Abstract
Vascular endothelial growth factor (VEGF) promotes the growth of solid tumor mainly via VEGF receptor-1 and receptor-2, which are expressed preferentially in proliferating endothelial cells. Therefore, a strategy for simultaneous blockage of both VEGF receptors may have a useful therapeutic effect in tumor growth. In this study, we utilized a fusion protein which is composed of receptor binding domain of VEGF-A (RBDV) and the constant region fragment (Fc) of a human immunoglobulin G1 (IgG1), to interfere with the growth of human umbilical vein endothelial cells (HUVECs) via VEGF receptors. The results showed that RBDV-IgG1 Fc was able to bind with both VEGF receptor-1 and receptor-2. In addition, RBDV-IgG1 Fc could decrease VEGF-induced proliferation and tube formation among HUVECs. Moreover, the cytotoxic test showed RBDV-IgG1 Fc could also enhance the cytotoxic activity of human natural killing cells. The data are suggesting that the fusion protein, RBDV-IgG1 Fc, may have potential as an angiogenesis antagonist for future tumor therapy.
Keywords: Vascular endothelial growth factor, Receptor binding domain of VEGF-A, Immunoglobulin, Fusion protein, Human umbilical vein endothelial cells
Introduction
Vascular endothelial growth factor (VEGF) is a potent mitogen that stimulates angiogenesis in vitro and in vivo (Asano et al. 1995) and has been shown to play a central role in tumor angiogenesis (Yuan et al. 1996; Gerber et al. 1998, 1999). VEGF binds to the two distinct receptor tyrosine kinases, VEGF receptor-1 (Flt-1) and VEGF receptor-2 (KDR) (Waltenberger et al. 1994), which are expressed preferentially in the proliferating endothelial cells of blood vessels (Plate et al. 1993). VEGF receptor-1 has a number of conserved kinase motifs and response by this receptor is an important part of blood vessel formation; deficiency in this receptor causes embryonic lethality (Fong et al. 1995). In contrast to VEGFR-1, VEGFR-2 is regarded as the major mediator of mitogenesis and survival of endothelial cells (Ferrara 2004). Both receptors are important for angiogenesis and therefore a drug that is able to interfere simultaneously with the activity of both VEGFR-1 and VEGFR-2 may have a significantly better therapeutic effect on tumor formation by causing major suppression of new vessel formation.
Many antagonists have been shown to successfully suppress angiogenesis processes either in vivo or in clinical trials. Certain molecules with a low molecular weight have been developed to inhibit the proliferation and migration of endothelial cells, one example being TNP-470 (Ingber et al. 1990; Moulton et al. 1999). Others have been developed to impair the activity of the tyrosine kinases of the VEGF receptors in order to abolish angiogenesis signalling (Smith et al. 2004). In addition, a humanized anti-VEGF monoclonal antibody (Willett et al. 2004), anti-VEGF receptor-2 antibody (Prewett et al. 1999) and a soluble VEGF receptor chimeric protein (Holash et al. 2002), have been developed with the aim of suppressing new vessel formation in tumors by interfering with the interactions between VEGF and the two VEGF receptors. Moreover, a recent study has indicated that recombinant therapeutic antibodies may be more useful than small chemical molecules because they provide a better anti-angiogenestic effect because of their longer half-life in vivo (Glennie and van de Winkel 2003).
In our previous study, a fusion protein composed of the “receptor binding domain of VEGF” (RBDV, from amino acids 1 to 109 of VEGF-A) and the constant region fragment (Fc) domain of immunoglobulin G1 (from the hinge region to the CH3 domain) was designed and constructed (Tseng et al. 2010). This fusion protein, RBDV-IgG1 Fc, could result in tumor inhibition by reducing angiogenesis in mouse model in vivo. Therefore, in this study, we examined the effects of RBDV-IgG1 Fc on human umbilical vein endothelial cells (HUVEC) as a model for future development of human drug.
