Abstract
Metabolic, infectious, and tumor cell-intrinsic noxae can all evoke the endoplasmic reticulum (ER) stress response in tumor cells, which is critical for tumor cell growth and cancer progression. Evidence exists that the ER stress response can drive a proinflammatory program in tumor cells and macrophages but, to our knowledge, a role for the tumor ER stress response in influencing macrophages and inflammation in the tumor microenvironment has not been suggested. Here we show that macrophages cultured in conditioned medium from ER-stressed tumor cells become activated, and themselves undergo ER stress with the up-regulation of Grp78, Gadd34, Chop, and Xbp-1 splicing, suggesting a general activation of the ER stress-signaling pathways. Furthermore, these macrophages recapitulate, amplify and expand the proinflammatory response of tumor cells. We term this phenomenon “transmissible” ER stress. Although neither Toll-like receptor (TLR)2 nor interleukin 6 receptor (IL6R) signaling is involved, a reduction was observed in the transmission of ER stress to TLR4 KO macrophages, consistent with the fact that a second signal through TLR4 combined with exposure to tumor ER stress-conditioned medium results in a faster ER stress response and an enhancement of proinflammatory cytokine production in macrophages. The injection of tumor ER stress-conditioned medium into WT mice elicited a generalized ER stress response in the liver. We suggest that transmissible ER stress is a mechanism through which tumor cells can control myeloid cells by directing them toward a proinflammatory phenotype, thus facilitating tumor progression.
Tumor- and tumor-associated macrophage-derived inflammation contributes to tumor growth, progression, and metastasis (1, 2). Inflammation has been linked to the transformation of premalignant into malignant cells, a process requiring NF-κB activation (3, 4), enhancement of growth factor-free survival of cancer cells (5), and tumor control of myeloid cells, leading to more rapid cancer growth and metastasis (6). Although the origin and nature of the signaling pathways involved in these processes are yet to be fully understood, studies of tumor-associated inflammation provide new clues as to how tumor cells seize control of the function of neighboring cells.
The endoplasmic reticulum (ER) is the initial checkpoint for the folding and modification of proteins that reside within the secretory pathway (7). The ER stress response is mediated by three initiator/sensor molecules, namely, inositol-requiring enzyme 1 (IRE1α), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6), which are normally associated with the 78 kDa glucose-regulated protein (GRP78) (8). Upon activation, PERK signals downstream effectors such as the growth arrest and DNA damage gene (GADD)34 and the C/EBP homologous protein (CHOP). IRE1α is an endoribonuclease that, upon activation, initiates the unconventional splicing of the mRNA encoding X-box–binding protein 1 (XBP-1). Spliced XBP-1 is a potent transcriptional activator that increases expression of a subset of the unfolded protein response (UPR)-related genes involved in efficient protein folding, maturation, and degradation in the ER (9).
Data suggest a direct relationship between the ER stress response and tumor growth and progression (10). In tumor cells the inactivation of ER stress signaling by mutations of PERK, or by a dominant-negative PERK, results in tumors that are smaller and less aggressive than their WT counterparts (11). Increased GRP78 expression in human prostate cancer correlates with recurrence and poor survival (12), and conditional deletion of Grp78 in the prostates of Pten-deficient mice abrogates prostate tumorigenesis (13). siRNA inhibition of XBP-1 in human fibrosarcoma cells inhibits growth and angiogenesis in a xenograft model (14). Recent evidence shows that the ER stress response is associated with the transcriptional activation of a proinflammatory cytokine program, both in tumor cells and in bone marrow-derived myeloid cells (15, 16). Based on the foregoing, we hypothesized that ER stress could be the mechanistic link between tumor cells and myeloid cells in fueling inflammation in the tumor microenvironment.
Results
Tumor ER Stress-Conditioned Medium Transfers ER Stress and Proinflammation to Macrophages.
