Abstract
The aim of the study was to develop a new strategy for the differentiation of hematopoietic stem cell (HSC) derived from UCB into hepatocyte like cells and also to estimate the effects of combination of fibroblast growth factor 4 (FGF 4) and hepatocyte growth factor (HGF) on hematopoietic stem cell differentiation. HSCs were isolated and purified by magnetic activated cell sorting. HSCs were induced to hepatocyte like cells under a 2-step protocol with combination of growth factors. Reverse transcription polymerase chain reaction was performed to detect multiple genes related to hepatocyte like cells development and function. Hepatocyte like morphology was illustrated by inverted repeat microscope and the secretion of albumin and α- fetoprotein by these cells was confirmed by enzyme linked immunosorbent assay. Hepatocyte like cells was observed at the end of the protocol (days 14). These differentiated cells were observed to show high expression of genes related to hepatocytes (tryptophan 2, 3-dioxygenase [TO], glucose 6-phosphate [G6P], cytokeratin 18 [CK 18], albumin and α- fetoprotein [AFP]). The quantities of albumin and AFP at day 0 were low and upon differentiation the cells were able to produce albumin and AFP at high levels. Our results show a new strategy for differentiation in a short duration, using a combination of growth factors for the differentiation of umbilical cord blood derived HSC into hepatocyte like cells under certain in vitro conditions. After further studies this approach has the potency, for widespread cell replacement therapy for liver diseases.
Keywords: Growth factors, Hematopoietic stem cell, Hepatocyte, Trans-differentiation, Umbilical cord blood
Introduction
Most liver diseases lead to hepatocyte dysfunction with the possibility of eventual organ failure. In India, mortality rate due to liver disease is high, 60% of patients admitted to the gastroenterology departments are with liver diseases. The most common liver diseases are fibrosis, cirrhosis, hepatitis, cancer, etc., occurring as a result of viral infection or alcohol abuse (Trey and Davidson 1970). The liver is a quiescent organ and the adult liver can regenerate by hepatocytes reentering cell cycle after surgical resection or injury. In several cases of liver injury, the proliferative capacity of liver cells is not sufficient to successfully restore organ function. In such situations, hepatocyte progenitor cells and stem cell of intrahepatic and/or extrahepatic organ may come into play in organ regeneration (Qin et al. 2004).
Orthotropic liver transplantation has proven to be effective in the treatment of a variety of life-threatening liver diseases; however, significant morbidity and mortality remains. In addition, the growing disparity between the number of donated organs and the disproportionately large number of patients awaiting transplantation has provided an impetus for developing alternative therapies for the treatment of liver failure. Novel strategies designed to increase the number of organs transplanted, such as the use of adult living donors, are not without significant risk to both the donor and recipient (Fox and Chowdhury 2004). The replacement of diseased hepatocyte and the stimulation of endogenous or exogenous regeneration by stem cells are the main aims of liver-directed cell therapy. There is growing evidence to suggest that reservoirs of stem cells may reside in several types of adult tissue (Visconti et al. 2006). These cells may retain the potential to trans-differentiate from one phenotype to another one, presenting exciting possibilities for cell therapy.
Within an adult tissue, locally resident stem cells were formally considered to be capable of only giving rise to the cell lineage(s) it is normally present in. However, adult hematopoietic stem cells (HSCs) in particular appear to be much more flexible; removed from their usual niche, they are capable of differentiating into all types of tissues including skeletal, cardiac, muscle, endothelia and a variety of epithelia including neuronal cells, pneumocytes and hepatocytes. Some hepatocytes were first revealed to be derived from circulating bone morrow cells in the rat (Forbes et al. 2002). There are three sources of HSC that are routinely used for medical treatments, and they are: the bone marrow of an adult person, the peripheral blood of an adult person and the umbilical cord blood of a newborn baby. As a source of HSC for regenerative medicine, cord blood (normally discarded) has certain advantages over bone marrow and peripheral blood. Umbilical cord blood (UCB) contains a high concentration of highly proliferative HSC and there are no ethical problems for basic studies and clinical application (McAdams et al. 1996). UCB cells can be collected without any harm to the newborn infant and it is immediately available for transplantation (Bromeyer 1995), having a lower rate of infection with cytomegalovirus. Stem cells in UCB are less mature than those in bone marrow and peripheral blood cells and they carry much lower incidence of graft verses host disease (GVHD).
