Abstract
Leber hereditary optic neuropathy (LHON) and autosomal-dominant optic atrophy (DOA) are the two most common inherited optic neuropathies in the general population. Both disorders share striking pathological similarities, marked by the selective loss of retinal ganglion cells (RGCs) and the early involvement of the papillomacular bundle. Three mitochondrial DNA (mtDNA) point mutations; m.3460G>A, m.11778G>A, and m.14484T>C account for over 90% of LHON cases, and in DOA, the majority of affected families harbour mutations in the OPA1 gene, which codes for a mitochondrial inner membrane protein. Optic nerve degeneration in LHON and DOA is therefore due to disturbed mitochondrial function and a predominantly complex I respiratory chain defect has been identified using both in vitro and in vivo biochemical assays. However, the trigger for RGC loss is much more complex than a simple bioenergetic crisis and other important disease mechanisms have emerged relating to mitochondrial network dynamics, mtDNA maintenance, axonal transport, and the involvement of the cytoskeleton in maintaining a differential mitochondrial gradient at sites such as the lamina cribosa. The downstream consequences of these mitochondrial disturbances are likely to be influenced by the local cellular milieu. The vulnerability of RGCs in LHON and DOA could derive not only from tissue-specific, genetically-determined biological factors, but also from an increased susceptibility to exogenous influences such as light exposure, smoking, and pharmacological agents with putative mitochondrial toxic effects. Our concept of inherited mitochondrial optic neuropathies has evolved over the past decade, with the observation that patients with LHON and DOA can manifest a much broader phenotypic spectrum than pure optic nerve involvement. Interestingly, these phenotypes are sometimes clinically indistinguishable from other neurodegenerative disorders such as Charcot-Marie-Tooth disease, hereditary spastic paraplegia, and multiple sclerosis, where mitochondrial dysfunction is also thought to be an important pathophysiological player. A number of vertebrate and invertebrate disease models has recently been established to circumvent the lack of human tissues, and these have already provided considerable insight by allowing direct RGC experimentation. The ultimate goal is to translate these research advances into clinical practice and new treatment strategies are currently being investigated to improve the visual prognosis for patients with mitochondrial optic neuropathies.
Keywords: Dominant optic atrophy, Glaucoma, Hereditary spastic paraplegia, Leber hereditary optic neuropathy, Mitochondrial DNA, Mitofusin, Multiple sclerosis, Neuroprotection, Optic neuritis, Optic neuropathy, Retinal ganglion cell
1. Introduction
Inherited optic neuropathies affect at least 1 in 10,000 individuals and as a group, they represent an important cause of chronic visual impairment (Man et al., 2003; Newman and Biousse, 2004; Schaefer et al., 2008; Yu-Wai-Man et al., 2010a). Historically, these inherited optic nerve disorders were classified according to their mode of inheritance, and whether they were isolated or part of a more complicated syndromal variant. The identification of the underlying genetic defects in a large number of these inherited optic neuropathies now allows for a more accurate molecular classification, which has greatly benefited diagnostic accuracy and genetic counselling. The two classical prototypes are Leber hereditary optic neuropathy (LHON) and autosomal-dominant optic atrophy (DOA), which are both characterised by the preferential loss of retinal ganglion cells (RGCs) (Carelli et al., 2009). LHON is due to primary mitochondrial DNA (mtDNA) mutations, whereas the majority of patients with DOA harbour pathogenic mutations within the OPA1 gene, which codes for a mitochondrial inner membrane protein (Yu-Wai-Man et al., 2009b; Fraser et al., 2010). As the genetic basis for other inherited optic neuropathies were uncovered, it became apparent that mitochondrial dysfunction is a recurrent molecular theme underlying the loss of RGCs in these disorders. In this review, we will cover basic aspects of mitochondrial biology and genetics, and how disruption of these critical biological systems contributes to optic nerve degeneration in different mitochondrial disease models.
2. The mitochondria
2.1. Evolutionary origin
Mitochondria are ubiquitous intracellular organelles and they fulfil a fundamental role by providing most of the adenosine triphosphate (ATP) requirements of eukaryotic cells (DiMauro and Schon, 2003). The prevailing endosymbiotic hypothesis suggests that mitochondria evolved from aerobic α-proteobacteria, which were then gradually assimilated by primitive glycolytic eubacteria in a symbiotic relationship (Margulis, 1971; Gray et al., 1999). During evolution, the α-proteobacteria gradually transferred the majority of their genetic material to the eubacteria’s nuclear chromosomes, creating the prototypal eukaryotic cell (Gabaldon and Huynen, 2004). Phylogenetic comparison of mtDNA between modern humans and other organisms, including Rickettsia prowazekii, an α-proteobacterium, supports this common evolutionary origin for the mitochondrial genome (Martin and Muller, 1998; Gray et al., 1999).
2.2. Structure
Mitochondria are tubular-shaped organelles bounded by an outer and an inner membrane, and these delineate two distinct compartments: an intermembrane space and an internal matrix space (Frey and Mannella, 2000). The outer membrane allows passive diffusion of low molecular weight molecules up to 10 kDa, and this permeability is conferred by a family of channel-forming proteins known as porins, or voltage dependent anion channels (VDAC). The inner membrane is highly convoluted and these multiple infoldings, known as cristae, greatly increase its effective surface area (Perkins et al., 1997). Compared to the outer membrane, the inner membrane is relatively impermeable except for specific active transport channels, allowing an electrochemical gradient to be established across this barrier. The inner membrane also contains a number of highly specialised proteins, including the respiratory chain complexes and members of the mitochondrial membrane protease family. The mitochondrial matrix compartment contains mtDNA molecules packaged within nucleoid structures, and it is also the site of multiple metabolic pathways essential for normal cellular function: the citric acid cycle, β-oxidation of fatty acids, steroid, amino acid, and pyrimidine biosynthesis (Raha and Robinson, 2000).
2.3. Oxidative phosphorylation
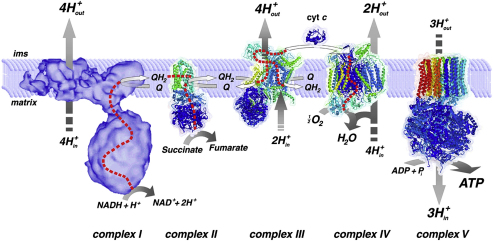
The mitochondrial respiratory chain comprises four multi-subunit polypeptide complexes (I–IV) which are embedded within the inner mitochondrial membrane (Fig. 1). The production of ATP is tightly regulated and it is the end-product of a process known as oxidative phosphorylation (OXPHOS). Acetyl-CoA, an intermediate product of glycolysis and β-oxidation, is metabolised further by the citric acid cycle to the reducing equivalents nicotinamide adenine dinucleotide hydrogen (NADH) and flavin adenine dinucleotide hydrogen (FADH2) (DiMauro and Schon, 2003). NADH and FADH2 are then re-oxidised by donating electrons to complexes I and II respectively. The energy released by the shuttling of these high energy electrons along the respiratory chain complexes allows protons to be pumped from the matrix compartment into the intermembrane space. Two additional carriers, ubiquinone (Co-enzyme Q10) and cytochrome c, also play critical roles in the efficient transfer of electrons through these successive oxidation–reduction reactions. The electrochemical gradient generated across the inner mitochondrial membrane is then used by complex V (ATP synthase) to catalyze the conversion of adenosine diphosphate (ADP) and inorganic phosphate (Pi) to ATP (Yoshida et al., 2001).
Fig. 1.
The mitochondrial respiratory chain and oxidative phosphorylation. Reproduced with permission from Nijtmans et al. (2004).
2.4. Mitochondrial genetics
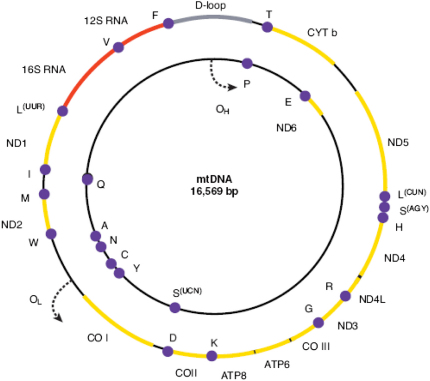
Mitochondria are unique in having their own genome in the form of a circular, double-stranded molecule 16,569 bp long (Fig. 2) (Anderson et al., 1981; Andrews et al., 1999b). It is a high-copy number genome with hundreds to thousands of mtDNA molecules per cell, depending on their specific energy requirements. MtDNA replicates continuously and this process is independent of nuclear genome replication, occurring in both mitotic and post-mitotic cells. The mitochondrial genome codes for 2 ribosomal RNAs (12S and 16S rRNA), 22 transfer RNAs (tRNAs), and 13 polypeptide subunits of the respiratory chain complexes. The majority of these genes is located on the H-strand, with only MTND6 and eight tRNA genes found on the L-strand (Taylor and Turnbull, 2005). MtDNA is a very compact molecule with overlapping gene regions devoid of introns, and a small 1.1 kb non-coding region, known as the D-loop, which is involved in mtDNA transcription and replication.
Fig. 2.
The human mitochondrial genome. Protein coding (yellow), rRNA (red), and tRNA (purple) genes are depicted on the heavy (H-, outer) and light (L-, inner) strands. The 22 tRNAs are indicated by their cognate amino acid letter code and the 2 rRNAs by their sedimentation coefficients (12S and 16S). The origins of mtDNA replication and the direction of synthesis are denoted by OH for the H-strand, and OL for the L-strand.
2.5. Mitochondrial haplogroups
The mitochondrial genome accumulates mutations at a significantly faster rate compared to the nuclear genome, and several factors contribute to this higher mutational rate: the absence of protective histones, the lack of effective repair mechanisms, the high mtDNA replication rate increasing the likelihood of errors, and the close proximity of mtDNA molecules to the respiratory chain complexes where they are exposed to high levels of reactive oxygen species (ROS) (Howell et al., 1996; Jazin et al., 1998; Raha and Robinson, 2000). The mutational rate varies between different mtDNA regions, and it is much faster within the two hypervariable regions (HVR I and II) of the D-loop where a mutation is estimated to occur every 30 maternal generations (Parsons et al., 1997; Siguroardottir et al., 2000). MtDNA is therefore highly polymorphic, and during human evolution, a number of relatively benign mitochondrial sequence variants have become fixed in different populations. As mtDNA is maternally inherited, these polymorphisms have accumulated sequentially along radiating female lineages, following the pattern of human migration from Africa into the various continents some 150,000 years ago (Cann, 2001). The human phylogenetic tree contains 18 major mtDNA haplogroups, and these comprise a total of 497 haplogroup-defining polymorphic variants (Torroni and Wallace, 1994; Herrnstadt et al., 2002). Individuals of European ancestry belong to one of nine haplogroups: H, I, J, K, T, U, V, W and X, with haplogroup H accounting for nearly half of all cases.
2.6. Heteroplasmy and mutational threshold
There are about 10,000 mtDNA molecules per cell, with each mitochondrion containing multiple copies. Two possible situations can therefore arise, known as homoplasmy and heteroplasmy (Lightowlers et al., 1997; Chinnery, 2002). In the heteroplasmic state, two or more mtDNA variants are present at a specific nucleotide position, and the same phenomenon can also occur for mtDNA re-arrangements such as deletions. Most mtDNA mutations are heteroplasmic, a feature which supports the concept of a mutational threshold for pathogenicity (Chinnery et al., 2000; Taylor and Turnbull, 2005). The relationship between mutational load and respiratory chain activity has been extensively investigated in different tissues, and the deleterious consequences of most mtDNA mutations on OXPHOS usually become apparent when the proportion of the mutant species exceeds 60–80% (Shoubridge et al., 1990; Bua et al., 2006; Durham et al., 2007). There are mutation- and tissue-specific variations in this biochemical threshold (Corral-debrinski et al., 1992; Chinnery et al., 1999; Wang et al., 2001; Nekhaeva et al., 2002), and although these could account for the pattern of organ involvement and clinical severity associated with a particular mtDNA defect, the molecular mechanisms are likely to be much more complex.
2.7. Nuclear-mitochondrial interactions
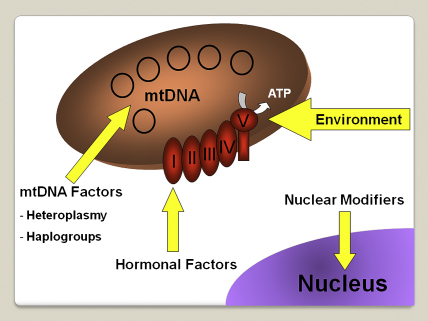
Mitochondria only have limited autonomy and they rely heavily on the nuclear genome for the majority of their structural and functional subunits (Fig. 3). Mitochondrial disorders can therefore arise secondary to both primary mtDNA mutations and nuclear genetic defects which disrupt mitochondrial-related proteins. In 2001, the first nuclear genes, POLG1 and PEO1, were identified among families with autosomal-dominant chronic progressive external ophthalmoplegia (CPEO) associated with multiple mtDNA deletions (Spelbrink et al., 2001; Van Goethem et al., 2001). Since then, the number of genes causing nuclear mitochondrial disorders has expanded continuously (Table 1), allowing significant progress to be made in elucidating the fundamental mechanisms that underpin mitochondrial physiology in both normal and disease states. As a result, we have gained a better understanding of the complex interactions between subunits of the respiratory chain complexes, and the crucial role played by accessory proteins in ensuring their proper assembly and stability along the inner mitochondrial membrane. These sometimes rare neurodegenerative and metabolic disorders have also provided important insights into the molecular components required for mtDNA maintenance, and the translational machinery that regulates intra-mitochondrial protein synthesis.
Fig. 3.
Mitochondrial and nuclear-encoded subunits of the mitochondrial respiratory chain complexes.
Table 1.
Nuclear mitochondrial disorders.
| Mutations involving structural subunits of the mitochondrial respiratory chain |
|---|
| Leigh syndrome: with complex I deficiency – mutations in NDUFS1, NDUFS4, NDUFS7, NDUFS8, NDUFV1; with complex II deficiency – mutations in SDHA |
| Cardiomyopathy and encephalopathy with complex I deficiency – mutations in NDFUS2 |
| Optic atrophy and ataxia with complex II deficiency–mutations in SDHA |
| Hypokalaemia and lactic acidosis with complex III deficiency – mutations in UQCRB |
| Mutations involving assembly factors of the mitochondrial respiratory chain |
| Leigh syndrome–mutations in SURF I and LRPPRC |
| Hepatopathy and ketoacidosis – mutations in SCO1 |
| Cardiomyopathy and encephalopathy – mutations in SCO2 |
| Leukodystrophy and renal tubulopathy – mutations in COX10 |
| Hypertrophic cardiomyopathy – mutations in COX15 |
| Encephalopathy, liver failure, and renal tubulopathy with complex III deficiency – mutations in BCS1L |
| Encephalopathy with complex V deficiency – mutations in ATP12 |
| Nuclear genetic disorders of intra-mitochondrial protein synthesis |
| Leigh syndrome, liver failure, and lactic acidosis – mutations in EFG1 |
| Lactic acidosis, developmental failure, and dysmorphism – mutations in MRPS16 |
| Myopathy and sideroblastic anaemia – mutations in PUS1 |
| Leukodystrophy and polymicrogyria – mutations in EFTu |
| Encephalomyopathy and hypertrophic cardiomyopathy – mutations in EFTs |
| Oedema, hypotonia, cardiomyopathy, and tubulopathy–mutations in MRPS22 |
| Hypotonia, renal tubulopathy, and lactic acidosis – mutations in RRM2B |
| Nuclear genetic disorders of mitochondrial protein import |
| Mohr–Tranebjaerg syndrome or deafness-dystonia-optic neuronopathy (DDON) syndrome – mutations in TIMM8A (DDP) |
| Early-onset dilated cardiomyopathy with ataxia (DCMA) or 3-methylglutaconic aciduria, type V–mutations in DNAJC19 |
| Nuclear genetic disorders of mitochondrial DNA maintenance |
| Chronic progressive external ophthalmoplegia – mutations in POLG1, POLG2, PEO1, SLC25A4, RRM2B, and OPA1) |
| Mitochondrial neurogastrointestinal encephalomyopathy – mutations in TYMP |
| Alpers syndrome–mutations in POLG1 and MPV17 |
| Infantile myopathy and spinal muscular atrophy – mutations in TK2 |
| Encephalomyopathy and liver failure – mutations in DGUOK |
| Hypotonia, movement disorder and/or Leigh syndrome with methylmalonic aciduria – mutations in SUCLA2 and SUCLG1 |
| Optic atrophy, deafness, chronic progressive external ophthalmoplegia, myopathy, ataxia, and peripheral neuropathy – mutations in OPA1 |
| Miscellaneous |
| Co-enzyme Q10 deficiency – mutations in PDSS2, APTX, COQ2, and ETFDH |
| Barth syndrome –mutations in TAZ |
| Cardiomyopathy and lactic acidosis associated with mitochondrial phosphate carrier deficiency – mutations in SLC25A3 |
Alpers syndrome: epilepsy, cortical blindness, micronodular hepatic cirrhosis, episodic psychomotor regression; Barth syndrome: cardiomyopathy, hypotonia, weakness, and neutropenia.
Nuclear mitochondrial disorders represent an important group of human diseases. They often pose significant diagnostic challenges related to their genetic and phenotypic heterogeneity, but they are increasingly being recognised, helped by greater clinical awareness and easier access to molecular genetic testing. A common feature shared by all these disorders is impaired mtDNA maintenance, which can lead to a reduction in mtDNA copy number, the accumulation of high levels of somatic mtDNA mutations, or both (Alberio et al., 2007; Chinnery and Zeviani, 2008; Spinazzola and Zeviani, 2009). The identification of these quantitative and qualitative mtDNA abnormalities in diagnostic specimens is therefore a key finding, suggesting an underlying nuclear defect, and helping to direct appropriate molecular investigations. MtDNA depletion is the pathological hallmark of several early-onset mitochondrial syndromes, and the clinical prognosis is often poor, due to the marked bioenergetic crisis caused by such a gross reduction in mtDNA copy number (Spinazzola et al., 2009). Interestingly, the observed mtDNA depletion can be highly tissue-specific, which partly explains the variability in disease presentation and severity.
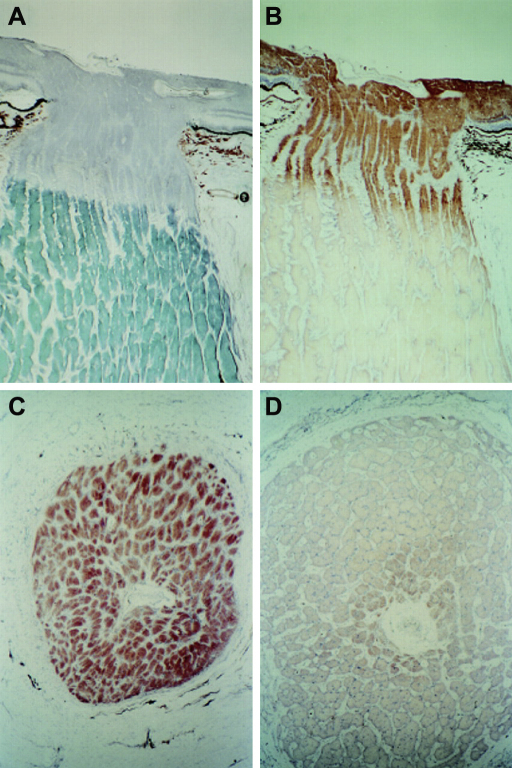
A mosaic pattern of cytochrome c oxidase (COX) deficient fibres is frequently observed in muscle biopsies of patients with both primary mtDNA and nuclear mitochondrial disorders, with some of these fibres exhibiting abnormal accumulation of mitochondria in the subsarcolemmal space, giving the classical appearance of “ragged-red fibres” (RRFs) (Fig. 4). For nuclear genetic defects involving POLG1 (Horvath et al., 2006; Tzoulis et al., 2006), POLG2 (Longley et al., 2006), PEO1 (Spelbrink et al., 2001), SLC25A4 (Kaukonen et al., 2000), TYMP (Nishino et al., 1999), and more recently OPA1 (Amati-Bonneau et al., 2008; Hudson et al., 2008; Stewart et al., 2008), the COX-defect is secondary to the accumulation of multiple mtDNA deletions, which have clonally expanded within individual cells to reach suprathreshold levels >70%. These deleted mtDNA species can be detected in homogenate DNA samples with Southern blot and long-range polymerase chain reaction (PCR), or more accurately quantified at the single-fibre level using real-time PCR assays (He et al., 2002; Taylor et al., 2004; Bua et al., 2006). As most mtDNA deletions involve critical tRNA and protein-encoding genes, OXPHOS is adversely affected, and this eventually leads to apoptotic cell loss and tissue dysfunction. Mutations in POLG1 and TYMP have also been linked with the accumulation of somatic mtDNA point mutations (Del Bo et al., 2003; Nishigaki et al., 2003; Wanrooij et al., 2004). Although still speculative and controversial, these point mutations could compromise the replication machinery located within the D-Loop, thereby contributing to the formation of mtDNA deletions. It is fascinating that different mutations within the same gene can result in such a varied spectrum of secondary mtDNA abnormalities. The clarification of the secondary factors which dictate whether depletion, deletions, or point mutations predominate will provide crucial insights into the underlying disease mechanisms in nuclear mitochondrial disorders (Chinnery and Zeviani, 2008).
Fig. 4.
Skeletal muscle sections illustrating the characteristic histochemical features of mitochondrial dysfunction: (A) Ragged-red muscle fibre identified using the modified Gomori trichome stain. The red component of the staining mixture is selectively sequestered by mitochondria, which have accumulated in the subsarcolemmal region, giving the fibre an irregular red outline, (B) Serial section of the same muscle fibre after SDH staining. This is a more specific assay for detecting the subsarcolemmal accumulation of mitochondria, SDH being a specific marker for complex II activity, (C) Abnormal COX–SDH histochemistry from a patient with chronic progressive external ophthalmoplegia (CPEO) due to a single 5 kb mtDNA deletion, showing normal COX-positive (Brown) and energy deficient, COX-negative (Blue) muscle fibres.
3. Leber hereditary optic neuropathy
3.1. Epidemiology
The North of England has been relatively stable in terms of migratory flux, with a population of about three million, predominantly white, inhabitants (Fig. 5). As a result of the nature of healthcare provision in this region, over the past 20 years, patients with unexplained visual failure and suspected inherited optic neuropathies have been referred to the neuro-ophthalmology (PYWM and PGG) and neurogenetics (PFC) services in Newcastle upon Tyne for further assessment. This centralised referral pattern, in addition to active contact tracing, allowed us to determine for the first time the prevalence of LHON in a defined geographical region (Man et al., 2003). We found a minimum estimate of 1 in 31,000, and fairly comparable prevalence figures of 1 in 39,000 and 1 in 50,000 have since been reported in Dutch and Finnish population studies, respectively (Spruijt et al., 2006; Puomila et al., 2007). In Australia, the cause of visual impairment for about 2% of individuals on the national blind registry was optic atrophy secondary to LHON (Mackey and Buttery, 1992).