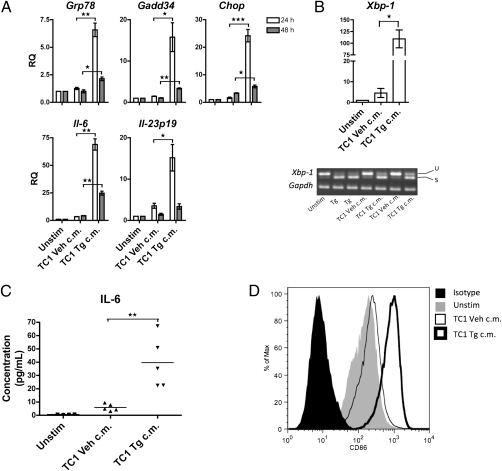
Murine prostate cancer TRAMP-C1 (TC1) cells treated with thapsigargin (Tg), a sesquiterpene lactone tumor promoter that specifically induces ER stress by inhibiting the sarco/endoplasmic reticulum Ca2+ ATPase (17), undergo ER stress and transcriptional activation of proinflammatory cytokines (18). We used 18-h TC1 ER stress-conditioned medium (Fig. S1A) to explore possible noncognate influences of ER-stressed tumor cells on myeloid cells in a model system in which cell-free ER stress-conditioned medium is transferred to macrophages (Fig. S1B). After 24 and 48 h culture, we noted that J774 macrophages up-regulate the ER stress response genes Grp78, Gadd34, and Chop (Fig. 1A). Detection of an increased level of Xbp-1 and its spliced Xbp-1s form indicated Ire1α activation (Fig. 1B). J774 macrophages also up-regulated the genes for the proinflammatory cytokine IL-6 and the unique p19 subunit of IL-23 (IL-23p19) (Fig. 1A), and secreted IL-6 (Fig. 1C), confirming the real-time quantitative PCR (RT-qPCR) findings. Taken together, these data indicate that tumor cells under ER stress secrete a mediator(s) that causes macrophages to initiate an ER stress response with concomitant transcriptional activation and secretion of tumor-promoting, proinflammatory cytokines. Exposure to the ER stress-conditioned medium also promoted the up-regulation of the costimulatory molecule CD86, indicating macrophage activation (Fig. 1D).
Fig. 1.
J774 macrophages experience ER stress, produce pro-inflammatory cytokines, and up-regulate the CD86 costimulatory molecule upon exposure to conditioned medium from ER-stressed tumor cells. J774 macrophages were cultured in conditioned medium of ER-stressed TC1 cells (TC1 Tg c.m.), control TC1 cells (TC1 Veh c.m.), or culture medium alone (Unstim) for 24 or 48 h. (A) RNA was isolated and analyzed by RT-qPCR for UPR activation and proinflammatory cytokine gene transcription. Columns indicate fold increase in transcript level (RQ) of each treatment group. The value of an unstimulated control was set arbitrarily to 1. Error bars represent SEM of two to three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired, two-tailed t test. (B) RNA (24-h) was isolated and analyzed by RT-qPCR for Xbp-1 transcription and splicing. (C) J774 supernatants from the 24- and 48-h timepoints were analyzed by multiplex cytometric bead assay for presence of IL-6. **P < 0.01, unpaired, two-tailed t test. (D) Flow cytometry analysis of J774 macrophages treated for 48 h as indicated. Results are of representative of three independent experiments. u, Unspliced; s, spliced.
To exclude the possibility of Tg carryover, we analyzed the ER stress-conditioned medium by mass spectroscopy and found it to contain no Tg (Fig. S2). Cell death was also ruled out as a contributing factor, as Annexin V positivity in Tg-treated TC1 cells was comparable to that of vehicle-treated TC1 cells (Fig. S3A). Furthermore, J774 macrophages cultured in medium from TC1 cells treated with staurosporine to cause equivalent levels of apoptosis failed to up-regulate either the ER stress response or the proinflammatory cytokine genes (Fig. S3B). Finally, the effects could not be attributed to mycoplasma-induced activation because TC1 cells tested repeatedly negative for this pathogen. These data suggest that the transmission of ER stress and proinflammation from tumor cells to macrophages is not due to Tg carryover, apoptosis, or contamination of TC1 “transmitter” cells.