Materials and methods
Construction of pAAV-MCS/IgG1 Fc and pAAV-MCS/RBDV-IgG1 Fc
RBDV-IgG1 Fc and IgG1 Fc fusion protein were constructed according the procedures in the previous study (Tseng et al. 2010). Briefly, the PCR fragments of RBDV were ligated prior to the fragment encoding the Fc portion of IgG1 and the chimeric transgene was further subcloned into the pAAV/MCS vector (Stratagene, La Jolla, CA, USA), with the poly his6-tag at the C terminals. For the IgG1 Fc, a DNA fragment encoding the IL-2 leader sequence was fused with the Fc portion of human IgG1 and the transgene was subcloned into the pAAV/MCS vector (Stratagene).
Cell Culture
Primary cultures of human umbilical vein endothelial cells (HUVECs) were kindly provided by Dr. Ko-Jiunn Liu (Nation Health Research Institute, Taipei, Taiwan) and were cultured in Medium 199 (Gibco Invitrogen Co., Grand Island, NY, USA) which was supplemented with substances according to the instructions of American Type Culture Collection (ATCC, Manassas, VA, USA). Human epithelial kidney (HEK) 293T cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Gaithersburg, MD, USA), supplemented with 10% fetal bovine serum (FBS; Biological Industries, Kibbutz Beit Haemek, Israel) and 1% penicillin–streptomycin amphotericin B solution (PSA; Biological Industries). NK-92 MI were grown in minimum essential medium eagle alpha modification medium (Sigma, St. Louis, MO, USA) supplemented with 0.2 mM inositol (Sigma), 0.2 mM 2-mercapto-ethanol (Sigma), 0.02 mM folic acid (Sigma), 12.5% horse serum (Gibco), 12.5% FBS. All cells were incubated in an incubator with 5% CO2 at 37 °C.
Expression and purification of chimeric proteins
RBDV-IgG1 Fc and IgG1 Fc fusion protein were produced and purified according the procedures in the previous study (Tseng et al. 2010). Briefly, pAAV-MCS/IgG1 Fc and pAAV-MCS/RBDV-IgG1 Fc vectors were respectively transfected into 293T cells and incubated for 48 h, and the supernatants of the transfectants were collected and purified by Protein G-Agarose (Upstate Inc., Lake Placid, NY, USA), and the eluted fractions were further purified by a nickel-charged HisTrap HP affinity column (Amersham Biosciences, Piscataway, NJ, USA). Finally, the buffer was exchanged to PBS using a Sephadex G-25 prepacked column (Amersham Biosciences, Uppsala, Sweden) and the recombinant proteins were concentrated using the Microcon Centrifugal Filter Unit (Millipore, Bedford, MA, USA).
In vitro binding assay for the receptors of VEGF
A 96-well micro-titer plate (Nunc, Roskilde, Denmark) was previously coated with 0.4 μg/well of the extracellular domain 1–3 of human VEGFR-1 (R&D systems, Minneapolis, MN, USA) or the extracellular domain 1–7 of human VEGFR-2 (Merck-Calbiochem, San Diego, CA, USA). The purified proteins (1 μg in 100 μL of PBS) were applied respectively into the wells of the coated plate and incubated for 1 h. After washing, the reactive recombinant proteins were detected with horseradish peroxidase (HRP)-conjugated anti-his-tagged antibodies (Novus Biologicals, Littleton, CO, USA) for 1 h. Then, the reactions were developed by the addition of TMB substrate (KPL, Gaithersburg, MD, USA) for 20 min, stopped with 1N HCl for 10 min and measured (OD450) using an ELISA reader (Sunrise™ Tecan Group Ltd., Männedorf, Switzerland). The index is the average OD450 value of (sample–background)/(the group of PBS–background).
Cell surface binding assay
HUVECs were washed with PBS, detached by versene (0.2% EDTA in PBS, pH = 7.4), then washed and resuspended with flow cytometry buffer (1% bovine serum albumin in PBS, pH = 7.4). Ten microgram of purified RBDV-IgG1 Fc or 8 mg of IgG1 Fc were respectively incubated with the cell suspension for 3 h at 4 °C, followed by 1 h of incubation with FITC conjugated goat anti-human IgG antibody (Acris Antibodies GmbH, Hiddenhausen, Germany). The cells were washed with ice-cold flow cytometer buffer after incubation for three times. Cell pellets were suspended in 1 ml flow cytometer buffer and analyzed with a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA). Negative control is the cell stained with secondary antibody alone without the primary antibody.