Fig. 5.
The minimum prevalence of inherited optic nerve disorders in the North of England.
3.2. Primary mitochondrial DNA mutations
The majority of patients with LHON (90–95%) harbour one of three primary mtDNA point mutations: m.3460G>A (Howell et al., 1991a; Huoponen et al., 1991), m.11778G>A (Wallace et al., 1988), and m.14484T>C (Johns et al., 1992; Mackey and Howell, 1992). The m.11778G>A mutation was identified in 1988 by Wallace et al. (1988), and it is of special historical significance, being the first mtDNA substitution confirmed to cause human disease. The most prevalent LHON mutation in Northern Europe, Australia, and the Far East is m.11778G>A (Mackey et al., 1996; Mashima et al., 1998; Yen et al., 2002), but as a result of a founder event, m.14484T>C is the most common mutation (87%) among French Canadians. A number of other pathogenic mtDNA LHON variants have since been reported (Table 2), with some still awaiting full confirmation for pathogenicity, having been identified in only single families (Taylor et al., 2003). The distribution of these rarer mtDNA defects is not uniform, and the MTND1 and MTND6 gene regions are thought to be “mutational hotspots”, harbouring other LHON-causing mutations, in addition to m.3460G>A and m.14484T>C (Chinnery et al., 2001b; Valentino et al., 2004; Fraser et al., 2010). On close questioning, up to 60% of affected individuals report other family members with a pattern of early-onset visual failure, and a detailed family history should always be sought in suspected inherited optic neuropathy cases. De novo m.3460G>A and m.14484T>C mutations have been reported in LHON, but these are rare (Biousse et al., 1997; Man et al., 2003). Most presumed singleton cases are therefore probably due to difficulties in tracing back a more extensive family history.
Table 2.
Pathogenic mtDNA LHON mutations.
These mtDNA mutations are definitely pathogenic. They have been confirmed in ≥2 independent LHON pedigrees and show segregation with affected disease status.
As a follow-on to our original epidemiological study, about 3000 umbilical cord blood samples from a local birth cohort in the North of England were screened for specific mtDNA point mutations (Elliott et al., 2008). Nine healthy neonates were found to harbour one of the three primary LHON mutations, about 1 in every 350 births. In six of these neonates, the mutation was heteroplasmic, and of these cases, four were present at mutational levels less than 70%. There is clearly a large pool of these primary LHON mutations in the general population, and for heteroplasmic variants, the “mitochondrial bottleneck” will introduce shift in mitochondrial allele frequencies among future generations, directly influencing the risk of disease expression. The “mitochondrial bottleneck” is thought to be a protective mechanism which allows the rapid removal of deleterious mtDNA mutations from the genetic pool (Khrapko, 2008; Cree et al., 2009). The fertilised oocyte contains over 100,000 mtDNA molecules and during the early stages of development, there is a dramatic reduction in mtDNA copy number, down to 200–2000 copies before mtDNA replication is re-initiated. This decrease in the number of mitochondrial genomes repopulating the offspring of the next generation causes a sampling effect and accounts for the rapid changes in heteroplasmy levels. A pathogenic mtDNA variant would either be lost during transmission to the next generation, or it would quickly reach suprathreshold levels within an oocyte, increasing the likelihood of developmental arrest and its elimination. Even if a mature oocyte carrying a high proportion of the mutant species is successfully fertilised and a live birth results, there is a high probability that the affected individual’s fertility will be subnormal, which again serves to limit the transmission of mtDNA mutations. This situation clearly does not apply to LHON, as there is no evidence that mutational carriers have reduced fertility, and the majority of individuals, both affected and unaffected, are homoplasmic for the mtDNA mutation (Section 3.4.1).
3.3. Clinical manifestations
3.3.1. Pre-symptomatic phase
In some asymptomatic LHON carriers, fundal abnormalities such as telangiectatic vessels around the optic discs, and fluctuating levels of retinal nerve fibre layer oedema have previously been observed (Nikoskelainen et al., 1996; Savini et al., 2005; Quiros et al., 2006). More detailed psychophysical testing can also uncover more subtle features of optic nerve dysfunction in some individuals, with loss of colour discrimination along the red–green axis, minimal central visual field changes on automated static perimetry, reduced contrast sensitivity, and subnormal visual electrophysiology (Salomao et al., 2004; Sadun et al., 2006; Sacai et al., 2010).
3.3.2. Acute phase
Disease onset among LHON carriers is characterised by acute, painless loss of central vision, which is bilateral in about 25% of cases (Johns et al., 1993a; Nikoskelainen, 1994; Harding et al., 1995a; Nikoskelainen et al., 1996). If unilateral, the fellow eye is usually affected within six to eight weeks. There are rare cases of unilateral optic neuropathy (Nikoskelainen et al., 1996; Sugisaka et al., 2007), but these are the exceptions, second-eye involvement in LHON occurring invariably within 1 year of disease onset. The majority of carriers become symptomatic in the second and third decades of life, and over 90% of carriers who will experience visual failure will do so before the age of 50 years (Man et al., 2003; Spruijt et al., 2006). However, visual deterioration can occur anytime during the first to the seventh decade of life and LHON should be part of the differential diagnosis for all cases of bilateral, simultaneous or sequential optic neuropathy, irrespective of age, and especially in male patients (Shah et al., 2008; Yu-Wai-Man et al., 2008; Decanini-Mancera et al., 2009; Giraudet et al., 2010). Although one report identified females harbouring the m.11778G>A mutation as having a slightly increased age of onset compared to other groups (Harding et al., 1995b), gender and mutational status are not thought to significantly influence the timing or initial severity of visual loss.
Visual loss worsens over a period of four to six week, and it is severe, dropping to levels of 6/60 or worse, with a dense central or centrocaecal scotoma, and marked impairment in colour vision. Importantly, the pupillary light reflexes are thought to be relatively preserved in affected LHON patients compared with the extent of visual loss, and this can be a useful clinical sign (Wakakura and Yokoe, 1995; Kawasaki et al., 2010). In the acute stage, dilated fundal examination can be particularly informative, classical LHON cases exhibiting several distinct abnormalities such as vascular tortuosity of the central retinal vessels, swelling of the retinal nerve fibre layer, and a circumpapillary telangiectatic microangiopathy (Fraser et al., 2010). However, it must be stressed that in about 20% of LHON cases, the optic disc looks entirely normal, and these patients are sometimes labelled as having functional visual loss (Nikoskelainen et al., 1977; Harding et al., 1995a; Nikoskelainen et al., 1996).
3.3.3. Chronic phase
Within six weeks, optic nerve pallor becomes apparent, initially more marked temporally due to early axonal loss within the papillomacular bundle. Pathological cupping of the optic disc can occur with more extensive loss of RGC axons, and it is not an uncommon finding in longstanding LHON cases. If a patient is first assessed at this late stage, it can be difficult to exclude compressive, infiltrative or inflammatory causes of a bilateral optic neuropathy, especially when there is no convincing maternal history of early-onset visual failure. The results of molecular genetic testing in some diagnostic laboratories can take up to 2 months, and in these cases, the appropriate investigations, including neuroimaging of the anterior visual pathways, should not be delayed in order to exclude the possibility of reversible causes.
3.3.4. Visual prognosis
LHON causes significant visual impairment and in the majority of cases, visual recovery is minimal, the patient remaining within the legal requirement for blind registration. In the first year following disease onset, visual fields can improve with the appearance of small islands of vision (Mackey and Howell, 1992; Stone et al., 1992; Nikoskelainen et al., 1996). These fenestrations can help with scanning vision, especially if the central scotoma becomes concurrently less dense. The likelihood of visual recovery is greatest with the m.14484T>C mutation, and least with the m.11778G>A mutation, the m.3460G>A mutation having an intermediate visual prognosis (Harding et al., 1995a; Yu-Wai-Man et al., 2009b; Fraser et al., 2010). To objectively document the level of visual handicap experienced by LHON patients, we used the well-validated Visual Function Index (VF-14) questionnaire in a large cohort of 125 LHON pedigrees (Kirkman et al., 2009a). LHON had a negative detrimental impact on most activities of daily living, and quality of life, as assessed by the overall VF-14 score, was the worst compared with other acquired and inherited ophthalmological disorders.
3.3.5. Extra-ocular LHON features
Although visual failure is the cardinal clinical feature, cardiac arrhythmias and neurological abnormalities such as peripheral neuropathy, myopathy, dystonia, and myoclonus have been reported to be more common among LHON carriers compared to controls (Bower et al., 1992; Nikoskelainen et al., 1994; Meire et al., 1995; Nikoskelainen et al., 1995; Mashima et al., 1996; McFarland et al., 2007; La Morgia et al., 2008). In a small number of families from Holland, Australia and North America, the reported extra-ocular features were particularly severe, with variable combinations of psychiatric disturbances, spastic dystonia, ataxia, and juvenile onset encephalopathy complicating the optic neuropathy. The phenotypic severity of these so-called “LHON plus” syndromes has been linked with specific mtDNA variants at m.4160T>C (Howell et al., 1991b), m.11696A>G and/or m.14596T>A (De Vries et al., 1996), and m.14459G>A (Jun et al., 1994; Gropman et al., 2004; Tarnopolsky et al., 2004). Two mtDNA point mutations affecting complex I activity, m.3376G>A and m.3697G>A, have also been identified in individuals with overlap clinical features of LHON and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) (Blakely et al., 2005; Spruijt et al., 2007).
Harding et al. (1992) originally described an intriguing association between the m.11778G>A primary mutation among female LHON carriers and demyelination. Following the onset of visual loss, these patients developed clinical and neuroimaging features indistinguishable from multiple sclerosis (MS), with characteristic periventricular white matter lesions on magnetic resonance imaging (MRI), and unmatched oligoclonal bands in their cerebrospinal fluid (Kellar-Wood et al., 1994; Jansen et al., 1996; Vanopdenbosch et al., 2000). Since this first description of Harding’s disease, further evidence has emerged in LHON and other mitochondrial disorders, which suggest that this association is unlikely to be a chance occurrence (Kovacs et al., 2005; Jaros et al., 2007; Carelli and Bellan, 2008; Verny et al., 2008; Yu-Wai-Man et al., 2010b). LHON female carriers are twice more likely to develop an MS-like illness compared with male carriers, and although there is a preponderance for the m.11778G>A mutation, this phenotype has also been observed with the m.3460G>A and m.14484T>C primary mutations (Sapey et al., 2001). More robust confirmatory epidemiological studies are required, but demyelination has been estimated to affect up to 1 in 20 LHON carriers (Palace, 2009), which is fifty times higher than the prevalence of MS in the general population (Fox et al., 2004; Koch-Henriksen and Sorensen, 2010). It has not yet been determined whether subclinical white matter MRI changes are present in asymptomatic LHON carriers or those who only manifest pure optic nerve involvement. If present, these again would suggest more widespread central nervous involvement in LHON, which becomes clinically manifest in only a subgroup of at-risk individuals (Inglese et al., 2001). Interestingly, the proton magnetic resonance spectroscopic (1H-MRS) profile of both affected and unaffected LHON carriers were found to be abnormal compared to healthy controls, with reduced creatine (Cr) and N-acetylaspartate (NAA) levels in normal-appearing white matter regions, suggesting an underlying mitochondrial metabolic deficit (Ostojic et al., 2009). A number of pathophysiological mechanisms have been put forward linking RGC loss, oligodendrocyte survival, and mitochondrial dysfunction in patients with LHON and MS-like features (Section 8).
3.4. Incomplete penetrance and gender bias
Two key features of LHON still remain unexplained; the marked incomplete penetrance and the significant gender bias in disease predisposition, with only 50% of male and 10% of female carriers eventually losing vision in their lifetime. The primary LHON mutation is a prerequisite, but secondary factors are clearly modulating the risk of visual loss. Their identification has proven challenging, and the accumulated evidence favours a complex disease model, with both genetic and environmental factors interacting to precipitate optic nerve dysfunction (Carelli et al., 2007b; Yu-Wai-Man et al., 2009b; Tonska et al., 2010).
3.4.1. Mitochondrial genetic factors
Among heteroplasmic LHON carriers, visual loss only occurs if the mutational load exceeds 60%, the threshold required for triggering a bioenergetic defect (Chinnery et al., 2001a). However, incomplete penetrance is still observed among heteroplasmic carriers harbouring suprathreshold mutational levels, and over 80% of all LHON pedigrees are homoplasmic for the primary mtDNA mutation (Smith et al., 1993; Harding et al., 1995b; Man et al., 2003). Another possible mitochondrial modulating factor is the haplogroup background on which the LHON mutation is segregating. In a meta-analysis of 159 Caucasian LHON pedigrees, there was a significantly increased risk of visual failure when the m.11778G>A and m.14484T>C mutations occurred on a haplogroup J background, whereas m.3460G>A carriers were more likely to experience visual loss if they belonged to haplogroup K (Hudson et al., 2007b). A protective effect was conferred by haplogroup H, but only among m.11778G>A mutational carriers. The mitochondrial background also influenced the clinical expression of the m.11778G>A mutation among mainland Chinese LHON carriers, with haplogroup M7b1’2 increasing the risk of disease conversion, and haplogroup M8a having a protective effect (Ji et al., 2008). MtDNA haplogroups are defined by combinations of various polymorphic substitutions within the mitochondrial genome (Section 2.5). Some of these are non-synonymous, and they result in amino acid changes within mitochondrially-encoded subunits of the respiratory chain. Although it is convenient to view them as separate entities (Fig. 1), mitochondrial respiratory chain complexes do not exist in isolation, but they interact closely with one another forming so-called supercomplexes (Dudkina et al., 2010). Although speculative, these amino acid changes could induce subtle conformational changes, which affect the assembly and stability of these putative supercomplexes (Dudkina et al., 2005; Carelli et al., 2006; Hudson et al., 2007b; Pello et al., 2008). In support of this hypothesis, cybrid cell lines harbouring the m.11778G>A mutation had a lower oxygen consumption and a longer doubling time on a haplogroup J background, compared with other mtDNA haplogroups (Vergani et al., 1995). However, the link between specific mtDNA haplogroups and the risk of visual failure in LHON is not entirely clear-cut. A study of South-East Asian m.11778G>A LHON pedigrees found no significant association between specific mtDNA polymorphisms and the risk of developing overt optic nerve dysfunction (Tharaphan et al., 2006). Similarly, using in vivo 31P-MRS measurements, haplogroup J did not induce a more pronounced mitochondrial biochemical defect in the brain and skeletal muscle of affected m.11778G>A mutational carriers (Lodi et al., 2000).
3.4.2. Nuclear genetic factors
The marked male bias seen in LHON cannot be explained by mitochondrial genetic factors. Based on an extensive analysis of 31 large pedigrees totalling more than 1200 individuals, Bu and Rotter (1991, 1992) have proposed a two-locus model for visual failure in LHON. The segregation pattern was consistent with a visual-loss susceptibility gene on the X-chromosome, acting in synergy with the primary mtDNA mutation to precipitate visual loss among at-risk carriers. Male carriers have only one X-chromosome, and unlike female carriers, they cannot compensate for the inheritance of a putative X-linked visual-loss susceptibility allele (Oostra et al., 1996; Pegoraro et al., 1996; Hudson et al., 2007a). Three studies using microsatellite markers have now confirmed significant linkage on the X-chromosome, with some of these candidate regions showing areas of overlap (Figs. 6 and 7) (Hudson et al., 2005; Shankar et al., 2008; Ji et al., 2010). The actual gene or genes involved have yet to be identified, and more sophisticated bioinformatic tools are currently being applied for candidate gene analysis and to narrow down specific areas of interest. LHON could be an even more complex disorder than originally considered and the existence of autosomal nuclear modifiers remains a distinct possibility. A recent genome-wide study of nine large m.11778G>A Thai pedigrees found evidence of significant linkage on areas of chromosomes 3, 12, 13, and 18. Candidate gene regions were analysed with a tagging single nucleotide polymorphism (SNP) methodology, and two SNPs, rs3749446 and rs1402000, located within PARL (Presenilin-associated rhomboid-like) were associated with a statistically increased risk of phenotypic expression among LHON carriers (Phasukkijwatana et al., 2010). However, the association between these two PARL SNPs and visual loss was not replicated in an independent cohort of Chinese m.11778G>A LHON pedigrees (Zhang et al., 2010).
Fig. 6.
Summary of linkage studies investigating the existence of putative LHON nuclear modifiers on the X-chromosome. A nonparametric LOD score (NPL) >2 is indicative of significant linkage, and these chromosomal areas possibly harbour susceptibility loci which influence the risk of visual loss among LHON carriers. The different studies are colour coded: red (Hudson et al., 2005), blue (Shankar et al., 2008), and black (Ji et al., 2010). Reproduced with permission from Ji et al. (2010).
Fig. 7.
The complex interaction of genetic, hormonal, and environmental factors in the pathophysiology of LHON.
3.4.3. Hormonal factors
Although much attention has been focused on possible secondary genetic modifiers in LHON, hormonal factors could also influence phenotypic expression. This hypothesis has recently been investigated by Giordano et al. (2010) using osteosarcoma-derived cybrid cell lines harbouring one of the three primary LHON mutations: m.3460G>A, m.11778G>A, and m.14484T>C. These mutant cybrids exhibited elevated ROS levels, decreased mitochondrial membrane potential, increased rates of apoptosis, and hyper-fragmented mitochondrial networks compared with controls. Interestingly, treatment with 17β-oestradiol had a mitigating effect on these pathological features. In addition, supplementation of these LHON cybrids with 17β-oestradiol led to increased cellular levels of the anti-oxidant enzyme superoxide dismutase (SOD) and to more efficient mitochondrial biogenesis. These results are very interesting, providing another explanation for the protective effect of female gender on LHON penetrance, and supporting the possible therapeutic use of oestrogen-like compounds in this disorder.
3.4.4. Environmental factors
Two pairs of discordant monozygotic twins have been described, where one sibling has remained visually unaffected on long-term follow-up (Johns et al., 1993b; Biousse et al., 1997). These rare observations support an environmental component to the pathophysiology of optic nerve dysfunction in LHON, and there is increasing evidence in the literature supporting this hypothesis. The role of smoking and alcohol in LHON has been studied in a number of relatively small case-control studies, with contradictory findings (Chalmers and Harding, 1996; Tsao et al., 1999; Sadun et al., 2003; Newman, 2009). In one study, which included affected and unaffected siblings from 80 LHON sibships, high alcohol and tobacco consumption were not linked with an increased likelihood of visual failure (Kerrison et al., 2000). To further clarify this important issue, we conducted a multi-centre study of potential triggers in LHON, comparing the environmental exposure between 196 affected and 206 unaffected carriers (Kirkman et al., 2009b). Smoking was strongly associated with an increased risk of visual loss, and interestingly, there was a dose-response relationship, with the risk of visual loss being much greater in heavy smokers compared to light smokers. There was also a trend towards an increased risk of visual failure among heavy drinkers, but this effect was not as strong as smoking. Based on these results, LHON carriers should be strongly advised not to smoke and to moderate their alcohol intake, especially avoiding binge drinking episodes. Although no functional studies were performed, smoking could further impair mitochondrial OXPHOS, either through a direct effect on complex I activity, or by reducing arterial oxygen concentration (Gvozdjak et al., 1987; Vanjaarsveld et al., 1992; Yang et al., 2007; Kirkman et al., 2009b; Newman, 2009). Several other environmental triggers have been reported in LHON, including head trauma, acute physical illness, psychological stress, occupational exposure to chemical toxins such as 2,5-hexanedione, antiretroviral drugs, and anti-tuberculous agents (Sadun et al., 2003; Sanchez et al., 2006; Carelli et al., 2007a; Hudson et al., 2007b; Kirkman et al., 2009b). Most of these reports are clinical descriptions and they do not provide conclusive evidence for a causal relationship. However, Ghelli et al. (2009) have shown that 2,5-hexanedione had a mitochondrial toxic effect on LHON cybrids harbouring the m.11778G>A and m.14484T>C primary mutations. Of particular interest, an increased sensitivity to undergo apoptosis was noted on the haplogroup J mtDNA background, further highlighting the possible synergistic interactions between environmental and genetic risk factors.
3.5. Biochemical defect in LHON
All the three primary LHON mutations involve complex I subunits and their impact on mitochondrial OXPHOS has been extensively investigated using a wide range of cell types and biochemical assays. These in vitro studies have not always been consistent regarding the extent of the respiratory chain defect in LHON, but the overall evidence supports a predominant impairment in complex I-driven ATP synthesis (Table 3). In vivo 31P-MRS studies have also confirmed an underlying biochemical defect in LHON, with the m.11778G>A mutation resulting in the most pronounced reduction in mitochondrial ATP synthesis, followed by m.14484T>C, and m.3460G>A (Barbiroli et al., 1995; Lodi et al., 1997, 2002). With the caveat that RGCs were not directly assessed, an interesting observation from all these studies is that no significant difference in biochemical profile has ever been clearly demonstrated between affected and unaffected LHON carriers.
Table 3.
Biochemical Consequences of the Primary LHON mutations.
| MtDNA Mutation |
In Vitroa |
In Vivob |
||
|---|---|---|---|---|
| Complex I Activity (%) | Respiratory Rate (%) | ATP Synthesis (%) | 31P-MRS (%) | |
| m.3460G>A | 60–80 | 30–35 | 90 | 0–40 |
| m.11778G>A | 0–50 | 30–50 | 35 | 75 |
| m.14484T>C | 0–65 | 10–20 | 90 | 50 |
% Decrease relative to controls
Oxidative stress leads to free radical production and ROS levels were found to be significantly increased in transmitochondrial cybrids carrying one of the three primary LHON mutations (Wong et al., 2002; Carelli et al., 2004b; Baracca et al., 2005; Floreani et al., 2005). LHON patients also have reduced α-tocopherol/lipid ratios and elevated 8-hydroxy-2-deoxygaunosine levels in their blood leukocytes, both of these biomarkers being indicative of increased ROS production (Klivenyi et al., 2001; Yen et al., 2004). LHON cybrids have impaired EAAT1 (Excitatory amino acid transporter 1) activity, which is highly relevant to RGC survival, as these transporters are actively involved in the uptake of glutamate into Muller cells of the inner retina (Beretta et al., 2004). The glutamate transport defect could be partially reversed by supplementing these LHON cybrids with antioxidants, supporting the use of these compounds in RGC neuroprotection (Ghelli et al., 2008; Sala et al., 2008). Increased ROS and glutamate excitotoxicity are potent inducers of apoptotic cell death, and in LHON, this process is likely to be both Fas-dependent and caspase-independent (Ghelli et al., 2003; Zanna et al., 2003).
