Abstract
Heart cells from the clam Ruditapes decussatus were routinely cultured with a high level of reproducibility in sea water based medium. Three cell types attached to the plastic after 2 days and could be maintained in vitro for at least 1 month: epithelial-like cells, round cells and fibroblastic cells. Fibroblastic cells were identified as functional cardiomyocytes due to their spontaneous beating, their ultrastructural characteristics and their reactivity with antibodies against sarcomeric α-actinin, sarcomeric tropomyosin, myosin and troponin T-C. Patch clamp measurements allowed the identification of ionic currents characteristic of cardiomyocytes: a delayed potassium current (IK slow) strongly suppressed (95%) by tetraethylammonium (1 mM), a fast inactivating potassium current (IK fast) inhibited (50%) by 4 amino-pyridine at 1 mM and, at a lower level (34%) by TEA, a calcium dependent potassium current (IKCa) activated by strong depolarization. Three inward voltage activated currents were also characterized in some cardiomyocytes: L-type calcium current (ICa) inhibited by verapamil at 5 × 10−4 M, T-type Ca2+ current, rapidly activated and inactivated, and sodium current (INa) observed in only a few cells after strong hyperpolarization. These two currents did not seem to be physiologically essential in the initiation of the beatings of cardiomyocytes. Potassium currents were partially inhibited by tributyltin (TBT) (1 μM) but not by okadaic acid (two marine pollutants). DNA synthesis was also demonstrated in few cultured cells using BrdU (bromo-2′-deoxyuridine). Observed effects of okadaic acid and TBT demonstrated that cultured heart cells from clam Ruditapes decussatus can be used as an experimental model in marine toxicology.
Keywords: Clam, Ruditapes decussatus, Cardiomyocyte, Patch clamp, Ionic currents, In vitro
Introduction
Numerous attempts in culturing cells have been performed from different marine or freshwater bivalves (Rinkevich 2005) such as clams (Cecil 1969; Wen et al. 1993b; Chen and Wen 1999), mussels (Chardonnet and Pérès 1963; Quinn et al. 2009), scallops (Le Marrec-Croq et al. 1998, 1999; Fritayre 2004; Talarmin et al. unpublished) and oysters (e.g. Le Deuff et al. 1994; Renault et al. 1995; Buchanan et al. 1999; Chen and Wen 1999; Domart-Coulon et al. 2000; Pennec et al. 2002, 2004; Droguet 2006; Talarmin et al. 2008). The aim of such attempts was to establish experimental models for applications in pathology and toxicology.
The heart of marine bivalves has been used as a target organ in most of these cell culture attempts due to limited risks of microbial contaminations. Such contaminations have been reported by many authors as one of the major sources of problems in establishing cell cultures from aquatic invertebrates. Cardiomyocytes e.g. from oysters and scallops can be now routinely maintained functional in culture up to 1 month (Domart-Coulon et al. 1994; Le Marrec-Croq et al. 1997, 1998, 1999; Pennec et al. 2002, 2004; Fritayre 2004; Droguet 2006; Talarmin et al. 2008). Primary cultures can be obtained from freshly isolated cardiomyocytes and from cryopreserved cells (Le Marrec-Croq et al. 1998; Cheng et al. 2001).
The usefulness of bivalves as sensitive bioindicators of environmental changes has been established for a long time, due to their high filtration rate, their ability to bioconcentrate toxicants, their widespread distribution and abundance. It has been demonstrated for example that clam, an economically important bivalve in many countries, such as in France, Tunisia and Portugal could be submitted to various pollutants of anthropogenic origin (Bebianno et al. 2004). Therefore, it was interesting to attempt to establish cell cultures from this species. Characterization of the ionic currents of these cardiomyocytes is also a preliminary step before using them in electrophysiological assays. This is the aim of the present work in order to provide a new suitable cell culture system with particular emphasis on its application in toxicology.
Materials and methods
Adult clams, R. decussatus, of 3–4 cm shell in length, were collected from a local fish farm. After brushing the shells, the animals were briefly washed with sterile seawater (SSW) and rinsed with ethanol 70%.
Heart cell cultures
Animals were carefully opened under sterile conditions by section of the adductor muscle. Tissues were rinsed twice with SSW. After removing of the pericardium, the heart was taken out with sterile forceps and treated 3 times for 5 min with antibiotics in decreasing concentrations (4X, 2X, 1X). 1X was a solution of streptomycin (13 g L−1), gentamycin (4 g L−1) and erythromycin (2 g L−1). The heart of several animals was dissected into pieces of around 1 mm3 and placed overnight in pronase 0.2% (W/V) in Hank’s balanced salt solution (osmolarity adjusted with sodium chloride to 900 mOsm), at 4 °C. The cell suspension was filtred through a 60 μm nylon mesh and centrifuged (500 g for 5 min). The pellet was washed twice in SSW and finally resuspended in a culture medium (SSW supplemented with 20% Leibovitz L15, 10% fetal calf serum, 10 mM HEPES buffer and antibiotics 1X. The pH was adjusted to 7.3 and the osmolality to 900 mOsm by addition of NaCl. Cell viability was evaluated by the trypan blue exclusion test adapted to the marine environment. Cells were seeded in Petri dishes at a density of 0.8 × 106 cells cm−2 and incubated at 20 °C. Microscopic observations were performed daily. Cells in culture were colored using May-Grunwald-Giemsa (MGG) procedure after 4 and 15 days and observed microscopically.