Based on these pervious findings, the aim of this study was to demonstrate that UCB derived HSC can be differentiated into hepatocyte like cells in vitro using a 2-step protocol with combination of growth factors. Step 1—conditioning step: L-DMEM + EGF + bFGF for 2 days) and step 2—differentiation and maturation step: H-DMEM + HGF + FGF 4 + dexamethasone for 14 days. It is important to note here that our study presents a short protocol for hepatic cell differentiation of about 14 days as compared to 21 days mentioned in earlier studies. This study provides support for continuing efforts utilizing UCB stem cells as a steady and renewable source of hepatocytes for cell based therapy. Moreover, the response to inductive extracellular signals and the role of growth factor in the differentiation process in vitro have been revealed.
Materials and methods
Collection of umbilical cord blood
Human UCB was obtained from local government hospital in Tamilnadu, India. Blood was collected from the umbilical cord vein with informed consent of the mother. A bag system containing 17 mL of anticoagulant (citrate, phosphate and dextrose) was used. All UCB units were processed within 3 h after delivery (Feng et al. 2008).
Isolation of mononuclear cells
UCB mononuclear cells (MNCs) were prepared from 40 to 50 mL UCB by density gradient separation using lymphocyte separation medium (1.077 g/mL). Cells were centrifuged at 400 × g for 30 min at room temperature (RT). The MNCs at the interface were washed with phosphate-buffered saline (PBS) and resuspended in PBS containing 2 mM EDTA (Yu et al. 2007).
Cell labeling and magnetic cell sorting
The portion of MNCs was further purified using magnetic activated cell sorting (MACS). Briefly, 1 × 106 cells were suspended in a final volume of 80 μL MACS (Miltenyi Biotech) buffer and labeled with 20 μL of microbeads with FITC (fluorescein isothiocyanate) conjugated mouse anti-human CD34 antibodies (QBEND/10). The cells were mixed well and incubated at 4 °C for 15 min in dark. After incubation the cells were washed thrice with 500 μL of MACS buffer by spinning at 300 × g for 10 min. The cells were resuspended in 500 μL of buffer and used for magnetic sorting. The column was washed with 500 μL of MACS buffer. The magnetically labeled cells were passed through the column. The cells with magnetic microbeads are retained within the column and those that are unlabelled will elute out. The eluted fraction was collected as negative fraction. The column was washed thrice with 500 μL of MACS buffer. Then the column was removed from the magnetic field. The retained cells in the column were firmly flushed out by applying pressure on the matrix of the column by a plunger supplied with the kit. These were the positive fractions which were washed twice with MACS buffer by spinning at 300 × g for 5 min and resuspended in 500 μL of MACS buffer.
Flow cytometric analysis of hematopoietic stem cells
Flow cytometric analysis was performed with a FACS (fluorescent activated cell sorting) caliber flow cytometer. Cells were resuspended in 1 × 106 in 200 μL PBS and incubated with respective conjugated antibodies, using isotype-matched controls (BD Biosciences). The ratio of fluorescence signals versus scatter signals were calculated by the EPICS XL/MCL flow cytometer (Beckman Coulter) this analysis was carried out after the initial samples were obtained from MACS (Feng et al. 2008).
Cell culture and hepatocyte differentiation
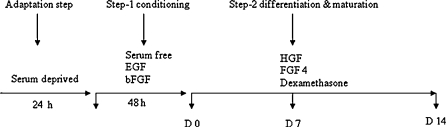
CD34+ cells (HSC) were suspended in DMEM (Sigma, St. Louis, MO, USA) supplemented with 100 mL/L FCS, 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were plated at a final concentration of 1 × 106 cells/mL. The culture at 85% confluency was used for differentiation assays. The cells were serum deprived for 24 h and pre-cultured in DMEM supplemented with 2 ng/mL EGF (Sigma, St. Louis, MO, USA) and 10 ng/mL bFGF (Sigma, St. Louis, MO, USA) (conditioning step), to stop the proliferation prior to induction of differentiation toward a hepatic phenotype. Then a second step differentiation and maturation of hepatocyte was performed by culturing in H-DMEM supplemented with 10 mL/L FBS, 20 ng/mL HGF (Sigma, St. Louis, MO, USA), 10 ng/mL FGF-4, 1 μmol/L dexamethasone to achieve cell differentiation and maturation for up to 14 days (Fig. 1). Medium was changed twice weekly and medium was collected at days 2, 4, 6, 8, 10 and 14 then stored at −20 °C for estimation of albumin and α-fetoprotein assay (Visconti et al. 2006).
Fig. 1.