In vitro proliferation assay for HUVEC
Five thousand HUVECs seeded on wells pre-coated with 50 μL 1% gelatine on a 96-well plate and were cultured with M199 containing 20% FBS, 25 units/ml heparin and 1% PSA for 16 h. The VEGF (8 ng/mL), IgG1 Fc (2.5 μg/mL), and RBDV-IgG1 Fc (2.5 μg/mL) were added to the wells respectively, and the proliferation rate of HUVECs was determined by MTS assay (Promega, Madison, WI, USA) after 3 days incubation. The survival percentages were calculated as the average of OD values for recombinant protein-treated cells/the average of OD values for the untreated cells (PBS group).
Five thousand HUVECs seeded on wells pre-coated with 50 μL 1% gelatine on a 96-well plate were cultured with M199 containing 20% FBS, 25 units/mL heparin and 1% PSA for 16 h. Subsequently, the purified recombinant proteins were added into the well 2 h before human VEGF (Upstate Inc.) treatment, which were supplied at a concentration of 8 ng/mL of VEGF. For the 72 h incubation, the proliferative responses were detected by MTS reagent (Promega) and measured by spectrophotometric analysis at 492 nm. The survival percentages were calculated as the average of OD values for recombinant protein-treated cells/the average of OD values for the untreated cells (PBS group).
Tube formation assay
Growth factor-reduced Matrigel 10 μL (BD Biosciences) was coated on each well of a 96-well culture plate, and allowed to polymerize for 2 h at 37 °C. PBS alone, IgG1 Fc (10 μg/mL) in PBS, and RBDV-IgG1 Fc (10 μg/mL) in PBS were respectively added, and incubated with 1.5 × 104 HUVECs in the gel-coated wells for 1 h, and VEGF (Upstate Inc.) was later added (final concentration was 15 ng/mL). As the control, HUVECs were treated with PBS without VEGF. The cells were incubated for a further 16 h at 37 °C in assay medium (M199 with 20% FBS and 25 units/mL heparin), and tube formations were observed and photographed under an inverted light microscope (Olympus, Tokyo, Japan). The total number of tubes were counted and analyzed.
NK killing assay
HUVECs were detached with 0.2% EDTA in PBS, then washed and resuspended in assay medium (M199 with 20% FBS and 25 units/mL of heparin). Ten thousand target cells per well were respectively incubated with recombinant proteins at a final concentration of 32 μg/mL of IgG1 Fc or 40 μg/mL of RBDV-IgG1 Fc for 1 h, or 1 μg/mL of VEGF for 20 min and then treated with 40 μg/mL of RBDV-IgG1 Fc for another 1 h. Simultaneously, NK 92-MI effector cells were washed and resuspended at 10,000 cells/well in 100 μL of minimum essential medium eagle alpha modification medium (as described before). And then effector cells were mixed with target cells, which have been cultured with recombinant proteins as described above, at an E/T ratio of 1:1 (N/N) and transferred to 1% gelatin coated 96-well round-bottom plates (Corning Inc., Corning, NY, USA). Plates were centrifuged for 4 min at 250 × g, and incubated for further 5 h and analyzed by MTS assay. As negative control, HUVECs were cultured with NK92-MI cells without recombinant proteins (NK cells groups). As system control, the group of target cells cultured in the condition containing neither effector cells nor recombinant proteins was present as 100% of proliferation rate of HUVECs.
Statistical analysis
Statistical analyses were performed using SPSS statistics software (SPSS Inc., Chicago, IL, USA). The nonparametric Wilcoxon Mann–Whitney test was used when comparing two independent samples. A value of p < 0.05 was considered significant (2-tailed).