Beating rhythm of cardiomyocytes
Beating rate of cultured cardiomyocytes was monitored on an inverted phase-contrast microscope coupled to high speed CCD camera and computer system. For each contractile cardiomyocyte, 5 successive countings were realized in standardized experimental conditions (20 °C) to determine the average frequency of the contractions in beats per minute (bpm). Beating rate of clam heart was also evaluated in vivo for comparison with in vitro data.
Immunocytochemistry
After 4 days of culture, the medium was pipetted off. Adherent cells were washed with phosphate buffered saline (PBS) and fixed with ice-cold methanol. Immunoreactivity of cardiomyocytes against sarcomeric α-actinin (Sigma A7811, 1:400), sarcomeric tropomyosin (Sigma T9283, 1:50), myosin (Sigma M 8421, 1:10) and troponin T-C (CT3) (Santa Cruz biotechnology sc-20025, 1:200) was investigated. For immunostaining, cells were treated by using an automated immunostainer (Ventana Medical Systems) with a Niew™ DAB detection kit (Ventana, 760-091). Negative control without primary antibodies were realized.
Ultrastructural study
For an ultrastructural study after 4 days of culture, cells were fixed in situ at 4 °C with 2.5% glutaraldehyde in 0.1 M phosphate buffer (PBS: pH 7.2—osmolarity adjusted to 900 mOsm by addition of sodium chloride) for 10 min and postfixed after washing with PBS in 1% osmium tetroxide in the same buffer for 30 min. They were then washed, dehydrated, and embedded in EMBed 812. Ultrathin sections were prepared by using conventional methods for transmission electron microscopy (TEM). They were contrasted with uranyl acetate and lead citrate (Reynolds 1963).
Cell proliferation
A RPN 20 kit supplied by Amersham was used to investigate DNA synthesis. A thymidine analog, the [5-bromo-2′-deoxyuridine] BrdU, was incorporated into replicating DNA. After 3 days of culture, cells were incubated with BrdU (1:1,000) during 24 h. Then, they were washed twice with PBS and fixed for 5 min at 4 °C in methanol with 1.5% H2O2. After washing 3 times with PBS for 5 min, cells were incubated with BSA (bovine serum albumin) 2% in PBS for 30 min. Cells were incubated in Nuclease/anti-BrdU for 1 h. They were then treated for 30 min with anti-mouse IgG2a antibody coupled with peroxydase. Mitotic cells were revealed using DAB (diaminobenzidine). Positive nuclei were dark. Cardiomyocytes were characterized by a double immunostaining using antibodies against BrdU and troponine. After saturation with BSA 2% in PBS, cells were incubated with anti-troponin T-C (CT3) antibody (1:200 in PBS-Tween (PBT) 0.05%-BSA 0.2%) for 1 h. Then, cells were washed twice with PBS and incubated with a secondary antibody coupled with peroxydase (Sigma, 1:500). After PBS washing (3 × 5 min), detection of immunocomplexes was carried out using DAB. Then, cells were treated for BrdU as described above. For negative controls, the anti-troponin T-C (CT3) antibody was omitted.
Electrophysiology
A macro-patch clamp technique was used to characterize the main ionic currents according to the method previously described (Pennec et al. 2004). The diameter of opening of the used pipettes (3 ± 0.02 μm) was checked by electron microscopy. The average resistance of pipettes filled with standard medium (modified seawater) was 1.5 Mohm. Junction potential was corrected before the realization of the seal. The microelectrodes were connected to an amplifier (Axon; Geneclamp 500 B, USA) via a current-to-voltage converter headstage (CV5-100MU). The signal was digitized at 48 kHz by an analog-to-digital converter (CED 1401 plus, UK). A program (WCP v. 3.52 from Strathclyde University, UK) was used to record the currents and to deliver sequences of programmed voltage pulses. A classical P/4 protocol was used to remove residual leak current, if any, and residual capacitance artefacts (Almers et al. 1983). The currents were analyzed off-line by using WCP to draw the current–voltage relationships and to calculate ionic conductances. Experiments were carried out at room temperature (20 ± 1 °C).
Pharmacological agents were used to selectively block ionic currents. They were: tetraethylammonium (TEA, Sigma: T2265), 4-aminopyridin (4-AP, Sigma, A0152), verapamil (Sigma, V4629). The stock solutions were achieved in distilled water then higher dilutions were made in SSW to be used with the cells. The effects of the drugs on the ionic currents were recorded after direct addition in the cell bath.