Hepatic differentiation protocol by sequential addition of exogenous factors. Passage to cultures at 85% of confluency were used for differentiation assays. Cells were serum deprived for 24 h. After that cells were pre-cultured in serum free medium supplemented with EGF and bFGF for 2 days (condition step), then cells were cultured in medium supplemented with HGF, FGF4 and dexamethasone for 14 days (differentiation and maturation). Medium was changed twice a week and hepatic differentiation was assessed at different time points
Cell viability assay
Trypan blue dye exclusion test was done by the method of Rosenberg et al. (1978). The test was based on the exclusion of trypan blue by viable cells, whereas dead cells are stained by this dye. Trypan blue solution and cell suspension were mixed in the ratio of 1:1. Then the cells were observed under microscope and counted. The percentage of viable cells was calculated as the number of viable cells divided by total number of cells (viable + dead cells) ×100.
Cytotoxicity assay
MTT assay was done as described by Mossman (1983). The reaction involves the conversion of tetrazolium salt (3- [4,5-dimethylthiozol-2yl]-2,5-dephenyl tetrazolium bromide), a pale yellow substrate to formazan which is a dark blue product, by the active mitochondria in living cells only. This was dissolved in isopropanol and the absorbance was measured spectrophotometrically at 540 nm. Cells were taken at a concentration of 1 × 106 cells/mL, and centrifuged at 1,000 rpm. The supernatant was removed and the cell pellet was incubated with 3 mL of MTT reagent at 37 °C for 2 h. The cells were again centrifuged and 1 mL of isopropanol was added to the cell pellet and incubated at room temperature for 20 min. They were again centrifuged and the purple color supernatant was transferred to a cuvette and read at 540 nm. The amount of formazan formed was expressed as μM/106 cells.
Functional assessment of differentiated cells
Cell morphology changes were investigated under microscope. Hepatic gene marker (TO, ALB, AFP, G6P and CK-18) expression was detected with reverse transcription polymerase chain reaction (RT–PCR), and albumin and α-fetoprotein were detected with enzyme linked immuno-sorbant assay (ELISA).
Enzyme-linked immunosorbent assay (ELISA)
The amounts of Human albumin and α-fetoprotein (AFP) secretion into the medium were measured by enzyme-linked immunosorbent assay. Standard human albumin and AFP, goat anti-human antibody, mouse anti-human antibody and peroxidase-conjugated goat immunoglobulin G fraction to human albumin, horseradish peroxidase-conjugated mouse monoclonal anti-AFP antibody were purchased from Omega Diagnostics Limited. Microplates were precoated with anti-human-albumin and AFP antibody (4 μg/mL) in PBS by incubating overnight at 4 °C. The buffer containing the unbound antibodies was drained from the plate and the wells were washed four times with PBS containing 0.05% Tween-20. The unbound sites on the wells were blocked by incubation of 200 μL of a block solution (2% (w/v) milk powder in PBS) in each well for 2 h at room temperature. The wells were then washed as described above. Human albumin and AFP standard was diluted with PBS buffer containing 0.05% Tween-20 and 0.1% milk. An aliquot (100 μL) of these human albumin standards was added to each well in duplicate and samples of media 100 μL) were added to test wells. The plates were incubated for 1 h at room temperature. The wells were the washed as described above. PBS containing polyclonal anti-human-albumin (3 μg/mL) and AFP antibody (3 μg/mL) conjugated with horseradish peroxidase were added to each well and incubated for 1 h, and the wells were washed again. Colour development was started by adding 100 μL of substrate solution prepared freshly [3, 3′, 5, 5′- tetramethylbenzidine (0.2 mg/mL) and H2O2 (0.3 mg/mL) in 0.1 M Na2HPO4 and citric acid buffer, pH 4.3]. The reaction was stopped by adding 100 μL of 1 M H2SO4, after which the absorbance was determined at 450 nm with a 96 well plate ELISA reader (Bio Rad).
RNA extraction and RT–PCR analysis
Total RNA was prepared from the undifferentiated and differentiated HSC by TRIzol (Acid guanidinium thiocyanate-phenol–chloroform) method followed by DNase treatment (Chomaczynski and Sacchi 1987). One entire plate of cells was used for each isolation of total RNA. Gene expression level of AFP, ALB, TO, CK18 and G6P were determined by RT–PCR. Reverse transcription was carried out using 1 μg total RNA, 20 mmol/L dNTP and 100 unit reverse transcriptase in a total volume of 20 μL. PCR was carried out using 2 μL cDNA in a total volume of 20 μL. PCR products were analyzed in 2% agarose gel. The name and sequences of the primers, cycling conditions and annealing temperature for each pair are listed in Table 1.
