Abstract
While use of mammography is limited, due to concerns related to radiation exposure, Dual Energy X-ray Absorptiometry (DXA), commonly available in medical care settings, is characterized by low radiation exposure. In the current paper, we compared breast density measured by DXA with mammographic density in 101 adult women who had a screening mammogram during the last 2 years. DXA scans of both breasts were taken using a clinical DXA system calibrated to measure breast density. The total projected breast area was manually delineated on each image and percent fibroglandular volume density (%FGV), absolute fibroglandular volume, total breast area and volume were computed. After digitizing mammographic films, total breast area, dense area, and percent density (PD) were estimated using computer-assisted mammographic density assessment. Both DXA and mammographic measures showed high correlations between left and right breasts ranging from 0.85 to 0.98 (p <0.0001). Mean %FGV was 38.8±14.3%, and mean percent density was 31.9±18.2% for craniocaudal views and 28.3±16.2% for mediolateral views. The correlation between the two measures was 0.76 for both views (p <0.0001). Associations with common risk factors showed similar patterns for DXA and mammographic densities; in particular, the inverse associations with BMI and age at menarche were evident for both methods. Multilinear regression with stepwise selection indicated an explained variance of 0.56 for %FGV alone and of 0.58 for %FGV plus number of children. Despite some differences in methodology, the current comparison suggests that DXA may provide a low-radiation option in evaluating breast density.
Keywords: DXA, mammographic density, mammogram, breast, adult, risk factors
Mammographic density, the distribution of fat, connective, and epithelial tissues in the female breast, is strongly associated with breast cancer and has been used as a biomarker for breast cancer risk among adult women [1, 2]. However, use of X-ray based mammography is limited due to concerns related to radiation exposure. In contrast, Dual Energy X-ray Absorptiometry (DXA) is characterized by low radiation exposure and commonly available in medical care settings. As shown among 17 women, a commercial DXA device, when it is calibrated to measure breast density, provides a precise measure of breast composition in comparison with mammography [3]. This suggests that DXA may provide an additional tool for evaluating breast cancer risk with minimal radiation exposure. For now, DXA breast imaging serves as a research tool to investigate breast development in girls and young women. However, in the future it may play a role in individualized risk prediction [4]. In the current paper, we compared breast density measured by DXA and by regular mammography in women who took part in a study that included mothers and their adolescent daughters 10-16 years of age. An additional objective of this report was to explore a prediction model for mammographic density using the DXA measure in combination with demographic, anthropometric, and reproductive information.
Materials and Methods
Study design and procedure
The current analysis was conducted as part of a study that measured breast density and body-fat composition in adult women and their daughters using DXA. The project was approved by the Committee on Human Studies at the University of Hawaii and the Institutional Review Board of Kaiser Permanente (KP) Hawaii. We recruited women aged 30 years and older who had received a normal mammogram (defined as BIRADS categories 1 through 3) during the last 2 years and their daughters aged 10-16 years through KP Hawaii, a large health maintenance organization. We mailed 3,915 invitation letters to women and to girls in the respective age ranges over the course of 11 months. Potential participants were selected from the membership data base according to the age and mammographic criteria. From the 304 respondents, we excluded mothers who had no mammograms, a previous history of breast cancer or surgery, an abnormal mammogram, a previous biopsy, breast implants, or chronic health conditions that interfered with study participation. We excluded girls without breast development and mother-daughter pairs who were not biologically related or did not reside on Oahu. A few daughters were recruited though KP whose biological mother was not a KP member.
Of the 138 eligible mother-daughter pairs, 102 pairs completed the study visit. Prior to DXA scans, all mothers signed informed consent, answered a demographic questionnaire, and completed height and weight measurements in duplicate. The six women whose screening mammograms were not performed at KP signed a mammogram release authorization form for the respective clinics. In the questionnaire, participants reported ethnicity and reproductive factors, i.e., age at menarche, age at first live birth, number of children, menopausal hormone use, and the most recent menstrual period. Women whose last menstrual period was >1 year ago were classified as postmenopausal. Body mass index (BMI) was calculated using the height and weight and classified as normal (18.5 - <25), overweight (25 - <30) and obese (≥30 kg/m2).