4. Autosomal-dominant optic atrophy
4.1. Epidemiology
DOA affects at least 1 in 35,000 individuals in the North of England, a figure which is comparable to LHON (Yu-Wai-Man et al., 2010a). Based on the Danish Registry for Hereditary Eye Diseases, the prevalence of this optic nerve disorder in Denmark has been estimated at 1 in 12,000 (Toomes et al., 2001), a higher figure which could reflect the methodological criteria used. This study was also performed in the pre-molecular era and patients were included based on a clinical diagnosis of DOA.
4.2. Clinical features
Visual loss in DOA is insidious, invariably starting in the first two decades of life, and with a mean age of onset of 6–10 years. Compared with LHON, it has a milder phenotype and up to 1 in 4 patients are visually asymptomatic, optic atrophy only being detected through contact tracing of an affected proband (Kline and Glaser, 1979; Hoyt, 1980). Mean visual acuities of 6/18–6/60 have been reported in various case series, with visual acuities ranging from 6/6 to the detection of hand movement (Kjer, 1959; Eliott et al., 1993; Votruba et al., 1998b; Puomila et al., 2005; Cohn et al., 2008). There is a marked inter- and intra-familial variability in the rate of disease progression, but a significant proportion of patients (50–75%) will experience further worsening of their visual function in later life (Cohn et al., 2008; Yu-Wai-Man et al., 2010a,c). Although the overall visual prognosis is better compared with LHON, DOA still causes significant visual impairment with half of all patients failing the driving standards and being registered legally blind (Kjer et al., 1996; Votruba et al., 1998a; Cohn et al., 2007).
Most patients with DOA have a generalised dyschromatopsia and only a minority (10%) has pure tritanopia, which used to be considered a pathognomonic feature of this optic nerve disorder (Berninger et al., 1991). Due to the primary involvement of the papillomacular bundle, the most common visual defects are central, centrocaecal, and paracentral scotomas, with sparing of the peripheral field (Votruba et al., 1998a; Yu-Wai-Man et al., 2010a,c). Both magnocellular and parvocellular pathways seem to be similarly affected in DOA. Similar to LHON, the retino-tectal fibres mediating the pupillary light reflex are relatively preserved, and patients do not normally exhibit an afferent pupillary defect (Bremner et al., 2001).
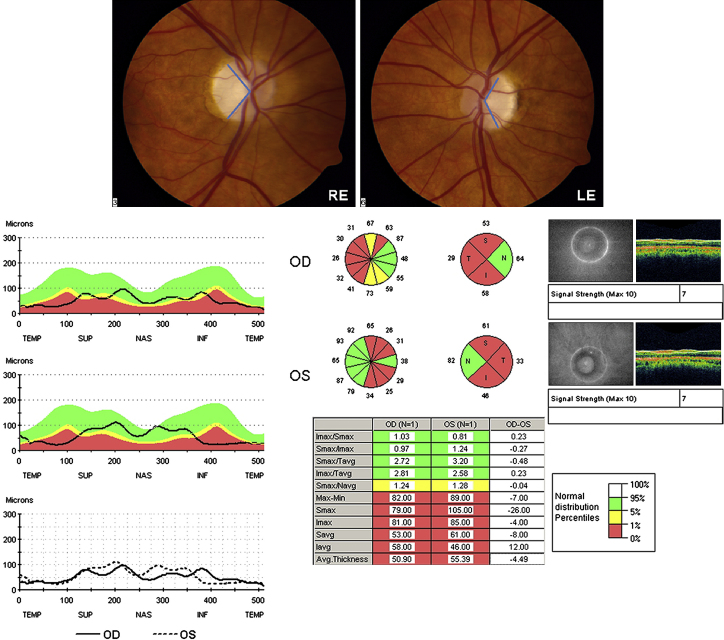
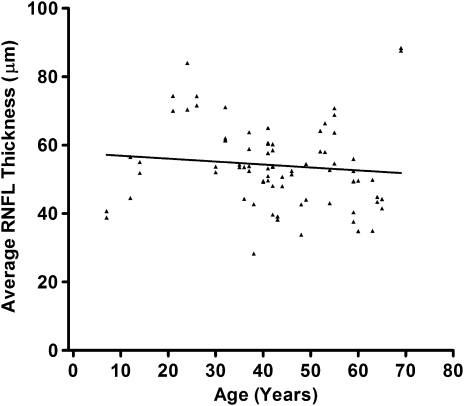
Optic disc pallor in DOA can be either diffuse, involving the entire neuro-retinal rim, or it can show a characteristic temporal wedge (Fig. 8). Pallor of the neuro-retinal rim can be subtle and in one report, nearly a third of all affected patients had normal looking optic discs on slit lamp biomicroscopy (Cohn et al., 2007). Based on our own experience, measurement of retinal nerve fibre layer (RNFL) thickness with optical coherence tomography (OCT) can be particularly helpful in equivocal cases, by confirming pathological RNFL thinning, especially in the temporal quadrant involving the papillomacular bundle (Ito et al., 2007; Kim and Hwang, 2007; Milea et al., 2010). Although not characteristic, other morphological disc features have been reported in DOA; saucerisation of the neuro-retinal rim, peripapillary atrophy, and enlarged cup-to-disc ratios greater than 0.5, the latter often leading to an erroneous diagnosis of normal tension glaucoma (Votruba et al., 1998a, 2003; Fournier et al., 2001; Yu-Wai-Man et al., 2010a,c).
Fig. 8.
The optic disc appearance of a patient with a confirmed pathogenic OPA1 mutation showing pallor of the neuro-retinal rim, which is more marked temporally. The bottom panels illustrate the pattern of retinal nerve fibre layer (RNFL) thinning seen in patients with OPA1 mutations, with relative sparing of the nasal peripapillary quadrant. The RNFL profile for each eye is superimposed on the normal distribution percentiles, and compared with each other (Bottom left panel). Various measurement parameters are automatically generated by the analysis software including sectorial RNFL thickness for each individual quadrant and clock hour, and an overall value for the average RNFL thickness (Bottom middle panel). The normal distribution indices are colour-coded: (i) red <1%, (ii) yellow 1–5%, (iii) green 5–95%, and (iv) white >95% (Bottom right panel).
4.3. Mutational spectrum
The majority (50–60%) of patients with DOA harbour mutations in the OPA1 gene (OMIM 165500), and over 200 pathogenic mutations have been identified (eOPA1 database at http://lbbma.univ-angers.fr/lbbma.php?id=9, Accessed 31st of August 2010) (Table 4). OPA1 consists of 30 exons spread over 100 kb of genomic DNA and alternative splicing of exons 4, 4b and 5b results in eight different mRNA isoforms (Davies and Votruba, 2006; Olichon et al., 2006; Lenaers et al., 2009). OPA1 mutations cluster in the GTPase domain (Exons 8–15) and dynamin central region (Exons 16–23), with single base-pair substitutions (69%) representing the most common mutational subtype, followed by deletions (26%), and insertions (5%) (Ferre et al., 2005, 2009). OPA1 testing is usually performed with PCR-based sequencing protocols, and although cost and access are important practical issues, about 10–20% of patients who are found to be negative using these screening methods will harbour large-scale OPA1 re-arrangements (Fuhrmann et al., 2009; Yu-Wai-Man et al., 2010a). The majority of OPA1 mutations result in premature termination codons, with truncated mRNAs which are unstable and mostly degraded by protective cellular mechanisms (Pesch et al., 2001; Schimpf et al., 2006; Zanna et al., 2008). The reduction in OPA1 protein level is a major disease mechanism in DOA, and the importance of haploinsufficiency is further emphasised by rare families with microdeletions which span the entire OPA1 coding region (Marchbank et al., 2002; Fuhrmann et al., 2009). However, about 30% of patients with DOA harbour missense OPA1 mutations, and those located within the catalytic GTPase domain are more likely to exert a dominant-negative effect (Section 4.4) (Amati-Bonneau et al., 2008; Ferraris et al., 2008; Hudson et al., 2008; Yu-Wai-Man et al., 2010b).
Table 4.
Primary inherited optic nerve disorders – Pattern of inheritance, reported loci, and causative genes.
| Inheritance | Locus | Gene | OMIM | Phenotypes | References |
|---|---|---|---|---|---|
| AD | 3q28-q29 | OPA1 | 165500 | Isolated optic atrophy and syndromal forms of dominant optic atrophy (DOA+) | (Delettre et al., 2000; Alexander et al., 2000; Yu-Wai-Man et al., 2010b) |
| 19q13.2-q13.3 | OPA3 | 165300 | Optic atrophy and premature cataracts (ADOAC) | (Garcin et al., 1961; Reynier et al., 2004) | |
| 18q12.2-q12.3 | OPA4 (Unknown) | 605293 | Optic atrophy | (Kerrison et al., 1999) | |
| 22q12.1-q13.1 | OPA5 (Unknown) | 610708 | Optic atrophy | (Barbet et al., 2005) | |
| 16q21-q22 | OPA8 (Unknown) | – | Optic atrophy and sensorineural deafness | (Carelli et al., 2007c) | |
| AR | 19q13.2-q13.3 | OPA3 | 606580 | 3-methylglutaconic aciduria type III (Costeff syndrome) | (Anikster et al., 2001) |
| 8q21-q22 | OPA6 (Unknown) | 258500 | Optic atrophy | (Barbet et al., 2003) | |
| 11q14.1-q21 | OPA7 (TMEM126A) | 612989 | Optic atrophy | (Hanein et al., 2009) | |
| XL | Xp11.4-p11.21 | OPA2 (Unknown) | 311050 | Optic atrophy | (Assink et al., 1997; Katz et al., 2006) |
| Mitochondrial | – | – | 53500 | Leber hereditary optic neuropathy and overlap mitochondrial syndromes | Table 1 |
Other genetic loci have been identified in families with DOA phenotypes (Table 4), but of these, only the causative gene for OPA3 has been characterised. OPA3 mutations were first identified in Iraqi Jewish families with Type III 3-methylglutaconic aciduria (Costeff syndrome); an autosomal recessive neurodegenerative disorder characterised by optic atrophy, progressive neurodegeneration, increased urinary levels of 3-methylglutaconic acid, and elevated plasma 3-methylglutaric acid levels (Costeff et al., 1989; Anikster et al., 2001). Pathogenic mutations were subsequently found in two independent French families segregating both optic atrophy and premature cataract in an autosomal-dominant mode of inheritance (ADOAC) (Reynier et al., 2004). However, OPA3 mutations are likely to be extremely rare, especially in isolated optic atrophy cases. We did not identify any pathogenic OPA3 variants in two large case series of OPA1-negative individuals, even with the use of a comparative genomic hybridization (CGH) assay to screen for large-scale OPA3 re-arrangements (Yu-Wai-Man et al., 2010a,c).
4.4. Expanding clinical phenotypes
Even in the pre-molecular era, a number of pedigrees with DOA were described where optic neuropathy occurred in parallel with other clinical features such as sensorineural deafness and CPEO (Kjer, 1956, 1959; Kline and Glaser, 1979; Hoyt, 1980; Meire et al., 1985). OPA1 mutations have been confirmed as the causative genetic defects in these syndromal forms of DOA, but until recently, these were thought to be rare manifestations among isolated families (Amati-Bonneau et al., 2003, 2005; Shimizu et al., 2003; Payne et al., 2004; Liguori, 2008). In our North of England inherited optic neuropathy cohort, DOA+ phenotypes were observed in 1 in 6 OPA1 carriers, which clearly indicate that these syndromal variants affect a significant patient subgroup (Yu-Wai-Man et al., 2010a). We then conducted a multi-centre study of 104 patients manifesting DOA+ to define the phenotypic spectrum and natural history of these additional neurological complications (Yu-Wai-Man et al., 2010b). Bilateral sensorineural deafness beginning in late childhood and early adulthood was the most frequently observed extra-ocular manifestation, affecting nearly two-thirds of all cases. Other prominent clinical features then developed from the third decade of life onwards; ataxia, myopathy, peripheral neuropathy and CPEO. Unexpectedly, OPA1 mutations were also identified in families previously labelled as having autosomal-dominant hereditary spastic paraplegia (HSP), and in individuals with visual failure complicated by a multiple sclerosis-like illness. Of note, there was a two- to three-fold increased risk of developing multi-system neurological disease with missense OPA1 mutations located within the GTPase domain, suggesting deleterious gain-of-function mechanisms. Although these syndromal DOA+ variants show significant phenotypic variability even within the same family, a consistent finding is a worse visual prognosis among this patient subgroup. These observations are of major pathophysiological importance, highlighting the widespread deleterious consequences of OPA1 mutations, not only for RGCs, but also for other neuronal populations, skeletal muscle, and extra-ocular muscle (Spinazzi et al., 2008; Zeviani, 2008; Mackey and Trounce, 2010). A key feature of these OPA1 mutations is the induction of secondary mtDNA deletions, and their possible roles in triggering multi-system cellular dysfunction are currently being investigated (Yu-Wai-Man et al., 2010d).
4.5. OPA1 and OPA3 protein functions
OPA1 is highly expressed within the RGC layer, but it is a ubiquitous protein, and abundant levels have also been identified in photoreceptors, and other non-ocular tissues such as the inner ear and the brain (Aijaz et al., 2004; Pesch et al., 2004; Bette et al., 2005; Kamei et al., 2005; Akepati, 2008). OPA1 belongs to a large family of mechanoenzymes, which is characterised by a highly conserved, dynamin GTPase domain (Davies and Votruba, 2006; Lenaers et al., 2009). OPA1 is a transmembrane protein embedded within the mitochondrial inner membrane, and it mediates several interrelated cellular functions. One important aspect relates to its pro-fusion properties and unsurprisingly, the pathological hallmark of OPA1 mutations is mitochondrial network fragmentation, the isolated mitochondria becoming dysmorphic, with aberrant balloon-like enlargements, disorganised cristae, and paracrystalline inclusion bodies (Kamei et al., 2005; Olichon et al., 2007; Amati-Bonneau et al., 2008; Chevrollier et al., 2008; Zanna et al., 2008). GTP hydrolysis activates membrane tubulation and this GTPase activity is thought to be enhanced following interaction of OPA1 with inner membrane phospholipids such as cardiolipin (Ban et al., 2010). Cytochrome c molecules are normally sequestered within the tight cristae junctions, and as they leach out into the cytosol, the apoptotic cascade is potentiated contributing to cell death and tissue dysfunction (Cipolat et al., 2006; Frezza et al., 2006; Suen et al., 2008). OPA1 also regulates OXPHOS by interacting directly with the respiratory chain complexes, controlling their delicate assembly, and facilitating the efficient shuttling of electrons between complexes (Zanna et al., 2008). Fibroblasts harbouring OPA1 mutations have reduced mitochondrial membrane potentials and ATP synthesis was found to be significantly impaired (Amati-Bonneau et al., 2005; Chevrollier et al., 2008), secondary to a predominantly complex I defect (Zanna et al., 2008). With the use of in vivo phosphorus magnetic resonance spectroscopy (31P-MRS), these biochemical defects have also been demonstrated in the calf muscles of patients harbouring OPA1 mutations (Lodi et al., 2004; Yu-Wai-Man et al., 2010f). Interestingly, it is now apparent that OPA1 plays a critical role in maintaining the integrity of the mitochondrial genome. High levels of COX-negative fibres have been identified in skeletal muscle biopsies from patients with DOA+ phenotypes, and this has been conclusively linked to the accumulation of multiple mtDNA deletions (Stewart et al., 2008; Yu-Wai-Man et al., 2010b,d).
OPA3 has a mitochondrial targeting domain, and it was thought to localise to the mitochondrial inner membrane (Anikster et al., 2001; Ho et al., 2008; Huizing et al., 2010). However, a recent study challenged this view, suggesting instead that OPA3 is an integral component of the mitochondrial outer membrane, with its C-terminus exposed to the cytosol (Ryu et al., 2010). Mitochondrial fragmentation was induced both with mutant forms of the protein, and following overexpression of the wild-type protein, indicating an important pro-fission role for OPA3 (Ryu et al., 2010). The cells studied had an increased sensitivity to various pro-apoptotic stimuli, a phenomenon which was also observed in fibroblasts collected from an affected patient belonging to one of the French ADOAC families (Reynier et al., 2004).
5. Other mitochondrial optic neuropathies
5.1. Charcot-Marie-Tooth disease
Charcot-Marie-Tooth (CMT) disease is a heterogeneous group of inherited peripheral neuropathies, and as a group they are one of the most common inherited human disorders, affecting at least 1 in 2500 individuals (Zuchner and Vance, 2006; Pareyson and Marchesi, 2009). Both motor and sensory nerves are affected resulting in distal limb weakness, sensory loss, decreased deep tendon reflexes, and foot deformities. A specific CMT subtype, hereditary motor and sensory neuropathy type VI (HMSN-VI, OMIM 601152), is caused by MFN2 mutations (1p36.2) (Zuchner et al., 2004). In addition to early onset, severe peripheral neuropathy, affected individuals develop progressive optic nerve dysfunction starting in later childhood (Zuchner et al., 2006). Visual acuity usually deteriorates to levels of 6/60 or worse, but a subset of patients with HMSN-VI can experience sometimes dramatic visual recovery in later life. MFN1 and MFN2 are mitochondrial outer membrane proteins with dynamin GTPase domains, and they share a remarkable degree of structural and functional complementarity with OPA1 (Cartoni and Martinou, 2009; Chen and Chan, 2009). These three proteins interact closely with each other to coordinate the various steps involved in mitochondrial membrane fusion. MFN2 is also thought to exert a direct influence on mitochondrial biogenesis by regulating the expression of nuclear-encoded respiratory chain subunits (Pich et al., 2005). Consistent with this hypothesis, fibroblasts from patients harbouring MFN2 mutations exhibit a mitochondrial coupling defect, with impaired membrane potential and reduced OXPHOS capacity (Loiseau et al., 2007).
5.2. Hereditary spastic paraplegia
The clinical hallmark of HSP is slowly progressive lower limb spasticity and weakness. Its prevalence has been estimated at 3–10 per 100,000 in Europe, and the age of onset varies according to the causative genetic defect (Salinas et al., 2008). HSP is classified into pure and complicated forms, depending on whether additional clinical features are present besides spastic paraplegia, such as optic atrophy, ataxia, peripheral neuropathy, extrapyramidal deficits, and cognitive decline (Harding, 1983). HSP is genetically heterogeneous with 41 mapped loci. So far, 17 genes have been identified, providing valuable insights into the various pathogenetic mechanisms which trigger axonal degeneration along the corticospinal tracts (Shy et al., 2002; Reid, 2003; Salinas et al., 2008).
OPA1 is cleaved by mitochondrial proteases following import into the mitochondrial intermembrane space, and these post-translational maturational steps are critical, generating long (L) and short (S) forms of the protein (Pellegrini and Scorrano, 2007; Lenaers et al., 2009; Martinelli and Rugarli, 2010). On their own, the L and S forms of OPA1 have little functional activity, but when co-expressed, they complement each other, triggering mitochondrial network fusion. One of these key mitochondrial proteases is paraplegin, which is encoded by SPG7 (16q24.3). Mutations in SPG7 have been identified in an autosomal recessive form of HSP, and in some patients, bilateral optic neuropathy is a prominent clinical feature further complicating the neurological phenotype (HSP-7, OMIM 607259) (Casari et al., 1998). Muscle biopsies from two severely affected individuals with HSP-7 showed classical histochemical changes of mitochondrial dysfunction with ragged-red fibres and COX-negative fibres. Transmission electron microscopy confirmed an accumulation of abnormal mitochondria containing paracrystalline inclusion bodies. Biochemical studies performed on cultured myoblasts harbouring SPG7 mutations have revealed a reduction in citrate synthase-corrected complex I activity, again suggesting that impaired OXPHOS plays an important role in the pathogenesis of HSP, and by extension the optic neuropathy observed in a subgroup of patients (Wilkinson et al., 2004).
5.3. Friedreich ataxia
Friedreich ataxia (FRDA) is an autosomal recessive disorder caused by pathological GAA trinucleotide repeat expansions in the FXN gene (9q13-q21.1, OMIM 229300) (Campuzano et al., 1996). The encoded protein frataxin is directed to the mitochondrial inner membrane and it is involved in the assembly of iron–sulphur clusters, which are critical components of the mitochondrial respiratory chain complexes (Rouault and Tong, 2005; Stemmler et al., 2010). FXN mutations therefore impair OXPHOS and they also result in abnormal accumulation of intra-mitochondrial iron, which eventually reaches toxic levels (Rotig et al., 1997, 1998). Because frataxin has anti-oxidant properties, cellular defences against ROS are impaired in FRDA, and this likely further exacerbates neuronal loss (Rustin et al., 1999; Rotig et al., 2002; Schmucker and Puccio, 2010).
Patients with FRDA usually become symptomatic in the second decade of life, with progressive gait ataxia, loss of the deep tendon reflexes, dysarthria, distal limb weakness, pes cavus, scoliosis, and arrhythmias secondary to hypertrophic cardiomyopathy (Durr et al., 1996). In a recent study of 26 patients with genetically-confirmed FRDA, all patients had evidence of optic nerve dysfunction, although only five were visually symptomatic (Fortuna et al., 2009). The optic neuropathy differed from that observed in LHON and DOA, with a diffuse and progressive pattern of RNFL loss, and no preferential involvement of the papillomacular bundle. Interestingly, the pathological process in FRDA extended to the post-geniculate optic radiations, and the involvement of the anterior and posterior visual pathways seemed to proceed independently of each other. Altogether, these findings suggest that FXN mutations probably cause RGC loss via other disease mechanisms compared with OPA1 and the primary mtDNA LHON mutations.
5.4. Autosomal recessive non-syndromal optic atrophy
Autosomal recessive optic neuropathies are rare and the visual phenotype is usually overshadowed by other more prominent neurodegenerative features. Using homozygosity mapping, Hanein et al. (2009) identified mutations in TMEM126A (11q14.1-q21, OMIM 612989) in a large, inbred, Algerian family segregating pure optic atrophy. TMEM126A is conserved in higher eukaryotes and it encodes a transmembrane mitochondrial protein, which is found at high levels within the RGC layer and optic nerve head. The exact localisation and function of this protein remain to be clarified, but TMEM126A mutations do not cause mitochondrial network fragmentation or mtDNA depletion. A novel nonsense TMEM126A mutation has since been reported where affected family members had subclinical auditory neuropathy in addition to progressive visual failure. The clinical phenotype associated with TMEM126A mutations could therefore expand further as more families with syndromal optic atrophy are screened for this particular gene (Meyer et al., 2010).