Table 1.
Primers for RT–PCR
| Genes | Sense | Antisense |
|---|---|---|
| ALB | GCTTTGCCGAGGAGGGTAA | GGTAGGCTGAGATGCTTTTAAATG |
| CK 18 | TGGTACTCTCCTCAATCTGCTG | CTCTGGATTGACTGTGGAAGT |
| TO | ATACAGAGACTTCAGGGAGC | TGGTTTGGGTTCATCTTCGGTATC |
| G6P | GCTGGAGTCCTGTCAGGCATTGC | TAGAGCTGAGGCGGAATCGGAG |
| AFP | TGCAGCCAAAGTGAAGAGGGAAGA | CATAGCGAGCAGCCCAAAGAAGAA |
Conditions Initial denaturation at 95 °C for 4 min. followed by 40 cycles of 94 °C, 1 min; 56 °C, 30 s: 72 °C, 1 min; A final extension at 72 °C for 10 min
Statistical analysis
The results were expressed as mean ± standard deviation (SD). The statistical significance of difference was assessed by the student’s t test. A value of p < 0.05 was considered statistically significant.
Results
Purification of hematopoietic stem cells
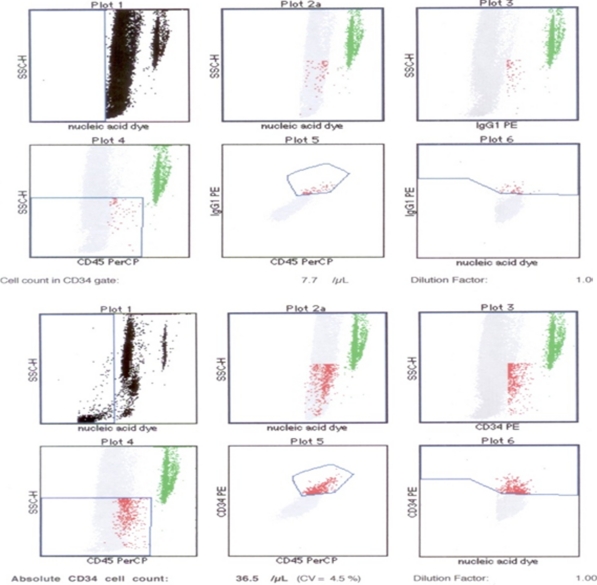
Hematopoietic and hepatic stem cells share characteristic markers such as CD34, c-kit, and thy1. CD45 antigen also expressed on the HSCs except for some mature cell types. Cells expressing CD45 and CD34 are well documented in HSCs and CD45+, CD34- cells are probably less mature HSCs. Based on the recent observations that hepatocytes may originate from hematopoietic stem cells, we investigated the potential of CD34+ umbilical cord hematopoietic cells in vitro. CD34+ cells were isolated from UCB MNC fractions by incubation with CD34 microbeads, followed by sequential passages through two MiniMACS columns. Fluorescence-activated cell sorting analysis with anti-CD34 antibody was performed to determine the percentage purity of the positive fraction. The result showed that CD34+ cells were enriched after magnetic cell sorting (36.5 ± 3 cells; Fig. 2).
Fig. 2.
Flow cytometric analysis of haematopoietic stem cell surface antigen (CD34) in the UCB derived HSC was performed using the labelled antibody anti-CD34 or control IgG1 as indicated. The fluorescence of the conjugated monoclonal antibodies (anti CD34-PE and IgG1-PE) as well as side scatter signals were measured on dot plot. The ratios were calculated by the EPICS XL/MCS flowcytometer. The upper 6 panel represent the CD34+ cell population (1%) in the control sample (before MACS). The lower 6 panel represent the CD34+ cell population enriched after MACS was 4.5% on the average
In vitro hepatic differentiation of cord blood-derived hematopoietic stem cells
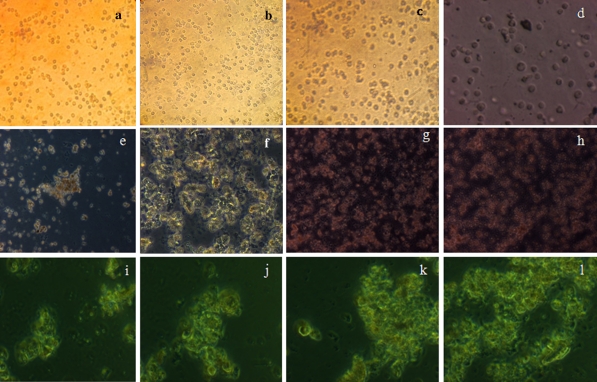
We analysed the morphological changes of UCB HSC during differentiation protocol stages in order to evaluate the effect of combining growth factors and hormone. When cells were precultured for 48 h in serum-free medium supplemented with EGF and bFGF, cell proliferation stopped. Cells before differentiation (day 0) exhibited an oval-like morphology (Fig. 3a). Cell morphology of UCB HSC did not change significantly during conditioning step-1, when cultures were treated with HGF, although the morphology was lost and cells developed a broadened flattened shape. However, a polygonal shape developed during differentiation step-2 when cells were exposed to medium containing FGF4 and dexamethasone (Fig. 3b–l). The protocol used includes the sequential addition of exogenous factors that have been reported to be implicated in liver development and proved to be effective to induce the hepatic differentiation of human HSC from UCB.