DXA data collection
At the exam, a urine test excluded pregnancy in all participants. We performed DXA scans of both breast, as well as of the whole body, using the research scan protocol and software version 10.1 on a GE Lunar Prodigy Bone Densitometer (GE Healthcare). Prodigy utilizes an ultra low radiation and cadmium-zinc-telluride detector to convert X-rays into an electronic signal without the intermediate conversion to light. We used a custom thickness-step phantom made of reference materials to recalibrate the DXA device. Details of this phantom and mathematical equations to compute breast density and thickness were described elsewhere [5, 6]. After changing into a hospital gown, breast scans were taken on both breasts in the decubitus mediolateral position with the nipple positioned in a true lateral profile. A duplicate breast scan of the left breast was performed on a random 10% of subjects for quality control.
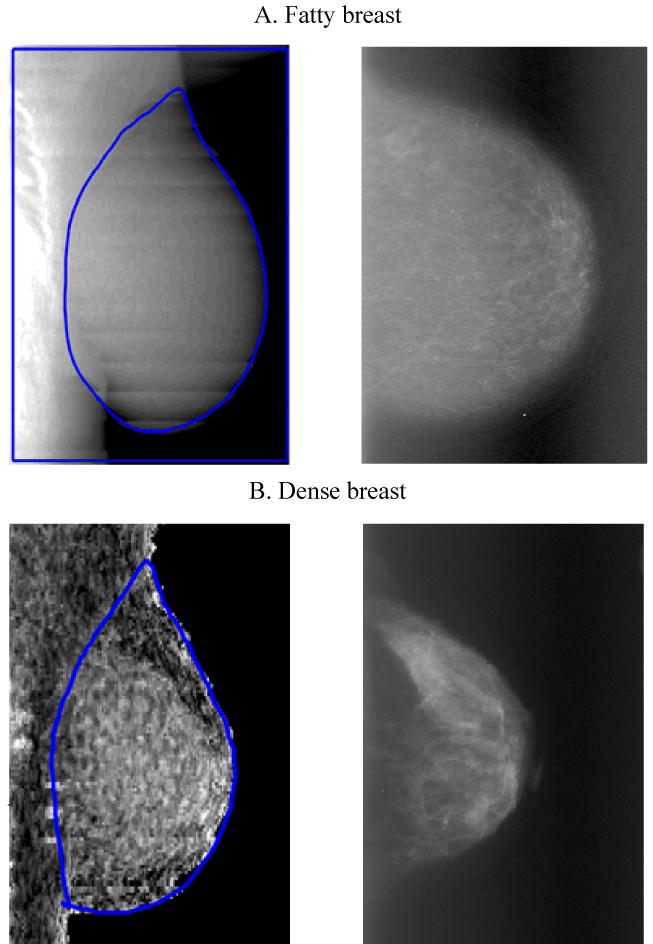
A low energy and high energy attenuation image was saved for each scan using the options available from GE Lunar for the research scan mode. These images were analyzed by the University of California at San Francisco using a Breast Density Workstation. The total projected breast area was manually delineated on each image by the same operator (Figure 1). From the bottom of the breast, the delineation followed the thoracic cage, then the pectoral muscle to continuously reach the exterior of the breast. Finally the external line was delineated. To calculate DXA density, a two-compartment model of adipose and fibroglandular tissue was used [7]. Scans of a calibrated phantom with known composition and thicknesses allowed the calculation of calibration curves. We computed total breast area, breast volume, absolute fibroglandular volume (FGV), and percent FGV (%FGV).
Figure 1. Breast images by DXA (left) and mammography (right).
For quality control, a calibrated phantom was scanned over 8 months, once every day if participants were scheduled, and once a week if no participant was scheduled. The phantom varied in thickness (2, 10, and 20 cm) and contained three %FGV values of 28%, 65%, and 100%. The phantom precision values ranged from 1.9% (10 cm in thickness and 65% composition) to 5.4% (2 cm in thickness and 65% composition). For the repeated breast scans, a root mean square standard deviation of 2.5 and a correlation of 0.975 were achieved.
Mammographic data
Craniocaudal (CC) and mediolateral (ML) views of screening mammograms taken within the last 2 years were scanned with a Kodak LS85 Film Digitizer for 101 women; one film could not be located. Ninety-five women had film mammography at KP, and 6 women had digital mammography at non-KP clinics and hospitals. All personal identifiers were removed from the scanned images. One of the authors (GM) performed computer-assisted density assessment using the Cumulus package [8, 9]; all mammograms for one woman were assessed during the same session. Using this interactive method, the reader selects a threshold value (gray scale on the screen) that best distinguishes the breast from the dark background and another threshold value, the gray value that best identifies the edges of the mammographically dense areas within the breast outline. The number of pixels in the two areas is then measured by the computer. The mammographic measures for our analysis included the total breast area and the dense area of the breast; percent density (PD) was calculated as the ratio of the two. In a sample of 49 duplicate mammographic readings, the correlations were 0.997 for the size of the total breast area and 0.959 for the dense breast area resulting in a coefficient of 0.955 for PD.