5.5. Mitochondrial protein-import disorders
Mohr–Tranebjaerg syndrome or deafness-dystonia-optic neuronopathy (DDON) syndrome is caused by loss-of-function mutations in the TIMM8A gene (Xq22, OMIM 300356) (Tranebjaerg et al., 1997). The clinical phenotype includes prelingual or postlingual sensorineural deafness, dystonia and ataxia in late childhood, visual failure with optic atrophy from the age of 20 years, and cognitive decline with psychiatric disturbances before the age of 50 years (Tranebjaerg et al., 1997; Tranebjaerg, 2003). Electrophysiological studies indicate that the visual loss in DDON is secondary to RGC loss, and the visual prognosis is poor, with most patients registered legally blind by the age of 40 years (Tranebjaerg et al., 1995, 2001; Ponjavic et al., 1996; Ujike et al., 2001). TIMM8A assembles as a 70 kDa hetero-oligomeric complex in the mitochondrial intermembrane space, and in conjunction with TIMM13, another mitochondrial membrane translocase, this complex facilitates the import and insertion of inner membrane proteins (MacKenzie and Payne, 2007). COX-negative fibres were not present in muscle biopsy specimens from affected patients and there was no alteration in mitochondrial network morphology (Binder et al., 2003; Blesa et al., 2007). However, there was evidence of an underlying mitochondrial biochemical defect, with reduced complex I–IV enzymatic activities.
Dilated cardiomyopathy with ataxia (DCMA) or 3-methylglutaconic aciduria, type V (OMIM 610198) is an autosomal recessive disorder found in the Dariusleut Hutterite population of Canada and the Northern United States. The clinical phenotype is characterised by growth failure, severe, early-onset cardiomyopathy, and a cerebellar syndrome with ataxia. Optic atrophy has been reported in some patients with DCMA, although it is not a cardinal feature of this syndrome (Davey et al., 2006). Homozygous mutations in the DNAJC19 gene (3q26.33) have recently been identified in these inbred families, a G–C transversion within the splice acceptor site of intron 3 (Davey et al., 2006). The DNAJC19 protein localises to the mitochondrial compartment and although its subcellular localisation requires further investigation, it is thought to be the human orthologue of the yeast Tim14 protein (Mokranjac et al., 2006). Tim14 is an integral mitochondrial inner membrane protein and together with other mitochondrial translocases, it actively participates in protein import into the mitochondrial matrix compartment. Both DDON and DCMA are therefore due to defective mitochondrial protein-import systems, and it is fascinating that in both disorders optic nerve involvement is observed, albeit as part of a more widespread multi-systemic degeneration (MacKenzie and Payne, 2007).
5.6. Mitochondrial encephalomyopathies
Optic neuropathy can also develop in the following classical mitochondrial syndromes, although it is usually a secondary feature, overshadowed by other more prominent neurological and ocular manifestations: MELAS, myoclonic epilepsy and ragged-red fibres (MERRF), CPEO, KSS, maternally inherited Leigh syndrome (MILS), and mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) (Gronlund et al., 2010).
5.7. Overlapping phenotypes
The concept of mitochondrial optic neuropathies has greatly expanded over the years and this trend is set to continue as new genes with mitochondrial-related functions are identified in other inherited optic nerve disorders. What is truly remarkable is the overlapping phenotypes seen with this group of disorders, for example among OPA1 carriers, some patients develop neurological features indistinguishable from HSP, others develop a pattern of peripheral neuropathy with a similar disease course to CMT, and others still will develop a prominent cerebellar syndrome consistent with FRDA. These shared clinical features also apply to the optic nerve phenotype, as seen with LHON and DOA, the two most common inherited optic neuropathies diagnosed in the general population. As described in earlier sections, LHON and DOA have distinct clinical presentations which usually allow for their easy differentiation, but with the greater availability of genetic testing, it is now clear that a degree of phenotypic overlap exists. Although unusual, some patients with genetically-confirmed OPA1 mutations have been described with acute and even reversible visual loss (Cornille et al., 2008; Nochez et al., 2009), and a subgroup of LHON patients can present with a slowly progressive optic neuropathy, more suggestive of DOA (Barboni et al., 2006).
6. Toxic optic neuropathies
6.1. Smoking, alcohol, and nutritional deprivation
Individuals with excessive alcohol and tobacco intake can develop a bilateral optic neuropathy, with slowly progressive visual loss, dyschromatopsia, and centrocaecal scotomas (Rizzo and Lessell, 1993; Lessell, 1998). This entity is sometimes referred to as “tobacco-alcohol amblyopia”, but there is persisting debate regarding its actual aetiology (Grzybowski, 2007; Grzybowski and Holder, 2010). It is possible that alcohol and tobacco per se are not the main or only causative factors, with other confounding variables such as nutritional deprivation contributing to the observed RGC-toxic effect. An epidemic of optic neuropathy was observed in Cuba in the early 1990s at a time of deteriorating socioeconomic conditions within this country (Sadun et al., 1994; Bern et al., 1995). This outbreak of optic neuropathy has been linked with chronic malnutrition combined with high levels of tobacco and cassava consumption. Cassava contains naturally-occurring cyanogenic glucosides and the occurrence of cyanide poisoning has been well described, especially during periods of famine. In terms of disease mechanisms, it is interesting that primary mtDNA LHON mutations have been detected in patients labelled as having “tobacco-alcohol amblyopia” (Cullom et al., 1993; Purohit and Tomsak, 1997). Moreover, smoking has recently been identified as a strong risk factor for visual loss among LHON mutational carriers, with heavy smokers having a higher risk than light smokers (Kirkman et al., 2009b). However, it should be noted that during the Cuban optic neuropathy outbreak, there was no evidence of increased disease penetrance in a large Cuban m.11778G>A family (Newman et al., 1994).
6.2. Chloramphenicol
With the advent of safe, broad spectrum antibiotics, systemic chloramphenicol is now rarely used for treating severe, disseminated infections. However, until the late 1970s, it was frequently used in the management of patients with cystic fibrosis, and a rare but serious complication noted in this group of patients was bilateral optic neuropathy (Godel et al., 1980; Yunis, 1989; Venegas-Francke et al., 2000). Post-mortem studies performed on patients who died shortly afterwards showed complete loss of RGCs within the distribution of the papillomacular bundle, with relative sparing of the nasal and peripheral retina (Cogan et al., 1973). There was no evidence of inflammation within the optic nerve but there was mild gliosis and demyelination in both the pre- and post-laminar segments. Transmission electron microscopy of haemopoetic cells collected from patients with chloramphenicol-induced bone marrow suppression revealed swollen mitochondria with disrupted cristae architecture, in addition to abnormal intra-mitochondrial iron deposits (Smith et al., 1970; Yunis and Smith, 1970; Skinnider and Ghadially, 1976). These findings strongly suggest that the pathological changes in chloramphenicol optic neuropathy are due to a similar mitochondrial toxic effect. Chloramphenicol is a powerful inhibitor of both bacterial and mitochondrial ribosomal protein synthesis, resulting in mitochondrial stress and a marked reduction in cellular ATP biosynthesis (Bhat et al., 1981; Li et al., 2010).
6.3. Linezolid
Linezolid is an oxazolidinone antibiotic that is increasingly being used to treat drug-resistant, gram positive organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) (De Vriese et al., 2006). Overall, it is a safe antibiotic agent when used for up to 28 days, with only minor side effects reported in large Phase III clinical trials. However, about 20 cases of linezolid-associated optic neuropathy (LION) have been reported in the literature, and a common feature of this rare association seems to be an extended duration of treatment (5–50 months) (McKinley and Foroozan, 2005; Saijo et al., 2005; Rucker et al., 2006; Javaheri et al., 2007). Patients present with typical features of a bilateral optic neuropathy and at first presentation, visual acuities ranging from 6/9 to counting fingers have been reported. Early in the course of the disease, optic disc swelling and marked thickening of the RNFL have also been documented, the latter indicating axonal RGC swelling and strikingly reminiscent of the pathological process in acute LHON. Unlike linezolid-induced peripheral neuropathy which is irreversible, full visual recovery is possible following discontinutation of linezolid therapy. It is therefore crucial for clinicians to be aware of this possibly devastating complication among patients receiving linezolid treatment beyond the 28-day safe limit. Intriguingly, mitochondrial dysfunction seems to be central to the pathophysiology of LION, and a number of hypotheses have been proposed based upon the mechanism of action of linezolid. The antibacterial effect of linezolid is due to inhibition of bacterial protein synthesis, with the active moiety binding to the 23S rRNA of the 50S ribosomal subunit, thereby preventing the formation of the 70S RNA initiation complex (Javaheri et al., 2007). Mammalian ribosomes lack the 50S component and they are therefore not affected by linezolid. Although speculative, it is possible that mitochondrial ribosomes are susceptible to the sustained effect of long-term linezolid because of their closer evolutionary similarities to bacterial ribosomes. Consistent with this mechanism, mitochondrial respiratory chain activity was significantly depressed in a range of tissues; muscle, liver, and kidney, biopsied from a patient who developed optic neuropathy, encephalopathy, skeletal myopathy, lactic acidosis, and renal failure after a 4-month course of linezolid (De Vriese et al., 2006). This finding was further substantiated in an experimental rat model, with linezolid inducing a dose- and time-dependent decrease in the activity of complex I, and to a lesser extent that of complex IV (De Vriese et al., 2006).
6.4. Erythromycin
Luca et al. (2004) reported on a 23-year-old male who developed bilateral optic neuropathy following treatment with erythromycin. His visual acuity was severely reduced to counting fingers in both eyes and bilateral diffuse optic nerve pallor was observed, more marked temporally. A homoplasmic m.11778G>A mutation was identified, and to investigate a possible link between erythromycin and the onset of visual failure in this LHON carrier, cybrid osteosarcoma cells were established harbouring the patient’s mtDNA. When cultured in media containing erythromycin, a marked impairment in mitochondrial protein synthesis was noted and this was a dose-dependent effect. Similar to linezolid, erythromycin binds to the 23S rRNA molecule of the 50S ribosomal subunit blocking the exit of the growing polypeptide chain (Thomas and Wilkie, 1968; Tok and Bi, 2003). Erythromycin and, by extension, other macrolide antibiotics are therefore potential mitochondrial toxins and their use should be avoided in patients with mitochondrial genetic disorders, especially if other alternatives are available.
6.5. Ethambutol
Ethambutol is an important first-line drug used for treating tuberculosis and 2–6% of patients receiving this anti-mycobacterial drug will develop optic neuropathy, a serious side effect which is duration- and dose-dependent (Melamud et al., 2003; Lee et al., 2008). Optic nerve involvement is rare with treatment duration of less than 2 months, and although visual function can improve following discontinuation of the drug, in some cases, it is irreversible (Kim and Hwang, 2009). Several studies have confirmed a direct, toxic effect of ethambutol on RGC survival, and various pathophysiological mechanisms have been proposed including glutamate excitotoxicity, zinc-mediated lysosomal membrane permeabilisation, and disruption of mitochondrial complex IV activity through a copper-chelating action (Heng et al., 1999; Chung et al., 2009). Dotti et al. (1998) were the first to describe the onset of bilateral, subacute visual loss in a LHON m.11778G>A carrier, 8 months following the initiation of treatment for pulmonary tuberculosis. Since then, other case reports of ethambutol-induced optic neuropathy among LHON carriers have been reported, suggesting a synergistic deleterious effect of this anti-mycobacterial drug on the background of a pre-existing pathogenic mtDNA mutation (De Marinis, 2001; Hwang et al., 2003; Ikeda et al., 2006; Pradhan et al., 2010; Seo et al., 2010). Against this argument, the m.11778G>A mutation did not further enhance ethambutol cytotoxicity in NT2/D1 teratoma-derived cybrids, but this could simply reflect a tissue-specific effect, the latter being more pronounced in RGCs (Pommer et al., 2008).
The link between ethambutol therapy and inherited mitochondrial optic neuropathies was further highlighted by Guillet et al. (2010). They unexpectedly identified a pathogenic OPA1 mutation in a male patient who developed bilateral, sudden, central visual loss 3 months after starting treatment for primary tuberculosis on a standard regimen of ehthambutol, isoniazid, and rifampicin. His visual acuity was severely affected, with caecocentral scotomas, and no visual recovery was noted following withdrawal of ethambutol. The acute presentation observed in this case is somewhat atypical for DOA and functional studies performed on cultured fibroblasts supported a role for ethambutol in precipitating an accelerated loss of RGCs. Compared with control fibroblasts similarly treated with ethambutol, a more marked biochemical uncoupling defect was observed, with loss of mitochondrial membrane potential, mitochondrial fragmentation, disturbed calcium homeostasis, and the accumulation of abnormal intracellular vacuoles (Guillet et al., 2010).
6.6. Antiretroviral drugs
There are suggestive reports of visual loss being triggered among LHON carriers following the initiation of highly active antiretroviral therapy (HAART) for newly diagnosed HIV infections (Shaikh et al., 2001; Mackey et al., 2003). These HIV patients were all male and harboured either the m.11778G>A or the m.14484T>C primary mtDNA mutations. The possible mechanisms have focused mainly on a particular class of antitretrovirals, the nucleoside analogue reverse transcriptase inhibitors (NRTIs), which can induce mitochondrial toxicity. NRTIs can inhibit mitochondrial polymerase gamma (POLG), a key component of the mtDNA replication machinery (Samuels, 2006; Scruggs and Naylor, 2008). A well reported side effect of NRTI therapy is mtDNA depletion, the resulting biochemical defect leading to lactic acidosis, hepatotoxicity, cardiomyopathy, generalised myopathy, and peripheral neuropathy (Cote et al., 2002; Frerichs et al., 2002; Maagaard et al., 2006). Fascinatingly, HAART has also been linked with the development of a CPEO phenotype in a limited number of HIV patients, with a gradual onset of ophthalmoplegia and ptosis (Pfeffer et al., 2009). One attractive hypothesis is that patients harbouring specific SNPs within POLG are at a higher risk of developing mitochondrial toxicity to NRTIs, with the HIV virus itself having an additive detrimental effect, further exacerbating mitochondrial dysfunction (Nolan and Mallal, 2004; Maagaard et al., 2006; Samuels, 2006). The mitochondrial protein profile of treatment-native HIV patients and those receiving HAART therapy have recently been investigated in blood leukocytes using a proteomic-based approach. Both groups of patients showed downregulated levels of prohibitins, important mitochondrial enzymes closely involved in OXPHOS and the maintenance of the mitochondrial network (Artal-Sanz and Tavernarakis, 2009; Ciccosanti et al., 2010). Additional studies are needed to determine whether fibroblasts from HIV patients, on or off HAART therapy, show any evidence of mitochondrial network fragmentation. Given the emerging links between HIV, HAART therapy, and mitochondrial dysfunction, it is not unreasonable to speculate that LHON carriers would be at an increased risk of visual loss in this particular context, and should therefore be adequately counselled should they require treatment with antiretrovirals.
7. Glaucoma
7.1. Normal tension glaucoma
Primary open angle glaucoma (POAG) affects over 60 million people worldwide and it is the second most common cause of legal blindness in developed countries (Bunce and Wormald, 2006; Quigley and Broman, 2006). RGCs are lost at an accelerated rate compared with normal ageing, resulting in pathological disc cupping and visual field defects. POAG is a complex disease and several genetic susceptibility loci have been identified in populations from different ethnic backgrounds (Libby et al., 2005; Wiggs, 2007; Ray and Mookherjee, 2009). The major risk factor for POAG is elevated intraocular pressure (IOP), but in about a third of patients, IOPs never exceed the statistical normal threshold of 21 mmHg (Boland and Quigley, 2007). Although lowering IOP can reduce visual field progression in normal tension glaucoma (NTG), additional IOP-independent risk factors are considered to be more important in the pathophysiology of NTG than in high tension glaucoma (HTG) (Alward et al., 1998; Schulzer et al., 1998; Cheng et al., 2009). The pattern of RGC loss in these two disease subgroups has been extensively investigated, and in most studies, NTG patients tended to have steeper, more focal field defects, which were located closer to fixation compared with HTG patients (Araie et al., 1997; Ahrlich et al., 2010). It is therefore not surprising that patients with DOA are often misdiagnosed as having NTG, especially if there is no family history of early-onset visual loss (Trobe et al., 1980; Fournier et al., 2001). Furthermore, disc pallor in DOA can be quite subtle, involving only the temporal segment, and about half of all patients have excavated discs, with cup-to-disc ratios greater than 0.5 (Votruba et al., 1998a, 2003). Some investigators have even argued, rather provocatively, that NTG is in actual fact an inherited optic neuropathy representing a forme fruste of DOA (Buono et al., 2002).
7.2. OPA1 polymorphisms
OPA1 was an obvious candidate disease-susceptibility gene in glaucoma and we recently carried out a systematic screen of the entire coding region using a well-characterised POAG cohort from the North of England (Yu-Wai-Man et al., 2010e). No pathogenic sequence variants were identified ruling out OPA1 as a monogenic risk determinant for this disorder. Instead, we found a statistically significant association between two specific intronic OPA1 SNPs, IVS8+4c>t and IVS8+32t>c, and the risk of developing NTG but not HTG. Three other studies have found a similar association between these two OPA1 SNPs and NTG, supporting a possible role of the OPA1 protein in modulating disease susceptibility to glaucoma (Aung et al., 2002; Powell et al., 2003; Mabuchi et al., 2007). However, these findings have not been replicated in other populations, and functional studies are needed to clarify the mechanisms by which OPA1 SNPs could exacerbate RGC loss and increase the risk of developing NTG (Sato et al., 2003; Woo et al., 2004; Kumaramanickavel et al., 2005; Yao et al., 2006; Wolf et al., 2009).
7.3. OPTN mutations
Mutations in four genes have been identified in families with POAG: optineurin (OPTN, OMIM 602432), myocilin (MYOC, OMIM 610652), CYP1B1 (OMIM 601771), and WDR36 (OMIM 609669). OPTN mutations are present in about 17% of families with autosomal-dominant NTG (Rezaie et al., 2002), but overall they are relatively rare, accounting for less than 2% of sporadic cases (Weisschuh et al., 2005; Hauser et al., 2006; Sripriya et al., 2006). These studies, including the original report of OPTN mutations in NTG, also suggest that the p.M98K OPTN variant could be an important disease risk factor, being present in 10–15% of glaucoma subjects compared with only 2–4% of normal controls. In one study, the p.M98K variant was not found to be associated with DOA and LHON, and it did not influence the severity or risk of visual loss (Craig et al., 2006).
Intriguingly, OPTN mutations have also recently been identified in Japanese individuals from consanguineous marriages and with a familial form of amyotrophic lateral sclerosis (ALS) (Maruyama et al., 2010). OPTN is a multifaceted protein and one of its important functions is to protect cells against high levels of oxidative stress by regulating the activation of NF-κB, an important component of the extrinsic apoptotic pathway (De Marco et al., 2006; Zhu et al., 2007; Chalasani et al., 2008). NF-κB is also found in both the cytosol and mitochondrial compartments and its upregulation inhibits cytochrome c oxidase and cytochrome b mRNA, disrupting the structural integrity of the mitochondrial respiratory chain and adversely affecting ATP synthesis (Chalasani et al., 2007). Furthermore, OPTN co-localises to the Golgi apparatus and it probably plays an important role in membrane trafficking and vesicular transport (del Toro et al., 2009). The neuropathology in ALS is characterised by the degeneration of upper and lower motor neurones, and it is intriguing that OPTN mutations seem to have a predilection for long neuronal populations, including RGCs, with mitochondrial dysfunction being one of the likely pathogenetic mechanisms (Shi et al., 2010).
8. Demyelination, optic neuritis, and multiple sclerosis
MS is an inflammatory demyelinating disease of the central nervous system and as the disease progresses, extensive axonal loss is observed in the brain and spinal cord (Frohman et al., 2005; Balcer, 2006). The prevalence of MS varies worldwide and in populations of Northern European extraction, the average quoted figure is 1 in 1000 (Fox et al., 2004; Koch-Henriksen and Sorensen, 2010). Up to half of all patients with MS will experience an episode of optic neuritis, and in 15–20% of cases, it is the first manifestation of an underlying demyelinative disorder (Arnold, 2005; Balcer, 2006). Optic neuritis classically presents acutely, with loss of central vision, marked dyschromatopsia, and pain on eye movement. Spontaneous visual recovery usually occurs two to three weeks after the first onset of symptoms and it is nearly complete within the next four weeks. The overall visual prognosis is very good and 1 year after an attack of optic neuritis, more than 90% of patients have recovered their premorbid level of visual acuity (Beck et al., 1993). However, in longitudinal studies of MS patients, including those without a previous history of optic neuritis, progressive RNFL thinning was noted on OCT, clearly indicating a chronic process of subclinical RGC loss (Frohman et al., 2008; Zaveri et al., 2008; Talman et al., 2010).
Female LHON carriers are twice more likely to develop an MS-like illness than male carriers, despite the fact that the risk of visual loss is five times higher among males (Section 3.3.5). We propose two main hypotheses to account for this association. It is possible that subclinical disease in at-risk LHON carriers results in immunogenic mitochondrial peptides being exposed, which trigger or potentiate an autoimmune cascade against oligodendrocytes (Loveland et al., 1990; Chalmers et al., 1995). Conversely, the autoimmune process could be the primary pathological factor and the deleterious consequences for oligodendrocytes are magnified by the presence of an underlying mtDNA defect, the latter putting additional stress on mitochondrial oxidative metabolism (Mahad et al., 2007; Mahad et al., 2009). The optic neuropathy in LHON patients who develop an MS-like illness is invariably severe with poor visual recovery and as such behaves differently from the natural history of acute demyelinative optic neuritis. Presumably, RGC axonal loss in these LHON-MS patients is amplified by dysfunctional oligodendrocytes leading to demyelination or failure of remyelination (Sapey et al., 2001; Mao and Reddy, 2010).
The link between mitochondrial dysfunction and MS is not limited to the primary mtDNA LHON mutations. We recently reported the occurrence of a more disseminated demyelinative process in two independent DOA families with genetically-confirmed OPA1 mutations. Compound heterozygous POLG1 mutations have also been recently identified in a 4-year-old child with presumed acute disseminated encephalomyelitis (ADEM) (Harris et al., 2010), and in two patients in their thirties presenting initially with unilateral optic neuritis, which subsequently evolved into clinically-definite MS with classical white matter MRI abnormalities and unmatched CSF oligoclonal bands (Echaniz-Laguna et al., 2010). In both adult MS cases, muscle biopsy revealed numerous COX-negative fibres, with prominent RRFs and multiple mtDNA deletions on long-range PCR analysis.