Fig. 3.
Morphology of UCB derived HSC differentiation protocol. Cells were induced to differentiate by using sequential addition of growth factors and hormone. Morphology of passaged HSCs, no significant morphological changes were observed upto day 6 (a–f). However HSCs significantly changed the morphology and developed a polygonal shape during the step-2 differentiation (g-l). (original magnification ×10 for all pictures)
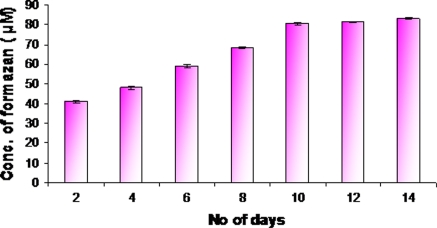
Cytotoxicity assay
The viability of cells was estimated by using the TBE assay. The result thus obtained revealed that the viability of the cells using TBE was significantly high. Further, attempts to ascertain the functional viability of the cells was studied by MTT assay, and the results showed that the mitochondrial activity increased in the 14 days culture compared to previous day’s culture (Fig. 4).
Fig. 4.
Cytotoxicity assay was performed by MTT assay, to analyse the effect of growth factor during differentiation of UCB-HSC into hepatocyte like cells (Day 0–Days 14)
Expression of hepatic marker protein analysis by ELISA
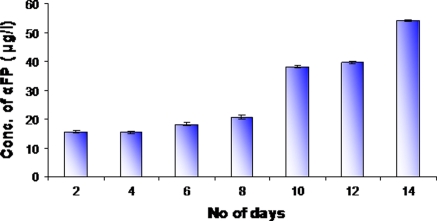
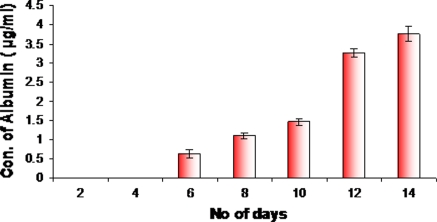
We examined the expression of hepatic protein markers such as albumin and α-fetoprotein by ELISA. Albumin and α-fetoprotein are the functional markers characteristic of liver cells and used to determine the population of hepatic cells. We found that AFP could be detected throughout the differentiation process because the medium contained a low level of α-fetoprotein. From days 12 the level of AFP increased significantly compared to control (Fig. 5), suggesting that HSCs could be trans-differentiated into hepatocyte like cells. It has been previously reported that AFP was produced by immature hepatocytes. That is to say, hepatocytes are immature on days 12. Before days 6 the level of albumin could not be measured because the amount of albumin was too low in the medium. When HSCs were differentiated to hepatocyte like cells albumin secretion started. Albumin expression started only after 6 days of culturing, and compared to days 6 the albumin level was increased on days 14 (Fig. 6). The presence of albumin is a prominent feature of mature hepatocytes, as liver is the predominant site for the synthesis of albumin protein.
Fig. 5.
AFP concentrations were measured by ELISA during the whole culture duration (day 0–day 14). Basal AFP secretion of preinduced HSC was low, but that of induced cells was dramatically increased
Fig. 6.
Albumin concentrations were measured by ELISA in cultures during the day 0–day 14 period. Basal albumin secretion of preinduced HSC could not be measured because the amount of albumin was too low in the medium (Day 0–Day 4), but that of induced cells was dramatically increased
RT–PCR analysis of hepatic gene expression of UCB differentiated cells
To determine whether differentiated cells show the characteristic expression of hepatic phenotype markers, total RNA from UCB HSC was isolated at day 0 and days 14 of the differentiation protocol and the mRNA levels of several hepatic genes were examined by RT–PCR. Undifferentiated cells were used as controls (day 0 of the differentiation protocol). RT–PCR analysis showed the expression of ALB and G6P by days 14. CK 18, AFP and TO was detected at both time points (Day 0 and days 14) and increased with the time of differentiation (Fig. 7), whereas undifferentiated cells did not express ALB, G6P but did express low levels of AFP and CK 18, and TO (data not shown).