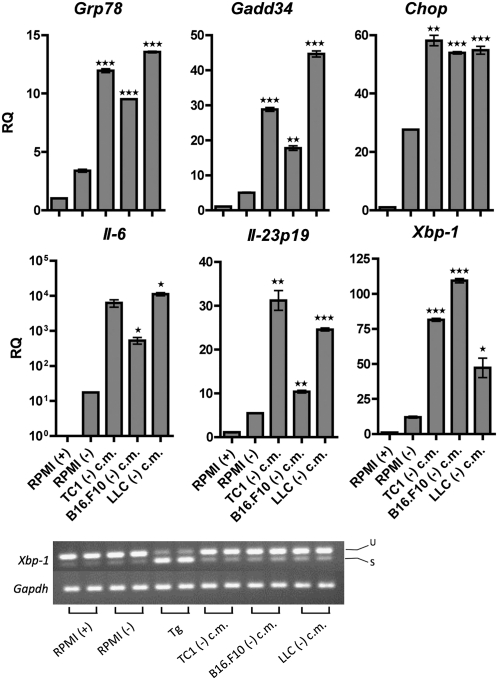
To validate this phenomenon, we then performed experiments to demonstrate that (i) it occurs in tumor cells other than TC1, and (ii) it occurs using a nonpharmacological physiological ER stressor. For the first case, we used the metastatic melanoma cell line B16.F10 and the Lewis lung carcinoma (LLC) cells. A comparable up-regulation of the ER stress and proinflammatory cytokine genes was readily observed in J774 macrophages cultured in ER stress-conditioned medium from either B16.F10 or LLC cells (Fig. S4 A and B). Similarly, CD86 up-regulation was observed in macrophages exposed to ER stress-conditioned medium from both cell lines (Fig. S4C). In the second case, all three cell lines were cultured for 24 h in medium lacking glucose, as glucose starvation is a condition demonstrated to elicit ER stress in the tumor microenvironment (19). J774 macrophages cultured in glucose-deficient tumor conditioned medium developed a robust ER stress response including activation of the PERK and IRE1α pathways, as evidenced by Chop, Gadd34, and Xbp-1 up-regulation (and splicing) as well as the transcriptional up-regulation of Il-6 and Il-23p19, compared with J774 macrophages cultured in glucose-deficient medium alone (Fig. 2).
Fig. 2.
Medium conditioned by tumor cells under physiological ER stress transmits ER stress and proinflammation to macrophages. J774 macrophages were cultured in conditioned medium of glucose-deprived tumor cells [TC1, B16.F10, LLC (-) c.m.], or culture medium with [RPMI (+)] or without [RPMI(-)] glucose for 24 h. RNA was isolated and analyzed by RT-qPCR for UPR activation and proinflammatory cytokine gene transcription. Columns indicate fold increase in transcript level (RQ) of each treatment group. The value of an RPMI (+) control was set arbitrarily to 1. Error bars represent SEM of two biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired, two-tailed t test. Test samples’ gene expression was compared with the gene expression of RPMI (-) cells.
Next, we determined that bone marrow-derived macrophages (BMDM) are also susceptible to “transmissible” ER stress. Similar to J774 macrophages, CD11b+ BMDM cultured in ER stress-conditioned medium experienced ER stress, and Il-6, Il-23p19, tumor necrosis factor-α (TNF-α) transcriptional up-regulation (Fig. S5A), as well as CD86 activation (Fig. S5B). BMDM cultured in tumor ER stress-conditioned medium also secreted large amounts of two inflammatory chemokines, macrophage inflammatory protein-1α (MIP-1α) and MIP-1β, which themselves are known to induce the synthesis and release of IL-6 and TNF-α (20) (Fig. 3D). Taken together, the results show that ER-stressed tumor cells release a soluble factor (or factors) that activates macrophages and initiates an ER stress response along with transcriptional activation and secretion of proinflammatory cytokines and chemokines.
Fig. 3.
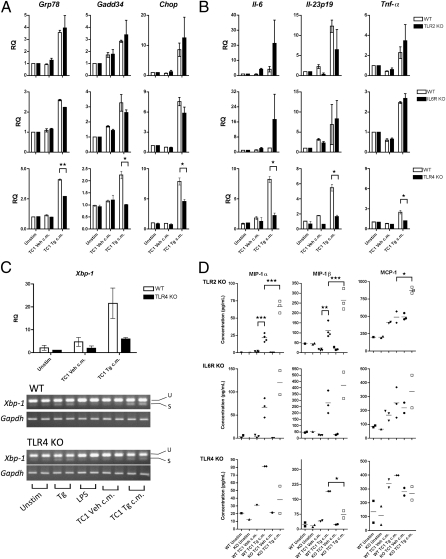
TLR4 senses transmissible ER stress, whereas TLR2 and IL6R do not. BMDM generated from WT (WT) C57BL/6 mice, TLR2 KO mice, IL6RKO, or TLR4 KO mice were cultured in ER-stressed conditioned medium from TC1 cells (TC1 Tg c.m.), control TC1 cells (TC1 Veh c.m.), or culture medium with or without (Unstim) LPS for 24 h. RNA was isolated from macrophages and analyzed by RT-qPCR for (A) UPR activation and (B) proinflammatory cytokine gene transcription. Columns indicate fold increase in transcript level (RQ) of each treatment group. For each genotype, the value of an unstimulated control was set arbitrarily to 1. Error bars represent SEM of two to four biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired, two-tailed t test. (C) Xbp-1 activation and splicing. u, Unspliced; s, spliced. (D) BMDM supernatants were tested for presence of chemokines using the Multiplex cytometric bead assay. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired, two-tailed t test.
