Abstract
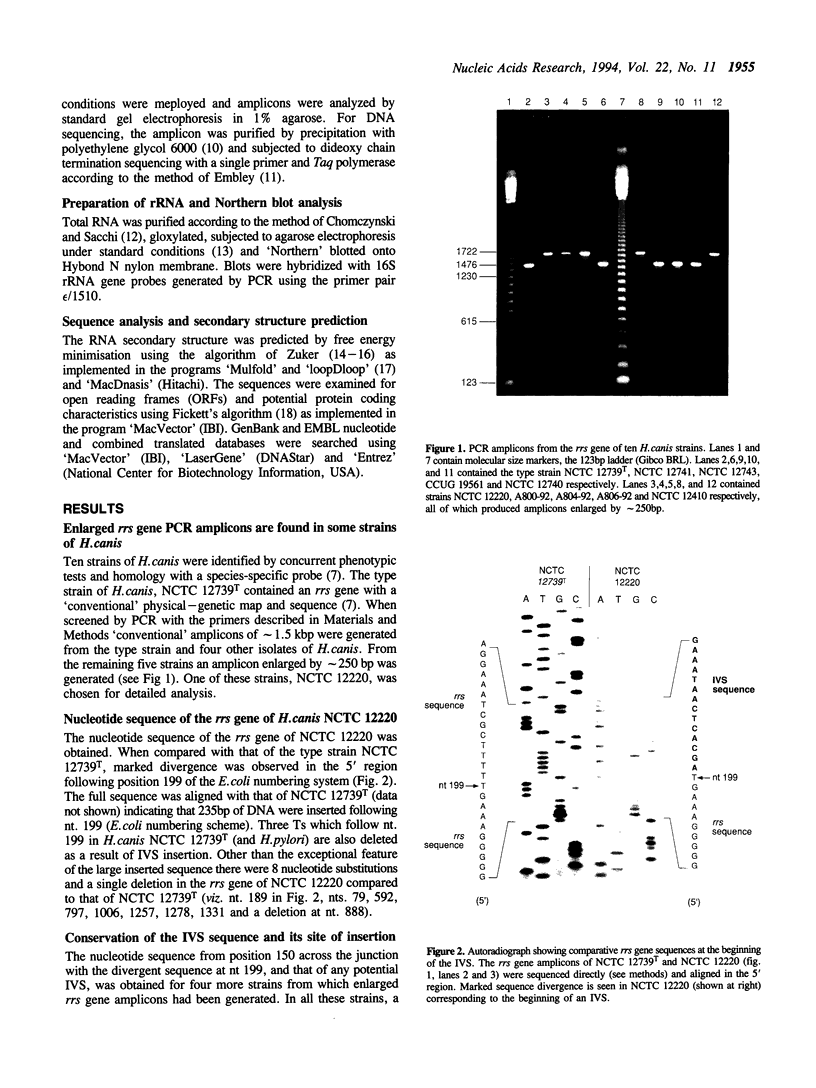
PCR amplicons enlarged by approximately 250bp were generated from the 16S rRNA (rrs) genes of certain strains of the recently described Helicobacter species, H. canis. The DNA sequence of the rrs gene of one such strain was determined, and it was shown that an intervening sequence (IVS) of 235bp followed nucleotide 199 in the rrs sequence. In four other H. canis strains, identical or similar IVSs were found, always at the same location in the rrs gene. The secondary structures of the RNA transcripts of the IVSs were predicted. They were characterised by the presence of a conserved stem-loop structure, a potential recognition site for RNA processing enzymes. Ribosomal RNA was compared from a strain of H. canis with and without the IVS-containing rrs gene. In the former 16S rRNA appeared as two fragments, whose sizes were consistent with cleavage at either side of the IVS, and which were not subsequently religated. The IVS sequence was not represented elsewhere in the H. canis genome. Its evolutionary significance is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopyanz N., Bukanov N. O., Westblom T. U., Berg D. E. PCR-based RFLP analysis of DNA sequence diversity in the gastric pathogen Helicobacter pylori. Nucleic Acids Res. 1992 Dec 11;20(23):6221–6225. doi: 10.1093/nar/20.23.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori: microbiology of a 'slow' bacterial infection. Trends Microbiol. 1993 Oct;1(7):255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- Burgin A. B., Parodos K., Lane D. J., Pace N. R. The excision of intervening sequences from Salmonella 23S ribosomal RNA. Cell. 1990 Feb 9;60(3):405–414. doi: 10.1016/0092-8674(90)90592-3. [DOI] [PubMed] [Google Scholar]
- Burnens A. P., Stanley J., Schaad U. B., Nicolet J. Novel Campylobacter-like organism resembling Helicobacter fennelliae isolated from a boy with gastroenteritis and from dogs. J Clin Microbiol. 1993 Jul;31(7):1916–1917. doi: 10.1128/jcm.31.7.1916-1917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Embley T. M. The linear PCR reaction: a simple and robust method for sequencing amplified rRNA genes. Lett Appl Microbiol. 1991 Sep;13(3):171–174. doi: 10.1111/j.1472-765x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D., Pan M. J., Zee Y. C., LeFebvre R. B. Unique ribosome structure of Leptospira interrogans is composed of four rRNA components. J Bacteriol. 1990 Jun;172(6):3478–3480. doi: 10.1128/jb.172.6.3478-3480.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D., Zee Y. C., Ingraham J., Shih L. M. Diversity of cleavage patterns of Salmonella 23S rRNA. J Gen Microbiol. 1992 Jan;138(1):199–203. doi: 10.1099/00221287-138-1-199. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Improved predictions of secondary structures for RNA. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7706–7710. doi: 10.1073/pnas.86.20.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusukawa N., Uemori T., Asada K., Kato I. Rapid and reliable protocol for direct sequencing of material amplified by the polymerase chain reaction. Biotechniques. 1990 Jul;9(1):66-8, 70, 72. [PubMed] [Google Scholar]