Results
The binding activities of RBDV-IgG1 Fc to human VEGF receptors
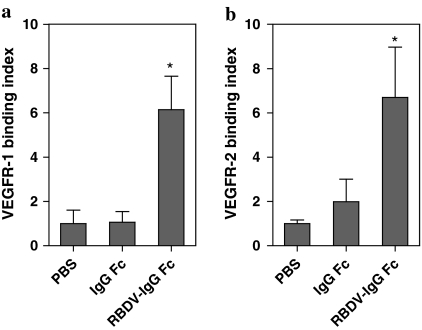
In the previous study, RBDV-IgG1 Fc was designed and constructed by fusing the receptor binding domain of human VEGF-A with the Fc portion of human IgG1, and this fusion protein could be effective in anti-angiogenesis in vitro and suppressing tumor growth in a mouse model (Tseng et al. 2010). To examine whether the RBDV-IgG1 Fc could also bind with human VEGFR-1 and VEGFR-2, immobilized human VEGFR-1 and VEGFR-2 were respectively incubated with the RBDV-IgG1 Fc fusion protein, and IgG1 Fc as negative control. The results showed that the RBDV-IgG1 Fc fusion protein was able to bind with both immobilized VEGFR-1 (Fig. 1a) and immobilized VEGFR-2 (Fig. 1b), but not IgG1 Fc. We therefore demonstrated that this fusion protein could bind with human VEGFRs via the RBDV as expected.
Fig. 1.
The binding activity of RBDV-IgG1 Fc to human VEGF receptors. The binding activity of the RBDV-IgG1 Fc with a immobilized hVEGFR-1or b hVEGFR-2 was measured by TMB substrate at 450 nm. *p < 0.05 as compared with the data of PBS group. Shown is the mean ± SD of two independent experiments performed in duplicate
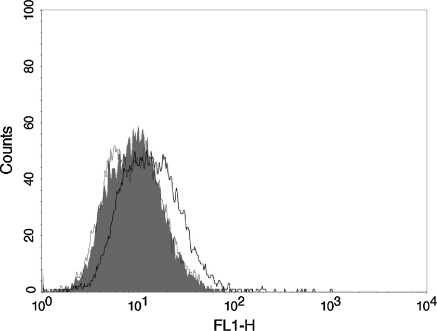
To further confirm the binding ability of RBDV-IgG1 Fc with VEGFRs, we stained HUVECs expressing the VEGFR-1 and VEGFR-2 with the RBDV-IgG1 Fc or IgG1 Fc. Figure 2 shows that the RBDV-IgG1 Fc but not IgG1 Fc could bind with HUVECs, suggesting that this binding was via the RBDV to VEGFRs.
Fig. 2.
The binding ability of RBDV-IgG1 Fc to HUVECs. The human umbilical vein endothelial cells (HUVECs) were stained with RBDV-IgG1 Fc (black thick line), IgG1 Fc (black thin line), or without fusion protein (grey shadow), followed by FITC-conjugated goat anti-human IgG antibody. Immunofluorescence of the stained cells was measured by flow cytometer
In vitro potency of RBDV-IgG1 Fc as antagonist
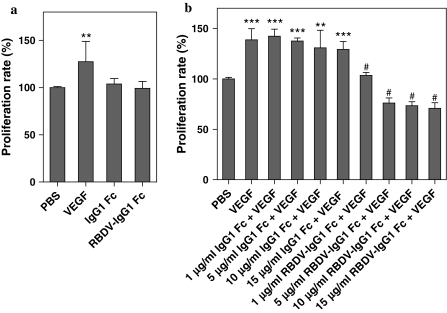
As mentioned above, RBDV-IgG1 Fc could bind with human VEGFRs, we therefore examined whether the RBDV-IgG1 Fc would have the undesired side effect to induce the proliferation of human endothelial cells upon binding to the VEGFRs on the cell surface. The results showed that the addition of VEGF to HUVECs caused an increase in cell proliferation. However, in the presence of RBDV-IgG1 Fc, as well as with IgG1 Fc alone, the HUVECs did not show any significant increase in proliferation. It is concluding that neither the RBDV nor IgG1 Fc portions would affect the proliferation of HUVECs.