9. Optic nerve head morphology and disease susceptibility
Unaffected LHON carriers have larger optic disc areas compared with affected carriers and normal controls. Among affected LHON carriers, visual recovery was associated with larger vertical but not horizontal disc diameters, and there was a trend towards a better visual outcome with larger optic disc areas (Ramos et al., 2009). About 10% of LHON carriers experience visual loss before the age of 10 years, and in these childhood cases, two patterns of visual loss have been described; an acute presentation similar to the one seen in adults, and a milder variant with a slowly progressive course (Barboni et al., 2006). Compared to the slowly progressive group, children with acute LHON had worse visual acuities, thinner peripapillary RNFL thickness, and significantly smaller optic disc areas. The results from these two studies therefore suggest a possible link between optic disc anatomy and the phenotypic expression of the primary mtDNA LHON mutations. Similar to anterior ischaemic optic neuropathy (AION), a small crowded optic disc could predispose LHON carriers to a higher risk of visual loss (Hayreh 2009a,b). Transient and segmental RNFL swelling have been observed in asymptomatic LHON carriers (Nikoskelainen et al., 1996, 1977), and with the advent of OCT imaging, thickening of the temporal RNFL has been documented more objectively, confirming these earlier clinical observations (Barboni et al., 2005, 2009; Savini et al., 2005; Seo et al., 2009). The swelling of these RGC axons is secondary to axoplasmic stasis and it reflects the compromised bioenergetic state within these neurones, which are struggling to maintain energy-dependent cytoskeletal transport mechanisms (Section 10.3). If the optic nerve axons are congenitally packed within a relatively confined space, under the right circumstances, a compartment syndrome could result, further impeding axonal transport. As the fine capillary network around the lamina cribosa becomes compressed by the surrounding tissue oedema, the resulting ischaemia could set up a vicious cycle, which eventually precipitates the onset of visual loss. The concept of a “disc-at-risk” is also attractive in that it could explain the role of environmental factors such as heavy smoking in increasing the risk of disease conversion among LHON carriers. Long-term heavy smokers are more likely to have atherosclerotic vascular changes and these could further exacerbate a local hypoxic insult at the optic nerve head.
In one study, OPA1 carriers had significantly smaller optic disc areas compared with normal controls (Barboni et al., 2010), and although speculative, these findings were ascribed to the role played by OPA1 in early embryonic development (Section 11.3.2). By accentuating developmental apoptosis, OPA1 mutations could result in a greater loss of RGCs and therefore a correspondingly smaller optic nerve head. In support of this hypothesis, OPA1 carriers have thinner peripapillary RNFL measurements than controls at all age groups, and extrapolating the data, it is likely that these patients had lower RGC counts at birth (Fig. 9) (Milea et al., 2010). If OPA1 does exert a developmental influence on optic nerve head morphology, this could have important implications for other ocular disorders such as NTG and optic nerve hypoplasia. One could speculate that specific combinations of OPA1 variants could have a subtle effect on the biomechanical characteristics of the lamina cribosa, which is the principal site of IOP-related damage to RGC axons (Burgoyne et al., 2005; Sigal and Ethier, 2009; Sigal et al., 2010). The mechanical effect of IOP on load-bearing connective tissues is accentuated at the lamina cribosa, and this will vary depending on the unique susceptibility conferred by an individual’s optic nerve head configuration. In individuals with high-risk OPA1 genotypes and relatively weak optic nerve head biomechanics, axonal damage could occur at relatively low IOPs, increasing the risk of developing NTG. The aetiology of optic nerve hypoplasia is poorly defined and in 20% of cases, this abnormality occurs in isolation, without more widespread cerebral malformations or hypothalamic dysfunction (Borchert and Garcia-Filion, 2008). It would therefore be of great interest to screen for OPA1 in a cohort of patients with isolated optic nerve hypoplasia to determine whether some patients harbour pathogenic mutations, or whether there is any association with specific polymorphic sequence variants.
Fig. 9.
Age-related decrease in average RNFL thickness, consistent with progressive retinal ganglion cell loss among OPA1 mutational carriers (n = 40). Spearman rank correlation coefficient = −0.2419, P = 0.0307 (PWYM, unpublished data).
10. Greater vulnerability of retinal ganglion cells
10.1. Anatomical considerations
Both OPA1 and the primary LHON mutations result in a mitochondrial biochemical defect, but the same situation applies to other cell types such as photoreceptors and substantia nigra neurones, which have similar, if not higher, oxidative requirements than RGCs. What other factors therefore contribute to this greater vulnerability of RGCs in mitochondrial optic neuropathies? The optic nerve has some rather peculiar anatomical constraints which could influence the pathological consequences of these genetic defects. One such feature is the fact that RGC axons only acquire a myelin sheath beyond the lamina cribosa. In the pre-laminar region, the generation and propagation of action potentials are therefore less efficient and require greater energetic input (Carelli et al., 2004a; Morgan, 2004). To investigate this further, we performed a series of histological studies of human optic nerves, first staining them for mitochondrial COX and succinate dehydrogenase (SDH) enzyme activities (Andrews et al., 1999a; Bristow et al., 2002). As we expected, the pre-laminar, unmyelinated optic nerve demonstrated much more intense COX staining compared with the post-laminar, myelinated segment, clearly indicating a differential concentration of mitochondria across the lamina cribosa (Fig. 10). The increased mitochondrial content within the unmyelinated RGC axons was associated with a high density of voltage gated sodium channels, reflecting the greater energy requirements for nerve conduction in the absence of myelination (Fig. 11) (Barron et al., 2004). The pre-laminar segment of the optic nerve could therefore be viewed as a vulnerable metabolic “chokepoint”, magnifying the consequences of any impairment in mitochondrial OXPHOS, however subtle. Another important point to consider is that parvocellular RGCs within the papillomacular bundle have relatively small cross-sectional areas, which could put them at an even greater physiological disadvantage in terms of their mitochondrial reserve, compared with the larger magnocellular RGCs (Sadun et al., 2000).
Fig. 10.
Optic nerve sections stained for myelin and mitochondrial COX activity: (A) Sudan black staining revealing the presence of myelin posterior to the lamina cribosa, (B) marked differential COX activity in the pre- and post-lamina cribosa segments, with intense COX staining in transverse sections taken from the pre-laminar region (C), and significantly lower levels of COX activity in the pos-tlaminar region (D). Reproduced with permission from Andrews et al. (1999a).
Fig. 11.
Histology, mitochondrial histochemistry and immunohistochemistry (IHC) performed on serial longitudinal optic nerve sections: (A) haematoxylin and eosin, (B) Van Gieson preparation for connective tissue fibres (Red), with the arrow pointing towards the lamina cribosa, (C) Weigert iron haematoxylin preparation for myelin (Dark blue), (D) COX activity, with a darker stain (Brown) evident in the unmyelinated pre-lamina cribosa segment of the optic nerve, (E) IHC for COX subunit IV revealing a pattern consistent with the level of mitochondrial enzyme activity, (F ) IHC for Nav 1.1 showing a greater concentration of these specific voltage gated Na+ channels in the pre-laminar region, (G) IHC for Nav 1.2 demonstrating a uniformly pale labelling pattern in both pre- and post-lamina cribosa areas, (H) IHC for Nav 1.6 with a strong staining reaction observed in the unmyelinated pre-laminar optic nerve, and (I) control optic nerve section with the primary antibody omitted. Reproduced with permission from Barron et al. (2004).
Another cranial nerve marked by such a dramatic transition between unmeylinated and myelinated segments is the cochlear nerve. Nearly two-thirds of all patients with DOA+ phenotypes develop sensorineural deafness (Yu-Wai-Man et al., 2010b). It is therefore very revealing that in two family members with optic atrophy and hearing loss due to a pathogenic OPA1 mutation, auditory investigations localised the defect to the terminal unmyelinated portions of cochlear nerve (Huang et al., 2009). Transtympanic electrocochleography revealed sub-threshold, abnormally-prolonged negative potentials within the unmyelinated auditory nerve terminals suggesting a failure to generate action potentials at the first node of Ranvier.
10.2. Mitochondrial network dynamics
The mitochondrial network is in a constant state of flux and it relies on a delicate balance between fusional and fissional forces. Disruption of mitochondrial fusion can have dramatic consequences on cell survival as clearly illustrated by OPA1 and MFN2 mutations. The role of mitochondrial network dynamics in maintaining cellular homeostatis was confirmed further when a heterozygous, dominant-negative, DLP1 mutation was identified in a female infant with a complex neurodegenerative phenotype characterised by microcephaly, abnormal brain development, optic atrophy, and disturbed metabolic function (Waterham et al., 2007). The mitochondrial network in the patient’s cultured fibroblasts was elongated, forming tangled tubular structures, which were clumped around the nucleus. DLP1 codes for DRP1, a pro-fission, dynamin-related GTPase protein, and the mutation leads to a dominant-negative loss of function, resulting in unopposed mitochondrial fusion. A dysfunctional mitochondrial network can obviously impact on other cellular processes such as axonal transport, but as such, it still does not explain the selective vulnerability of RGCs. Some important clues were uncovered by Kamei et al. (2005), who transfected cultured rat RGCs and cerebellar granule cells (CGCs) with short interfering RNAs (siRNAs) against Opa1 transcripts. RGCs were much more susceptible to these Opa1-blocking siRNAs and they exhibited a greater degree of mitochondrial fragmentation compared with CGCs, reflecting the predominant optic nerve phenotype seen in patients with DOA.
10.3. Mitochondrial–cytoskeletal interactions
Mitochondria do not exist in isolation and an intricate cytoskeletal system is essential for their proper localisation to areas of increased energetic demands (Fig. 12) (Das et al., 2010). These mitochondrial–cytoskeletal interactions apply not only to the lamina cribosa, but also to other sites requiring the maintenance of a differential mitochondrial gradient such as the nodes of Ranvier. Axonal transport along the relatively long RGC axons is mainly dependent on the microtubule tracks, which are powered by the energy-dependent dynein and kinesin family of motor proteins (Yaffe et al., 2003; Boldogh and Pon, 2007). Conceptually, abnormal axonal transport could therefore be disrupted by two processes, either a mitochondrial biochemical defect or a primary problem with the assembly and maintenance of the microtubule network, as seen in the HSP group of disorders (Salinas et al., 2008). The distinction is somewhat artificial in that one will precipitate the other, setting up a vicious circle leading to axonal swelling due to axoplasmic stasis, and ultimately axonal degeneration marked by the onset and progression of clinical symptoms (Morfini et al., 2009).
Fig. 12.
The importance of the cytoskeleton in maintaining the differential concentration of mitochondria in the pre- and post-lamina cribosa segments of the optic nerve. The left panel shows a longitudinal section of a human optic nerve stained sequentially for COX and SDH. The pre-lamina, unmyelinated segment has a much darker COX staining consistent with the higher concentration of mitochondria. The right panel is a schematic representation of the cytoskeletal–mitochondrial interactions which facilitate the transport, distribution, and localisation of mitochondria to different areas of the optic nerve.
10.4. Light and mitochondrial toxicity
RGCs are constantly being exposed to light in the 400–760 nm wavelength spectra, the cornea and the lens effectively blocking ultraviolet light below 400 nm. The blue component of visible light has the shortest wavelength and in studies of the retinal pigment epithelium and photoreceptors, it had the greatest potential for inducing cellular ROS production. Photoreceptors are partially shielded by macular lutein and zeoxanthin pigments (Chen et al., 2001; Landrum and Bone, 2001), whereas mitochondria within the pre-laminar, unmyelinated RGC axons are directly exposed to light and do not have the benefit of these protective mechanisms. It is therefore possible that chronic light exposure could tip the balance in RGCs already compromised by a genetically-determined mitochondrial respiratory chain defect. This hypothesis has been partially tested in RGC-5 cell lines exposed to incremental light intensities, and cultured in both normal media and conditions of serum deprivation (Lascaratos et al., 2007; Osborne et al., 2008). Light exposure resulted in the generation of a significant amount of ROS in these RGC-5 cells, an effect which was intensity-dependent and exacerbated in conditions of nutritional deprivation. This light-induced elevation in ROS levels affected cellular viability by suppressing the expression of RGC-specific mRNAs and triggering the apoptotic cascade. Additional studies are therefore warranted to investigate the consequences of light exposure on RGC survival in mitochondrial optic nerve disorders, and for that purpose, both patient cell lines and existing animal models could be used (Lascaratos et al., 2007; Osborne et al., 2008).
10.5. Melanopsin retinal ganglion cells
Much attention has focused on the selective vulnerability of RGCs in mitochondrial optic neuropathies. However, immunohistochemical analysis of post-mortem eyes from patients with LHON and DOA has revealed relative sparing of a specific subpopulation of melanopsin-containing RGCs (La Morgia et al., 2010). These melanopsin RGCs constitute only about 1% of the total RGC population, but they subserve an important evolutionary role, regulating the body’s circadian rhythm as part of the retino-hypothalamic tract. The relative sparing of these melanopsin RGCs in LHON and DOA also explain to a certain extent the normally preserved pupillary light reflexes in these two disorders. The inherent resistance of melanopsin RGCs to mitochondrial dysfunction is tantalising and it could reveal key protective mechanisms that could be applied to neuroprotection of the larger pool of susceptible RGCs.
11. Experimental disease models
11.1. Drosophila dOpa1 mutants
Yarosh et al. (2008) have recently established a Drosophila model harbouring a specific dOpa1 mutation (CG8479), and the ocular phenotypes associated with heterozygous and homozygous carriers were determined. Homozygous mutant flies developed a rough and glossy eye phenotype due to the loss of hexagonal lattice cells, and decreased lens and pigment deposition. The dOpa1 mutation caused an increase in ROS levels and mitochondrial fragmentation, which damaged both cone and pigment cells. In a series of elegant experiments, these investigators were then able to demonstrate that the rough and glossy eye phenotype could be partially reversed by dietary supplementation with SOD-1 and vitamin E, and by genetic overexpression of human SOD1. Heterozygous adult flies did not exhibit any ocular abnormalities, but similar to the homozygous mutants, they also demonstrated elevated ROS levels and a greater susceptibility to oxidative stress. The heteterozygous drosophila carriers showed irregular and dysmorphic mitochondria in their muscle, and they had a significantly shortened lifespan, which was only partially restored by anti-oxidant treatment (Shahrestani et al., 2009; Tang et al., 2009).
11.2. Zebrafish opa3 mutants
High levels of opa3 mRNA transcripts are present in the central nervous system of zebrafish (Danio rerio), including the optic nerve and retinal layers. Zebrafish embryos were therefore engineered harbouring a 5.2 kb retroviral DNA insertion immediately downstream of the mitochondrial leader signal, which disrupts the opa3 reading frame and results in a premature termination codon (Pei et al., 2010). Five-days-old homozygous mutant embryos (opa3ZM/ZM) had increased MGA levels, recapitulating the biochemical signature of patients with Costeff syndrome (Section 4.3). A significant loss of RGCs together with a reduction in optic nerve diameter was also observed on histological analysis of 1-year-old opa3ZM/ZM zebrafish mutants, confirming the role of opa3 in preserving RGC function. In addition, these homozygous mutants demonstrated extra-ocular deficits with dramatic alterations in swimming behaviour secondary to ataxia, loss of buoyancy control, and hypokinesia.
11.3. Current murine models
11.3.1. LHON
There is still no animal model where the primary LHON mutations have been successfully introduced into the mitochondrial genome. In spite of these technical difficulties, four experimental techniques have been developed which can replicate the optic nerve degeneration seen in LHON: (i) intravitreal injection of a respiratory chain poison such as rotenone (Zhang et al., 2002), (ii) stereotactic injection of biodegradable rotenone-loaded microspheres into the optical layer of the superior colliculus (Marella et al., 2010), (iii) downregulation of nuclear-encoded complex I subunits (e.g. NFUFA1) with specific mRNA-degrading ribozymes (Qi et al., 2003), and (iv) allotopic expression of mutant subunits (e.g. MTND4) which are then imported into the mitochondria (Qi et al., 2007b; Ellouze et al., 2008). These disease models will be indispensable when testing the feasibility of gene therapy in LHON, and different strategies are currently being pursued (Section 12.4).
11.3.2. Opa1
Two DOA mouse models have been developed which harbour pathogenic mutations in exon 8 (c.1051C>T) and intron 10 (c.1065+5g>a) of the Opa1 gene (Alavi et al., 2007; Davies et al., 2007). These mutations are truncative in nature and they result in a 50% reduction in the overall expression of the Opa1 protein. In both models, homozygous mutant mice (Opa1−/−) died in utero during embryogenesis, highlighting the central role played by the Opa1 protein in early development. Heterozygous Opa1+/− mice faithfully replicated the human phenotype exhibiting a slowly progressive optic neuropathy and demonstrating objective reduction in visual function on psychophysical testing. Visual evoked potential (VEP) measurements showed significantly reduced amplitudes, but no change in latencies, supporting an ascending progress of degeneration from the soma towards the axon (Heiduschka et al., 2010). Histological and retrograde labelling experiments confirmed a gradual loss of RGCs and an associated thinning of the retinal nerve fibre layer. The surviving optic nerve axons had an abnormal morphology with swelling, distorted shapes, irregular areas of demyelination and myelin aggregates. These features of optic nerve degeneration were seen as early as 9 months, but they were much more visible by 24 months. Loss of dendritic arborisation was observed for on- but not off-centre RGCs, these early features of neuronal dysfunction preceding the onset of axonal loss (Williams et al., 2010). An increased number of autophagosomes was also noted in the RGC layer of heterozygous Opa1+/− mice at later time points, probably due to the accumulation of dysfunctional mitochondria (White et al., 2009). Mitochondria within these axons showed disorganised cristae structures on transmission electron microscopy and cultured fibroblasts showed increased mitochondrial network fragmentation. To further investigate the extent of mitochondrial dysfunction, we carried out additional histochemical and molecular genetic studies on both these DOA mouse models (Yu-Wai-Man et al., 2009a). COX deficiency and multiple mtDNA deletions were not detected in sketelal muscle and the RGC layer of heterozygous mutant mice, implicating other Opa1-related disease mechanisms in precipitating optic nerve degeneration.
A third Opa1 mouse model is currently being characterised harbouring a heterozygous c.2708-2711delTTAG mutation in exon27. Heterozygous mutant mice are showing signs of optic nerve degeneration from the age of 6 months, with subnormal VEPs, and abnormal mitochondrial distribution, especially in the lamina cribosa region (Dr Guy Lenaers, Montpellier, personal communication). All these Opa1 mouse models only manifest pure optic atrophy, and it will be important to create a DOA+ mouse model in order to dissect the mechanisms which contribute to multi-system organ involvement.
The role played by Opa1 in early mouse embryogenesis was recently confirmed in the lilR3 mutant mouse where a proportion of embryos was noted to die at midgestation (E11.5) displaying growth retardation, exencephaly, and abnormal patterning along the anterior–posterior axis (Moore et al., 2010). Using a meiotic mapping strategy, Moore et al. (2010) uncovered the genetic basis for this embryonic lethality by identifying a homozygous splice site mutation within intron 19 of the Opa1 gene, which results in a 6-bp intronic sequence insertion. As a result of this Opa1 mutation, the mutant Opa1 protein is mislocalised, remaining within the cytosol instead of being properly translocated to the mitochondrial inner membrane.
11.3.3. Opa3
An Opa3 mouse model carrying a c.365T>C (p.L122P) mutation has been reported (Davies et al., 2008). Heterozygous Opa3+/− mice were not compromised, whereas homozygous Opa3−/− mice developed multi-system organ failure with cachexia, dilated cardiomyopathy, extrapyramidal features, and a reduced lifespan of less than 4 months. These mice had severely impaired visual function, and although all the retinal layers were affected, cell loss was much more prominent within the RGC layer, further reinforcing the selective vulnerability of this specific cell type.
11.3.4. Glaucoma
A series of papers has been reported by the Weinreb group exploring the relationships between Opa1 and IOP-mediated RGC loss in murine glaucoma models (Ju et al., 2008a,b, 2009a,b, 2010). Several interesting observations were made: (i) increased IOPs led to apoptotic RGC loss, which was preceded by mitochondrial network fragmentation, and marked Opa1 and cytochrome c release into the cytosol, (ii) the IOP effect was mediated by glutamate receptor activation, which could be blocked by the N-methyl-d-aspartate (NMDA) glutamate receptor antagonist memantine, and (iii) overexpression of Opa1 with an adenovirus-associated virus (AAV) vector was protective against RGC death in glaucomatous optic neuropathy.
11.4. In vivo imaging of retinal ganglion cells
Analysis of fixed retinal whole mount preparations is time-consuming and rather limited given that the animal in question needs to be sacrificed. A better understanding of the complex pathological mechanisms underlying inherited optic neuropathies will therefore require more sophisticated in vivo techniques for visualising RGCs, with the need for both time-lapse imaging and long-term serial monitoring of various cellular processes. Apoptosis has been implicated in all mitochondrial optic neuropathies and a non-invasive system which allows RGC death to be visualised directly would be a major research tool. Glaucoma investigators are working on different experimental paradigms, which include (i) retrograde labelling of RGCs, with tracer injection at the superior colliculus (Kobbert et al., 2000; Gray et al., 2008), (ii) transduction of RGCs following intravitreal injection of a recombinant AAV vector carrying GFP (Folliot et al., 2003), and (iii) DARC (Detection of apoptosing retinal cells) with intravitreal injection of fluorescently-labelled annexin 5, which binds to exposed phosphatidylserine in early stages of apoptosis (Cordeiro et al., 2004; Guo and Cordeiro, 2008).
All these apoptosis-imaging techniques are invasive and they require repeated injections, with the possibility of introducing non-disease-related artefacts. To overcome these technical issues, various groups have generated transgenic mice which express a fluorescent marker such as GFP, under a specific RCG promoter such as Thy-1 (Walsh and Quigley, 2008; Leung and Weinreb, 2009). Although further improvement in transfection efficiency is needed, RGCs which are successfully transfected can be visualised using a standard confocal scanning laser ophthalmoscope (CSLO), with the mouse anaesthesised on an appropriately adapted microscope stage. Using this protocol, extremely detailed RGC images can be obtained including cell body morphology and the extent of dendritic arborisation. Furthermore, coupled with the intravitreal injections of tracer agents, time-lapsed sequences would allow axonal transport to be quantified both in the pre- and post-laminar segments of the optic nerve. The application of these new imaging modalities to both existing and future animal models of mitochondrial optic neuropathies will be a major breakthrough, allowing for example early subclinical RGC abnormalities to be detected, and the therapeutic potential of putative neuroprotective agents to be assessed at a more functional level.