Chemicals
Experiments were carried out to determine using the macro-patch clamp technique the effect of a phycotoxin, the okadaic acid (OA) (Alexis Biochemicals, ALX-350-003-C100), and of tributyltin chloride (TBT) (Sigma, T5,020-2) on the ionic currents in clam heart cells after 7 days of culture. Stock solutions of OA (100 μM) and TBT (10−2 M) were prepared in dimethyl sulfoxide (DMSO 4‰). Chemicals were then diluted in the culture medium to 1 μM. Cells were exposed to pollutants for 30 min.
Results
Cultured adherent cells
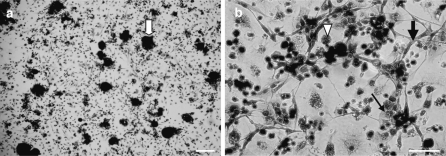
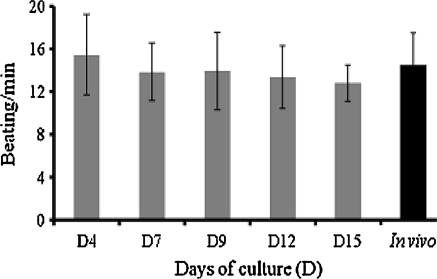
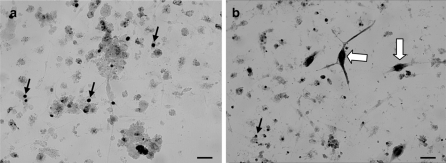
Ninety percent of cell viability was routinely obtained after dissociation of clam heart with pronase. 48 h after seeding, isolated cells and cells in clusters were attached to the substrate. Cultured cells were maintained alive for up to 1 month. Three types of adherent cells could be observed microscopically: fibroblastic cells, epithelial-like cells (Fig. 1) and round cells. Some round cells have numerous cytoplasmic inclusions (Fig. 1). Fibroblastic cells were organized in networks (Fig. 1) and were spontaneously contractile 3 days after seeding. Beatings were observed during at least 1 month of culture. The beating rate (Fig. 2) in vitro (15 ± 4 bpm) remained stable throughout the first 15 days of culture and was quite similar to what was observed in vivo (14 ± 3 bpm).
Fig. 1.
Heart cells in culture colored by using MGG after 4 days (a) and 15 days (b). Small clusters (  ), epithelial-like cells (
), epithelial-like cells (  ), fibroblastic cells (
), fibroblastic cells ( 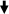 ) and round cells (▽). Scale bar 160 μm (a); 100 μm (b)
) and round cells (▽). Scale bar 160 μm (a); 100 μm (b)
Fig. 2.
Spontaneous beating rhythm of cardiomyocytes measured in vitro and in vivo. Values are means ± SD
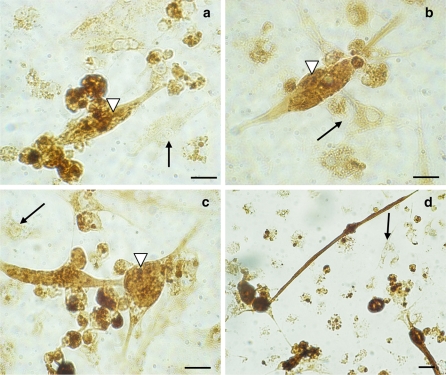
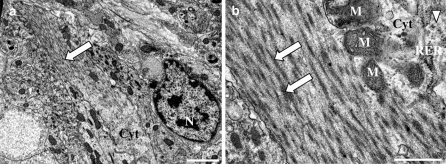
Immunostaining performed by using the anti-troponin antibody showed positive fibroblastic cells (Fig. 3). These cells also slightly cross reacted with the sarcomeric α-actinin, tropomyosin and myosin antibodies. TEM study of fibroblastic cells after 4 days of culture showed myofilaments characteristic for cardiomyocytes (Fig. 4a, b). The dense area connecting myofibrilla give the cardiomyocyte a striated appearance (Fig. 4a, b). Mitochondria and glycogen-like particules were abundant in the cytoplasm.
Fig. 3.
Cardiomyocytes positively immunostained (▽) with anti-sarcomeric α-actinin- (a), anti-myosin- (b), anti-sarcomeric tropomyosin- (c), anti-troponin- (d) antibodies. Uncolored fibroblastic cells as negative controls ( 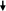 ). Scale bar 40 μm
). Scale bar 40 μm
Fig. 4.
