α = 0.81). The DES consists of five items. Participants rate the likelihood that each of the listed harmful events (e.g., “you might slip and fall over the guard rail on the observation deck”) will pass through their minds while being in a height situation on five-point scales [not likely at all (1), probably not (2), maybe (3), quite likely (4), or definitely (5), range 5–25] (iv). The AES, German version (55) to assess anxiety symptoms in a height situation. The AES consists of 10 items describing anxiety symptoms (e.g., “you could feel dizzy”). Participants rate the likelihood of experiencing these symptoms while being in a height situation on the same five-point scale as described above (range 10–50).
Acute fear in height situations.
During the behavioral test in VR, participants rated their anxiety while going up with the elevator by means of verbal SUDs on a 100-point scale from 0 = “no anxiety at all” to 100 = “extreme anxiety.” Pretreatment, posttreatment, and during the follow-up assessment, participants performed a BAT consisting of a real-life heights situation (going up an outdoor staircase with three levels). During the BAT, the performance was rated on a scale from 0 to 6. One point was given for each completed level and another point for looking down for 30 s at each level.
Trait anxiety and depression.
Anxiety and depressive symptoms were assessed with the German versions of the state-trait anxiety inventory (STAI, German version) (56), the anxiety sensitivity index (ASI, German version) (57), and the Beck depression inventory (BDI, German version) (58).
Presence.
At pretreatment assessment, participants answered five questions taken from the immersive tendencies questionnaire (ITQ), which measures, by means of ratings on a 7-point scale based on the semantic differential principle, differences in disposition to experience presence. Like the semantic differential, each ITQ item is anchored at the ends by opposing descriptors. Unlike the semantic differential the ITQ scale includes a midpoint anchor (52).
Treatment credibility.
Treatment credibility/expectancy (score ranging from 0 to 30) were completed by all of the participants after the psychoeducational part at pretreatment assessment and the first and last exposure session (59). Participants were asked at each exposure session and at posttreatment and follow-up on a scale from −5 to 5 (placebo to cortisol) to indicate whether they believed they were assigned to active medication or placebo. We did not systematically obtain reports of adverse effects although the subjects were routinely asked at the beginning and end of each session if they were experiencing any difficulties.
Skin Conductance Level.
Electrodermal activity was measured using 11-mm Ag/AgCl electrodes filled with isotonic electrode paste (60) attached to the volar surfaces of the medial index and middle fingers (with a constant 0.5 V passed between the electrodes). Body movement was assessed using an accelerometer attached to the right shoulder to allow identification of movement artifacts. All physiological channels were sampled continuously at a rate of 1,000 Hz using a BIOPAC MP150 amplifier and Acqknowlege software (Biopac Systems) that was installed on a 1.7 GHz Intel personal computer. Autonomic Nervous System Laboratory (ANSLAB) software (Available at the software repository http://www.sprweb.org) was used to edit the raw signals for artifacts and extract physiologically meaningful parameters: The raw signal of electrodermal activity was edited for artifacts and 1-Hz lowpass filtered to extract the average SCL during the phases of the experiment. Mean values were calculated for four time segments during the behavioral test in VR (60-s period on the floor, 17-s period elevator upward, 60-s period on the roof, 17-s period elevator downward). Additionally, a difference score was built by subtracting the mean value of a physiological variable during the 60-s period on the floor from the mean value of a physiological variable during the 60-s period on the roof after going up with the elevator.
Statistics.
Data were entered into the SPSS statistics package for Macintosh (SPSS, 17.0) by research assistants blind to condition. Group differences in demographic and clinical characteristics and cortisol levels at baseline, before and after VR exposure, were analyzed with unpaired t tests or X2 tests.
VR exposure effects.
To analyze VR-induced symptom change from pretreatment to posttreatment and follow-up, variables of interest (AQ, DES, AES, ATHQ, BAT, and SUD) were analyzed with repeated-measures ANCOVAs with symptoms at certain time points as within-subject factors, age as covariate, and sex and treatment condition as cofactors. The controlled effect size (Cohen's d) was calculated for significant variables via the formula: (meanposttreatment/follow-up − meanpretreatment)/[(SDposttreatment/follow-up + SDpretreatment)/2] (61, 62).
Drug-dependent analyses: Pretreatment, posttreatment, and follow-up.
To compare the outcome of the two treatment conditions (exposure + placebo vs. exposure + cortisol), we used univariate ANCOVAs with treatment condition as between-subject factor, posttreatment, and follow-up measures as dependent variables (AQ, DES, AES, BAT, and SUD) and corresponding pretreatment baseline measures as covariate (63, 64). Furthermore, we included age as covariate and sex as cofactor. The controlled effect size (Cohen's d) was calculated for significant variables via the formula: (meanexposure+cortisol − meanexposure+placebo)/[(SDexposure+cortisol + SDexposure+placebo)/2] (61, 62).