Transmissible ER Stress Is Not Sensed by TLR2 or IL-6R.
To delineate the mechanism by which transmission of ER stress could occur in “receiver” macrophages, we considered Toll-like receptor 2 (TLR2) and IL-6 receptor (R). In the first case, we reasoned that a role for TLR2 was plausible for the following reasons: (i) TLR2 is selectively up-regulated under ER stress (16); (ii) TLR2 is induced in dendritic cells and monocytes under hypoxic conditions (21); and (iii) TLR2 mediates macrophage activation and IL-6 and TNF-α production in myeloid cells ligated by tumor cell-derived versican (6). BMDM from TLR2 KO mice treated with TC1 ER stress-conditioned medium did not down-regulate either the ER stress or the proinflammatory cytokine responses (Fig. 3 A and B). Of note, we found that TLR2 KO BMDM secreted increased amounts of MIP-1α, MIP-1β, and monocyte chemoattractant protein-1 (MCP-1) in response to the ER stress-conditioned medium relative to WT BMDM, suggesting that TLR2 may normally function as a negative regulator in response to proinflammatory stimuli (22, 23). Next, we turned our attention to the IL-6R based on the following: (i) both cancer cells and macrophages secrete IL-6, a proliferative antiapoptotic proinflammatory cytokine; (ii) IL-6 production can be amplified by autocrine/paracrine mechanisms via IL-6 receptor (IL-6R) signaling (2); and (iii) the supernatant of LLC cells up-regulates IL-6 production in myeloid cells (6). As shown in Fig. 3 A and B, IL-6R KO BMDM exposed to ER stress-conditioned medium show no decrease in the ER stress response and proinflammatory cytokine transcription compared with WT BMDM. Collectively, these results argue that neither TLR2 nor IL-6R is involved in sensing transmissible ER stress.
TLR4 Senses and Potentiates Transmissible ER Stress.
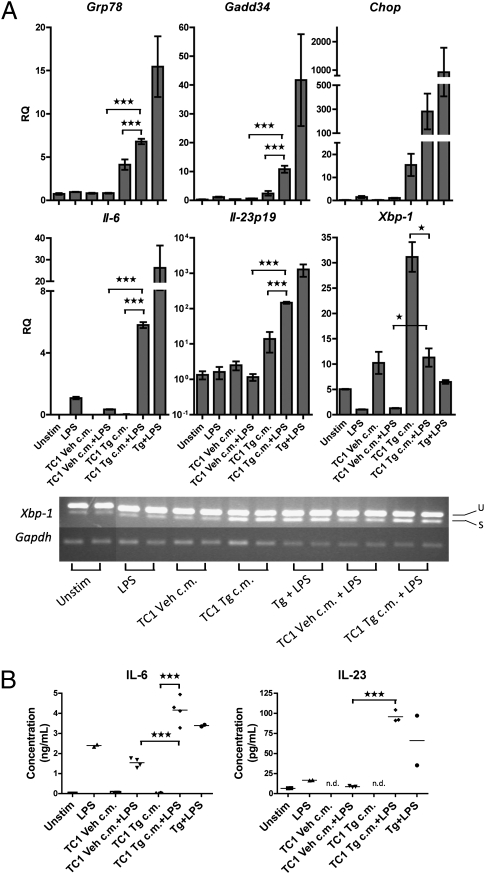
Next, we studied the response of J774 macrophages to transmissible ER stress in conjunction with activation by bacterial LPS, a canonical TLR4 activator. It was recently demonstrated that, in macrophages, the ER stress response and TLR4 signaling synergize to cause IL-6 and IL-23 production at levels greater than those observed after either signaling event alone (24, 25). Thus, we reasoned that receiver macrophages undergoing transmissible ER stress would experience a more rapid or greater ER stress and proinflammatory cytokine response if concomitantly stimulated with LPS. TC1 ER stress-conditioned medium plus LPS (0.1 μg/mL) caused accelerated up-regulation of the ER stress response genes Grp78, Gadd34, and Chop, as well as proinflammatory cytokine genes, which peaked at 6 h, compared with the ER stress-conditioned medium alone (Fig. 4A), whose maximal effect on transcription occurs at 24 h (Fig. 1A). Likewise, macrophages secreted increased amounts of IL-6 and IL-23 (Fig. 4B and Fig. S6). In agreement with Martinon et al. (24), who showed that UPR-mediated splicing of Xbp-1 is reduced by LPS treatment, we observed that Xbp-1 up-regulation and splicing were blunted in macrophages cotreated with TC1 ER stress-conditioned medium plus LPS compared with ER stress-conditioned medium alone (Fig. 4A). Taken together, these data suggest that, like canonical ER stress, transmissible ER stress in macrophages is potentiated by a second signal through TLR4.