Next, the antagonistic potential of RBDV-IgG1 Fc in the presence of VEGF was determined. The HUVECs were pre-cultured with RBDV-IgG1 Fc or IgG1 Fc, and then supplemented with VEGF. The results indicated that 1 μg/ml RBDV-IgG1 Fc was able to reduce the level of cellular proliferation in the presence of VEGF as a supplement, and the trend of decrease in proliferation of HUVEC was in dose-dependent manner (Fig. 3b). In contrast, IgG1 Fc had no significant effect in inhibiting the cell proliferation with treatment of VEGF.
Fig. 3.
The effect of RBDV-IgG1 Fc on the proliferation of HUVECs. a HUVECs were incubated with different proteins for 72 h and proliferation profile was determined by MTS assay. **p < 0.01 as compared with the data of PBS group as a control (100%). b HUVECs were pre-cultured in the presence of different amounts of IgG1 Fc or RBDV-IgG1 Fc. After treating with VEGF for 72 h, the proliferation profile was determined by MTS assay. PBS: treatment with PBS without VEGF as negative control. VEGF: treatment with PBS and then VEGF as positive control. **p < 0.01 and ***p < 0.001 as compared with the data of PBS group; #p < 0.001 as compared with the data of VEGF group. Shown is the mean ± SD of two independent experiments performed in duplicate
Taken together, RBDV-IgG1 Fc fusion protein retained the receptor-binding activity but have lost the ability to trigger the angiogenic signal.
In vitro potency of RBDV-IgG1 Fc in inhibiting angiogenesis
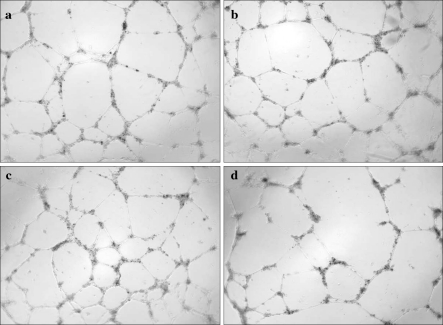
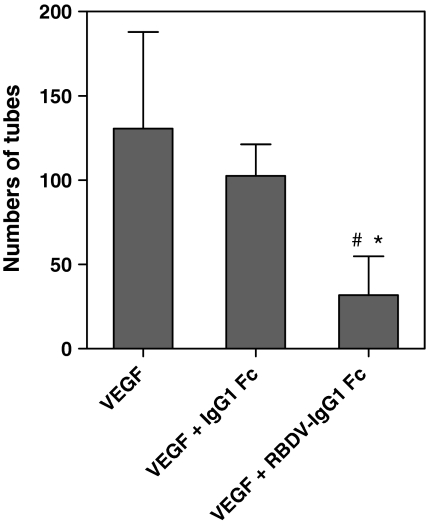
As mentioned before, the inhibition of angiogenesis could suppress tumor growth. Therefore, we examined the antagonistic effect of RBDV-IgG1 Fc on tube formation of endothelial cells by microscopy in vitro. The photographs showed that HUVECs could form a network under normal condition (Fig. 4a), and VEGF could increase network numbers of tube formation (Fig. 4b). However, the network numbers of capillary-like tube formation in HUVECs were inhibited by RBDV-IgG1 Fc (Fig. 4d) but not IgG1 Fc (Fig. 4c). And gathering statistics, Fig. 5 indicated that the RBDV-IgG1 Fc could significantly inhibit the VEGF-induced tube formation.
Fig. 4.
The effect of RBDV-IgG1 Fc to HUVECs on tube formation. a Under normal conditions, HUVECs form a network of tubes as seen under a microscope (100 X magnification). HUVECs were also treated with b PBS, c IgG1 Fc, and d RBDV-IgG1 Fc, then, incubated in medium containing VEGF for 16 h to monitor the effects on tube formation
Fig. 5.
