Abstract
Pluripotent human embryonic stem cell (hESC) lines are a promising model system in developmental and tissue regeneration research. Differentiation of hESCs towards the three germ layers and finally tissue specific cell types is often performed through the formation of embryoid bodies (EBs) in suspension or hanging droplet culture systems. However, these systems are inefficient regarding embryoid body (EB) formation, structural support to the EB and long term differentiation capacity. The present study investigates if agarose, as a semi solid matrix, can facilitate EB formation and support differentiation of hESC lines. The results showed that agarose culture is able to enhance EB formation efficiency with 10% and increase EB growth by 300%. The agarose culture system was able to maintain expression of the three germ layers over 8 weeks of culture. All of the four hESC lines tested developed EBs in the agarose system although with a histological heterogeneity between cell lines as well as within cell lines. In conclusion, a 3-D agarose culture of spherical hESC colonies improves EB formation and growth in a cost effective, stable and non-laborious technique.
Keywords: Human embryonic stem cell, In vitro differentiation, Embryoid body, Agarose, In vitro culture
Introduction
Human embryonic stem cells are pluripotent cells derived from the inner cell mass of the blastocyst (Bongso et al. 1994; Thomson et al. 1998; Reubinoff et al. 2000). The pluripotency of hESC is often confirmed by the ability of the cells to form teratoma that comprises derivatives of the three embryonic germ layers; endoderm, mesoderm and ectoderm when transplanted intramuscularly, subcutaneously or under the renal capsule in severe combined immunodeficiency (SCID) mice (Amit et al. 2000; Cooke et al. 2006; Prokhorova et al. 2008). In vitro differentiation of hESC often requires culture of spontaneously formed spherical structures, i.e. EBs, in suspension culture prior to the formation of the desired adult cell type (Itskovitz-Eldor et al. 2000; Dang et al. 2002; Martin and Evans 1975; Keller 1995; Hopfl et al. 2004). Suspension culture of EBs is known to have a low EB formation efficiency (below 50%) due to cell death and to loss of individual EBs because of adhesion to the culture dish, aggregation of individual EBs, i.e. agglomeration, or dissolved EBs (Dang et al. 2002; Reubinoff et al. 2000).
A way of avoiding EB agglomeration and EB adhesion to the culture dish is to grow single EBs in hanging droplets. However, the hanging droplet system does not solve the problem of dissolved EBs and technically challenging medium changes make it difficult, if not impossible, to perform long-term culture experiments with the hanging droplet system.
Embryoid body cultures could be considerably more efficient if all the problems with cell death, adhesion to the culture dish, uncontrolled agglomeration and dissolved EBs could be solved. One way of counteracting these problems would be to physically separate and encapsulate the EBs in a three dimensional (3-D) semi solid matrix during culture. Such a 3-D culture system can also enhance the cellular differentiation and form an in vitro supplement for the 3-D supportive tissues that evolves during embryogenesis in vivo (Benya and Shaffer 1982; Yu et al. 1999). Further, 3-D environments have been reported to influence differentiation of embryonic stem cells in vitro (Chen et al. 2003; Levenberg et al. 2003; Gerecht et al. 2007).
Several laboratories have developed new techniques to encapsulate and thereby separate EBs in suspension cultures (Sakai et al. 2008; Dang et al. 2004). However, these 3-D culturing techniques have drawbacks when it comes to easily study embryonic stem cell differentiation in that they still have a limited EB formation yield and does not provide an easy way of studying development within single EBs due to the suspension based system. Further the 3-D environment, interesting for differentiation studies, provided by the encapsulation inevitably disappears when the formed EBs hatch from the encapsulation.
The aim of this study was to develop an easy and effective technique for 3-D EB culture based on agarose. We hypothesized that EBs grown with a static physical 3-D support would support development of single EBs during a longer time period compared to suspension and hanging droplet techniques. EB cultures of 4 different hESC lines in 3-D agarose were compared with regular suspension cultures and hanging droplet cultures.
Here we report that agarose cultures of EBs give a high EB formation efficiency and growth compared to the regular culturing techniques. Furthermore the agarose cultures support EB formation during a long period of time with robust pluripotent differentiation as shown by development of the three germ layers. We also show and would like to attend to that although identical culturing technique was used with different hESC lines there is a differentiation heterogeneity both within and between hESC lines.