Fig. 4.
TLR4 signaling potentiates the effect of transmissible ER stress on macrophages. Macrophages were cultured in either conditioned medium of ER-stressed TC1 cells (TC1 Tg c.m.) or control TC1 cells (TC1 Veh c.m.), with or without LPS (100 ng/mL), culture medium containing LPS or thapsigargin (Tg, 300 nM), or culture medium alone (Unstim) for 6 h. Macrophages cultured in medium containing Tg (300 nM) plus LPS serve as a positive control. (A) RNA was isolated from macrophages and analyzed by RT-qPCR for UPR activation and proinflammatory cytokine gene transcription. Columns indicate fold increase in transcript level (RQ) of each treatment group. LPS-treated control was set arbitrarily to 1. Error bars represent SEM of two to four biological replicates. *P < 0.05, ***P < 0.001, unpaired, two-tailed t test. u, Unspliced; s, spliced. (B) Supernatants from macrophages in A were analyzed by Multiplex cytometric bead array for presence of IL-6 and IL-23. n.d., Not detectable. ***P < 0.001, unpaired, two-tailed t test.
In light of these findings, we then tested the direct involvement of TLR4 in sensing transmissible ER stress. TLR4 KO BMDM treated with TC1 ER stress-conditioned medium showed decreased activation of the ER stress response and proinflammatory cytokine transcription compared with WT BMDM controls (Fig. 3 A–C). In addition, TLR4 KO BMDM secreted lower amounts of MIP-1α and MIP-1β (53% and 61%, respectively) (Fig. 3D). Of note, this diminished effect was not due to an intrinsic defect in the ability of TLR4 KO BMDM to mount a UPR, as these cells respond to Tg treatment comparably to WT BMDM (Fig. S7), a result consistent with the observation that TLR4 mutant (C3H/HeJ) BMDM undergo similar level of Xbp-1 splicing as TLR4 competent (C3H/HeOuJ) BMDM in response to tunicamycin (24). Thus, it appears that TLR4 may be involved both in sensing and potentiating transmissible ER stress in receiver macrophages.
Transmission of ER Stress in Vivo.
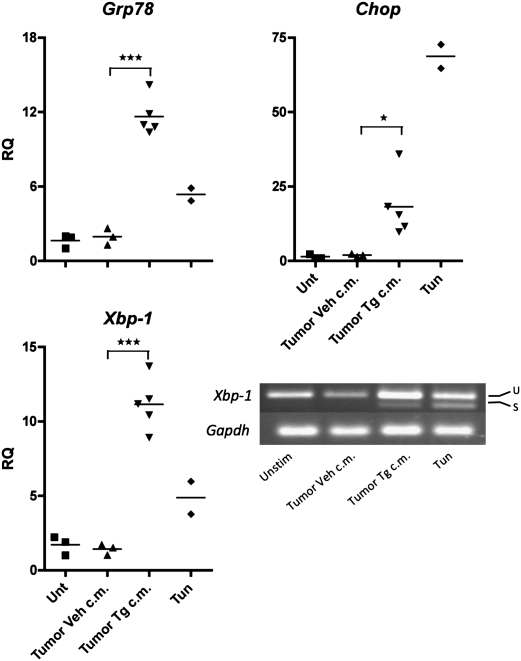
To validate the in vitro data in an in vivo context, we injected C57BL/6 mice with tumor ER stress-conditioned medium seeking the induction of ER stress in the liver, an organ highly sensitive to ER stress. Mice were injected with ER stress-conditioned medium from either TC1, B16.F10 or LLC cells to minimize the impact of cell line-specific factors. As shown in Fig. 5, all five mice given an i.p. injection of tumor ER stress-conditioned medium developed an ER stress response characterized by the up-regulation of Grp78, Chop, and Xbp-1 and spliced Xbp-1, which was not observed in naive mice or in mice injected with vehicle control medium. Taken together, these results indicate that tumor ER stress-conditioned medium is able to trigger the activation of several ER stress signaling pathways in vivo.
Fig. 5.