Ultrastucture of clam cultured cells after 4 days. Myofilaments ( 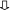 ) in a cardiomyocyte (a). Details of a cardiomyocyte with longitudinal myofibrilla of striated appearance (b). Cyt cytoplasm, M mitochondria, N nucleus, RER rough endoplasmic reticulum, glycogen-like particles (▽). Scale bars 20 μm for a and 5 μm for b
) in a cardiomyocyte (a). Details of a cardiomyocyte with longitudinal myofibrilla of striated appearance (b). Cyt cytoplasm, M mitochondria, N nucleus, RER rough endoplasmic reticulum, glycogen-like particles (▽). Scale bars 20 μm for a and 5 μm for b
DNA synthesis
Around 10% of cells cultured for 3 days were positively marked (Fig. 5) after 24 h of incubation with BrdU. Positive dark nuclei were observed in fibroblastic cells and especially in round cells. Some of them cross reacted with the anti-troponin T-C (CT3) antibody. That shows that some of the cultured cells are progressing in the cell cycle.
Fig. 5.
DNA synthesis evidenced by using BrdU. Dark positive nuclei ( 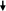 ) (a); double BrdU-troponin immunostained cells (
) (a); double BrdU-troponin immunostained cells ( 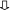 ) (b). Scale bar 40 μm
) (b). Scale bar 40 μm
Electrophysiology
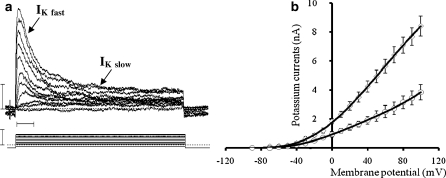
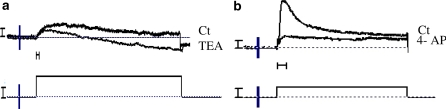
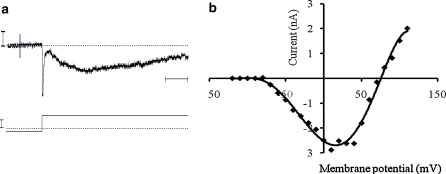
A slow activating, non inactivating potassium outward current (IKslow) similar to the classical delayed potassium current observed in numerous cell types was measured (Fig. 6a). The current was triggered by depolarizing voltage steps and increased rapidly. The current–voltage relationship (I/V curve) showed a strong rectification (Fig. 6b). Its maximal conductance, calculated by linear regression performed on the linear part of the I/V relationship, was 0.057 ± 0.008 μS (n = 18). This delayed potassium current was significantly inhibited (76%) by TEA (10−5 M), the percentage of inhibition reached 95% with 1 mM TEA (Fig. 7a).
Fig. 6.
Example of potassium currents recorded in cultured clam heart cells (a) showing fast potassium outward current (IKfast) and delayed potassium current (IKslow). Lower trace voltage step applied to the membrane. Vertical bars 1 nA (top) and 100 mV (bottom). Horizontal bar 10 ms. Current–voltage relationship for respectively delayed and fast potassium (b) currents showing a strong outward rectification. Open circlesIKslow, open diamondsIKfast. Values are means ± SEM
Fig. 7.
Example of current inhibition of both the delayed and the fast inactivating current by TEA 1 mM (a). Lower trace voltage step applied to the membrane. Vertical bars 1 nA (top) and 100 mV (bottom). Horizontal bar 2 ms. Example of inhibition of the fast inactivating potassium current by 4-amino pyridine (4-AP) 10−3 M (b). The fast potassium current is totally inhibited; only remains the delayed potassium current. Vertical bars 0.1 nA (top) and 1 mV (bottom). Horizontal bar 10 ms
A fast activating and spontaneously inactivating outward current identified as IK fast, was elicited by depolarizing pulses in hyperpolarized cells (holding potential = −120 mV) (Fig. 6a). The mean value of maximum conductance was 0.1 ± 0.006 μS (n = 22) (Fig. 6b). This current was lightly inhibited by 10−5 M TEA (about 27% of inhibition) and a little more by 1 mM TEA (about 34% of inhibition) (Fig. 7a). The conductance was reduced to 0.016 ± 0.007 μS compared to 0.024 ± 0.008 μS.
IK fast was strongly inhibited (50%) by 4-AP 10−3 M (Fig. 7b) which did not modify the slow inward current.
In most cells, a large outward current elicited by strong depolarizing pulses was identified as a calcium dependent potassium current (IKCa), having a large conductance of 0.94 ± 0.05 μS (n = 8). This current was also inhibited by preventing intracellular calcium increase by a calcium channel inhibitor: verapamil at 5 × 10−4 M (data not shown).
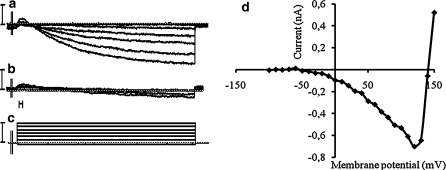
In TEA treated cells, a long lasting inward current, not inactivating throughout the 100 ms pulse duration, was observed (Fig. 8a). The maximum conductance was: 0.46 ± 0.14 μS (n = 5) (Fig. 8d). The inversion potential could not be systematically determined because large depolarizing pulses induced patch rupture. This current was inhibited by verapamil at 5 × 10−4 M (Fig. 8b, c). As it showed no noticeable inactivation, it was identified as a slow inward calcium current of the L-Type. In few cells, when strong hyperpolarisation was applied to the membrane: holding potential of −40 to −60 mV below the normal resting potential, a transient inward current could also be observed (Fig. 9a) suggesting the possibility of a fast inactivating calcium current of the T-type in some cells.