Materials and methods
Human embryonic stem cells
The 5 hESC lines (SA002, AS034, AS038, SA121, and SA167) used in this study were established at Cellartis AB, Gothenburg, Sweden (Heins et al. 2004; Sjogren-Jansson et al. 2005). All work with the hESCs was conducted according to the ISSCR Guidelines for the Conduct of Human Embryonic Stem Cell Research and with the approval of the local ethics committees at the University of Gothenburg and the University of Uppsala, Sweden.
Human embryonic stem cell culture
Human embryonic stem cells were derived, characterized and cultured as described previously (Heins et al. 2004). Briefly the hESCs were cultured using mitomycin C inactivated mouse embryonic fibroblast (MEF) feeder layers and VitroHES™ medium (Vitrolife AB, Kungsbacka, Sweden) supplemented with 4 ng/mL human recombinant basic fibroblast growth factor (bFGF) (Invitrogen Co., Carlsbad, CA) at +37°C, 5% CO2 and 90–95% humidity. Undifferentiated hESC were passaged every 4–5 days by mechanical dissociation using a Stem Cell Cutting Tool™ (Swemed by Vitrolife AB, Kungsbacka, Sweden).
Embryoid body formation
To induce EB formation, whole hESC colonies were removed using a stem cell cutting tool (Swemed by Vitrolife AB) and placed in different culture conditions as described below. The colonies were cut with a margin to the MEFs in order to avoid mouse embryo fibroblast contamination and to achieve an even size of the starting colony.
Each experiment replicate was performed with 5–15 colonies from each hESC lines. The first experiment was performed with EBs from the hESC lines; SA002, AS034, AS038 and SA121 cultured in agarose, hanging droplet and suspension in two individual replicates in order to evaluate the EB formation efficiency and growth for 1 week. The EB formation efficiency was calculated by dividing the number of formed EBs from the individual experiments with the number of colonies originally placed in each culture condition. The second experiment of EBs cultured in agarose was performed in three individual replicates, with the hESC lines; SA002, AS034, SA121 and SA167, in order to evaluate the EB differentiation potential of the agarose system when cultured for 8 weeks. The EBs were analyzed with an inverted light microscope (Nikon Diaphot 300, Nikon, NY, USA). Pictures were taken with a digital camera (Nikon digital camera DXM1200, Nikon) using Nikon ACT-1 version 2.12 software.
Suspension culture
The detached hESC colonies were placed in VitroHES™ medium, without bFGF supplement, in bacteriological Petri dishes (BD Falcon, San Jose, CA USA) and cultured for 7 days at +37°C, 5% CO2 and 90–95% humidity.
Hanging droplet cultures
The hanging droplet cultures were prepared inside a Petri dish lid using 25 μL droplets of VitroHES™ medium without bFGF. One hESC colony was placed into each droplet. Subsequently, the lid was gently inverted and placed on the Petri dish, which contained 10 mL VitroHES™ medium without bFGF to prevent evaporation from the droplets. The EBs were cultured for 7 days at +37°C, 5% CO2 and 90–95% humidity.
Agarose culture
The detached hESC colonies were placed in +37°C VitroHES™ medium without bFGF for 1 h in a Petri dish, to allow the colonies to form spherical structures. The formed spheres were subsequently transferred to agarose culture. Autoclaved agarose was prepared in sterile flasks at +100°C in two stock solutions at concentrations of 1% (Agarose standard low MT, Biorad, Hercules, CA) and 2% (Certified™ low melt agarose, Biorad). The spherical hESC colonies were transferred to room tempered VitroHES™ before the seeding procedure. The 1% agarose was cooled to +60°C and poured into a Petri dish to coat the inside. Surplus 1% agarose was discarded immediately. The 2% agarose was cooled down to +60°C and quickly mixed 1:1 with room tempered VitroHES™. The tempered agarose/VitroHES™ solution was immediately mixed 1:1 with the spherical hESC colonies suspended in VitroHES™. The agarose/hESC-colony suspension was subsequently poured into the 1% agarose coated Petri dish. The above described procedures were carefully conducted without generating bubbles. The agarose culture was left to settle at +4°C for 30 min and the semisolid agarose layer was subsequently overlaid with VitroHES™ medium without bFGF (Fig. 1a, b). The culture medium was changed once a week and the EBs were cultured for 7 days and 8 weeks, respectively, at +37°C, 5% CO2 and 90–95% humidity.