Tumor ER stress-conditioned medium elicits an ER stress response in vivo. 10× Conditioned medium of ER-stressed (Tumor Tg c.m.) or control (Tumor Veh c.m.) tumor cells in 1 mL was injected i.p into C57BL/6 mice. Tunicamycin-treated (Tun, 2 mg/kg) and untreated (Unt) mice served as controls. Two mice were given TC1 Tg c.m. or B16.F10 Tg c.m., respectively, and one mouse received LLC Tg c.m. Control conditioned medium derived from each cell line was injected into one mouse each (three). Livers were harvested 8 h after injection. RNA was isolated and analyzed by RT-qPCR for UPR activation. Columns indicate the fold increase in transcript level (RQ) of each treatment group. The value of a single untreated control was set arbitrarily to 1. *P < 0.05, ***P < 0.001, unpaired, two-tailed t test. u, Unspliced; s, spliced.
Discussion
The phenomenon described herein links tumor cells and macrophages in a new functional interplay that underscores the tumor cell's effort to seize control of myeloid cells in the tumor microenvironment, ostensibly leading to tumor growth and progression. We demonstrate that cancer cells under pharmacological or physiological ER stress (transmitters) can condition macrophages (receivers) to mirror the behavior of cancer cells (i.e., ER stress and transcriptional up-regulation of a tumorigenic, proinflammatory response). This scenario is plausible based on our demonstration that tumor ER stress-conditioned medium is active in vivo, inducing ER stress in the liver of injected mice.
Instructive models in biological systems such as “quorum sensing” in bacteria (26) and “infectious” transplantation tolerance in mice (27), both of which have effects on gene expression and cell regulation via wireless cell-to-cell communication, constitute precedents to our observation. Further support is provided by the observation that tumor cells under ER stress can secrete Par-4, which induces an ER stress response in neighboring tumor cells upon binding to surface Grp78 (28). Although the molecule(s) involved in this phenomenon remain elusive, initial studies suggest that the activity of the transmissible factor(s) is heat resistant, and may actually be potentiated by heat treatment (Fig. S8). Of note, we found that the transmissible factor(s) released by ER-stressed tumor cells is sensed by TLR4 and is also amplified through concomitant TLR4 signaling by LPS. This suggests that endogenous TLR4 ligands (oxidized phospholipids, tenascin-C, β-defensin, heat shock proteins, etc) (29) or infection by Gram-negative bacteria, could be cofactors in the tumorigenic effects of ER stress-initiated inflammation in the tumor microenvironment.
That transmissible ER stress conditions macrophages to a proinflammatory phenotype is relevant to a better understanding of the tumor–microenvironment interplay, and adds further complexity to the apparent paradox on the coexistence of pro-inflammation and anti-inflammation in the tumor microenvironment, which includes myeloid cells recruited to the tumor site that are believed to be prevalently anti-inflammatory/suppressive cells (30). However, tumor-associated macrophages can secrete inflammatory factors such as cytokines, chemokines, and metalloproteinases that promote tumorigenesis (6). In an attempt to distinguish receiver macrophages into M1 or M2 cells (31), we found that they up-regulate CD86 (Fig. 1D, Fig. S4C, and Fig. S5B), but fail to modulate CD64, CD16/32, or CD14 expression (Fig. S9A). Together with the up-regulation of Il-6, Il-23p19, and Tnf-α, these data suggest that the likely functional phenotype of receiver macrophages is that of inflammatory myeloid cells. This is consistent with the fact that they do not up-regulate transcription of Il-10 (Fig. S9B). Confirming their identity as tumor-associated macrophages, receiver macrophages up-regulate arginase (31) transcription (although variably) (Fig. S9C). Relevant to our finding is the fact that macrophages infiltrating adipose tissue, where stromal adipocytes experience high levels of ER stress, mount a proinflammatory response (32).
Finally, it is tempting to suggest that the phenomenon described herein might contribute to negative regulation of natural antitumor T-cell responses as implied elsewhere (33). We note that the expression of Il23p19, which is increased in human cancer tissues and was found to inhibit cytotoxic T-cell function (34), also inhibits innate NK-mediated antitumor immunity (35), and is associated with the plastic differentiation of CD4 T cells into FoxP3+/IL-10–secreting regulatory T cells, which suppress antitumor T-cell responses (36). Furthermore, IL-6 and IL-23 are important factors in the differentiation and maintenance of Th17 cells, which are considered to contribute to inflammation in the tumor microenvironment. In conclusion, our studies suggest that ER stress in tumor cells may take its tumor-promoting toll by priming macrophages to recapitulate, amplify, and expand inflammation. Because it appears that the tumor cell ER stress response is the driver of this process, tumor cell ER stress signaling components and/or the molecule(s) mediating transmissible ER stress may serve as attractive therapeutic targets for broad-spectrum anticancer therapy.