Statistical Analysis
All statistical analyses were performed using the SAS statistical software package version 9.2 (SAS Institute, Inc., Cary, NC). Correlations between right and left breasts measures were computed for both DXA and mammographic measures. The correlation between the two methods was evaluated using their corresponding measures, i.e., total area (cm2) by DXA and total area (cm2) by mammography, FGV (cm3) and dense area (cm2), and %FGV and PD. Because of the small sample size, subjects were grouped into 3 major ethnic categories: Caucasian, Asian (Japanese, Chinese, Filipino, Korean, and Other Asian), and Other (Hawaiian and other Pacific Islanders, Black, Native American, Hispanic, and Other). Correlation coefficients and analysis of variance were used to detect statistically significant differences in demographic characteristics by ethnic category. Mean DXA and mammographic measures were calculated by ethnicity and relevant risk factors, i.e., age, BMI, age at menarche, age at first live birth, number of children, menopausal status, and menopausal hormone use. To develop a prediction formula, we performed multilinear regression using the stepwise selection method with PD as dependent variable and %FGV and all covariates as independent variables. An α-level of 0.15 was used to select independent predictors for inclusion in the final model.
Results
The ethnic background of the study population was 31% Caucasian, 46% Asian, and 23% Other (Table 1). With one exception (age of 64 years), the ages ranged between 39 and 58 years. The three ethnic groups differed in BMI, age at menarche, and age at first live birth. The mean BMI was highest in the Other group (p = 0.01), while the mean age at menarche was lowest among Asians and highest among Others (p <0.001). Age at first live birth was higher in Asian and Caucasian than in Other women (p = 0.01). Mammographic density, DXA measures, menopausal status, number of children, and menopausal hormone use did not differ significantly by ethnicity although PD for the CC view was marginally significant (p = 0.08).
Table 1. Characteristics of study participants by ethnicity.
| Ethnic category | Caucasian | Asian | Other | All | p valuea |
|---|---|---|---|---|---|
| N | 32 | 47 | 23 | 102 | -- |
| Age (years) | 48.0±4.4 | 48.3±5.3 | 46.2±3.8 | 47.7±4.8 | 0.19 |
| BMI (kg/m2) | 26.6±5.8 | 26.5±5.6 | 31.1±5.8 | 27.5±5.9 | <0.01 |
| Age at menarche (years) | 12.9±1.6 | 11.9±1.2 | 13.3±1.9 | 12.5±1.6 | <0.001 |
| Age at first live birth (years) | 30.0±5.4 | 30.1±5.8 | 26.0±5.4 | 29.1±5.8 | 0.01 |
| Number of children (N) | 2.5±1.0 | 2.3±0.8 | 2.9±1.3 | 2.5±1.0 | 0.07 |
| Postmenopausal status (N)b | 9 | 12 | 7 | 28 | 0.89 |
| Menopausal hormone use (N) | 5 | 13 | 4 | 21 | 0.38 |
| Overweight/Obese (N)c | 9/8 | 17/8 | 7/12 | 33/28 | 0.02 |
| DXA total volume (cm3) | 819.5±521.0 | 746.6±448.2 | 1005.7±535.5 | 827.9±497.6 | 0.12 |
| total area (cm2) | 95.6±43.4 | 85.2±35.5 | 103.3±38.2 | 92.5±39.0 | 0.16 |
| FGV (cm3) | 275.9±103.7 | 259.9±112.8 | 298.6±102.3 | 273.7±107.7 | 0.37 |
| %FGV | 40.4±14.0 | 40.1±15.3 | 33.9±11.8 | 38.8±14.3 | 0.18 |
| Mammographic PD CC view | 30.9±19.6 | 35.8±17.4d | 25.5±16.2 | 31.9±18.2 | 0.08 |
| ML view | 29.8±18.4 | 29.9±15.6d | 23.3±13.5 | 28.3±16.2 | 0.23 |
p value based on analysis of variance or chi-square test
Last menstrual period more than one year ago; one woman had missing data.