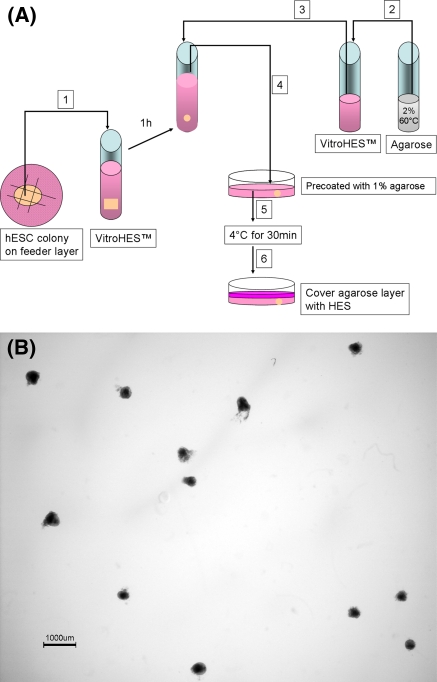
Fig. 1.
Overview of the agarose culture. a Schematic procedure according to materials and methods. The numbers in boxes indicates the procedure order. b Low magnification image of a 2 days old agarose culture of EBs from the SA002 hESC line. Each EB is fixed in place and can be followed individually through out the culture time and harvested individually at any time point. Bar 1,000 μm
In vivo teratoma
In vivo teratoma of the SA002, AS034, SA121, SA167 hESC lines were performed as previously described (Heins et al. 2004). Briefly, undifferentiated hESC colonies were cut and injected under the kidney capsule or in the testicular lumen in SCID mice. The resulting tumors were allowed to develop for 8 weeks before the experiment was terminated. The teratomas were further processed as described bellow for morphological analysis.
Morphological analysis
Spherical or partly spherical EBs ≥200 μm in size were fixed in 4% paraformaldehyde for 2 h and embedded in paraffin. Teratomas were fixed in 4% paraformaldehyde over night. The agarose-cultured EBs were carefully dissected from the agarose and washed in phosphate buffered saline (PBS) before fixation. Paraffin embedded 4 μm thick serial sections of EBs were stained with Alcian Blue/van Gieson (1 week EBs) and Hematoxylin-Eosin (8 weeks EBs) in order to examine the histology. Sections were made in the middle of the EBs. Serial sections of the in vivo teratomas were stained with Hematoxylin-Eosin. The sections were analyzed using light microscopy (Nikon optiphot 2-pol). Paraffin embedding and sectioning were done by Histocenter, Gothenburg, Sweden.
Immunohistochemical staining and analysis
The EBs were stained for specific proteins characteristically expressed in the individual embryonic germ layers; alpha fetoprotein (AFP) for endoderm, vimentin for mesoderm and β-tubulin for ectoderm. 4′,6-diamidino-2-phenylindole (DAPI) (Sigma–Aldrich, St. Louis, MO) was used as nuclear stain.
The serial sections from all 4 hESC lines were stained simultaneously. The EBs were fixed as above, embedded in paraffin and cut into 4 μm sections. The slides were deparaffinised in xylene (Merck, Damstadt, Germany) and hydrated through a series of 99, 95 and 70% ethanol baths (Kemetyl, Stockholm, Sweden) and subsequently incubated in PBS for 10 min. The sections were boiled in 0.1 M TRIS-buffer pH 9 (Merck) for first 8 min in 750 W and subsequently for 15 min in 350 W in a microwave oven for antigen retrieval, left to cool down to room temperature and rinsed in PBS. Endogenous peroxidase was quenched with 3% H2O2 (Merck) for 10 min. The slides were washed in PBS before individual treatment with one of the three germ layers marker antibodies as described below. Isotype control IgG was used as negative control on each analyzed slide and applied in the same concentration as the primary antibody. Negative control antibody was: monoclonal mouse isotype IgG1 (Biolegend, CA, USA).
Alpha fetoprotein
Permeabilisation of the cell membrane for AFP staining was done with 99.5% ethanol (Kemetyl) for 2 min. Blocking was done with blocking reagent supplied in the TSA™ Cyanine 3 System kit (PerkinElmer, Boston, MA, USA) for 30 min at room temperature. The monoclonal IgG1 mouse anti-human-AFP antibody (Invitrogen) was mounted and incubated overnight in humidity chamber at +4°C. At day 2, the sections were rinsed three times in PBS for 5 min each before they were incubated with the horse radish peroxidase coupled rabbit anti-mouse IgG1 antibody (Invitrogen) for 45 min in humidity chamber at room temperature. The slides were rinsed in PBS and incubated with fluorophore tyramide working solution supplied in the TSA™ Cyanine 3 System kit according to manufactures instructions. The sections were counterstained with DAPI for 3 min, rinsed in PBS and mounted in p-phenylenediamine anti-fade solution (PPD11).