12. Treatment strategies for inherited optic neuropathies
12.1. Genetic counselling
Male carriers harbouring mtDNA point mutations can be reassured that their children are not at risk of inheriting their genetic defect. Female carriers will transmit the mutation to all their offspring, but if the mutation is heteroplasmic, it is not possible to reliably predict the mutational level that will be transmitted. As discussed earlier, significant variations in heteroplasmy levels can occur as a result of the mitochondrial genetic bottleneck. The use of amniocentesis or chorionic villus sampling for prenatal testing is therefore limited in this situation, as the mutational load detected in amniocytes and chorionic villus cells could differ from other foetal tissues, especially those at greater risk from a specific mtDNA point mutation such as RGCs (Brown et al., 2006). Although it is not possible to accurately predict whether a LHON carrier will eventually lose vision, individuals can be counselled based on the two major identifiable risk factors in this disorder, age and gender. Male carriers have about a 50% lifetime risk of visual failure compared with only 10% for female carriers, and most patients will experience visual loss in their late teens and twenties. The probability of becoming affected decreases subsequently and disease conversion in LHON is rare over the age of 50 years. The risks of transmission for nuclear mitochondrial disorders follow the laws of Mendelian inheritance, but if a specific nuclear defect has not been identified, only an approximate risk can be provided based on the family history. As a result of the marked inter- and intra-familial phenotypic variability seen with both pure and syndromal inherited optic neuropathies, genetic counselling for patients and their families remains a challenging area of practice.
12.2. Supportive measures
The treatment options for patients with inherited optic neuropathies are currently limited (Chinnery et al., 2006). However, there are several practical steps that can be taken as part of a multi-disciplinary team to improve a patient’s quality of life, and minimise long-term morbidity (Yu-Wai-Man et al., 2009b; Fraser et al., 2010). Depending on their needs, these patients should be provided with access to facilities such as low visual aids and occupational therapy, and clinicians can help with financial assistance through their local social services. It is also important to aggressively manage related medical problems such as diabetes and epilepsy, and clinicians need to be vigilant to the development of new complications such as cardiomyopathy and sensorineural deafness, which could be amenable to therapeutic intervention. Patients with mitochondrial disorders should be strongly advised not to smoke and to minimise their alcohol intake, not only as a general health measure, but smoking, and to a lesser extent excessive alcohol intake, have been linked with an increased risk of visual loss among LHON carriers (Kirkman et al., 2009b). Patients with CPEO often get significant benefit from simple conservative measures such as ptosis props or Fresnel prisms for symptomatic ocular misalignment (Richardson et al., 2005). In some cases, strabismus and ptosis surgery are indicated but these should be performed by experienced surgeons (Shorr et al., 1987; Ahn et al., 2008), because of the increased risk of complications such as corneal exposure secondary to poor orbicularis oculi function and impaired Bell’s phenomenon (Daut et al., 2000).
12.3. Neuroprotection
On the basis of limited, mostly anecdotal evidence, various combinations of pharmacological agents have been used to treat patients with mitochondrial disorders including multi-vitamin supplements, co-enzyme Q10 (CoQ10) and its derivatives, and putative free radical scavengers (Horvath et al., 2008; Tarnopolsky, 2008; Schon et al., 2010). There is relatively more clinical data on the use of CoQ10, which has shown a clear benefit for patients with primary CoQ10 deficiency. In one randomised, double-blind, crossover study of 16 patients with mitochondrial cytopathies, a combination of CoQ10 with creatine monohydrate and α-lipoic acid, reduced the levels of resting plasma lactate and oxidative stress markers (Rodriguez et al., 2007).
Idebenone is a synthetic analogue of CoQ10, and it is currently being investigated as a treatment option for LHON and other neurodegenerative disorders such as FRDA. Idebenone is able to operate under low oxygen tension and it is thought to have anti-oxidant properties, in addition to optimising ATP production by the respiratory chain complexes (Tonon and Lodi, 2008; Sacconi et al., 2010). Initial reports of idebenone therapy in FRDA showed promising results with an improvement in both cardiac and neurological status (Rustin et al., 2004; Di Prospero et al., 2007). However, preliminary data released from two recently completed randomised controlled trials have proven rather disappointing, with no significant difference in primary cardiac and neurological endpoints between the idebenone-treated and placebo groups. (http://www.santhera.com/index.php?docid=212&vid=&lang=en&newsdate=201005&newsid=1417424&newslang=en, Accessed 1st of December 2010). In a retrospective study of 28 affected LHON patients, half of whom received a combination of idebenone, vitamin B2, and vitamin C for at least 1 year, the treated group showed a faster rate of visual recovery (Mashima et al., 2000). However, in a more recent report, megadoses of idebenone, vitamin C, and riboflavin did not prevent second-eye involvement in two m.11778G>A LHON carriers treated after the onset of unilateral visual loss, and both patients showed no improvement in visual function (Barnils et al., 2007). The benefits of idebenone in LHON therefore remain unclear, but being a safe drug, it is often recommended by clinicians or self-prescribed by patients. In collaboration with clinical partners in Germany and Canada, we recently completed a double-blind randomised placebo-controlled trial of idebenone in LHON. The preliminary results suggest a beneficial effect, especially among patients with recent disease onset, and relatively good remaining visual acuity in the second eye to become affected. (http://www.santhera.com/index.php?docid=212&vid=&lang=enamp;newsdate=201006&newsid=1424223&newslang=en, Accessed 1st of December 2010). If these initial findings are confirmed, idebenone could also be considered as a neuroprotective agent for other mitochondrial optic neuropathies such as DOA, although long-term follow-up will be required given the relatively slower rate of RGC loss in this disorder.
Brimonidine is a topical α-2 agonist used in the management of glaucoma for its IOP-lowering properties. In addition, studies based on models of optic nerve ischaemia have suggested that brimonidine could have an anti-apoptotic effect, mitigating RGC loss (Wheeler et al., 2003). On this basis, topical brimonidine has been tested as prophylactic agent for second-eye involvement in an open-labelled study of 9 patients with unilateral acute vision from LHON (Newman et al., 2005). Brimonidine failed to prevent fellow eye involvement and there was no evidence of improved visual benefit following the onset of visual loss. In the long term, neuroprotection for LHON remains an attractive treatment option given the easy accessibility of ocular tissues to various forms of manipulation. Various agents have been shown to have RGC-neuroprotective properties in other models of optic nerve degeneration such as memantine, valproic acid (Biermann et al., 2010), prostaglandin J2 (PGJ2) (Touitou et al., 2010), and SIRT-1 activators (Shindler et al., 2007). It is not inconceivable an effective agent will soon become available in clinical practice, which could be safely delivered intravitreally, providing an instantaneous local protective effect.
12.4. Gene therapy
Classical gene therapy is proving very challenging for primary mitochondrial disorders, because the tools required for successful gene transfer into the mitochondrial genome is still not available (DiMauro and Mancuso, 2007; Kyriakouli et al., 2008; DiMauro and Rustin, 2009). The mitochondrial inner membrane represents a significant physical barrier that needs to be overcomed, and given the large number of mitochondria per cell, a highly efficient vector will be required to achieve an adequate level of transfection. To bypass these difficulties, allotopic strategies have been developed where the gene of interest is transfected into the nuclear genome, usually with an AAV vector (Manfredi et al., 2002; Qi et al., 2007a). The protein product has a mitochondrial targeting sequence and as a result, it gets imported into the mitochondrial compartment, either replacing the missing protein or complementing the dysfunctional mutant protein.
In both in vitro and in vivo experimental LHON models, RGC loss was dramatically reduced by transfecting them with an AAV vector containing the human SOD2 gene (Qi et al., 2004, 2007a). The increased expression of the superoxide dismutase enzyme is thought to improve RGC survival by minimising free radical damage, and decreasing the cell’s susceptiblity to apoptosis. Allotopic rescue, with the replacement of the defective mitochondrial complex subunit, is another attractive option for gene therapy in LHON. Proof-of-principle for this approach has been demonstrated in a rat model expressing a defective ND4 gene containing the m.11778A>G primary LHON mutation (Ellouze et al., 2008). The loss of visual function in these rats was reversed by transfecting RGCs with the wild-type ND4 gene, using an in vivo electroporation technique instead of an AAV vector. The transgene became stably integrated within the nuclear genome and the level of expression achieved was sufficient for successful RGC neuroprotection. These early studies of allotopic rescue in LHON are promising, but the results need to be replicated in larger animals, and long-term safety data is essential before human clinical trials can be contemplated (Friedmann, 2000; Miller, 2008). The same caveats will apply to proposed gene therapy strategies for nuclear mitochondrial disorders. Issues of safety and efficacy are especially relevant given the significant concerns that have been raised recently about the limitations of allotopic rescue in the treatment of primary mtDNA disorders (Figueroa-Martinez et al., 2010; Perales-Clemente et al., 2010). As yet, there is no conclusive experimental evidence that allotopically-expressed mitochondrial subunits can be properly integrated into fully-assembled OXPHOS complexes. Mitochondrially-encoded complex I subunits are also highly hydrophobic and if a proportion of these polypeptides is not imported, they could have deleterious consequences by physically aggregating into the cytosol or triggering an inappropriate immune response.
12.5. Preventing transmission of pathogenic mutations
Following successful fertilisation, two distinct structures can be observed within the fertilised oocyte, known as the male and female pronuclei (Brown et al., 2006). Given the lack of treatment for mitochondrial disorders, pronuclear transfer is currently being investigated as a method to prevent the transmission of mtDNA mutations in human embryos (Tachibana et al., 2009; Craven et al., 2010). This strategy involves the replacement of the entire mitochondrial population, by transferring karyoplast containing the pronuclei from a donor zygote to an enucleated recipient zygote. In a landmark study using abnormally-fertilised human zygotes, Craven et al. (2010) have shown that pronuclear transfer resulted in minimal carry-over of donor zygote mtDNA, and it was compatible with successful progression to the blastocyst stage. Preimplantation genetic diagnosis for nuclear defects is well established and it does not involve the same degree of complexity faced with mtDNA mutations.
13. Future directions
The past decade has seen significant advances in the way both clinicians and basic scientists approach inherited optic neuropathies. With greater access to molecular genetic testing, the phenotypic spectrum of classical optic nerve disorders such as LHON and DOA has expanded to encompass a much wider range of clinical features, some overlapping with other neurodegenerative disorders such as CMT, HSP, and even MS. Mitochondrial optic neuropathies could therefore be used as models to understand key pathophysiological aspects of these complex disorders, the optic nerve being relatively accessible and amenable to direct measurements with non-invasive imaging modalities. A fundamental change in our understanding is the realisation that in a significant number of genetically-determined optic nerve disorders, including glaucoma, mitochondrial dysfunction is likely to be part of a final common pathway mediating RGC loss. However, there are still more questions than answers – What combination of secondary factors will eventually prove to be the basis for the marked incomplete penetrance and male bias in LHON? What disease mechanisms explain multi-system tissue involvement in OPA1 carriers with DOA+ phenotypes, when other family members harbouring the same mutation only have pure optic nerve involvement? Conversely, why is it that only some patients with MFN2 and SPG7 mutations develop visual failure and optic atrophy? With rapid advances in genetic technology, bioinformatics, and molecular biology, the next decade will be an exciting time in this area of research. We are hopeful that these future breakthroughs will translate into much-needed, effective treatment strategies for LHON, DOA, and other mitochondrial optic neuropathies.
Conflicts of interest
None of the authors have any financial interests to disclose.
Acknowledgements
This work was supported by a Medical Research Council (MRC, UK) Clinical Research Fellowship in Neuro-Ophthalmology to PYWM (G0701386). PFC is a Wellcome Trust Senior Fellow in Clinical Science and an NIHR Senior Investigator. PFC also receives funding from Parkinson’s UK, the Association Française contre les Myopathies, the Medical Research Council Translational Muscle Centre, and the UK NIHR Biomedical Research Centre in Ageing and Age-related Disease.
References
- Ahn J., Kim N.J., Choung H.K., Hwang S.W., Sung M., Lee M.J. Frontalis sling operation using silicone rod for the correction of ptosis in chronic progressive external ophthalmoplegia. British Journal of Ophthalmology. 2008;92:1685–1688. doi: 10.1136/bjo.2008.144816. [DOI] [PubMed] [Google Scholar]
- Ahrlich K.G., De Moraes C.G.V., Teng C.C., Prata T.S., Tello C., Ritch R. Visual field progression differences between normal-tension and exfoliative high-tension glaucoma. Investigative Ophthalmology & Visual Science. 2010;51:1458–1463. doi: 10.1167/iovs.09-3806. [DOI] [PubMed] [Google Scholar]
- Aijaz S., Erskine L., Jeffery G., Bhattacharya S.S., Votruba M. Developmental expression profile of the optic atrophy gene product: OPA1 is not localized exclusively in the mammalian retinal ganglion cell layer. Investigative Ophthalmology & Visual Science. 2004;45:1667–1673. doi: 10.1167/iovs.03-1093. [DOI] [PubMed] [Google Scholar]
- Akepati V.R. Characterization of OPA1 isoforms isolated from mouse tissues. Journal of Neurochemistry. 2008;106:372–383. doi: 10.1111/j.1471-4159.2008.05401.x. [DOI] [PubMed] [Google Scholar]
- Alavi M.V., Bette S., Schimpf S., Schuettauf F., Schraermeyer U., Wehrl H.F. A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain. 2007;130:1029–1042. doi: 10.1093/brain/awm005. [DOI] [PubMed] [Google Scholar]
- Alberio S., Mineri R., Tiranti V., Zeviani M. Depletion of mtDNA: syndromes and genes. Mitochondrion. 2007;7:6–12. doi: 10.1016/j.mito.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Alexander C., Votruba M., Pesch U.E.A., Thiselton D.L., Mayer S., Moore A. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nature Genetics. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Alward W.L., Feldman F., Cashwell L.F., Wilensky J., Geijssen H.C., Greeve E. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. American Journal of Ophthalmology. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- Amati-Bonneau P., Guichet A., Olichon A., Chevrollier A., Viala F., Miot S. OPA1 R445H mutation in optic atrophy associated with sensorineural deafness. Annals of Neurology. 2005;58:958–963. doi: 10.1002/ana.20681. [DOI] [PubMed] [Google Scholar]
- Amati-Bonneau P., Odent S., Derrien C., Pasquier L., Malthiery Y., Reynier P. The association of autosomal dominant optic atrophy and moderate deafness may be due to the R445H mutation in the OPA1 gene. American Journal of Ophthalmology. 2003;136:1170–1171. doi: 10.1016/s0002-9394(03)00665-2. [DOI] [PubMed] [Google Scholar]
- Amati-Bonneau P., Valentino M.L., Reynier P., Gallardo M.E., Bornstein B., Boissiere A. OPA1 mutations induce mitochondrial DNA instability and optic atrophy plus phenotypes. Brain. 2008;131:338–351. doi: 10.1093/brain/awm298. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H., Coulson A.R., Drouin J. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Andrews R.M., Griffiths P.G., Johnson M.A., Turnbull D.M. Histochemical localisation of mitochondrial enzyme activity in human optic nerve and retina. British Journal of Ophthalmology. 1999;83:231–235. doi: 10.1136/bjo.83.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews R.M., Kubacka I., Chinnery P.F., Lightowlers R.N., Turnbull D.M., Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nature Genetics. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- Anikster Y., Kleta R., Shaag A., Gahl W.A., Elpeleg O. Type III 3-methylglutaconic aciduria (optic atrophy plus syndrome, or Costeff optic atrophy syndrome): identification of the OPA3 gene and its founder mutation in Iraqi Jews. American Journal of Human Genetics. 2001;69:1218–1224. doi: 10.1086/324651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araie M., Kitazawa M., Koseki N. Intraocular pressure and central visual field of normal tension glaucoma. British Journal of Ophthalmology. 1997;81:852–856. doi: 10.1136/bjo.81.10.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold A.C. Evolving management of optic neuritis and multiple sclerosis. American Journal of Ophthalmology. 2005;139:1101–1108. doi: 10.1016/j.ajo.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Artal-Sanz M., Tavernarakis N. Prohibitin and mitochondrial biology. Trends in Endocrinology and Metabolism. 2009;20:394–401. doi: 10.1016/j.tem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Assink J.J.M., Tijmes N.T., ten Brink J.B., Oostra R.J., Riemslag F.C., deJong P. A gene for X-linked optic atrophy is closely linked to the Xp11.4-Xp11.2 region of the X chromosome. American Journal of Human Genetics. 1997;61:934–939. doi: 10.1086/514884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung T., Ocaka L., Ebenezer N.D., Morris A.G., Krawczak M., Thiselton D.L. A major marker for normal tension glaucoma: association with polymorphisms in the OPA1 gene. Human Genetics. 2002;110:52–56. doi: 10.1007/s00439-001-0645-7. [DOI] [PubMed] [Google Scholar]
- Balcer L.J. Optic neuritis. New England Journal of Medicine. 2006;354:1273–1280. doi: 10.1056/NEJMcp053247. [DOI] [PubMed] [Google Scholar]
- Ban T., Heymann J.A.W., Song Z.Y., Hinshaw J.E., Chan D.C. OPA1 disease alleles causing dominant optic atrophy have defects in cardiolipin-stimulated GTP hydrolysis and membrane tubulation. Human Molecular Genetics. 2010;19:2113–2122. doi: 10.1093/hmg/ddq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracca A., Solaini G., Sgarbi G., Lenaz G., Baruzzi A., Schapira A.H. Severe impairment of complex I-driven adenosine triphosphate synthesis in leber hereditary optic neuropathy cybrids. Archives of Neurology. 2005;62:730–736. doi: 10.1001/archneur.62.5.730. [see comment] [DOI] [PubMed] [Google Scholar]
- Barbet F., Gerber S., Hakiki S., Perrault I., Hanein S., Ducroq D. A first locus for isolated autosomal recessive optic atrophy (ROA1) maps to chromosome 8q. European Journal of Human Genetics. 2003;11:966–971. doi: 10.1038/sj.ejhg.5201070. [DOI] [PubMed] [Google Scholar]
- Barbet F., Hakiki S., Orssaud C., Gerber S., Perrault I., Hanein S. A third locus for dominant optic atrophy on chromosome 22q. Journal of Medical Genetics. 2005:42. doi: 10.1136/jmg.2004.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiroli B., Montagna P., Cortelli P., Iotti S., Lodi R., Barboni P. Defective brain and muscle energy-metabolism shown by in-vivo P-31 magnetic-resonance spectroscopy in nonaffected carriers of 11778-mtDNA mutation. Neurology. 1995;45:1364–1369. doi: 10.1212/wnl.45.7.1364. [DOI] [PubMed] [Google Scholar]
- Barboni P., Carbonelli M., Savini G., Foscarini B., Parisi V., Valentino M.L. OPA1 mutations associated with dominant optic atrophy influence optic nerve head size. Ophthalmology. 2010;117:1547–1553. doi: 10.1016/j.ophtha.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Barboni P., Carbonelli M., Savini G., Ramos C.D., Carta A., Berezovsky A. Natural history of Leber’s hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2009 doi: 10.1016/j.ophtha.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Barboni P., Savini G., Valentino M.L., La Morgia C., Bellusci C., De Negri A.M. Leber’s hereditary optic neuropathy with childhood onset. Investigative Ophthalmology & Visual Science. 2006;47:5303–5309. doi: 10.1167/iovs.06-0520. [DOI] [PubMed] [Google Scholar]
- Barboni P., Savini G., Valentino M.L., Montagna P., Cortelli P., De Negri A.M. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology. 2005;112:120–126. doi: 10.1016/j.ophtha.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Barnils N., Mesa E., Munoz S., Ferrer-Artola A., Arruga J. Response to idebenone and multivitamin therapy in Leber’s hereditary optic neuropathy. Archivos de la Sociedad Española de Oftalmología. 2007;82:377–380. doi: 10.4321/s0365-66912007000600012. [DOI] [PubMed] [Google Scholar]