Skin conductance level.
Due to technical failure, not all physiological measurements could be used, resulting in a subject sample of 25 for posttreatment (11, placebo group; 14, cortisol group) and 20 at follow-up (9, placebo group; 11, cortisol group). We conducted ANCOVAs with treatment condition as between-subject factor, posttreatment, and follow-up physiological measures (i.e., difference score SCL) as dependent variable and the corresponding pretreatment baseline measure as covariate. Furthermore, we included age as covariate and sex as cofactor.
Because age and sex have been shown to influence glucocorticoid effects on memory and emotional processes (65–68), we included a priori age as covariate and sex as cofactor in the behavioral and SCL analyses. All tests were two-tailed and a P value < 0.05 was considered statistically significant. All variables were normally distributed (Kolmogorov–Smirnov test: P > 0.1 for all variables). All reported results were corrected by using the Greenhouse–Geisser procedure, where appropriate.
Supplementary Material
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation (PP00P3-123391 to D.J.-F.d.Q.) and the Basel Scientific Society (to F.H.W.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 6343.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018214108/-/DCSupplemental.
References
- 1.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 2.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 3.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Brewin CR, Holmes EA. Psychological theories of posttraumatic stress disorder. Clin Psychol Rev. 2003;23:339–376. doi: 10.1016/s0272-7358(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbert BN, et al. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- 7.Lang PJ, Tuma AH, Maser JD. Anxiety and the Anxiety Disorders. Hillsdale, NJ: Erlbaum; 1985. The cognitive psychophysiology of emotion: Fear and anxiety; pp. 131–170. [Google Scholar]
- 8.Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 9.Alpers GW, Abelson JL, Wilhelm FH, Roth WT. Salivary cortisol response during exposure treatment in driving phobics. Psychosom Med. 2003;65:679–687. doi: 10.1097/01.psy.0000073872.85623.0c. [DOI] [PubMed] [Google Scholar]
- 10.Bentz D, Michael T, de Quervain DJ, Wilhelm FH. Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. J Anxiety Disord. 2010;24:223–230. doi: 10.1016/j.janxdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 11.McNally RJ. Mechanisms of exposure therapy: How neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biol Psychiatry. 2006;60:361–368. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Hamm AO. Specific phobias. Psychiatr Clin North Am. 2009;32:577–591. doi: 10.1016/j.psc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann SG, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 17.Ressler KJ, et al. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 18.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 21.Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 22.de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 23.de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 24.de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 25.Barrett D, Gonzalez-Lima F. Behavioral effects of metyrapone on Pavlovian extinction. Neurosci Lett. 2004;371:91–96. doi: 10.1016/j.neulet.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 26.Bohus B, Lissák K. Adrenocortical hormones and avoidance behaviour of rats. Int J Neuropharmacol. 1968;7:301–306. doi: 10.1016/0028-3908(68)90012-9. [DOI] [PubMed] [Google Scholar]
- 27.Cai WH, Blundell J, Han J, Greene RW, Powell CM. Postreactivation glucocorticoids impair recall of established fear memory. J Neurosci. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 29.Soravia LM, et al. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci USA. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emmelkamp PM, et al. Virtual reality treatment versus exposure in vivo: A comparative evaluation in acrophobia. Behav Res Ther. 2002;40:509–516. doi: 10.1016/s0005-7967(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 31.Krijn M, et al. Treatment of acrophobia in virtual reality: The role of immersion and presence. Behav Res Ther. 2004;42:229–239. doi: 10.1016/S0005-7967(03)00139-6. [DOI] [PubMed] [Google Scholar]
- 32.Rothbaum BO, Hodges L, Smith S, Lee JH, Price L. A controlled study of virtual reality exposure therapy for the fear of flying. J Consult Clin Psychol. 2000;68:1020–1026. doi: 10.1037//0022-006x.68.6.1020. [DOI] [PubMed] [Google Scholar]
- 33.Rothbaum BO, et al. Effectiveness of computer-generated (virtual reality) graded exposure in the treatment of acrophobia. Am J Psychiatry. 1995;152:626–628. doi: 10.1176/ajp.152.4.626. [DOI] [PubMed] [Google Scholar]
- 34.Wiederhold BK, Wiederhold MD. Virtual Reality Therapy for Anxiety Disorders. Washington, D.C.: American Psychological Association; 2005. [Google Scholar]