Fig. 8.
Example of typical recording of the calcium inward current alone (a very small fast potassium current is not totally abolished at the start of recordings) (a). Inhibition of the calcium current by verapamil 5 × 10−4 M (b). Voltage steps imposed to the membrane (c). Vertical bars 10 nA (top) and 200 mV (bottom). Horizontal bar 2 ms. Current–voltage relationship corresponding to the calcium current (d)
Fig. 9.
Example of a transient inward calcium current following a rapid voltage gated sodium current after inhibition of potassium currents and application of a 40 mV hyperpolarizing holding potential to the cell (a). Vertical bars 1 nA (top) and 0.1 V (bottom). Horizontal bar 10 ms. Current–voltage relationship of the sodium current recorded from isolated cardiomyocytes (b); such a current was observed in only 2% of cells
A fast activating and inactivating inward current identified as a voltage gated sodium current could also be observed in few cells (2%), in the same conditions than the calcium current (Fig. 9a). The maximum conductance, obtained from the slope of the linear portion of the curve was 0.056 ± 0.031 μS (n = 3) (Fig. 9b).
Effect of pollutants on ionic currents
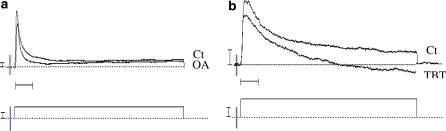
Results showed that ionic currents were not significantly modulated after 30 min of exposure to OA 1 μM as compared with untreated cells (Fig. 10a). Concerning TBT 1 μM a significant decrease of potassium currents (Fig. 10b) and a significant increase of L-type calcium currents were observed after 30 min of exposure.
Fig. 10.
Example of currents recorded after treatment with 1 μM of OA (a) and TBT (b) for 30 min. Vertical bars 1 nA (top) and 0.1 V (bottom). Horizontal bar 20 and 10 ms, respectively for for OA and TBT
Discussion
The main purpose of this study was to establish primary cultures of clam heart cells. Our experiments showed that cells isolated from R. decussatus using pronase, according to a protocol adapted from the procedure defined by Le Marrec-Croq et al. (1997) for scallop, allowed good cell viability and routinely functional primary cultures. Clam cultured cells remained viable for up to 1 month. Analogous results were previously obtained in our laboratory for scallop and oyster (Le Marrec-Croq et al. 1999; Fritayre 2004; Pennec et al. 2002, 2004; Droguet 2006).
To our knowledge it is the first time that pronase has been used to dissociate clam heart. In the literature, previous work showed that cell cultures from cardiac tissue of the surf clam Spisula solidissima can also be obtained using trypsin-EDTA (Cecil 1969) or collagenase for Meretrix lusoria (Chen and Wen 1999), the latter authors considering that treatment with collagenase was better than trypsin at dissociating mollusc tissue fragments for in vitro culture. This is not in accordance with our results after having tested, in preliminary experiments, the three enzymes for R. decussatus heart. A higher mortality was observed in cells isolated by trypsin or collagenase in comparison with pronase.
Clam cultures are heterogenous as reported for other marine bivalves (Chen and Wen 1999; Cheng et al. 2001; Fritayre 2004; Droguet 2006). The round cells are usually considered as haemocytes (Wen et al. 1993b; Renault et al. 1995). We can hypothesize that epithelial like cells have been isolated from the external simple prismatic epithelium covering the heart of bivalves (epicardium). Concerning fibroblastic cells, some of them are fibroblasts isolated from the connective tissue and the others are cardiomyocytes. The characterisation of these fibroblastic cells was the second aim of the present study.
To our knowledge, characterization of such cells from the clam has not been previously documented. In the literature; data obtained using patch clamp and/or immunostaining, ultrastructural studies concerned cultured cardiomyocytes from scallop, oyster and mussel (Wen et al. 1993a; Le Deuff et al. 1994; Curtis et al. 1999; Le Marrec-Croq et al. 1999; Pennec et al. 2002, 2004; Droguet 2006). Chen and Wen (1999) for oyster and Ellington (1993) for clam concluded that cultured fibroblastic cells were cardiomyocytes only by distinctive rhythmic contraction in vitro. In the present work proof that almost all adherent fibroblastic cells were cardiomyocytes was based on (1) the spontaneous beatings of fibroblastic cells observed in vitro with a rate similar to this measured in vivo, (2) results of immunological staining, showing positive reaction of these cells after treatment with antibodies against troponin, tropomyosin, myosin and sarcomeric actinin, (3) their ultrastructural characteristics and (4) results of electrophysiological analyses showing ionic channels and regulatory mechanisms very similar to those observed in cardiomyocytes of invertebrates and other marine bivalves (Pennec et al. 2004; Curtis et al. 1999). The other objective of the present work was to carry out the electrophysiological characterization of the cultured cardiomyocytes.