- Barron M.J., Griffiths P., Turnbull D.M., Bates D., Nichols P. The distributions of mitochondria and sodium channels reflect the specific energy requirements and conduction properties of the human optic nerve head. British Journal of Ophthalmology. 2004;88:286–290. doi: 10.1136/bjo.2003.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R.W., Cleary P.A., Trobe J.D., Kaufman D.I., Kupersmith M.J., Paty D.W. The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple-sclerosis. New England Journal of Medicine. 1993;329:1764–1769. doi: 10.1056/NEJM199312093292403. [DOI] [PubMed] [Google Scholar]
- Beretta S., Mattavelli L., Sala G., Tremolizzo L., Schapira A.H.V., Martinuzzi A. Leber hereditary optic neuropathy mtDNA mutations disrupt glutamate transport in cybrid cell lines. Brain. 2004;127:2183–2192. doi: 10.1093/brain/awh258. [DOI] [PubMed] [Google Scholar]
- Bern C., Philen R.M., Fredman D., Bowman B.A., Newman N.J., Gerr F. Epidemic optic neuropathy in cuba – clinical characterization and risk-factors. New England Journal of Medicine. 1995;333:1176–1182. doi: 10.1056/NEJM199511023331803. [DOI] [PubMed] [Google Scholar]
- Berninger T.A., Jaeger W., Krastel H. Electrophysiology and color perimetry in dominant infantile optic atrophy. British Journal of Ophthalmology. 1991;75:49–52. doi: 10.1136/bjo.75.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besch D., Leo-Kottler B., Zrenner E., Wissinger B. Leber’s hereditary optic neuropathy: clinical and molecular genetic findings in a patient with a new mutation in the ND6 gene. Graefes Archive for Clinical & Experimental Ophthalmology. 1999;237:745–752. doi: 10.1007/s004170050307. [DOI] [PubMed] [Google Scholar]
- Bette S., Schlaszus H., Wissinger B., Meyermann R., Mittelbronn M. OPA1, associated with autosomal dominant optic atrophy, is widely expressed in the human brain. Acta Neuropathologica. 2005;109:393–399. doi: 10.1007/s00401-004-0970-8. [DOI] [PubMed] [Google Scholar]
- Bhat N.K., Niranjan B.G., Avadhani N.G. The complexity of mitochondrial translation products in mammalian-cells. Biochemical and Biophysical Research Communications. 1981;103:621–628. doi: 10.1016/0006-291x(81)90496-4. [DOI] [PubMed] [Google Scholar]
- Biermann J., Grieshaber P., Goebel U., Martin G., Thanos S., Di Giovanni S. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Investigative Ophthalmology & Visual Science. 2010;51:526–534. doi: 10.1167/iovs.09-3903. [DOI] [PubMed] [Google Scholar]
- Binder J., Hofmann S., Kreisel S., Wohrle J.C., Bazner H., Krauss J.K. Clinical and molecular findings in a patient with a novel mutation in the deafness-dystonia peptide (DDP1) gene. Brain. 2003;126:1814–1820. doi: 10.1093/brain/awg174. [DOI] [PubMed] [Google Scholar]
- Biousse V., Brown M.D., Newman N.J., Allen J.C., Rosenfeld J., Meola G. De novo 14484 mitochondrial DNA mutation in monozygotic twins discordant for Leber’s hereditary optic neuropathy. Neurology. 1997;49:1136–1138. doi: 10.1212/wnl.49.4.1136. [DOI] [PubMed] [Google Scholar]
- Blakely E.L., de Silva R., King A., Schwarzer V., Harrower T., Dawidek G. LHON/MELAS overlap syndrome associated with a mitochondrial MTND1 gene mutation. European Journal of Human Genetics. 2005;13:623–627. doi: 10.1038/sj.ejhg.5201363. [DOI] [PubMed] [Google Scholar]
- Blesa J.R., Solano A., Briones P., Prieto-Ruiz J.A., Hernandez-Yago J., Coria F. Molecular genetics of a patient with Mohr–Tranebjaerg syndrome due to a new mutation in the DDPI gene. Neuromolecular Medicine. 2007;9:285–291. doi: 10.1007/s12017-007-8000-3. [DOI] [PubMed] [Google Scholar]
- Boland M.V., Quigley H.A. Risk factors and open-angle glaucoma: classification and application. Journal of Glaucoma. 2007;16:406–418. doi: 10.1097/IJG.0b013e31806540a1. [DOI] [PubMed] [Google Scholar]
- Boldogh I.R., Pon L.A. Mitochondria on the move. Trends in Cell Biology. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Borchert M., Garcia-Filion P. The syndrome of optic nerve hypoplasia. Current Neurology and Neuroscience Reports. 2008;8:395–403. doi: 10.1007/s11910-008-0061-7. [DOI] [PubMed] [Google Scholar]
- Bower S.P., Hawley I., Mackey D.A. Cardiac arrhythmia and Leber’s hereditary optic neuropathy. Lancet. 1992;339:1427–1428. doi: 10.1016/0140-6736(92)91257-9. [letter] [DOI] [PubMed] [Google Scholar]
- Bremner F.D., Tomlin E.A., Shallo-Hoffmann J., Votruba M., Smith S.E. The pupil in dominant optic atrophy. Investigative Ophthalmology & Visual Science. 2001;42:675–678. [PubMed] [Google Scholar]
- Bristow E.A., Griffiths P.G., Andrews R.M., Johnson M.A., Turnbull D.M. The distribution of mitochondrial activity in relation to optic nerve structure. Archives of Ophthalmology. 2002;120:791–796. doi: 10.1001/archopht.120.6.791. [DOI] [PubMed] [Google Scholar]
- Brown D.T., Herbert M., Lamb V.K., Chinnery P.F., Taylor R.W., Lightowlers R.N. Transmission of mitochondrial DNA disorders: possibilities for the future. Lancet. 2006;368:87–89. doi: 10.1016/S0140-6736(06)68972-1. [DOI] [PubMed] [Google Scholar]
- Brown M.D., Starikovskaya E., Derbeneva O., Hosseini S., Allen J.C., Mikhailovskaya I.E. The role of mtDNA background in disease expression: a new primary LHON mutation associated with Western Eurasian haplogroup. Journal of Human Genetics. 2002;110:130–138. doi: 10.1007/s00439-001-0660-8. [DOI] [PubMed] [Google Scholar]
- Brown M.D., Torroni A., Reckord C.L., Wallace D.C. Phylogenetic analysis of Leber’s hereditary optic neuropathy mitochondrial DNA’s indicates multiple independent occurrences of the common mutations. Human Mutation. 1995;6:311–325. doi: 10.1002/humu.1380060405. [DOI] [PubMed] [Google Scholar]
- Brown M.D., Trounce I.A., Jun A.S., Allen J.C., Wallace D.C. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber’s hereditary optic neuropathy mitochondrial DNA mutation. Journal of Biological Chemistry. 2000;275:39831–39836. doi: 10.1074/jbc.M006476200. [DOI] [PubMed] [Google Scholar]
- Brown M.D., Zhadanov S., Allen J.C., Hosseini S., Newman N.J., Atamonov V.V. Novel mtDNA mutations and oxidative phosphorylation dysfunction in Russian LHON families. Human Genetics. 2001;109:33–39. doi: 10.1007/s004390100538. [DOI] [PubMed] [Google Scholar]
- Bu X., Rotter J.I. Leber hereditary optic neuropathy: estimation of number of embryonic precursor cells and disease threshold in heterozygous affected females at the X-linked locus. Clinical Genetics. 1992;42:143–148. doi: 10.1111/j.1399-0004.1992.tb03226.x. [DOI] [PubMed] [Google Scholar]
- Bu X.D., Rotter J.I. X chromosome-linked and mitochondrial gene control of Leber hereditary optic neuropathy: evidence from segregation analysis for dependence on X chromosome inactivation. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8198–8202. doi: 10.1073/pnas.88.18.8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bua E., Johnson J., Herbst A., Delong B., McKenzie D., Salamat S. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. American Journal of Human Genetics. 2006;79:469–480. doi: 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce C., Wormald R. Leading causes of certification for blindness and partial sight in England & Wales. BMC Public Health. 2006;6:58. doi: 10.1186/1471-2458-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono L.M., Foroozan R., Sergott R.C., Savino P.J. Is normal tension glaucoma actually an unrecognized hereditary optic neuropathy? New evidence from genetic analysis. Current Opinion in Ophthalmology. 2002;13:362–370. doi: 10.1097/00055735-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Burgoyne C.F., Downs J.C., Bellezza A.J., Suh J.K.F., Hart R.T. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Progress in Retinal and Eye Research. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Campuzano V., Montermini L., Molto M.D., Pianese L., Cossee M., Cavalcanti F. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- Cann R.L. Genetic clues to dispersal in human populations: retracing the past from the present. Science. 2001;291:1742–1748. doi: 10.1126/science.1058948. [DOI] [PubMed] [Google Scholar]
- Carelli V., Achilli A., Valentino M.L., Rengo C., Semino O., Pala M. Haplogroup effects and recombination of mitochondrial DNA: novel clues from the analysis of Leber hereditary optic neuropathy pedigrees. American Journal of Human Genetics. 2006;78:564–574. doi: 10.1086/501236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli V., Bellan M. Myelin, mitochondria, and autoimmunity – what’s the connection? Neurology. 2008;70:1075–1076. doi: 10.1212/01.wnl.0000307668.75233.35. [DOI] [PubMed] [Google Scholar]
- Carelli V., Franceschini F., Venturi S., Barboni P., Savini G., Barbieri G. Grand rounds: could occupational exposure to n-hexane and other solvents precipitate visual failure in Leber hereditary optic neuropathy? Environmental Health Perspectives. 2007;115:113–115. doi: 10.1289/ehp.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli V., Ghelli A., Ratta M., Bacchilega E., Sangiorgi S., Mancini R. Leber’s hereditary optic neuropathy: biochemical effect of 11778/ND4 and 3460/ND1 mutations and correlation with the mitochondrial genotype. Neurology. 1997;48:1623–1632. doi: 10.1212/wnl.48.6.1623. [DOI] [PubMed] [Google Scholar]
- Carelli V., La Morgia C., Iommarini L., Carroccia R., Mattiazzi M., Sangiorgi S. Mitochondrial optic neuropathies: how two genomes may kill the same cell type? Bioscience Reports. 2007;27:173–184. doi: 10.1007/s10540-007-9045-0. [DOI] [PubMed] [Google Scholar]
- Carelli V., La Morgia C., Valentino M.L., Barboni P., Ross-Cisneros F.N., Sadun A.A. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochimica et Biophysica Acta-Bioenergetics. 2009;1787:518–528. doi: 10.1016/j.bbabio.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Carelli V., Ross-Cisneros F.N., Sadun A.A. Mitochondrial dysfunction as a cause of optic neuropathies. Progress in Retinal and Eye Research. 2004;23:53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Carelli V., Rugolo M., Sgarbi G., Ghelli A., Zanna C., Baracca A. Bioenergetics shapes cellular death pathways in Leber’s hereditary optic neuropathy: a model of mitochondrial neurodegeneration. Biochimica et Biophysica Acta-Bioenergetics. 2004;1658:172–179. doi: 10.1016/j.bbabio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Carelli V., Schimpf S., Valentino M.L., Fuhrmann N., Papke M., Schaich S. Dominant optic atrophy (DOA) and sensorineural hearing loss: clinical, biochemical, spectroscopic and molecular genetic study of a large Italian pedigree linked to a new locus an chromosome 16. Neurology. 2007;68:A42. [Google Scholar]
- Cartoni R., Martinou J.C. Role of mitofusin 2 mutations in the physiopathology of Charcot-Marie-Tooth disease type 2A. Experimental Neurology. 2009;218:268–273. doi: 10.1016/j.expneurol.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Casari G., De Fusco M., Ciarmatori S., Zeviani M., Mora M., Fernandez P. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell. 1998;93:973–983. doi: 10.1016/s0092-8674(00)81203-9. [DOI] [PubMed] [Google Scholar]
- Chalasani M.L., Balasubranianian D., Swarup G. Focus on molecules: optineurin. Experimental Eye Research. 2008;87:1–2. doi: 10.1016/j.exer.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Chalasani M.L., Radha V., Gupta V., Agarwal N., Balasubramanian D., Swarup G. A glaucoma-associated mutant of optineurin selectively induces death of retinal ganglion cells which is inhibited by antioxidants. Investigative Ophthalmology & Visual Science. 2007;48:1607–1614. doi: 10.1167/iovs.06-0834. [DOI] [PubMed] [Google Scholar]
- Chalmers R.M., Harding A.E. A case-control study of Leber’s hereditary optic neuropathy. Brain. 1996;119:1481–1486. doi: 10.1093/brain/119.5.1481. [DOI] [PubMed] [Google Scholar]
- Chalmers R.M., Robertson N., Kellarwood H., Compston D.A.S., Harding A.E. Sequence of the human homolog of a mitochondrially encoded murine transplantation antigen in patients with multiple-sclerosis. Journal of Neurology. 1995;242:332–334. doi: 10.1007/BF00878877. [DOI] [PubMed] [Google Scholar]
- Chen H., Chan D.C. Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Human Molecular Genetics. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.F., Chang Y., Wu J.C. The spatial distribution of macular pigment in humans. Current Eye Research. 2001;23:422–434. doi: 10.1076/ceyr.23.6.422.6963. [DOI] [PubMed] [Google Scholar]
- Cheng J.W., Cai J.P., Wei R.L. Meta-analysis of medical intervention for normal tension glaucoma. Ophthalmology. 2009;116:1243–1249. doi: 10.1016/j.ophtha.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Chevrollier A., Guillet V., Loiseau D., Gueguen N., de Crescenzo M.A., Verny C. Hereditary optic neuropathies share a common mitochondrial coupling defect. Annals of Neurology. 2008;63:794–798. doi: 10.1002/ana.21385. [DOI] [PubMed] [Google Scholar]
- Chinnery P.F. Modulating heteroplasmy. Trends in Genetics. 2002;18:173–176. doi: 10.1016/s0168-9525(01)02636-1. [DOI] [PubMed] [Google Scholar]
- Chinnery P.F., Andrews R.M., Turnbull D.M., Howell N. Leber hereditary optic neuropathy: does heteroplasmy influence the inheritance and expression of the G11778A mitochondrial DNA mutation? American Journal of Medical Genetics. 2001;98:235–243. doi: 10.1002/1096-8628(20010122)98:3<235::aid-ajmg1086>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Chinnery P.F., Brown D.T., Andrews R.M., Singh-Kler R., Riordan-Eva P., Lindley J. The mitochondrial ND6 gene is a hot spot for mutations that cause Leber’s hereditary optic neuropathy. Brain. 2001;124:209–218. doi: 10.1093/brain/124.1.209. [DOI] [PubMed] [Google Scholar]
- Chinnery P.F., Majamaa K., Turnbull D., Thorburn D. Treatment for mitochondrial disorders. Cochrane Database of Systematic Reviews. 2006 doi: 10.1002/14651858.CD004426.pub2. [DOI] [PubMed] [Google Scholar]
- Chinnery P.F., Thorburn D.R., Samuels D.C., White S.L., Dahl H.H.M., Turnbull D.M. The inheritance of mitochondrial DNA heteroplasmy: random drift, selection or both? Trends in Genetics. 2000;16:500–505. doi: 10.1016/s0168-9525(00)02120-x. [DOI] [PubMed] [Google Scholar]
- Chinnery P.F., Zeviani M. 155th ENMC workshop: polymerase gamma and disorders of mitochondrial DNA synthesis, 21–23 September 2007, Naarden, The Netherlands. Neuromuscul Disord. 2008;18:259–267. doi: 10.1016/j.nmd.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Chinnery P.F., Zwijnenburg P.J.G., Walker M., Howell N., Taylor R.W., Lightowlers R.N. Nonrandom tissue distribution of mutant mtDNA. American Journal of Medical Genetics. 1999;85:498–501. [PubMed] [Google Scholar]
- Chung H., Yoon Y.H., Hwang J.J., Cho K.S., Koh J.Y., Kim J.G. Ethambutol-induced toxicity is mediated by zinc and lysosomal membrane permeabilization in cultured retinal cells. Toxicology and Applied Pharmacology. 2009;235:163–170. doi: 10.1016/j.taap.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Ciccosanti F., Corazzari M., Soldani F., Matarrese P., Pagliarini V., Iadevaia V. Proteomic analysis identifies prohibitin down-regulation as a crucial event in the mitochondrial damage observed in HIV-infected patients. Antiviral Therapy. 2010;15:377–390. doi: 10.3851/IMP1530. [DOI] [PubMed] [Google Scholar]
- Cipolat S., Rudka T., Hartmann D., Costa V., Serneels L., Craessaerts K. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cock H.R., Cooper J.M., Schapira A.H. The 14484 ND6 mtDNA mutation in Leber hereditary optic neuropathy does not affect fibroblast complex I activity. American Journal of Human Genetics. 1995;57:1501–1502. [letter] [PMC free article] [PubMed] [Google Scholar]
- Cock H.R., Cooper J.M., Schapira A.H. Functional consequences of the 3460-bp mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Journal of the Neurological Sciences. 1999;165:10–17. doi: 10.1016/s0022-510x(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Cock H.R., Tabrizi S.J., Cooper J.M., Schapira A.H. The influence of nuclear background on the biochemical expression of 3460 Leber’s hereditary optic neuropathy. Annals of Neurology. 1998;44:187–193. doi: 10.1002/ana.410440208. [DOI] [PubMed] [Google Scholar]
- Cogan D.G., Truman J.T., Smith T.R. Optic neuropathy, chloramphenicol, and infantile genetic agranulocytosis. Investigative Ophthalmology. 1973;12:534–537. [PubMed] [Google Scholar]
- Cohn A.C., Toomes C., Hewitt A.W., Kearns L.S., Inglehearn C.F., Craig J.E. The natural history of OPA1-related autosomal dominant optic atrophy. British Journal of Ophthalmology. 2008;24:24. doi: 10.1136/bjo.2007.134726. [DOI] [PubMed] [Google Scholar]
- Cohn A.C., Toomes C., Potter C., Towns K.V., Hewitt A.W., Inglehearn C.F. Autosomal dominant optic atrophy: penetrance and expressivity in patients with OPA1 mutations. American Journal of Ophthalmology. 2007;143:656–662. doi: 10.1016/j.ajo.2006.12.038. [DOI] [PubMed] [Google Scholar]
- Cordeiro M.F., Guo L., Luong V., Harding G., Wang W., Jones H.E. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornille K., Milea D., Amati-Bonneau P., Procaccio V., Zazoun L., Guillet V. Reversible optic neuropathy with OPA1 exon 5b mutation. Annals of Neurology. 2008;63:667–671. doi: 10.1002/ana.21376. [DOI] [PubMed] [Google Scholar]
- Corral-debrinski M., Horton T., Lott M.T., Shoffner J.M., Beal M.F., Wallace D.C. Mitochondrial-DNA deletions in human brain – regional variability and increase with advanced age. Nature Genetics. 1992;2:324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- Cortelli P., Montagna P., Pierangeli G., Lodi R., Barboni P., Liguori R. Clinical and brain bioenergetics improvement with idebenone in a patient with Leber’s hereditary optic neuropathy: a clinical and 31P-MRS study. Journal of the Neurological Sciences. 1997;148:25–31. doi: 10.1016/s0022-510x(96)00311-5. [DOI] [PubMed] [Google Scholar]
- Costeff H., Gadoth N., Apter N., Prialnic M., Savir H. A familial syndrome of infantile optic atrophy, movement disorder, and spastic paraplegia. Neurology. 1989;39:595–597. doi: 10.1212/wnl.39.4.595. [DOI] [PubMed] [Google Scholar]
- Cote H.C.F., Brumme Z.L., Craib K.J.P., Alexander C.S., Wynhoven B., Ting L.L. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. New England Journal of Medicine. 2002;346:811–820. doi: 10.1056/NEJMoa012035. [DOI] [PubMed] [Google Scholar]
- Craig J.E., Hewitt A.W., Dimasi D.P., Howell N., Toomes C., Cohn A.C. The role of the Met98Lys optineurin variant in inherited optic nerve diseases. British Journal of Ophthalmology. 2006;90:1420–1424. doi: 10.1136/bjo.2006.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven L., Tuppen H.A., Greggains G.D., Harbottle S.J., Murphy J.L., Cree L.M. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465:82–U89. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree L.M., Samuels D.C., Chinnery P.F. The inheritance of pathogenic mitochondrial DNA mutations. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2009;1792:1097–1102. doi: 10.1016/j.bbadis.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullom M.E., Heher K.L., Miller N.R., Savino P.J., Johns D.R. Lebers hereditary optic neuropathy masquerading as tobacco-alcohol amblyopia. Archives of Ophthalmology. 1993;111:1482–1485. doi: 10.1001/archopht.1993.01090110048021. [DOI] [PubMed] [Google Scholar]
- Das M., Chiron S., Verde F. Microtubule-dependent spatial organization of mitochondria in fission yeast. Methods Cell Biol. 2010;97:203–221. doi: 10.1016/S0091-679X(10)97012-X. [DOI] [PubMed] [Google Scholar]
- Daut P.M., Steinemann T.L., Westfall C.T. Chronic exposure keratopathy complicating surgical correction of ptosis in patients with chronic progressive external ophthalmoplegia. American Journal of Ophthalmology. 2000;130:519–521. doi: 10.1016/s0002-9394(00)00558-4. [DOI] [PubMed] [Google Scholar]
- Davey K.M., Parboosingh J.S., McLeod D.R., Chan A., Casey R., Ferreira P. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. Journal of Medical Genetics. 2006;43:385–393. doi: 10.1136/jmg.2005.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies V., Votruba M. Focus on molecules: the OPA1 protein. Experimental Eye Research. 2006;83:1003–1004. doi: 10.1016/j.exer.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Davies V.J., Hollins A.J., Piechota M.J., Yip W., Davies J.R., White K.E. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Human Molecular Genetics. 2007;16:1307–1318. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- Davies V.J., Powell K.A., White K.E., Yip W., Hogan V., Hollins A.J. A missense mutation in the murine Opa3 gene models human Costeff syndrome. Brain. 2008;131:368–380. doi: 10.1093/brain/awm333. [DOI] [PubMed] [Google Scholar]
- De Marco N., Buono M., Troise F., Diez-Roux G. Optineurin increases cell survival and translocates to the nucleus in a Rab8-dependent manner upon an apoptotic stimulus. Journal of Biological Chemistry. 2006;281:16147–16156. doi: 10.1074/jbc.M601467200. [DOI] [PubMed] [Google Scholar]
- De Marinis M. Optic neuropathy after treatment with antituberculous drugs in a subject with Leber’s hereditary optic neuropathy mutation. Journal of Neurology. 2001;248:818–819. doi: 10.1007/s004150170103. [DOI] [PubMed] [Google Scholar]
- De Vries D.D., Went L.N., Bruyn G.W., Scholte H.R., Hofstra R.M., Bolhuis P.A. Genetic and biochemical impairment of mitochondrial complex I activity in a family with Leber hereditary optic neuropathy and hereditary spastic dystonia. American Journal of Human Genetics. 1996;58:703–711. [PMC free article] [PubMed] [Google Scholar]
- De Vriese A.S., Van Coster R., Smet J., Seneca S., Lovering A., Van Haute L.L. Linezolid-induced inhibition of mitochondrial protein synthesis. Clinical Infectious Diseases. 2006;42:1111–1117. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- Decanini-Mancera A., Harrison A.R., Lee M.S. Another case of Leber hereditary optic neuropathy in an octogenarian. Journal of Neuro-Ophthalmology. 2009;29:159–160. doi: 10.1097/WNO.0b013e3181a591b5. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M., Carelli V., Ghelli A., Ratta M., Crimi M., Sangiorgi S. Functional alterations of the mitochondrially encoded ND4 subunit associated with Leber’s hereditary optic neuropathy. FEBS Letters. 1994;352:375–379. doi: 10.1016/0014-5793(94)00971-6. [DOI] [PubMed] [Google Scholar]
- Del Bo R., Bordoni A., Sciacco M., Di Fonzo A., Galbiati S., Crimi M. Remarkable infidelity of polymerase gamma A associated with mutations in POLG1 exonuclease domain. Neurology. 2003;61:903–908. doi: 10.1212/01.wnl.0000092303.13864.be. [DOI] [PubMed] [Google Scholar]