Vimentin and β-Tubulin
The procedure for the vimentin and β-tubulin analysis was performed in the same way as for AFP with the following modifications. Cell membrane permeabilisation was conducted with 0.5% Triton-X (Sigma–Aldrich) in PBS for 5 min and the primary antibodies were: monoclonal IgG1 mouse anti-human-vimentin antibody (Sigma–Aldrich) for vimentin and monoclonal IgG1 mouse anti-β-tubulin antibody (Invitrogen) for β-tubulin.
The sections were counterstained with DAPI for 3 min, rinsed in PBS and mounted in PPD11.
All sections were finally analyzed and photo-documented using a Nikon Eclipse 90i fluorescence microscope (Nikon) with the NIS elements D 3.0 imaging software (Nikon).
Statistical analysis
Statistical analysis was performed using the Chi Squared test for trend in Fig. 2a and the two-sample student’s t test assuming equal variances in Fig. 2b.
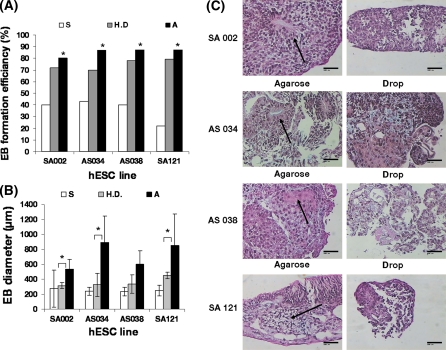
Fig. 2.
The agarose 3-D matrix support EB formation efficiency and EB growth. a EB formation efficiency of the hESC lines SA002, AS034, AS038 and SA121 when cultured for 1 week in suspension (S), hanging droplet (H.D) or agarose (A), respectively. Each bar represents the mean of two individual replicates where formation efficiency was calculated by dividing the number of EBs formed after 1 week by the number of colonies originally placed into the culture system. Each replicate was performed with 5–15 colonies, per hESC line, placed in each culture system. The Chi Squared test for trend was statistically significant for each hESC line (χ2 = 4,228; p < 0.050 for hESC line SA002, χ2 = 6,936; p < 0.010 for hESC line AS034, χ2 = 6,646; p < 0.010 for hESC line AS038 and χ2 = 15,026; p < 0.001 for hESC line SA121) indicating that EB formation efficiency increased when a more supportive culture milieu was used, i.e. EBs are hindered to adhere to the culture plate, agglomerate and dissolve. b EB size of the hESC lines SA002, AS034, AS038 and SA121 when cultured for 1 week in suspension (S), hanging droplet (H.D) or agarose (A), respectively. Each bar represents the mean of two individual replicates with 3 representative EBs, n = 6. *p < 0.05 with a two sample student’s t test. c The agarose 3-D matrix support differentiation of EBs from hESC. Histological sections stained with hematoxylin-eosin were made of EBs from the 4 hESC lines; SA002, AS034, AS038 and SA121 grown in agarose or hanging droplets for 1 week. The EBs cultured in agarose showed higher developed structures and higher extracellular matrix production (arrows) than EBs cultured in the hanging droplet system. Bar 100 μm
Statistical significance was set to p < 0.05. All statistical analyses were performed in SPSS 14.0 for windows (SPSS inc. Chicago, USA).
Results
Agarose 3-D matrix supports formation and growth of EBs from hESC
Two separate experiments with suspension, hanging droplet and agarose cultures with 4 hESC lines were performed in order to determine which method yielded the best EB formation efficiency and growth after 1 week of culture. After 1 week of culture the EB formation efficiency was significantly more stabile in the agarose system with EB formation efficiency between 80–90% compared to 70–80% in the hanging droplet culture and 20–40% in the suspension culture depending on the cell line analyzed (Fig. 2a). Furthermore, agarose culture yields EBs with up to 3 times larger diameter than the suspension or hanging droplet method (Fig. 2b). The 4 individual hESC lines exhibited different EB growth potentials where EBs from the AS034 and SA121 hESC lines grew up to 1.5 times larger in diameter than the SA002 and AS038 hESC line when cultured in agarose (Fig. 2b). Both the suspension and hanging droplet cultures suffered from dissolved EBs while the agarose culture did not (data not shown). The suspension cultures showed EBs with pronounced attachment to the culture dish as well as agglomeration between EBs while the agarose and hanging droplet cultures did not (data not shown).