Fig. 7.
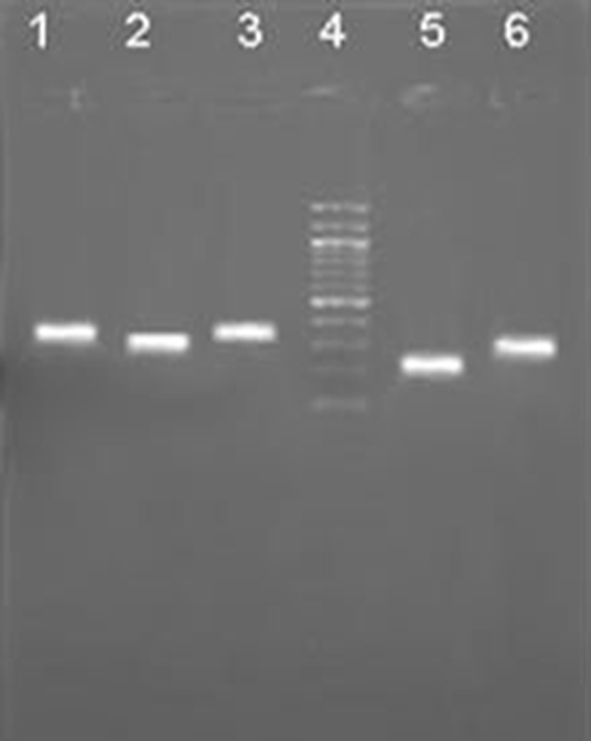
Gene expressions during UCB-HSCs differentiation. Lane 1: TO (299 bp); Lane 2: ALB (265 bp); Lane 3: G6P (350 bp); Lane 4: DNA marker; Lane 5: AFP (157 bp); Lane 6: CK 18 (271 bp)
Discussion
Over recent years, stem cells have generated great interest given their potential therapeutic use. HSCs obtained from cord blood have been shown to be a suitable alternative to adult bone marrow or peripheral blood in transplants for the treatment of leukemia, lymphoma, aplastic anemia, and inherited disorders of immunity and metabolism (Talens et al. 2006). It has been reported that umbilical cord blood cells (UBCs) contain many more hematopoietic stem/progenitor cells than peripheral blood (Nakahata and Ogawa 1982; Broxmeyer et al. 1989; Gluckman et al. 1989; Mayani and Lansdorp 1998; Glimm et al. 2002). In the clinic, umbilical cord blood (UCB) is transplanted into patients with various hematopoietic diseases and the therapeutic effect of the transplantation is widely recognized, with a relatively low incidence of graft versus host disease (Wagner et al. 1995; Kurtzberg et al. 1996). Therefore, UBCs may become a hopeful candidate for a hepatocyte progenitor source like BMCs. It has been reported that CD34+ hematopoietic stem cells and C1qRp-positive hematopoietic stem cells, which were isolated from UCB, differentiated into hepatocytes after transplantation into mouse recipients (Danet et al. 2002; Wang et al. 2003; Di Campli et al. 2004). As for UCB, CD34+ cells isolated from bone marrow were differentiated into hepatocytes in a culture containing HGF and FGF2. On the other hand, Ishikawa et al. (2003) showed that a CD45+ subpopulation of UBCs was capable of generating hepatocytes. Taken together, not only hematopoietic stem cells, but also some progenitor cells derived from hematopoietic stem cells may contribute to the cellular reconstitution. In this context, Lee et al. (2004) reported that mesenchymal stem cells isolated from UCB are capable of differentiating into functional hepatocytes in vitro.