HUVEC network formation as function of treatment with VEGF, VEGF + IgG1 Fc or VEGF + RBDV-IgG1 Fc. Three random fields were counted per well and total network numbers of tube formation in HUVECs were counted after treatment of different proteins and VEGF. #p < 0.05 as compared with the data of VEGF group; *p < 0.05 as compared with the data of VEGF + IgG1 Fc group. Shown is the mean ± SD of two independent experiments performed in duplicate
The effect of RBDV-IgG1 Fc to human natural killer cells
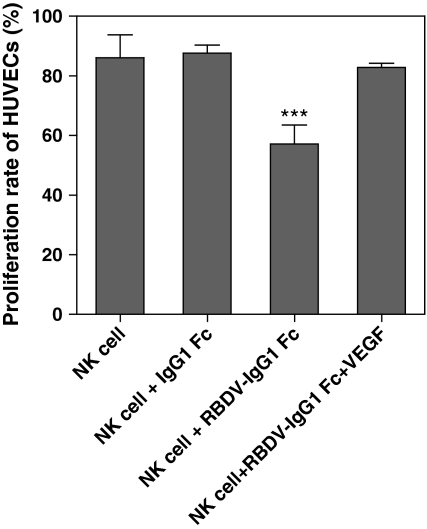
Humanized antibodies, such as rituximab or rituxan, have been shown in clinical cancer therapy, that its effectiveness relied on the cytotoxicity functions of human natural killer cells (NK cells) (Eisenbeis et al. 2004; Gluck et al. 2004). We therefore examined whether the Fc portion of RBDV-IgG1 Fc could also enhance the cytotoxicity of NK cells. The targeted cells, HUVECs, were pre-cultured with RBDV-IgG1 Fc and mixed with NK effector cells. The results revealed that NK cells could slightly decrease the survival rates of HUVECs; however, the RBDV-IgG1 Fc fusion protein but not IgG1 Fc could significantly increase the cytotoxic activity of NK cells against HUVECs (Fig. 6). In addition, this phenomenon could be reversed when pre-culturing the HUVECs with VEGF before treatment with RBDV-IgG1 Fc, suggesting the cytotoxicity of NK cells was enhanced via the binding of the RBDV domain of RBDV-IgG1 Fc to VEGFRs.
Fig. 6.
The effect of RBDV-IgG1 Fc on human natural killer cells (NK cells). NK-92MI cells (effector cells) mixed with HUVECs (targeted cells) at E/T ratio of 1:1 were co-incubated with IgG1 Fc, RBDV-IgG1 Fc, or VEGF plus RBDV-IgG1 Fc for 5 h, and the cytotoxic activity was determined by MTS assay. The HUVECs alone were used as system control (100%). ***p < 0.001 as compared with the data of NK cells group. Shown is the mean ± SD of two independent experiments performed in duplicate
Discussions
Inhibition of the VEGF/VEGFR signal pathway has been shown to efficiently suppress pathological angiogenesis in tumors and this has led to the development of various clinical VEGF inhibitors. In the previous study, we designed and produced a new chimeric protein, an RBDV-IgG1 Fc, by fusing a DNA fragment encoding the receptor binding domain of human VEGF-A (from amino acid 8 to 109 of VEGF-A) (Keyt et al. 1996; Muller et al. 1997) with a DNA fragment encoding the Fc portion of human IgG1 (Tseng et al. 2010). This antagonistic fusion protein was found to abolish the physiological interactions between VEGF and its two receptors, human VEGFR-1 and VEGFR-2 (Fig. 1). In addition, RBDV-IgG1 Fc seemed to retain the receptor-binding ability without the activity to trigger the angiogenic signal (Figs. 2, 3a). One possible reason for this is that a fusion protein did not possess the heparin binding region (amino acid 110–165) of VEGF, which has been demonstrated to simultaneously increase signal amplitude and duration when VEGF binds to its receptors (Ito and Claesson-Welsh 1999).