Agarose 3-D matrix supports differentiation of EBs from hESC and enables long-period cultures
The EBs derived from the SA002, AS034, AS038 and SA121 hESC lines cultured during 1 week with the 3-D support from agarose developed a more differentiated morphology and produced more extracellular matrix than the EBs cultured for 1 week in the hanging droplet system (Fig. 2c). Suspension cultured EBs were too small or too few to analyze after 1 week of culture due to adhesion to the culture dish or dissolved EBs. Prolonged culture over 1 week in the hanging droplet system resulted in dissolved or non-growing EBs.
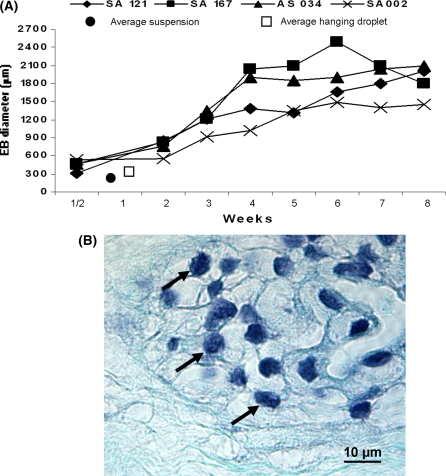
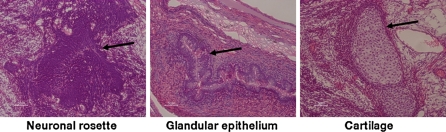
The 4 hESC lines; SA002, AS034, SA121 and SA167 were therefore only cultured in agarose for 8 weeks in order to evaluate the culture potential of the agarose system during long term cultures. The EB formation efficiency in the long term agarose culture experiments was 85 ± 5% after 8 weeks of culture depending on the used cell line (data not shown). The EBs from the 4 hESC lines grew to a medium size of 1,838 ± 248 μm in diameter after 8 weeks of agarose culture (Fig. 3a). Cells in the 8 weeks old EBs showed to have intact round shaped nuclei, which indicates the absence of necrotic or apoptotic cells (Fig. 3b). Morphology analysis showed different cell types both within the EBs from the same hESC line and between the EBs from the different hESC lines (Fig. 4). All hESC lines, except the SA002 cell line, developed cystic structures (data not shown). The corresponding 8 weeks old in vivo teratomas from the 4 hESC lines SA002, AS034, SA121 and SA167 developed the common morphological structures that indicate pluripotency, i.e. neuronal rosettes for ectoderm, cartilage for mesoderm and glandular epithelium for endoderm (Fig. 5).
Fig. 3.
Diagram of the EB growth of the 4 hESC lines when cultured in agarose 3-D matrix for 8 weeks. a Growth of the hESC lines SA002, AS034, SA167, SA121 when cultured in agarose for 8 weeks. All measure points represent the mean of six representative EBs. (filled circle) symbols the average size of the 4 hESC lines after 1 week of suspension culture from Fig. 2. (Open square) symbols the average size of the 4 hESC lines after 1 week of hanging droplet culture from Fig. 2. b The EBs from the 4 hESC lines; SA002, AS034, SA121 and SA167 showed all intact nuclei (black arrows) in the middle of the EBs cultured in agarose 3-D matrix for 8 weeks, here represented by the AS0034 hESC line. Staining = Hematoxylin-Eosin. Bar 10 μm
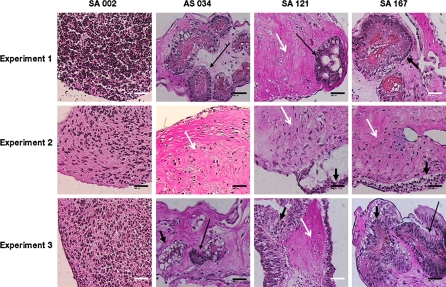
Fig. 4.