In this study we have investigated the induction to the hepatogenic differentiation of human umbilical cord hematopoietic stem cell. Seo et al. (2005) first showed that adipose stem cell (ADSC) can be differentiated into hepatocyte-like cells by treatment with cytokine mixtures (HGF and OSM) and DMSO in serum-free medium. However, we have used a 2-step differentiation protocol with a sequential addition of growth factors (EGF, bFGF and FGF4) and hormone (dexamethasone), which has been reported to be involved in the development and differentiation of hepatocytes (Lazaro et al. 2003; Sakai et al. 2002). The choice of exogenous factors and the time course to induce hepatogenic trans-differentiation are based on previous reports on BMSC differentiation (Lee et al. 2004). As previously mentioned, HGF plays an essential role in the development and regeneration of the liver (Wang et al. 2004). The differentiation of BMSCs into hepatocyte-like phenotypes in vitro by induction with HGF has been reported earlier (Lazaro et al. 2003; Sakai et al. 2002). Other reports showed differentiation of bone marrow derived MAPC toward hepatocyte-like cells induced by FGF4, however the degree of differentiation was higher when cells were also treated with HGF. This is consistent with the fact that FGF4 may play a role in endoderm specification (Oh et al. 2000) and that HGF induces differentiation of hepatocytes that are not actively proliferating. The bFGF is required to induce a hepatic fate in the foregut endoderm, whereas dexamethasone significantly enhances the in vitro maturation of fetal liver cells (Wang et al. 2004; Wells and Melton 2000).
In the present study the morphologic features and gene expression changes in HSC were evaluated. Under hepatogenic conditions, the morphology of HSCs gradually progressed toward the polygonal morphology of hepatocytes in a time-dependent manner and became apparent by days 14 postinduction. However, the mature cuboidal morphology with granulated structures is not fully developed until d 8 post-induction. Finally, the role of key hepatic growth factors in the regulation of the trans-differentiation process has been investigated using HGF and FGF4. The results show that HSCs are capable of giving rise to a hepatogenic trans-differentiation in response to a sequential addition of growth factors, assessed by an examination of morphology and hepatocyte-specific markers. It should be highlighted that we have established the culture conditions of human UCB HSC, as well as the differentiation protocol under adequate conditions for a suitable supply of hepatocyte like resources for the potential use in human cell transplantation therapy. To achieve this purpose, we have used only fetal calf serum for cell cultures. Cells were serum deprived for 24 h prior to inducing hepatic trans-differentiation, and were then cultured in serum-free conditions. Furthermore, we always used passage 2 cultures for differentiation assays, as it is convenient to differentiate cells for clinical use in low passages to avoid spontaneous differentiation.
In vitro functional assays on differentiated cells at different time points were consistent with the morphological changes. With the exception of AFP, which is strongly detectable by ELISA at all time points, other assays for functionality were found to be negative at days 4 postinduction but gradually became evident by days 6 postinduction. By days 10 postinduction, differentiated cells acquired complete functionality based on assays performed and were sustained till days 14 postinduction or later. AFP and albumin are the first secreted proteins produced by the embryonic liver (Lee et al. 2004). Fiegel and Lioznov (2003) also demonstrated the isolation of hepatic progenitors cells by using AFP as one of the markers for the progenitor cells. The data showed that the AFP expression was very high. In our experiments, we found that AFP could be detected throughout the differentiating process because the medium contained a low level of AFP. At day 12 the level of AFP increased significantly compared to day 0, suggesting that HSC can secrete AFP.
We also determined the expression of gene responsible for HSC (TO, CK18 and AFP) as well as the differentiation markers (G6P and ALB) of adult hepatic genotypic in UCB derived HSC. We compared expression levels by RT–PCR at the initial and final times of the differentiation procedure. Levels of the expression of ALB, and G6P was observed at days 14. The expression of CK18, AFP and TO was detected at both (day 0 and days 14) time points and increased with time of differentiation, whereas undifferentiated cells did not express ALB and G6P but did express low levels of AFP and CK 18 and TO.
In summary, our findings indicate that HSCs derived from human umbilical cord blood can differentiate into functional hepatocyte-like cells in vitro, in addition to mesodermal and ectodermal lineages. Also, we have demonstrated a protocol wherein the differentiation of cells to hepatocytes could be easily achieved in 14 days, as compared to 21 days reported earlier. This could have huge clinical significance, given the urgent requirements for cells for transplantation and therapy, and we are conducting transplantation experiments in animal models of liver disease and injury to demonstrate the effectiveness of the differentiated cells. The results of this study continue to challenge the perspective of the restricted differentiation potential of adult-derived stem cells. Most important of all, HSCs may serve as a cell source for tissue engineering or cell therapy of liver.