- 35.Wiederhold BK, Wiederhold MD. A review of virtual reality as a psychotherapeutic tool. Cyberpsychol Behav. 1998;1:45–52. [Google Scholar]
- 36.Vansteenwegen D, et al. The repeated confrontation with videotapes of spiders in multiple contexts attenuates renewal of fear in spider-anxious students. Behav Res Ther. 2007;45:1169–1179. doi: 10.1016/j.brat.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Cohen DC. Comparison of self-report and overt-behavioral procedures for assessing acrophobia. Behav Ther. 1977;8:17–23. [Google Scholar]
- 38.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 39.Flood JF, et al. Memory facilitating and anti-amnesic effects of corticosteroids. Pharmacol Biochem Behav. 1978;8:81–87. doi: 10.1016/0091-3057(78)90127-2. [DOI] [PubMed] [Google Scholar]
- 40.Kovács GL, Telegdy G, Lissák K. Dose-dependent action of corticosteroids on brain serotonin content and passive avoidance behavior. Horm Behav. 1977;8:155–165. doi: 10.1016/0018-506x(77)90032-0. [DOI] [PubMed] [Google Scholar]
- 41.Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav Neurosci. 2006;120:217–223. doi: 10.1037/0735-7044.120.1.217. [DOI] [PubMed] [Google Scholar]
- 42.Wolf OT, et al. Cortisol differentially affects memory in young and elderly men. Behav Neurosci. 2001;115:1002–1011. doi: 10.1037//0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]
- 43.Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Aerni A, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- 45.de Quervain DJ, Aerni A, Roozendaal B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am J Psychiatry. 2007;164:967–969. doi: 10.1176/ajp.2007.164.6.967. [DOI] [PubMed] [Google Scholar]
- 46.Kuhlmann S, Kirschbaum C, Wolf OT. Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol Learn Mem. 2005;83:158–162. doi: 10.1016/j.nlm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: Dependence upon training intensity. Neurobiol Learn Mem. 2008;89:178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kindt M, Soeter M, Vervliet B. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 50.Schneider S, Margraf J. DIPS diagnostisches Interview bei psychischen Störungen. Vol. 3. Berlin: Springer; 2006. p. 173. [Google Scholar]
- 51.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: APA; 1994. [Google Scholar]
- 52.Witmer BG, Singer MJ. Measuring presence in virtual environments: A presence questionnaire. Presence (Camb Mass) 1998;7:225–240. [Google Scholar]
- 53.Abelson JL, Curtis GC. Cardiac and neuroendocrine responses to exposure therapy in height phobics: Desynchrony within the ‘physiological response system.’. Behav Res Ther. 1989;27:561–567. doi: 10.1016/0005-7967(89)90091-0. [DOI] [PubMed] [Google Scholar]
- 54.Mühlberger A. Gefahrenerwartungsbogen bei Flugreisen. In: Hoyer J, Margraf J, editors. Angstdiagnostik. Berlin: Springer; 2003. pp. 443–445. [Google Scholar]
- 55.Mühlberger A. Angerwartungsbogen bei Flugreisen. In: Hoyer J, Margraf J, editors. Angstdiagnostik. Berlin: Springer; 2003. pp. 406–408. [Google Scholar]
- 56.Laux L. State-Trait-Angstinventar theoretische Grundlagen und Handanweisung. Weinheim, Germany: Beltz Testgesellschaft; 1981. Deutsche Ausgabe Ed p 1 Testmappe. [Google Scholar]
- 57.Ehlers A, Margraf J. “Angst vor der Angst”: Ein neues Konzept in der Diagnostik der Angststorungen (“Fear of fear”: A new concept in the assessment of anxiety disorders) Verhaltenstherapie. 1993;3:14–24. [Google Scholar]
- 58.Hautzinger MBM, Worall H, Keller F. Beck Depressionsinventar (BDI) Bern: Huber; 1994. [Google Scholar]
- 59.Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3:257–260. [Google Scholar]
- 60.Fowles DC, et al. Committee report. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18:232–239. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- 61.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 62.Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1:170–177. [Google Scholar]
- 63.Crager MR. Analysis of covariance in parallel-group clinical trials with pretreatment baselines. Biometrics. 1987;43:895–901. [PubMed] [Google Scholar]
- 64.Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: A simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Almela M, et al. The impact of cortisol reactivity to acute stress on memory: Sex differences in middle-aged people. Stress. 2011;14:117–127. doi: 10.3109/10253890.2010.514671. [DOI] [PubMed] [Google Scholar]
- 66.Kukolja J, Thiel CM, Wolf OT, Fink GR. Increased cortisol levels in cognitively challenging situations are beneficial in young but not older subjects. Psychopharmacology (Berl) 2008;201:293–304. doi: 10.1007/s00213-008-1275-8. [DOI] [PubMed] [Google Scholar]
- 67.Kuhlmann S, Wolf OT. Cortisol and memory retrieval in women: Influence of menstrual cycle and oral contraceptives. Psychopharmacology (Berl) 2005;183:65–71. doi: 10.1007/s00213-005-0143-z. [DOI] [PubMed] [Google Scholar]
- 68.Merz CJ, et al. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35:33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.