Materials and Methods
Mice, Preparation of BMDM, and Cell Culture Procedures.
C57BL/6 mice were purchased from Charles River and housed at the Moores Cancer Center Animal Facility. TLR2 KO and TLR4 KO mice were the kind gift of M. Karin and M. Corr, respectively (University of California at San Diego) with permission from S. Akira. All mice were handled in accordance with University of California at San Diego Animal Subjects Program Guidelines. Femurs, tibias, and fibulas from IL6R−/− mice (37) were contributed by A. F. Drew. Bone marrow cells were flushed from the femurs and cultured for 7 d in 30% L929 cell-conditioned medium (38) to obtain bone marrow-derived macrophages (BMDM). The yield of CD11b+ macrophages was >95%. TRAMP-C1, B16.F10, and LLC cells were originally obtained from A. Weinberg (Providence Portland Medical Center), D. Carson, and J. Varner (University of California at San Diego), respectively. J774 cells were a kind gift from A. Timmer and V. Nizet (University of California at San Diego). All lines were grown under standard conditions in RPMI 1640 medium or DMEM (Mediatech) supplemented with Hepes buffer, l-glutamine, sodium pyruvate, MEM nonessential amino acids (Gibco), penicillin/streptomycin, 2-mercaptoethanol and 10% FBS (HyClone: Lot No. KSJ30470 with an LPS content < 0.06 EU/mL by LAL test) at 37 °C in 5% CO2 atmosphere, except for addition of conditioned media. All cells were routinely tested for mycoplasma using the luminescence-based MycoAlert kit (Lonza), and were confirmed to be mycoplasma free.
Conditioned Media.
ER stress-conditioned medium was generated as follows. Briefly, tumor cells were induced to undergo ER stress by culture in 300 nM thapsigargin (Tg) (>99% pure by HPLC; Alexis Chemicals) for 2 h. Control cells were similarly treated with an equal volume of vehicle (100% ethanol). Cells were washed 2× with Dulbecco's PBS (Mediatech), and then incubated in fresh medium for 16 h. Conditioned medium was centrifuged for 10 min at 2,000 rpm and then filtered through a 0.22-μm filter (Millipore). Conditioned medium from apoptotic or control cells was generated from TC1 cells treated with 100 nM staurosporine (Sigma) or vehicle (DMSO), respectively. To generate glucose-deprived tumor conditioned medium, tumor cells were cultured for 24 h in basal RPMI 1640 or DMEM lacking glucose supplemented with 10% dialyzed FBS (10,000 MWCO; HyClone), l-glutamine, and penicillin/streptomycin. Conditioned medium was harvested, centrifuged at 2000 rpm for 10 min, and passed through a 0.22-μm syringe filter (Millipore) before use. Bacterial LPS (Sigma, catalog no. L2654) was used at a final concentration of 100 ng/mL. For heat inactivation experiments, basal medium and tumor cell conditioned media were heated to 37 °C, 56 °C, or 95 °C for 30 min. Before addition to macrophages, heated media were briefly cooled on ice to room temperature.
Injection of Tumor ER Stress-Conditioned Media in Vivo.
A 10-mL quantity of Tg-conditioned medium or EtOH vehicle control medium from TC1, B16.F10, and LLC tumor cells, was concentrated by lyophilization for 24–36 h, resuspended in 1 mL sterile, DNA/RNA-free water (Mediatech). C57BL/6 mice 14–18 wk of age were injected i.p. with 10× tumor ER stress-conditioned medium, 10× vehicle control medium, or tunicamycin (2 mg/kg) in a final volume of 1 mL dPBS. Eight hours later, mice were killed, and livers surgically removed and passed through a 40-μm cell strainer. Liver samples were washed once in dPBS, aliquoted for RNA isolation, and gene expression quantified by RT-qPCR as described below.
RT-qPCR.