An outward potassium current, identified as a delayed rectifying potassium current (IKslow), and a fast activating and inactivating potassium current (IK fast), classically observed in cardiac cells of mammalian and other marine molluscs such as oyster (Pennec et al. 2004), mussel (Curtis et al. 1999), snail (Yeoman and Benjamin 1999), squid (Ödblom et al. 2000) and sea hare (Souza et al. 2002) were observed in clam cells. The main role of IKslow is to initiate and terminate cardiac repolarization (Varro and Papp 1992). It is classically inhibited by TEA which is known to block a variety of K channel types with possible differences in their sensitivity to this compound (Ödblom et al. 2000). In clam, IKslow was more sensitive to TEA, compared to other species including bivalves such as oyster (Pennec et al. 2004) and mussel (Curtis et al. 1999), as it was almost totally abolished (95%) by TEA 1 mM. IK fast, which is involved in the initiation of the early fast repolarization process of cardiac action potentials and in the rate of repolarization (Varro and Papp 1992) was less sensitive to TEA than IKslow. However, IK fast was more sensitive in clam than in oyster or in mussel. IK fast was inhibited by 4-AP, in agreement with data reported in many other species (Ödblom et al. 2000; Pennec et al. 2004; Tseng et al. 1987). Then TEA and 4-AP could be used to separate the fast and the delayed potassium currents in clam cardiomyocytes. The question remains for the clam of a possible role in the modulation of cardiac frequency by IK fast.
Calcium-activated outward potassium current, activated by large depolarizing pulses and largely sensitive to calcium influx, was also observed in clam cardiomyocytes as in vertebrates (Siegelbaum and Tsien 1980; Kenyon and Sutko 1987; Giles and Imaizumi 1988) or invertebrates (Pennec et al. 2004; Ödblom et al. 2000). This current has a role in the regulation of cell contractility and intracellular calcium regulation. It is activated by increased intracellular calcium and speeds up the cell repolarization, thus reducing the duration of calcium inward current.
The clam myocytes also showed an inward current, rapidly activated and inhibited by verapamil, identified as a long duration calcium current (L-type). Such a calcium current, classically observed in vertebrate cardiac tissue (Hagiwara et al. 1988) has been identified in other molluscs (Curtis et al. 1999; Yeoman et al. 1999; Ödblom et al. 2000; Pennec et al. 2004). L-type channel, in molluscs as in lower vertebrates, is thought to be the primary pathway for Ca2+ entry for the excitation-contraction mechanism (Ferrier et al. 2000; Bers and Perez-Reyes 1999, Ödblom et al. 2000).
In some cells, when a strong hyperpolarization was applied to the membrane before the test pulse, another calcium inward current (T-type) was observed in the clam as in oyster (Pennec et al. 2004) and snail (Yeoman et al. 1999), but not in mussel (Curtis et al. 1999). We can hypothesize, as Pennec et al. (2004), Varro and Papp (1992), Yeoman et al. (1999), that this current can be involved in pacemaker function. The lack of transient calcium current and sodium current in most clam cardiac cells, despite of their spontaneous beatings when they are cultured, suggests that these channels are not essential in the automaticity of the cultured cells as reported by Curtis et al. (1999) in mussel without presuming the role of these currents in vivo. It was also reported that calcium released from the sarcoplasmic reticulum can be triggered by the T-type current, but this mechanism is less physiologically important in excitation–contraction coupling than the mechanism related to the L-type calcium channel (Bers and Perez-Reyes 1999). Moreover, the ventricular cells are supposed to lack the T-type calcium current (Bers and Perez-Reyes 1999) as most of the cultured cells are of ventricular origin, explaining why the transient calcium current was not frequently observed.
A sodium current was only found in few clam cells as evidenced in cardiac tissue of other molluscs (Pennec et al. 2004; Curtis et al. 1999; Ödblom et al. 2000). Varro and Papp (1992) showed that this current is responsible not only for the fast depolarization, but, in part also, for the maintenance of the plateau phase of the action potential. In the clam, the small size of this current in vitro can be explained by the fact that the fast sodium current like the T-type calcium current is not essential to the triggering of automaticity. Moreover it should be mostly inactivated at the normal resting potential. Thus, in clam cultured heart cells the action potential appears to be mostly based on the inflow of calcium ions, rather than sodium ions as suggested elsewere (Curtis et al. 1999).
DNA synthesis in few clam heart cells in culture was proven by BrdU uptake. Some of these cells could be identified as cardiomyocytes due to their double positive staining BrdU-troponin. BrdU incorporation was also demonstrated in cultured heart cells of scallop (Le Marrec-Croq et al. 1999) and oyster (Droguet 2006). In vitro mitoses were also observed under inverted microscope in cardiac tissue from surf clam (Cecil 1969) and hard clam (Wen et al. 1993a; Chen and Wen 1999). We can hypothesize that there were few unipotent stem cells in the population of adherent cardiac cells.