EBs from the 4 hESC lines; SA002, AS034, SA121 and SA167 cultured in agarose for 8 weeks developed heterogeneous differentiation patterns. Histological analysis with Alcian Blue-van Gieson staining showed differentiation heterogeneity both between EBs from the 4 hESC lines and between different EBs from the same hESC line. EBs from the AS034, SA121 and SA167 in three individual replicates developed highly differentiated areas with cell types from the three germ layers (thin black arrow for neuronal rosettes like structures representing ectoderm, white arrow for connective tissue representing mesoderm and thick black arrow for epithelia representing endoderm). The EBs from the SA002 cell line did not develop highly differentiated structures but produced extracellular matrix. Bar 100 μm
Fig. 5.
Representative in vivo teratoma structures of the three germ layers. Derivation of neuronal rosettes, glandular epithelium and cartilage represents ectodermal, endodermal and mesodermal germ layers, respectively. Each structure is marked by arrow and here derived from the SA 121 hESC line. Bar 50 μm
Expression of alpha fetoprotein, vimentin and β-tubulin in EBs from hESC grown in agarose for 8 weeks
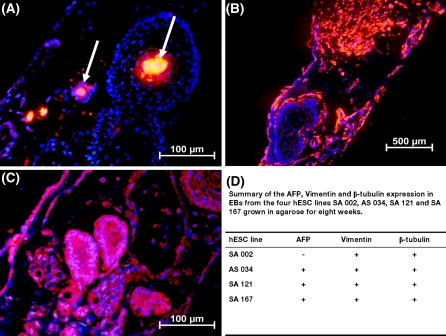
We analyzed the expression of three commonly used markers for the three germ layers, i.e. AFP for endoderm, vimentin for mesoderm and β-tubulin for ectoderm, in order to assess the development of the three germ layers in EBs from the 4 hESC lines grown in agarose 3-D matrix for 8 weeks. The immunohistochemistry analysis showed expression of vimentin and β-tubulin in all EBs from the 4 hESC lines SA002, AS034, SA121 and SA167 grown in agarose for 8 weeks. AFP expression was found in all EBs from the AS034, SA121 and SA167 hESC lines but was undetectable in the SA002 hESC line (Fig. 6). Negative control antibody showed no staining (data not shown). In summary, 3 out of 4 hESC lines expressed proteins representing the three germ layers after 8 weeks in agarose culture.
Fig. 6.
Agarose culture of EBs support formation of the three germ layers. Representative immunohistochemistry staining of a AFP (white arrow), b vimentin and c β-tubulin in EBs from the SA121 hESC line. Nuclear staining with DAPI. d Summary of AFP, vimentin and β-tubulin expression after 8 weeks of agarose EB culture
Discussion
This study demonstrates that EB formation and growth of 4 hESC lines is enhanced in an agarose based 3-D environment compared to the hanging droplet system and the regular suspension culture in bacteriological Petri dishes. The agarose matrix prevents the cell clusters from attaching to the culture dish and agglomerate. Further, agarose matrix provides mechanical stability and support, which enables the EBs to form without the risk of dissolving into smaller unproliferative cell clusters or single cells. These properties of agarose culture enhance the EB formation efficiency and enable clonal EB formation as compared to suspension culture. Additionally, the agarose system makes these improvements possible in a very convenient system, achievable in every laboratory, with uncomplicated medium changes as compared to the very laborious medium changes in the hanging droplet system. Dang et al. addressed the problem with non-clonal EB cultures due to agglomeration by culturing agarose encapsulated EBs in a stirred suspension system (Dang et al. 2004). However, this system was limited by an EB formation efficiency of 42 ± 15% and the EBs hatched from the encapsulation after 8 days, which makes this system inappropriate when performing long term cultures with external 3-D support. Agarose microcapsules have similarly been addressed as a culturing method for enclosed EBs (Sakai et al. 2008). Although these methods are functional they require new laborious techniques not present in all laboratories.