Overweight: BMI 25-30 kg/m2; Obese: BMI ≥30 kg/m2
N=46; one film could not be located.
When comparing left and right breasts, both DXA and mammographic measures showed high correlations (Table 2) ranging from 0.85 to 0.98 (p <0.0001) with a stronger association for CC than ML views. In addition, correlation coefficients were higher for DXA measures (0.96 to 0.98) than mammographic measures (0.85 to 0.95).
Table 2. Summary of DXA and mammographic measures for left and right breasts.
| Lefta | Righta | rb | p | ||
|---|---|---|---|---|---|
| DXA (N=102) | Total volume (cm3) | 834.6±508.3 | 821.2±490.5 | 0.98 | <0.0001 |
| Total area (cm2) | 93.2±39.0 | 91.8±39.7 | 0.97 | <0.0001 | |
| FGV (cm3) | 275.0±109.8 | 272.3±108.1 | 0.96 | <0.0001 | |
| %FGV | 38.7±14.3 | 38.8±14.5 | 0.97 | <0.0001 | |
| Mammography (N=101) | Total area (cm2) CC view | 122.7±57.6 | 112.8±52.7 | 0.90 | <0.0001 |
| ML view | 130.8±53.6 | 125.8±54.1 | 0.95 | <0.0001 | |
| Dense area (cm2) CC view | 34.2±22.3 | 31.1±18.1 | 0.88 | <0.0001 | |
| ML view | 32.3±20.2 | 32.7±21.7 | 0.85 | <0.0001 | |
| PD (%) CC view | 31.7±18.4 | 32.2±18.8 | 0.90 | <0.0001 | |
| ML view | 28.1±16.9 | 28.6±16.7 | 0.87 | <0.0001 | |
| DXA and mammography | Total area (CC view) | 0.83 | <0.0001 | ||
| Total area (ML view) | 0.89 | <0.0001 | |||
| FGV and dense area (CC view) | 0.30 | <0.01 | |||
| FGV and dense area (ML view) | 0.36 | <0.001 | |||
| %FGV and mammographic PD (CC view) | 0.76 | <0.0001 | |||
| %FGV and mammographic PD (ML view) | 0.76 | <0.0001 |
Mean and standard deviations
Spearman rank order correlation coefficient.
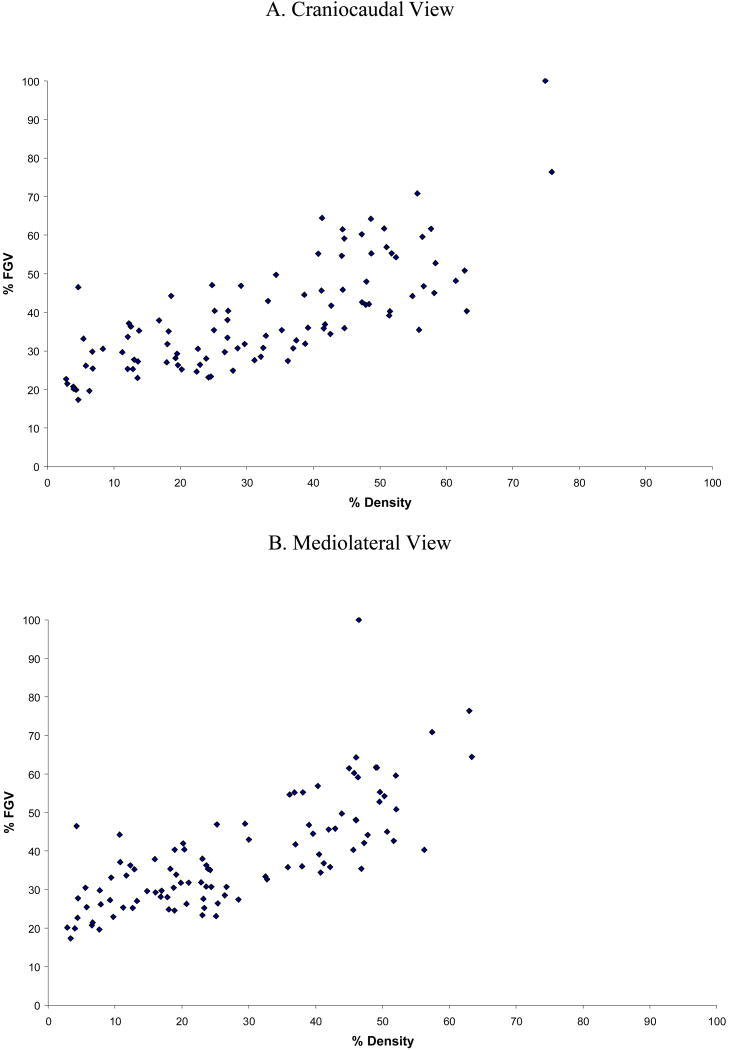
For the overall population, mean %FGV was 38.8±14.3, and mean PD was 31.9±18.2 for CC and 28.3±16.2 for ML views. PD in CC and ML views were strongly correlated with %FGV (Figure 2). The associations between DXA and mammographic measures were >0.80 for the total area and 0.76 for PD (p <0.0001 for both). However, the correlation coefficients between DXA FGV (cm3) and dense area (cm2), which possessed different dimensional characteristics, were 0.30 to 0.36 for CC and ML views (p <0.01 and <0.001, respectively).