To summarize, the present work shows that clam heart cells can be maintained functional in primary culture for up to 1 month. The characterization of cardiomyocyte physiological properties was a prerequisite before using such cells in culture as tools for toxicological studies as illustrated by results obtained after clam heart cell exposure to two major marine pollutants. This work showed that primary cultures of clam heart cell can be used as experimental models in marine toxicology and the interest of patch clamp technique as a sensitive analytical method.
References
- Almers W, Stanfield PR, Stuhmer W. Slow changes in currents through sodium channels in frog muscle membrane. J Physiol. 1983;339:253–271. doi: 10.1113/jphysiol.1983.sp014715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebianno MJ, Geret F, Hoarau P, Serafim MA, Coelho MR, Gnassia-Barelli M, Romeo M. Biomarkers in Ruditapes decussatus: a potential bioindicator species. Biomarkers. 2004;9:305–330. doi: 10.1080/13547500400017820. [DOI] [PubMed] [Google Scholar]
- Bers DM, Perez-Reyes E. Ca channels in cardiac myocytes: structure and function in Ca influx and intracellular Ca release. Cardiovasc Res. 1999;42:339–360. doi: 10.1016/S0008-6363(99)00038-3. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, La Peyre JF, Cooper RK, Tiersch TR. Improved attachment and spreading in primary cell cultures of the eastern oyster, Crassostrea virginica. In Vitro Cell Dev Biol Anim. 1999;35:593–598. doi: 10.1007/s11626-999-0097-2. [DOI] [PubMed] [Google Scholar]
- Cecil JT. Mitoses in cell cultures from cardiac tissue of the surf clam Spisula solidissima. J Invertebr Pathol. 1969;14:407–410. doi: 10.1016/0022-2011(69)90170-0. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y, Pérès G. Essai de culture de cellules provenant d’un mollusque: Mytilus gallovincialis. L C R Soc Biol Lyon. 1963;157:1593–1595. [PubMed] [Google Scholar]
- Chen SN, Wen CM. Establishment of cell lines derived from oyster, Crassostrea gigas Thunberg and hard clam, Meretrix lusoria Roding. Methods Cell Sci. 1999;21:183–192. doi: 10.1023/A:1009829807954. [DOI] [PubMed] [Google Scholar]
- Cheng TC, La Peyre JF, Buchanan JT, Tiersch TR, Cooper RK. Cryopreservation of heart cells from the eastern oyster. In Vitro Cell Dev Biol Anim. 2001;37:237–244. doi: 10.1007/BF02577536. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Depledge MH, Williamson R. Voltage-activated currents in cardiac myocytes of the blue mussel, Mytilus edulis. Comp Biochem Physiol A. 1999;124:231–241. [Google Scholar]
- Domart-Coulon I, Doumenc D, Auzoux-Bordenave S, Le Fichant Y. Identification of media supplements that improve the viability of primarily cell cultures of Crassostrea gigas oysters. Cytotechnology. 1994;16:109–120. doi: 10.1007/BF00754613. [DOI] [PubMed] [Google Scholar]
- Domart-Coulon I, Auzoux-Bordenave S, Doumenc D, Khalanski M. Cytotoxicity assessment of antibiofouling compounds and by-products in marine bivalve cell cultures. Toxicol In Vitro. 2000;14:245–251. doi: 10.1016/S0887-2333(00)00011-4. [DOI] [PubMed] [Google Scholar]
- Droguet M (2006) Etude des caractéristiques fonctionnelles des cardiomyocytes d’huître en culture. Thèse de doctorat de biologie. Université de Bretagne Occidentale, p 211
- Ellington WR. Studies of intracellular pH regulation in cardiac myocytes from the marine bivalve mollusk, Mercenaria campechiensis. Biol Bull. 1993;184:209–215. doi: 10.2307/1542228. [DOI] [PubMed] [Google Scholar]
- Ferrier GR, Redondo IM, Mason CA, Mapplebeck C, Howlett SE. Regulation of contraction and relaxation by membrane potential in cardiac ventricular myocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1618–H1626. doi: 10.1152/ajpheart.2000.278.5.H1618. [DOI] [PubMed] [Google Scholar]
- Fritayre P (2004) Culture des cellules atriales de coquille Saint-Jacques, Pecten maximus: valeur et limites du modèle. Applications en toxicologie. Thèse en Océanologie Biologique et Environnement marin. Université de Bretagne Occidentale, p 230
- Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H, Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon JL, Sutko JL. Calcium- and voltage-activated plateau currents of cardiac Purkinje fibers. J Gen Physiol. 1987;89:921–958. doi: 10.1085/jgp.89.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Deuff RM, Lipart C, Renault T. Primary culture of pacific oyster, Crassostrea gigas, heart cells. J Tissue Cult Methods. 1994;16:67–72. doi: 10.1007/BF01404838. [DOI] [Google Scholar]
- Le Marrec-Croq F, Dorange G, Chesné C (1997) Procédé de culture de cellules d’invertébrés marins et cultures obtenues, French Patent no 9506921; no of publication: 2735146 (1996)
- Le Marrec-Croq F, Fritayre P, Chesn GuillouzoA, Dorange G. Cryopreservation of Pecten maximus heart cells. Cryobiology. 1998;37:200–206. doi: 10.1006/cryo.1998.2113. [DOI] [PubMed] [Google Scholar]
- Le Marrec-Croq F, Glaise D, Guguen-Guillouzo C, Chesne C, Guillouzo A, Boulo V, Dorange G. Primary cultures of heart cells from the scallop Pecten maximus (mollusca-bivalvia) In Vitro Cell Dev Biol Anim. 1999;35:289–295. doi: 10.1007/s11626-999-0073-x. [DOI] [PubMed] [Google Scholar]
- Ödblom MP, Williamson R, Jones MB. Ionic currents in cardiac myocytes of squid, Alloteuthis subulata. J Comp Physiol B. 2000;170:11–20. doi: 10.1007/s003600050002. [DOI] [PubMed] [Google Scholar]
- Pennec JP, Gallet M, Gioux M, Dorange G. Cell culture of bivalves: tool for the study of the effects of environmental stressors. Cell Mol Biol (Noisy-le-grand) 2002;48:351–358. [PubMed] [Google Scholar]
- Pennec JP, Talarmin H, Droguet M, Giroux-Metges MA, Gioux M, Dorange G. Characterization of the voltage-activated currents in cultured atrial myocytes isolated from the heart of the common oyster Crassostrea gigas. J Exp Biol. 2004;207:3935–3944. doi: 10.1242/jeb.01221. [DOI] [PubMed] [Google Scholar]
- Quinn B, Costello MJ, Dorange G, Wilson JG, Mothersill C. Development of an in vitro culture method for cells and tissues from the zebra mussel (Dreissena polymorpha) Cytotechnology. 2009;59:121–134. doi: 10.1007/s10616-009-9202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault T, Flaujac G, Le Deuff RM. Isolation and culture of heart cells from the European flat oyster, Ostrea edulis. Methods Cell Sci. 1995;17:199–205. doi: 10.1007/BF00996127. [DOI] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich B. Marine invertebrate cell cultures: new millennium trends. Mar biotechnol (NY) 2005;7:429–439. doi: 10.1007/s10126-004-0108-y. [DOI] [PubMed] [Google Scholar]
- Siegelbaum SA, Tsien RW. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza MM, Stucchi-Zucchi A, Cassola AC, Scemes E. Electrophysiology of cardiac myocytes of Aplysia brasiliana. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:161–168. doi: 10.1016/S1095-6433(02)00151-4. [DOI] [PubMed] [Google Scholar]
- Talarmin H, Droguet M, Pennec JP, Schröder HC, Muller WEG, Gioux M, Dorange G. Effects of a phycotoxin, okadaic acid, on oyster heart cell survival. Toxicol Environ Chem. 2008;90:153–168. doi: 10.1080/02772240701382131. [DOI] [Google Scholar]
- Tseng GN, Robinson RB, Hoffman BF. Passive properties and membrane currents of canine ventricular myocytes. J Gen Physiol. 1987;90:671–701. doi: 10.1085/jgp.90.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A, Papp JG. The impact of single cell voltage clamp on the understanding of the cardiac ventricular action potential. Cardioscience. 1992;3:131–144. [PubMed] [Google Scholar]
- Wen CM, Kou GH, Chen SN. Cultivation of cells from the heart of the hard clam, Meretrix lusoria (RODING) J Tissue Cult Methods. 1993;15:123–130. doi: 10.1007/BF02388265. [DOI] [Google Scholar]
- Wen CM, Kou GH, Chen SN. Establishment of cell lines from the Pacific oyster. In Vitro Cell Dev Biol Anim. 1993;29A:901–903. doi: 10.1007/BF02634224. [DOI] [PubMed] [Google Scholar]
- Yeoman MS, Benjamin PR. Two types of voltage-gated K(+) currents in dissociated heart ventricular muscle cells of the snail Lymnaea stagnalis. J Neurophysiol. 1999;82:2415–2427. doi: 10.1152/jn.1999.82.5.2415. [DOI] [PubMed] [Google Scholar]
- Yeoman MS, Brezden BL, Benjamin PR. LVA and HVA Ca(2+) currents in ventricular muscle cells of the Lymnaea heart. J Neurophysiol. 1999;82:2428–2440. doi: 10.1152/jn.1999.82.5.2428. [DOI] [PubMed] [Google Scholar]