- Linton D., Dewhirst F. E., Clewley J. P., Owen R. J., Burnens A. P., Stanley J. Two types of 16S rRNA gene are found in Campylobacter helveticus: analysis, applications and characterization of the intervening sequence found in some strains. Microbiology. 1994 Apr;140(Pt 4):847–855. doi: 10.1099/00221287-140-4-847. [DOI] [PubMed] [Google Scholar]
- Ralph D., McClelland M. Intervening sequence with conserved open reading frame in eubacterial 23S rRNA genes. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6864–6868. doi: 10.1073/pnas.90.14.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Toivanen P. Intervening sequences (IVSs) in the 23S ribosomal RNA genes of pathogenic Yersinia enterocolitica strains. The IVSs in Y. enterocolitica and Salmonella typhimurium have a common origin. Mol Microbiol. 1991 Mar;5(3):585–593. doi: 10.1111/j.1365-2958.1991.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Smith N. H., Crichton P. B., Old D. C., Higgins C. F. Ribosomal-RNA patterns of Escherichia coli, Salmonella typhimurium and related Enterobacteriaceae. J Med Microbiol. 1988 Jul;26(3):223–228. doi: 10.1099/00222615-26-3-223. [DOI] [PubMed] [Google Scholar]
- Stanley J., Burnens A. P., Linton D., On S. L., Costas M., Owen R. J. Campylobacter helveticus sp. nov., a new thermophilic species from domestic animals: characterization, and cloning of a species-specific DNA probe. J Gen Microbiol. 1992 Nov;138(11):2293–2303. doi: 10.1099/00221287-138-11-2293. [DOI] [PubMed] [Google Scholar]
- Stanley J., Linton D., Burnens A. P., Dewhirst F. E., Owen R. J., Porter A., On S. L., Costas M. Helicobacter canis sp. nov., a new species from dogs: an integrated study of phenotype and genotype. J Gen Microbiol. 1993 Oct;139(10):2495–2504. doi: 10.1099/00221287-139-10-2495. [DOI] [PubMed] [Google Scholar]
- Sunday G. J., Gillespie M. J., Motley S. T., Zambon J. J. Atypical structure of the 23S ribosomal RNA molecule in certain oral bacteria. J Dent Res. 1991 Jun;70(6):961–965. doi: 10.1177/00220345910700061001. [DOI] [PubMed] [Google Scholar]
- Vandamme P., Falsen E., Rossau R., Hoste B., Segers P., Tytgat R., De Ley J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol. 1991 Jan;41(1):88–103. doi: 10.1099/00207713-41-1-88. [DOI] [PubMed] [Google Scholar]
- Winkler M. E. Ribosomal ribonucleic acid isolated from Salmonella typhimurium: absence of the intact 23S species. J Bacteriol. 1979 Sep;139(3):842–849. doi: 10.1128/jb.139.3.842-849.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]