Figure 2. Correlation of breast densities measured by DXA and mammography.
In evaluating the relation of DXA and mammographic measures with characteristics that have previously been found to be associated with breast density, we observed highly significant inverse associations of BMI with %FGV and PD (Table 3). The respective differences between normal weight and obese women were 22% and 25% (p <0.001 for both). As to reproductive characteristics, we observed non-significant, higher %FGV and PD in menopausal hormone users than non-users and significantly higher %FGV and PD among pre- than postmenopausal women. Age at menarche was positively associated with %FGV and PD (p <0.0001 and 0.04, respectively). Age and ethnicity did not show significant associations. DXA and mammographic density were non-significantly lower with more children (p = 0.47 and 0.09, respectively).
Table 3. DXA and mammographic measures by breast cancer risk factors.
| Risk factor | Category | N | FGV (%)a | pb | N | PD (%)a | pb |
|---|---|---|---|---|---|---|---|
| Age (years) | <45 | 34 | 41.1±16.7 | 33 | 32.0±18.0 | ||
| 45-49 | 37 | 36.9±11.7 | 37 | 33.9±17.5 | |||
| ≥50 | 31 | 38.5±14.3 | 0.45 | 31 | 29.5±19.4 | 0.59 | |
| Ethnicity | Caucasian | 32 | 40.4±14.0 | 32 | 30.9±19.6 | ||
| Asian | 47 | 40.1±15.3 | 46 | 35.8±17.4 | |||
| Other | 23 | 33.9±11.8 | 0.18 | 23 | 25.5±16.2 | 0.08 | |
| Body mass index (kg/m2) | 18.5-<25 | 41 | 48.8±15.0 | 41 | 42.7±15.8 | ||
| 25-<30 | 33 | 36.3±8.1 | 33 | 30.0±16.8 | |||
| ≥30 | 28 | 27.1±7.0 | <0.0001 | 27 | 17.8±12.0 | <0.0001 | |
| Age at menarche (years) | <13 | 60 | 34.5±10.5 | 59 | 28.8±16.7 | ||
| ≥13 | 42 | 44.9±16.6 | <0.001 | 42 | 36.2±19.4 | 0.04 | |
| Age at first live birth (years) | <30 | 60 | 38.4±15.2 | 59 | 29.2±18.2 | ||
| ≥30 | 42 | 39.2±13.3 | 0.76 | 42 | 34.9±17.9 | 0.11 | |
| Number of children (N) | 1-2 | 59 | 39.7±15.8 | 59 | 34.5±18.7 | ||
| ≥3 | 43 | 37.6±11.8 | 0.47 | 42 | 28.3±17.0 | 0.09 | |
| Menopausal statusc | Pre | 73 | 40.5±14.7 | 72 | 34.6±17.2 | ||
| Post | 28 | 34.3±12.5 | 0.05 | 28 | 25.8±19.3 | 0.03 | |
| Menopausal hormone use | Yes | 22 | 38.6±13.4 | 22 | 29.9±18.2 | ||
| No | 80 | 38.8±14.6 | 0.95 | 79 | 32.5±18.2 | 0.56 |
Mean and standard deviations of left and right breast measures (CC view for mammographic density)
Calculated by ANOVA as the trend across categories
One women had missing data; N=101
Multilinear regression with stepwise selection indicated an adjusted r2 of 0.56 for %FGV to PD both for the CC and the MLO views, and an adjusted r2 of 0.58 when the number of children was added. Other demographic and reproductive variables including menopausal hormone use did not contribute to the model. We also included a time variable describing the number of days between mammography and DXA. The mean time difference was 5.6 ± 4.2 months and inclusion of the time variable did not modify the regression estimates.