The stimulation of endothelial cells by growth factors, such as VEGF, is required for the process of angiogenesis (Shibusa et al. 1998). HUVECs express the two major VEGF receptors on their cell surface: VEGFR-1 is expressed at a few thousand copies per cell, while VEGFR-2 is relatively abundant on the cell surface (Waltenberger et al. 1994). Furthermore, VEGFR-2 is a tyrosine kinase and the binding of VEGF to this receptor leads to activation through phosphorylation of a tyrosine residue (Heldin 1995). Therefore, the mode of action of RBDV-IgG1 Fc during the protein’s inhibition of VEGF-stimulated mitogenesis (Fig. 3b) might be caused by a direct blocking of VEGF/VEGFR-2 interaction. In addition, Fong et al. (1995) and Hiratsuka et al. (2005) have proposed that disturbance of VEGFR-1 activity may result in vascular malformation. RBDV-IgG1 Fc also has the ability to decrease tube formation by endothelial cells (Figs. 4, 5), which may be a result of blocking of the VEGF/VEGFR-1 interaction. In conclusion, the VEGF-mediated angiogenic responses that occur with HUVECs were strikingly decreased by RBDV-IgG1 Fc in this study, which agrees with the hypothesis that this chimeric protein is able to bind and affect the activity of both receptors.
Several strategies that focus on VEGF binding antagonists have been reported; these include an anti-VEGF antibody (Asano et al. 1995; Mesiano et al. 1998), an anti-VEGFR-2 receptor antibody (Prewett et al. 1999), RNA-based aptamers (Ruckman et al. 1998) and various peptides (Bae et al. 2000; Binetruy-Tournaire et al. 2000). Here, we described an antagonistic fusion protein, RBDV-IgG1 Fc, which possesses a theoretical advantage over the above approaches because it targets both VEGF receptors. Furthermore, this IgG1-like fusion protein may be a better choice than smaller molecules, such as peptides, because it should provide not only a longer pharmacokinetic half-life in vivo, but also other immunological functions such as antibody-dependent cellular cytotoxicity (ADCC) and complement reactions, which have been proven in a mouse model in vitro and in vivo (Tseng et al. 2010). In addition, the RBDV-IgG1 Fc recombinant protein would seem to form a tetramer that is stabilized by disulfide bridges between the receptor binding domains of VEGF (Muller et al. 1997). Caron et al. (1992) and Wolff et al. (1993) proposed that IgG homodimerization by chemical coupling can increase the antitumor activity of antibodies against several different tumor antigens and that this involves ADCC, complement-dependent cytotoxicity, or direct killing. Furthermore, in clinical cancer therapy, the humanized antibodies, Rituximab or Rituxan, have been shown to have a cytotoxic effect with NK cells (Eisenbeis et al. 2004; Gluck et al. 2004). Figure 6 indeed showed RBDV-IgG1 Fc also could enhance cytotoxic activity of NK cells. Therefore, we suggest that RBDV-IgG1 Fc may also be able to inhibit tumor growth in clinical trials due to the Fc domain of protein acting directly against the endothelial cells of the tumor’s blood vessels.
Taken together, our results indicated that fusion of the receptor binding domain of the VEGF sequence to the human IgG1 Fc region produced a protein that was able to significantly bind with both human VEGF receptor-1 and receptor-2, and therefore antagonized the activity of VEGF. This protein also displayed anti-angiogenesis and anti-tube formation to the human epithelium cells, and could enhance the cytotoxic activity of human NK cells. In summary, our study suggests that RBDV-IgG1 Fc is worth for future development as clinical therapeutic agents against tumors.
Acknowledgments
This study was supported by grants from the National Science Council, Taipei, Taiwan (NSC97-2313-B-009-002, NSC-97-2320-B-040-005-MY3) and in part by the ATU-MOE project. This study was also supported by National Chiao Tung University (VGHUST94-P5-28) and Hualien Armed Forces Hospital.
Footnotes
Feng-Jen Tseng, Yen-Ku Liu, Yo-Shong Chung are the authors contributed equally to this work.