The EB-diameter was up to approximately 3 times larger for EBs grown in agarose than for EBs grown in hanging droplets for 1 week. We speculate that the enhanced EB growth could be due to the physical structural support from the agarose matrix that may transduce cytoskeletal signals as well as autocrine and/or paracrine signaling. The enhanced EB growth in the agarose system gives a considerable advantage as compared to the suspension and hanging droplet systems, as larger EBs are easier to analyze due to the larger size. Moreover, the larger mass makes the EBs easier to physically handle and may enable the researcher to study the local effect of injected growth factors and compare the effect to non-treated cells within the same EB. Another important advantage of the agarose culture is that, in contrast to suspension culture, the EBs are maintained in the same position throughout the whole differentiation experiment and can easily be followed during their development by several techniques, e.g. time-lapse studies or gene expression using confocal microscopy. The agarose system makes it possible to grow EBs for a longer time period with stable formation efficiency even after 8 weeks (85 ± 5%). This is possible because the EBs are kept physically intact and isolated from the culture dish and each other. The essential medium exchange in long term cultures is also easily done with the agarose system which is in contrast to the hanging droplet system where medium exchange is technically challenging, if not impossible. These methodological improvements make it practicable in the future to perform and evaluate long term studies of factors affecting EB differentiation, e.g. growth factors, signaling pathways and oxygen pressure (Schuldiner et al. 2000; Powers et al. 2008).
Pluripotency of embryonic stem cells is often concluded with the formation of in vivo teratoma, which contains cells from the three germ layers, when injecting clusters of embryonic stem cells in SCID mice (Prokhorova et al. 2008; Strom et al. 2007; Valbuena et al. 2006). The drawbacks of this technique is that in vivo teratoma does not show the differentiation potential of one single hESC colony clone as several hESC clusters are injected into the host animal. The teratoma could therefore be an effect of several hESC colonies contributing in concert to form the various cell types within the teratoma tissue. We evaluated if EBs grown in agarose for the same period of time as in vivo teratoma in SCID mice could form the three germ layers and thus conclude pluripotency of the used hESC line without the use of animal tests. Not surprisingly the results from the 8 weeks agarose study showed that the in vivo teratomas give more macroscopically developed tissue structures compared to the structures from the three germ layers developed by the corresponding agarose-cultured EBs. However, the results from the histological and immunochemistry analysis of long term EB cultures show that EBs grown in agarose are able to develop into the three germ layers in vitro. These results together with the possibility to follow the differentiation of one single colony make the agarose culture system an interesting in vitro alternative to in vivo teratoma when characterizing the pluripotency of many hESC lines.
The microenvironment can vary between individual EBs and different hESC lines have donor inherited genome diversities that may affect the differentiation of the EB (Wu et al. 2008). Immunohistochemical analysis of the EBs cultured in agarose for 8 weeks showed β-tubulin and vimentin expression in all EBs from the 4 hESC lines while AFP expression was found in EBs from the AS034, SA121 and SA167 hESC lines but was undetectable in the SA002 hESC line, which may be an effect of trisomy 13 in the SA002 hESC line (Heins et al. 2004). The absence of AFP expression in the SA002 hESC line is consistent with the histological analysis as, in this experiment, the SA002 hESC line did not develop into cystic structures that generally contains endodermal cell types. This result further emphasize the heterogeneity of differentiation properties between different hESC lines in different differentiation systems as the in vivo teratoma of the SA002 cell line gave good quality endodermal tissues.
The hESC lines in this experiment behaved in general similarly throughout the experiments with some exceptions. The histological analysis showed that there is a differentiation and growth heterogeneity both between different hESC lines and within EBs derived from the same hESC line. The differentiation and growth heterogeneity both between and within hESC lines indicate that there is a stochastic element in the EB culture system that affects the differentiation process within the same hESC line and that the genome variability between different hESC lines probably affects the differentiation potential of hESC derived from different donors. Our data supports the differentiation diversity demonstrated for neuronal differentiation where different hESC lines develop into different neuronal subtypes under the same differentiation conditions (Wu et al. 2007). These phenomena should be taken into consideration in all hESC differentiation studies and can be closely evaluated with the agarose culture system developed in this study.
In summary, the results from this study emphasize the necessity to consider the EB formation method used in differentiation studies of embryonic stem cells. We propose that agarose culture is a more appropriate EB culture system than the hanging droplet system and suspension culture, as the agarose 3-D system supports a higher EB formation efficiency, growth and has the advantage of studying individual EBs during a prolonged culture time.
Acknowledgments
The study was funded by: Swedish Research Council grant no. 2005-7544, The ALF/LUA research grant from the Sahlgrenska University Hospital. The Inga Britt and Arne Lundberg Research Foundation.