RNA was isolated from cells using the RNeasy (Qiagen), RNeasy Plus (Qiagen), or Nucleospin II kits (Macherey-Nagel). Concentration and purity of RNA was determined by analysis on a NanoDrop spectrophotometer (Thermo Scientific). cDNA was obtained using the High Capacity cDNA Synthesis kit (Life Technologies/Applied Biosystems), and RT-qPCR was performed on an ABI StepOne system using TaqMan reagents for 50 cycles using universal cycling conditions. Target gene expression was normalized to β-actin, and analyzed using the –ΔΔCt relative quantification method. Validated FAM-labeled mouse Il-23p19(a), Il-6, Tnf, Ddit3 (Chop), Myd116 (Gadd34), Hspa5 (Grp78), Arg1, and VIC-labeled mouse β-actin TaqMan primer/probe sets (Life Technologies/Applied Biosystems) were used. Mouse Xbp-1 FAM-labeled qPCR probe/primer sets was obtained from Integrated DNA Technologies. For gel-based PCR, Xbp-1 amplicons were amplified using the following cycling conditions: 96 °C for 2 min, followed by 30 cycles of 96 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, and a 1-min 72 °C extension, resolved on a 2.5% agarose gel, and imaged using a BioRad GelDoc system. Primers are listed in Table S1.
Flow Cytometry.
Single cell suspensions were stained with fluorochrome-labeled anti-CD86 (BD Pharmingen, clone GL1), anti-CD64 (BD Biosciences, clone ×54-5/7.1), anti-CD16/32 (BD Biosciences, clone 2.4G2), anti-CD14 (BD Biosciences, clone rmC5-3), anti-CD11b (eBioscience, clone M1/70), and Annexin V (BD Pharmingen) antibodies, or appropriate isotype controls. Viability was determined by 7-AAD (CalBiochem) exclusion. A FACSCalibur cytometer (Becton Dickinson) was used for acquisition of data, and CellQuest Pro (BD Biosciences) and FlowJo software (Tree Star) were used for data analysis.
Multiplex Cytometric Bead Assay.
BD CBA Flex set assays were used to measure mouse IL-6, IL-23, TNF-α, MIP-1α, MIP-1β, and MCP-1 (BD Pharmingen). Following acquisition of sample data using a BD FACSArray bioanalyzer flow cytometer, the sample results are generated in graphical and tabular formats using the FCAP Array Software.
Supplementary Material
Acknowledgments
The authors thank Vicky Chau and Stephanie Widmann for expert assistance in developing and performing the multiplex cytometric bead array assay for this paper. This work was supported in part by a grant from the University of California at San Diego (UCSD) Academic Senate. N.R.M. acknowledges support from the UCSD Medical Scientist Training Program and National Institute on Drug Abuse (NIDA) T32 Training Grant DA007315-07A2.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008942108/-/DCSupplemental.
References
- 1.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pikarsky E, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 4.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 8.Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 9.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: Friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 11.Bi M, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daneshmand S, et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, et al. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Ramirez L, et al. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl Oncol. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodall JC, et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci USA. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler MC, et al. KDEL-retained antigen in B lymphocytes induces a proinflammatory response: A possible role for endoplasmic reticulum stress in adaptive T cell immunity. J Immunol. 2008;181:256–264. doi: 10.4049/jimmunol.181.1.256. [DOI] [PubMed] [Google Scholar]
- 17.Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahadevan NR, Fernandez A, Rodvold J, Almanza G, Zanetti M. Prostate cells undergioing ER stress in vitro and in vivo activate transcription of pro-inflammatory cytokines. J Inflam Res. 2010;3:99–103. doi: 10.2147/JIR.S11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiotto MT, et al. Imaging the unfolded protein response in primary tumors reveals microenvironments with metabolic variations that predict tumor growth. Cancer Res. 2010;70:78–88. doi: 10.1158/0008-5472.CAN-09-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Driscoll KE. Macrophage inflammatory proteins: Biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- 21.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benson SA, Ernst JD. TLR2-dependent inhibition of macrophage responses to IFN-gamma is mediated by distinct, gene-specific mechanisms. PLoS ONE. 2009;4:e6329. doi: 10.1371/journal.pone.0006329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puliti M, Uematsu S, Akira S, Bistoni F, Tissi L. Toll-like receptor 2 deficiency is associated with enhanced severity of group B streptococcal disease. Infect Immun. 2009;77:1524–1531. doi: 10.1128/IAI.00965-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLay ML, et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–2643. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 27.Qin S, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 28.Burikhanov R, et al. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377–388. doi: 10.1016/j.cell.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erridge C. Endogenous ligands of TLR2 and TLR4: Agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 30.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 32.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: Modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci USA. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langowski JL, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 35.Teng MW, et al. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci USA. 2010;107:8328–8333. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kortylewski M, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFarland-Mancini MM, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Goncalves R, Mosser DM. Unit 14 11. The isolation and characterization of murine macrophages. Current Protocols in Immunology. November 1, 2008 doi: 10.1002/0471142735.im1401s83. 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.