References
- Asano M, Yukita A, Matsumoto T, Kondo S, Suzuki H. Inhibition of tumor growth and metastasis by an immunoneutralizing monoclonal antibody to human vascular endothelial growth factor/vascular permeability factor121. Cancer Res. 1995;55:5296–5301. [PubMed] [Google Scholar]
- Bae DG, Gho YS, Yoon WH, Chae CB. Arginine-rich anti-vascular endothelial growth factor peptides inhibit tumor growth and metastasis by blocking angiogenesis. J Biol Chem. 2000;275:13588–13596. doi: 10.1074/jbc.275.18.13588. [DOI] [PubMed] [Google Scholar]
- Binetruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, Plouet J, Derbin C, Perret G, Mazie JC. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000;19:1525–1533. doi: 10.1093/emboj/19.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron PC, Laird W, Co MS, Avdalovic NM, Queen C, Scheinberg DA. Engineered humanized dimeric forms of IgG are more effective antibodies. J Exp Med. 1992;176:1191–1195. doi: 10.1084/jem.176.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbeis CF, Grainger A, Fischer B, Baiocchi RA, Carrodeguas L, Roychowdhury S, Chen L, Banks AL, Davis T, Young D, Kelbick N, Stephens J, Byrd JC, Grever MR, Caligiuri MA, Porcu P. Combination immunotherapy of B-cell non-Hodgkin’s lymphoma with rituximab and interleukin-2: a preclinical and phase I study. Clin Cancer Res. 2004;10:6101–6110. doi: 10.1158/1078-0432.CCR-04-0525. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Glennie MJ, Winkel JG. Renaissance of cancer therapeutic antibodies. Drug Discov Today. 2003;8:503–510. doi: 10.1016/S1359-6446(03)02714-4. [DOI] [PubMed] [Google Scholar]
- Gluck WL, Hurst D, Yuen A, Levine AM, Dayton MA, Gockerman JP, Lucas J, Denis-Mize K, Tong B, Navis D, Difrancesco A, Milan S, Wilson SE, Wolin M. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-hodgkin’s lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin Cancer Res. 2004;10:2253–2264. doi: 10.1158/1078-0432.CCR-1087-3. [DOI] [PubMed] [Google Scholar]
- Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Kataoka Y, Nakao K, Nakamura K, Morikawa S, Tanaka S, Katsuki M, Maru Y, Shibuya M. Vascular endothelial growth factor A (VEGF-A) is involved in guidance of VEGF receptor-positive cells to the anterior portion of early embryos. Mol Cell Biol. 2005;25:355–363. doi: 10.1128/MCB.25.1.355-363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- Ito N, Claesson-Welsh L. Dual effects of heparin on VEGF binding to VEGF receptor-1 and transduction of biological responses. Angiogenesis. 1999;3:159–166. doi: 10.1023/A:1009008926710. [DOI] [PubMed] [Google Scholar]
- Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996;271:5638–5646. doi: 10.1074/jbc.271.10.5638. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998;153:1249–1256. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci USA. 1997;94:7192–7197. doi: 10.1073/pnas.94.14.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- Prewett M, Huber J, Li Y, Santiago A, O’Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Welsh L, Janjic N. 2’-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273:20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- Shibusa T, Shijubo N, Abe S. Tumor angiogenesis and vascular endothelial growth factor expression in stage I lung adenocarcinoma. Clin Cancer Res. 1998;4:1483–1487. [PubMed] [Google Scholar]
- Smith JK, Mamoon NM, Duhe RJ. Emerging roles of targeted small molecule protein-tyrosine kinase inhibitors in cancer therapy. Oncol Res. 2004;14:175–225. doi: 10.3727/000000003772462298. [DOI] [PubMed] [Google Scholar]
- Tseng FJ, Chen YC, Lin YL, Tsai NM, Lee RP, Chung YS, Chen CH, Liu YK, Huang YS, Hwang CH, Lai YK, Liao KW (2010) A fusion protein with the receptor binding domain of vascular endothelial growth factor-A (VEGF-A) is an antagonist of angiogenesis in cancer treatment: Simultaneous blocking of VEGF receptor-1 and 2. Cancer Biol Ther 10:865–873 [DOI] [PubMed]
- Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EA, Schreiber GJ, Cosand WL, Raff HV. Monoclonal antibody homodimers: enhanced antitumor activity in nude mice. Cancer Res. 1993;53:2560–2565. [PubMed] [Google Scholar]
- Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]