Discussion
The current study compared the breast density of adult women calculated from DXA images with mammographic density from screening mammograms. We observed moderate to high correlations between the two methods: 0.83-0.89 for total area, 0.30-0.36 for FGV and dense area and 0.76 for %FGV and PD. Moreover, the associations with demographic, BMI, and reproductive characteristics showed very similar patterns for DXA and mammographic density, in particular, the strong inverse association of PD with BMI was evident for both methods. In comparison with a previous report that computed a correlation of 0.52 between PD and %FGV [3], the current analysis evaluated DXA in a larger sample of women and found a stronger association between DXA and mammographic measures. Correlations between left and right measures were comparable in both DXA and mammography, and the linear association between DXA and mammographic measures were also strong. The correlation between FGV and dense area was considerably lower. This may be in part due to the breast compression used for mammography but not for DXA, but primarily it is due to the 3-dimensonial nature of FGV in contrast to the 2-dimensional mammograms. Other volumetric density methods suggest that percent density may be lower when assessed as a volumetric measure than as the 2-dimensional percentage of the breast area [10, 11]. As apparent from Figure 2, the lowest DXA density was approximately 20% while several women had mammographic density values below 20%. The reason for this is that DXA measures the breast as compartments of fibroglandular tissue and fat. In DXA, fat is used as a reference since the water content of adipose tissue changes and is not a stable reference. Thus, the breast does not get leaner than about 20%FGV, the fraction of water in adipose tissue. A similar result was presented by a report using magnetic resonance images (MRI) volume of fat and water to measure breast density [12].
Similar to DXA, a number of different methods are currently under investigation to examine breast density without radiation. Boyd et al [12] evaluated the use of MRI in 400 mother-daughter pairs and found a strong correlation (r = 0.85, p <0.0001) between percent breast water (as surrogate for fibroglandular-tissue level) calculated from MRI and mammographic density among 100 mothers. The same study also evaluated the use in young women 15-30 years and compared the results with density measures of their mothers. Investigators in Minnesota evaluated the use of ultrasound tomography images to assess breast density and found positive associations with mammographic density [13, 14]. Both MRI and ultrasound tomography are non-radiation methods and require no breast compression. One major advantage of DXA as compared to the other methods, both mammography and other novel approaches, is that it is widely available in clinics and hospitals, more than 30,000 systems worldwide, and relatively inexpensive. Unlike mammography, it provides three-dimensional data for density calculation, does not require a subjective interpretation of results [7], and has been explored as a research tool among young girls during pubertal development [6]. Furthermore, DXA provides additional information on whole body composition that may be useful in assessing additional breast cancer risk factors.
Some of the limitations of our analysis include the narrow age range of subjects, the exclusion of nulliparous women, and the lack of data in breast cancer cases. The combination of the restricted age range, the inclusion of only parous women, the high proportion of women who reported more than one ethnic background, and the small sample size are probably responsible for a lack of association of age and ethnicity with breast density in this study. Thus, our results need to be replicated in broader populations and should be interpreted with caution. Despite the great stability of mammographic density over time [15], breast density may change, especially during the menopausal transition or due to menopausal hormone therapy [16, 17]. Therefore, it was not ideal that we compared DXA and mammogram images taken at different times, but the time difference was not a significant predictor of PD in a multiple regression model with %FGV as an independent variable. Based on our previous study among premenopausal women [18], the mean annual change in PD is less than 2%; for 94 out of 101 women in the current analysis the time difference was less than one year. Thus, the differences should be very small. A strength of our study is the excellent quality control result. Duplicate readings of a subset of mammograms showed high reproducibility with a coefficient of 0.955 for PD. The calibration data and the repeated breast scans indicated high precision of the DXA data. Our multiethnic population strengthens the generalization of our results.