Abbreviations
- hESC
Human embryonic stem cell
- EB
Embryoid body
- EBs
Embryoid bodies
- SCID
Severe combined immunodeficiency
- 3-D
Three dimensional
- MEF
Mouse embryonic fibroblast
- PBS
Phosphate buffered saline
- AFP
Alpha fetoprotein
- DAPI
4′,6-diamidino-2-phenylindole
- Bfgf
Basic fibroblast growth factor
- PPD11
p-Phenylenediamine anti-fade solution
- IgG
Immuno globulin G
Footnotes
All work with the human embryonic stem cells was conducted according to the ISSCR Guidelines for the Conduct of Human Embryonic Stem Cell Research and with the approval of the local ethics committees at the University of Gothenburg and the University of Uppsala.
References
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bongso A, Fong CY, Ng SC, Ratnam S. Isolation and culture of inner cell mass cells from human blastocysts. Hum Reprod. 1994;9:2110–2117. doi: 10.1093/oxfordjournals.humrep.a138401. [DOI] [PubMed] [Google Scholar]
- Chen SS, Revoltella RP, Papini S, Michelini M, Fitzgerald W, Zimmerberg J, Margolis L. Multilineage differentiation of rhesus monkey embryonic stem cells in 3-D culture systems. Stem Cells. 2003;21:281–295. doi: 10.1634/stemcells.21-3-281. [DOI] [PubMed] [Google Scholar]
- Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15:254–259. doi: 10.1089/scd.2006.15.254. [DOI] [PubMed] [Google Scholar]
- Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins N, Englund MC, Sjoblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367–376. doi: 10.1634/stemcells.22-3-367. [DOI] [PubMed] [Google Scholar]
- Hopfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mol Biol. 2004;254:79–98. doi: 10.1385/1-59259-741-6:079. [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on 3-D polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci USA. 1975;72:1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers DE, Millman JR, Huang RB, Colton CK. Effects of oxygen on mouse embryonic stem cell growth, phenotype retention, and cellular energetics. Biotechnol Bioeng. 2008;101:241–254. doi: 10.1002/bit.21986. [DOI] [PubMed] [Google Scholar]
- Prokhorova TA, Harkness LM, Frandsen U, Ditzel N, Burns JS, Schroeder HD, Kassem M. Teratoma Formation by Human Embryonic Stem Cells is site-dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2008;18:47–54. doi: 10.1089/scd.2007.0266. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Sakai S, Hashimoto I, Kawakami K. Production of cell-enclosing hollow-core agarose microcapsules via jetting in water-immiscible liquid paraffin and formation of embryoid body-like spherical tissues from mouse ES cells enclosed within these microcapsules. Biotechnol Bioeng. 2008;99:235–243. doi: 10.1002/bit.21624. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren-Jansson E, Zetterstrom M, Moya K, Lindqvist J, Strehl R, Eriksson PS. Large-scale propagation of four undifferentiated human embryonic stem cell lines in a feeder-free culture system. Dev Dyn. 2005;233:1304–1314. doi: 10.1002/dvdy.20459. [DOI] [PubMed] [Google Scholar]
- Strom S, Inzunza J, Grinnemo KH, Holmberg K, Matilainen E, Stromberg AM, Blennow E, Hovatta O. Mechanical isolation of the inner cell mass is effective in derivation of new human embryonic stem cell lines. Hum Reprod. 2007;22:3051–3058. doi: 10.1093/humrep/dem335. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Valbuena D, Galan A, Sanchez E, Poo ME, Gomez E, Sanchez-Luengo S, Melguizo D, Garcia A, Ruiz V, Moreno R, Pellicer A, Simon C. Derivation and characterization of three new Spanish human embryonic stem cell lines (VAL −3 −4 −5) on human feeder and in serum-free conditions. Reprod Biomed Online. 2006;13:875–886. doi: 10.1016/S1472-6483(10)61038-3. [DOI] [PubMed] [Google Scholar]
- Wu H, Xu J, Pang ZP, Ge W, Kim KJ, Blanchi B, Chen C, Sudhof TC, Sun YE. Integrative genomic and functional analyses reveal neuronal subtype differentiation bias in human embryonic stem cell lines. Proc Natl Acad Sci USA. 2007;104:13821–13826. doi: 10.1073/pnas.0706199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Kim KJ, Mehta K, Paxia S, Sundstrom A, Anantharaman T, Kuraishy AI, Doan T, Ghosh J, Pyle AD, Clark A, Lowry W, Fan G, Baxter T, Mishra B, Sun Y, Teitell MA. Copy number variant analysis of human embryonic stem cells. Stem Cells. 2008;26:1484–1489. doi: 10.1634/stemcells.2007-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Dillon GP, Bellamkonda RB. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5:291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]