References
- Bromeyer HE. Questions to be answered regarding umbilical cord blood hematopoietic stem and progenitor cells and their use in transplantation. Transfusion. 1995;35:694–702. doi: 10.1046/j.1537-2995.1995.35895357903.x. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomaczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:196–199. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Danet GH, Luongo JL, Butler G, et al. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc Natl Acad Sci USA. 2002;99:10441–10445. doi: 10.1073/pnas.162104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Campli C, Piscaglia AC, Pierelli L, et al. A human umbilical cord stem cell rescue therapy in a murine model of toxic liver injury. Dig Liver Dis. 2004;36:603–613. doi: 10.1016/j.dld.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Feng G, De-Quan W, Yah-Hua H, et al. Extracellular matrix gel is necessary for in vitro cultivation of insulin producing cells from human umbilical cord blood derived mesenchymal stem cells. Chinese Med J. 2008;121:811–818. [PubMed] [Google Scholar]
- Fiegel HC, Lioznov MV. Cortes-Dericks liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98–104. doi: 10.1634/stemcells.21-1-98. [DOI] [PubMed] [Google Scholar]
- Forbes S, Vig P, Poulsom R, et al. Hepatic stem cells. J Pathol. 2002;197:510–518. doi: 10.1002/path.1163. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Chowdhury JR. Hepatocyte transplantation. Amer J Transplant. 2004;4:7–13. doi: 10.1111/j.1600-6135.2004.0340.x. [DOI] [PubMed] [Google Scholar]
- Glimm H, Tang P, Clark-Lewis I, et al. Ex vivo treatment of proliferating human cord blood stem cells with stroma-derived factor-1 enhances their ability to engraft NOD/SCID mice. Blood. 2002;99:3454–3457. doi: 10.1182/blood.V99.9.3454. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Broxmeyer HE, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Drake CJ, Yang S, et al. Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Ann NY Sci. 2003;996:174–185. doi: 10.1111/j.1749-6632.2003.tb03245.x. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- Lazaro CA, Croager EJ, Mitchell C, et al. Establishment, characterization, and long-term maintenance of cultures of human fetal hepatocytes. Hepatology. 2003;38:1095–1106. doi: 10.1053/jhep.2003.50448. [DOI] [PubMed] [Google Scholar]
- Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16:153–165. doi: 10.1002/stem.160153. [DOI] [PubMed] [Google Scholar]
- McAdams TA, Miller WM, Papoutsakis ET. Hematopoietic cell culture therapies. Trends Biotechnol. 1996;14:388–396. doi: 10.1016/0167-7799(96)10054-8. [DOI] [PubMed] [Google Scholar]
- Mossman T. Rapid colorimetric assay of cellular growth and survival: a application to proliferation and cytotoxicity assays. J Immunol Method. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nakahata T, Ogawa M. Hemopoietic colony-forming cells in umbilical cord blood with extensive capability to generate mono- and multipotential hemopoietic progenitors. J Clin Invest. 1982;70:1324–1328. doi: 10.1172/JCI110734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Miyazaki M, Kouchi H, et al. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500–504. doi: 10.1006/bbrc.2000.3985. [DOI] [PubMed] [Google Scholar]
- Qin AL, Zhou XQ, Zhang WQ, et al. Characteriation an enrichment of hepatic progenitor cells in adult liver. World J Gastroenterol. 2004;10:1480–1486. doi: 10.3748/wjg.v10.i10.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg IL, Russel CW, Giles GR. Cell viability studies on the exfoliated colonic cancer cell. British J Sur. 1978;65:188–190. doi: 10.1002/bjs.1800650314. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Jiang J, Kojima N, et al. Enhanced in vitro maturation of fetal mouse liver cells with oncostatin M, nicotinamide, and dimethyl sulfoxide. Cell Transplant. 2002;11:435–441. [PubMed] [Google Scholar]
- Seo MJ, Suh SY, Bae YC, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- Talens R, Bonora A, Jover R, et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrowmesenchymal stem cells. World J Gastroenterol. 2006;12:5834–5845. doi: 10.3748/wjg.v12.i36.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–298. [PubMed] [Google Scholar]
- Visconti RT, Bonora A, Jover R, et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006;28:5834–5845. doi: 10.3748/wjg.v12.i36.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE, Kernan NA, Steinbuch M, et al. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet. 1995;346:214–219. doi: 10.1016/S0140-6736(95)91268-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Ge S, McNamara G, et al. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PP, Wang JH, Yan P, et al. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem Biophys Res Commun. 2004;320:712–716. doi: 10.1016/j.bbrc.2004.05.213. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Yu S, Li C, Xin-Guo H, et al. Differentiation of bone marrow-derived mesenchymal stem cell from diabetic patients into insulin-producing cells in vitro. Chinese Med J. 2007;120:771–776. [PubMed] [Google Scholar]