Our findings suggest that DXA has potential to provide a low-radiation option for evaluating breast density and may be useful as part of breast cancer risk prediction although it is unlikely to play a role in the detection of abnormalities [4, 19]. Its current use is restricted to being a research tool, but in the future, DXA imaging may allow the identification of high-risk girls and young women and make it possible to offer them early detection approaches and/or interventions to reduce breast cancer risk. The method has advantages of relative low cost, broad availability, and simultaneous provision body composition data. However, we need more imaging information from younger women and from breast cancer cases to evaluate the method.
Acknowledgments
The current project was supported by grant BC060615 from the Breast Cancer Research program of the Department of Defense and by a Research Centers in Minority Institutions award (P20 RR11091) from the National Center for Research Resources, National Institutes of Health. We thank all women and their daughters who participated in this study; Aleli Vinoya at KP Hawaii for her assistance with participant recruitment and database management; Jane Yakuma at the University of Hawaii's Clinical Research Center for data collection.
Footnotes
Conflict of Interest Statement: No conflicts of interest.
References
- 1.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 2.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd JA, Herve L, Landau J, Fan B, Kerlikowske K, Cummings SR. Clinical comparison of a novel breast DXA technique to mammographic density. Med Phys. 2006;33(5):1490–1498. doi: 10.1118/1.2193691. [DOI] [PubMed] [Google Scholar]
- 4.Tice JA, Cummings SR, Ziv E, Kerlikowske K. Mammographic breast density and the gail model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94(2):115–122. doi: 10.1007/s10549-005-5152-4. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd JA, Herve L, Landau J, Fan B, Kerlikowske K, Cummings SR. Novel use of single X-ray absorptiometry for measuring breast density. Technol Cancer Res Treat. 2005;4(2):173–182. doi: 10.1177/153303460500400206. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd JA, Malkov S, Fan B, Laidevant A, Novotny R, Maskarinec G. Breast density assessment in adolescent girls using dual-energy X-ray absorptiometry: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1709–1713. doi: 10.1158/1055-9965.EPI-08-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd JA, Kerlikowske KM, Smith-Bindman R, Genant HK, Cummings SR. Measurement of breast density with dual X-ray absorptiometry: feasibility. Radiology. 2002;223(2):554–557. doi: 10.1148/radiol.2232010482. [DOI] [PubMed] [Google Scholar]
- 8.Byng JW, Yaffe MJ, Jong RA, et al. Analysis of mammographic density and breast cancer risk from digitized mammograms. Radiographics. 1998;18(6):1587–1598. doi: 10.1148/radiographics.18.6.9821201. [DOI] [PubMed] [Google Scholar]
- 9.Gram IT, Bremnes Y, Ursin G, Maskarinec G, Bjurstam N, Lund E. Percentage density, Wolfe's and Tabar's mammographic patterns: agreement and association with risk factors for breast cancer. Breast Cancer Res. 2005;7(5):R854–R861. doi: 10.1186/bcr1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe MJ, Boone JM, Packard N, et al. The myth of the 50-50 breast. Med Phys. 2009;36(12):5437–5443. doi: 10.1118/1.3250863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kataoka M, Atkinson C, Warren R, et al. Mammographic density using two computer-based methods in an isoflavone trial. Maturitas. 2008;59(4):350–357. doi: 10.1016/j.maturitas.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Boyd N, Martin L, Chavez S, et al. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol. 2009;10(6):569–580. doi: 10.1016/S1470-2045(09)70078-6. [DOI] [PubMed] [Google Scholar]
- 13.Glide C, Duric N, Littrup P. Novel approach to evaluating breast density utilizing ultrasound tomography. Med Phys. 2007;34(2):744–753. doi: 10.1118/1.2428408. [DOI] [PubMed] [Google Scholar]
- 14.Glide-Hurst CK, Duric N, Littrup P. Volumetric breast density evaluation from ultrasound tomography images. Med Phys. 2008;35(9):3988–3997. doi: 10.1118/1.2964092. [DOI] [PubMed] [Google Scholar]
- 15.Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15(4):732–739. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- 16.Guthrie JR, Milne RL, Hopper JL, Cawson J, Dennerstein L, Burger HG. Mammographic densities during the menopausal transition: a longitudinal study of Australian-born women. Menopause. 2007;14(2):208–215. doi: 10.1097/01.gme.0000232278.82218.1f. [DOI] [PubMed] [Google Scholar]
- 17.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95(1):30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 18.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134(11):3089–3094. doi: 10.1093/jn/134.11.3089. [DOI] [PubMed] [Google Scholar]
- 19.Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101(6):384–398. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]