- del Toro D., Alberch J., Lazaro-Dieguez F., Martin-Ibanez R., Xifro X., Egea G. Mutant huntingtin impairs Post-golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the golgi apparatus. Molecular Biology of the Cell. 2009;20:1478–1492. doi: 10.1091/mbc.E08-07-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C., Lenaers G., Griffoin J.M., Gigarel N., Lorenzo C., Belenguer P. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nature Genetics. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Di Prospero N.A., Baker A., Jeffries N., Fischbeck K.H. Neurological effects of high-dose idebenone in patients with Friedreich’s ataxia: a randomised, placebo-controlled trial. Lancet Neurology. 2007;6:878–886. doi: 10.1016/S1474-4422(07)70220-X. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Mancuso M. Mitochondrial diseases: therapeutic approaches. Bioscience Reports. 2007;27:125–137. doi: 10.1007/s10540-007-9041-4. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Rustin P. A critical approach to the therapy of mitochondrial respiratory chain and oxidative phosphorylation diseases. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2009;1792:1159–1167. doi: 10.1016/j.bbadis.2008.10.015. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Schon E.A. Mechanisms of disease: mitochondrial respiratory-chain diseases. New England Journal of Medicine. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- Dotti M.T., Plewnia K., Cardaioli E., Manneschi L., Rufa A., Alema G. A case of ethambutol-induced optic neuropathy harbouring the primary mitochondrial LHON mutation at nt 11778. Journal of Neurology. 1998;245:302–303. doi: 10.1007/s004150050223. [DOI] [PubMed] [Google Scholar]
- Dudkina N.V., Eubel H., Keegstra W., Boekema E.J., Braun H.P. Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3225–3229. doi: 10.1073/pnas.0408870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkina N.V., Kouril R., Peters K., Braun H.P., Boekema E.J. Structure and function of mitochondrial supercomplexes. Biochimica et Biophysica Acta-Bioenergetics. 2010;1797:664–670. doi: 10.1016/j.bbabio.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Durham S.E., Samuels D.C., Cree L.M., Chinnery P.F. Normal levels of wild-type mitochondrial DNA maintain cytochrome c oxidase activity for two pathogenic mitochondrial DNA mutations but not for m.3243A->G. American Journal of Human Genetics. 2007;81:189–195. doi: 10.1086/518901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr A., Cossee M., Agid Y., Campuzano V., Mignard C., Penet C. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. New England Journal of Medicine. 1996;335:1169–1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- Echaniz-Laguna A., Chassagne M., de Seze J., Mohr M., Clerc-Renaud P., Tranchant C. POLG1 variations presenting as multiple sclerosis. Archives of Neurology. 2010;67:1140–1143. doi: 10.1001/archneurol.2010.219. [DOI] [PubMed] [Google Scholar]
- Eliott D., Traboulsi E.I., Maumenee I.H. Visual prognosis in autosomal dominant optic atrophy (Kjer Type) American Journal of Ophthalmology. 1993;115:360–367. doi: 10.1016/s0002-9394(14)73589-5. [DOI] [PubMed] [Google Scholar]
- Elliott H.R., Samuels D.C., Eden J.A., Relton C.L., Chinnery P.F. Pathogenic mitochondrial DNA mutations are common in the general population. American Journal of Human Genetics. 2008;83:254–260. doi: 10.1016/j.ajhg.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouze S., Augustin S., Bouaita A., Bonnet C., Simonutti M., Forster V. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. American Journal of Human Genetics. 2008;83:373–387. doi: 10.1016/j.ajhg.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser S., Leo-Kottler B., Besch D., Luberichs J. Confirmation of the 14568 mutation in the mitochondrial ND6 gene as causative in Leber’s hereditary optic neuropathy. Ophthalmic Genetics. 2002;23:191–197. doi: 10.1076/opge.23.3.191.7881. [DOI] [PubMed] [Google Scholar]
- Fauser S., Luberichs J., Besch D., Leo-Kottler B. Sequence analysis of the complete mitochondrial genome in patients with Leber’s hereditary optic neuropathy lacking the three most common pathogenic DNA mutations. Biochemical and Biophysical Research Communications. 2002;295:342–347. doi: 10.1016/s0006-291x(02)00672-1. [DOI] [PubMed] [Google Scholar]
- Ferraris S., Clark S., Garelli E., Davidzon G., Moore S.A., Kardon R.H. Progressive external ophthalmoplegia and vision and hearing loss in a patient with mutations in POLG2 and OPA1. Archives of Neurology. 2008;65:125–131. doi: 10.1001/archneurol.2007.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre M., Amati-Bonneau P., Tourmen Y., Malthiery Y., Reynier P. eOPA1: An online database for OPA1 mutations. Human Mutation. 2005;25:423–428. doi: 10.1002/humu.20161. [DOI] [PubMed] [Google Scholar]
- Ferre M., Bonneau D., Milea D., Chevrollier A., Verny C., Dollfus H. Molecular screening of 980 cases of suspected hereditary optic neuropathy with a report on 77 novel OPA1 mutations. Human Mutation. 2009;30:E692–E705. doi: 10.1002/humu.21025. [DOI] [PubMed] [Google Scholar]
- Figueroa-Martinez, F., Vazquez-Acevedo, M., Cortes-Hernandez, P., Garcia-Trejo, J.J., Davidson, E., King, M.P., et al., 2010. What limits the allotopic expression of nucleus-encoded mitochondrial genes? The case of the chimeric Cox3 and Atp6 genes. Mitochondrion, Epub ahead of print. [DOI] [PubMed]
- Floreani M., Napoli E., Martinuzzi A., Pantano G., De Riva V., Trevisan R. Antioxidant defences in cybrids harboring mtDNA mutations associated with Leber’s hereditary optic neuropathy. FEBS Journal. 2005;272:1124–1135. doi: 10.1111/j.1742-4658.2004.04542.x. [DOI] [PubMed] [Google Scholar]
- Folliot S., Briot D., Conrath H., Provost N., Cherel Y., Moullier P. Sustained tetracycline-regulated transgene expression in vivo in rat retinal ganglion cells using a single type 2 adeno-associated viral vector. Journal of Gene Medicine. 2003;5:493–501. doi: 10.1002/jgm.367. [DOI] [PubMed] [Google Scholar]
- Fortuna F., Barboni P., Liguori R., Valentino M.L., Savini G., Gellera C. Visual system involvement in patients with Friedreichs ataxia. Brain. 2009;132:116–123. doi: 10.1093/brain/awn269. [DOI] [PubMed] [Google Scholar]
- Fournier A.V., Damji K.F., Epstein D.L., Pollock S.C. Disc excavation in dominant optic atrophy. Ophthalmology. 2001;108:1595–1602. doi: 10.1016/s0161-6420(01)00696-0. [DOI] [PubMed] [Google Scholar]
- Fox C.M., Bensa S., Bray I., Zajicek J.P. The epidemiology of multiple sclerosis in Devon: a comparison of the new and old classification criteria. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:56–60. [PMC free article] [PubMed] [Google Scholar]
- Fraser J.A., Biousse V., Newman N.J. The neuro-ophthalmology of mitochondrial disease. Survey of Ophthalmology. 2010;55:299–334. doi: 10.1016/j.survophthal.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs F.C.P., Dingemans K.P., Brinkman K. Cardiomyopathy with mitochondrial damage associated with nucleoside reverse-transcriptase inhibitors. New England Journal of Medicine. 2002;347:1895–1896. doi: 10.1056/NEJM200212053472320. [DOI] [PubMed] [Google Scholar]
- Frey T.G., Mannella C.A. The internal structure of mitochondria. Trends in Biochemical Sciences. 2000;25:319–324. doi: 10.1016/s0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- Frezza C., Cipolat S., de Brito O.M., Micaroni M., Beznoussenko G.V., Rudka T. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Friedmann T. Medical ethics – principles for human gene therapy studies. Science. 2000;287:2163–2165. doi: 10.1126/science.287.5461.2163. [DOI] [PubMed] [Google Scholar]
- Frohman E.M., Frohman T.C., Zee D.S., McColl R., Galetta S. The neuro-ophthalmology of multiple sclerosis. Lancet Neurology. 2005;4:111–121. doi: 10.1016/S1474-4422(05)00992-0. [DOI] [PubMed] [Google Scholar]
- Frohman E.M., Fujimoto J.G., Frohman T.C., Calabresi P.A., Cutter G., Balcer L.J. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nature Clinical Practice Neurology. 2008;4:664–675. doi: 10.1038/ncpneuro0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann N., Alavi M.V., Bitoun P., Woernle S., Auburger G., Leo-Kottler B. Genomic rearrangements in OPA1 are frequent in patients with autosomal dominant optic atrophy. Journal of Medical Genetics. 2009;46:136–144. doi: 10.1136/jmg.2008.062570. [DOI] [PubMed] [Google Scholar]
- Gabaldon T., Huynen M.A. Shaping the mitochondrial proteome. Biochimica et Biophysica Acta-Bioenergetics. 2004;1659:212–220. doi: 10.1016/j.bbabio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Garcin R., Raverdy P., Delthil S., Man H.X., Chimenes H. On a familial hereditary disorder comprising cataracts, optic atrophy, extra-pyramidal signs, and certain stigmata of Friedreich’s ataxia. Revista de Neurologia [Paris] 1961;104:373–379. [Google Scholar]
- Ghelli A., Porcelli A.M., Zanna C., Martinuzzi A., Carelli V., Rugolo M. Protection against oxidant-induced apoptosis by exogenous glutathione in Leber hereditary optic neuropathy cybrids. Investigative Ophthalmology & Visual Science. 2008;49:671–676. doi: 10.1167/iovs.07-0880. [DOI] [PubMed] [Google Scholar]
- Ghelli A., Porcelli A.M., Zanna C., Vidoni S., Mattioli S., Barbieri A. The background of mitochondrial DNA haplogroup J increases the sensitivity of Leber’s hereditary optic neuropathy cells to 2,5-hexanedione toxicity. PLoS One. 2009:4. doi: 10.1371/journal.pone.0007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelli A., Zanna C., Porcelli A.M., Schapira A.H.V., Martinuzzi A., Carelli V. Leber’s hereditary optic neuropathy (LHON) pathogenic mutations induce mitochondrial-dependent apoptotic death in transmitochondrial cells incubated with galactose medium. Journal of Biological Chemistry. 2003;278:4145–4150. doi: 10.1074/jbc.M210285200. [DOI] [PubMed] [Google Scholar]
- Giordano, C., Montopoli, M., Perli, E., Orlandi, M., Fantin, M., Ross-Cisneros, F.N., et al., 2010. Oestrogens ameliorate mitochondrial dysfunction in Leber’s hereditary optic neuropathy. Brain, Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Giraudet S., Lamirel C., Amati-Bonneau P., Reynier P., Bonneau D., Milea D. Never too old to harbour a young man’s disease? British Journal of Ophthalmology. 2010;2010:31. doi: 10.1136/bjo.2009.161539. [DOI] [PubMed] [Google Scholar]
- Godel V., Nemet P., Lazar M. Chloramphenicol optic neuropathy. Archives of Ophthalmology. 1980;98:1417–1421. doi: 10.1001/archopht.1980.01020040269011. [DOI] [PubMed] [Google Scholar]
- Gray D.C., Wolfe R., Gee B.P., Scoles D., Geng Y., Masella B.D. In vivo imaging of the fine structure of rhodamine-labeled macaque retinal ganglion cells. Investigative Ophthalmology & Visual Science. 2008;49:467–473. doi: 10.1167/iovs.07-0605. [DOI] [PubMed] [Google Scholar]
- Gray M.W., Burger G., Lang B.F. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- Gronlund M.A., Honarvar A.K.S., Andersson S., Moslemi A.R., Oldfors A., Holme E. Ophthalmological findings in children and young adults with genetically verified mitochondrial disease. British Journal of Ophthalmology. 2010;94:121–127. doi: 10.1136/bjo.2008.154187. [DOI] [PubMed] [Google Scholar]
- Gropman A., Chen T.J., Perng C.L., Krasnewich D., Chernoff E., Tifft C. Variable clinical manifestation of homoplasmic G14459A mitochondrial DNA mutation. American Journal of Medical Genetics: Part A. 2004;124:377–382. doi: 10.1002/ajmg.a.20456. [DOI] [PubMed] [Google Scholar]
- Grzybowski A. Tobacco amblyopia: does it really exist? Eye. 2007;21:1448–1449. doi: 10.1038/sj.eye.6702950. [DOI] [PubMed] [Google Scholar]
- Grzybowski, A., Holder, G.E., 2010. Tobacco optic neuropathy (TON) – the historical and present concept of the disease. Acta Ophthalmologica, Epub ahead of print. [DOI] [PubMed]
- Guillet V., Chevrollier A., Cassereau J., Letournel F., Gueguen N., Richard L. Ethambutol-induced optic neuropathy linked to OPA1 mutation and mitochondrial toxicity. Mitochondrion. 2010;10:115–124. doi: 10.1016/j.mito.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Guo L., Cordeiro M.F. Assessment of neuroprotection in the retina with DARC. Glaucoma: an Open Window to Neurodegeneration and Neuroprotection. 2008;173:437–450. doi: 10.1016/S0079-6123(08)01130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gvozdjak J., Gvozdjakova A., Kucharska J., Bada V. The effect of smoking on myocardial metabolism. Czechoslovak Medicine. 1987;10:47–53. [PubMed] [Google Scholar]
- Hanein S., Perrault I., Roche O., Gerber S., Khadom N., Rio M. TMEM126A, encoding a mitochondrial protein, is mutated in autosomal-recessive nonsyndromic optic atrophy. American Journal of Human Genetics. 2009;84:493–498. doi: 10.1016/j.ajhg.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A.E. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1:1151–1155. doi: 10.1016/s0140-6736(83)92879-9. [DOI] [PubMed] [Google Scholar]
- Harding A.E., Riordan-Eva P., Govan G.G. Mitochondrial DNA diseases: genotype and phenotype in Leber’s hereditary optic neuropathy. Muscle & Nerve. 1995;3:S82–S84. doi: 10.1002/mus.880181417. [DOI] [PubMed] [Google Scholar]
- Harding A.E., Sweeney M.G., Govan G.G., Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. American Journal of Human Genetics. 1995;57:77–86. [PMC free article] [PubMed] [Google Scholar]
- Harding A.E., Sweeney M.G., Miller D.H., Mumford C.J., Kellar-Wood H., Menard D. Occurrence of a multiple sclerosis-like illness in women who have a Leber’s hereditary optic neuropathy mitochondrial DNA mutation. Brain. 1992;115:979–989. doi: 10.1093/brain/115.4.979. [DOI] [PubMed] [Google Scholar]
- Harris M.O., Walsh L.E., Hattab E.M., Golomb M.R. Is it ADEM, POLG, or both? Archives of Neurology. 2010;67:493–496. doi: 10.1001/archneurol.2010.36. [DOI] [PubMed] [Google Scholar]
- Hauser M.A., Sena D.F., Flor J., Walter J., Auguste J., LaRocque-Abramson K. Distribution of optineurin sequence variations in an ethnically diverse population of low-tension glaucoma patients from the United States. Journal of Glaucoma. 2006;15:358–363. doi: 10.1097/01.ijg.0000212255.17950.42. [DOI] [PubMed] [Google Scholar]
- Hayreh S.S. Ischemic optic neuropathy. Progress in Retinal and Eye Research. 2009;28:34–62. doi: 10.1016/j.preteyeres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hayreh S.S. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. Archives of Ophthalmology. 2009;127:1082–1083. doi: 10.1001/archophthalmol.2009.199. [DOI] [PubMed] [Google Scholar]
- He L.P., Chinnery P.F., Durham S.E., Blakely E.L., Wardell T.M., Borthwick G.M. Detection and quantification of mitochondrial DNA deletions in individual cells by real-time PCR. Nucleic Acids Research. 2002;30 doi: 10.1093/nar/gnf067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiduschka P., Schnichels S., Fuhrmann N., Hofmeister S., Schraermeyer U., Wissinger B. Electrophysiological and histologic assessment of retinal ganglion cell fate in a mouse model for OPA1-associated autosomal dominant optic atrophy. Investigative Ophthalmology & Visual Science. 2010;51:1424–1431. doi: 10.1167/iovs.09-3606. [DOI] [PubMed] [Google Scholar]
- Heng J.E., Vorwerk C.K., Lessell E., Zurakowski D., Levin L.A., Dreyer E.B. Ethambutol is toxic to retinal ganglion cells via an excitotoxic pathway. Investigative Ophthalmology & Visual Science. 1999;40:190–196. [PubMed] [Google Scholar]
- Herrnstadt C., Elson J.L., Fahy E., Preston G., Turnbull D.M., Anderson C. Reduced-median-network analysis of complete mitochondrial DNA coding-region sequences for the major African, Asian, and European haplogroups. American Journal of Human Genetics. 2002;70:1152–1171. doi: 10.1086/339933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho G., Walter J.H., Christodoulou J. Costeff optic atrophy syndrome: new clinical case and novel molecular findings. Journal of inherited metabolic disease. 2008;7:7. doi: 10.1007/s10545-008-0981-z. [DOI] [PubMed] [Google Scholar]
- Hofhaus G., Johns D.R., Hurko O., Attardi G., Chomyn A. Respiration and growth defects in transmitochondrial cell lines carrying the 11778 mutation associated with Leber’s hereditary optic neuropathy. Journal of Biological Chemistry. 1996;271:13155–13161. doi: 10.1074/jbc.271.22.13155. [DOI] [PubMed] [Google Scholar]
- Horvath J., Horvath R., Karcagi V., Komoly S., Johns D.R. Sequence analysis of Hungarian LHON patients not carrying the common primary mutations. Journal of Inherited Metabolic Disease. 2002;25:323–324. doi: 10.1023/a:1016518811940. [DOI] [PubMed] [Google Scholar]
- Horvath R., Gorman G., Chinnery P.F. How can we treat mitochondrial encephalomyopathies? Approaches to therapy. Neurotherapeutics. 2008;5:558–568. doi: 10.1016/j.nurt.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R., Hudson G., Ferrari G., Futterer N., Ahola S., Lamantea E. Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain. 2006;129:1674–1684. doi: 10.1093/brain/awl088. [DOI] [PubMed] [Google Scholar]
- Howell N., Bindoff L.A., McCullough D.A., Kubacka I., Poulton J., Mackey D. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. American Journal of Human Genetics. 1991;49:939–950. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Halvorson S., Burns J., McCullough D.A., Paulton J. When does bilateral optic atrophy become Leber hereditary optic neuropathy? American Journal of Human Genetics. 1993;53:959–963. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Xu M., McCullough D.A. Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation. American Journal of Human Genetics. 1991;48:935–942. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Mackey D.A. How rapidly does the human mitochondrial genome evolve? American Journal of Human Genetics. 1996;59:501–509. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Mackey D.A. Low-penetrance branches in matrilineal pedigrees with leber hereditary optic neuropathy. American Journal of Human Genetics. 1998;63:1220–1224. doi: 10.1086/302049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Oostra R.J., Bolhuis P.A., Spruijt L., Clarke L.A., Mackey D.A. Sequence analysis of the mitochondrial genomes from Dutch pedigrees with Leber hereditary optic neuropathy. American Journal of Human Genetics. 2003;72:1460–1469. doi: 10.1086/375537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt C.S. Autosomal dominant optic atrophy – a spectrum of disability. Ophthalmology. 1980;87:245–251. doi: 10.1016/s0161-6420(80)35247-0. [DOI] [PubMed] [Google Scholar]
- Huang T.S., Santarelli R., Starr A. Mutation of OPA1 gene causes deafness by affecting function of auditory nerve terminals. Brain Research. 2009;1300:97–104. doi: 10.1016/j.brainres.2009.08.083. [DOI] [PubMed] [Google Scholar]
- Hudson G., Amati-Bonneau P., Blakely E.L., Stewart J.D., He L.P., Schaefer A.M. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain. 2008;131:329–337. doi: 10.1093/brain/awm272. [DOI] [PubMed] [Google Scholar]
- Hudson G., Carelli V., Horvath R., Zeviani M., Smeets H.J., Chinnery P.F. X-Inactivation patterns in females harboring mtDNA mutations that cause Leber hereditary optic neuropathy. Molecular Vision. 2007;13:2339–2343. [PubMed] [Google Scholar]
- Hudson G., Carelli V., Spruijt L., Gerards M., Mowbray C., Achilli A. Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background. American Journal of Human Genetics. 2007;81:228–233. doi: 10.1086/519394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G., Keers S., Man P.Y.W., Griffiths P., Huoponen K., Savontaus M.L. Identification of an X-chromosomal locus and haplotype modulating the phenotype of a mitochondrial DNA disorder. American Journal of Human Genetics. 2005;77:1086–1091. doi: 10.1086/498176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M., Dorward H., Ly L., Klootwijk E., Kleta R., Skovby F. OPA3, mutated in 3-methylglutaconic aciduria type III, encodes two transcripts targeted primarily to mitochondria. Molecular Genetics and Metabolism. 2010;100:149–154. doi: 10.1016/j.ymgme.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huoponen K., Lamminen T., Juvonen V., Aula P., Nikoskelainen E., Savontaus M.L. The spectrum of mitochondrial DNA mutations in families with Leber hereditary optic neuroretinopathy. Human Genetics. 1993;92:379–384. doi: 10.1007/BF01247339. [DOI] [PubMed] [Google Scholar]
- Huoponen K., Vilkki J., Aula P., Nikoskelainen E.K., Savontaus M.L. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. American Journal of Human Genetics. 1991;48:1147–1153. [PMC free article] [PubMed] [Google Scholar]
- Hwang J.M., Kim J., Park S.S. Leber’s hereditary optic neuropathy mutations in ethambutol-induced optic neuropathy. Journal of Neurology. 2003;250:87–89. doi: 10.1007/s00415-003-0960-0. [DOI] [PubMed] [Google Scholar]
- Ikeda A., Ikeda T., Ikeda N., Kawakami Y., Mimura O. Leber’s hereditary optic neuropathy precipitated by ethambutol. Japanese Journal of Ophthalmology. 2006;50:280–283. doi: 10.1007/s10384-005-0308-7. [DOI] [PubMed] [Google Scholar]
- Inglese M., Rovaris M., Bianchi S., La Mantia L., Mancardi G.L., Ghezzi A. Magnetic resonance imaging, magnetisation transfer imaging, and diffusion weighted imaging correlates of optic nerve, brain, and cervical cord damage in Leber’s hereditary optic neuropathy. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:444–449. doi: 10.1136/jnnp.70.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Nakamura M., Yamakoshi T., Lin J., Yatsuya H., Terasaki H. Reduction of inner retinal thickness in patients with autosomal dominant optic atrophy associated with OPA1 mutations. Investigative Ophthalmology & Visual Science. 2007;48:4079–4086. doi: 10.1167/iovs.07-0024. [DOI] [PubMed] [Google Scholar]
- Jansen P.H., van der Knaap M.S., de Coo I.F. Leber’s hereditary optic neuropathy with the 11 778 mtDNA mutation and white matter disease resembling multiple sclerosis: clinical, MRI and MRS findings. Journal of the Neurological Sciences. 1996;135:176–180. doi: 10.1016/0022-510x(95)00287-c. [DOI] [PubMed] [Google Scholar]
- Jaros E., Mahad D.J., Hudson G., Birchall D., Sawcer S.J., Griffiths P.G. Primary spinal cord neurodegeneration in leber hereditary optic neuropathy. Neurology. 2007;69:214–216. doi: 10.1212/01.wnl.0000265598.76172.59. [DOI] [PubMed] [Google Scholar]
- Javaheri M., Khurana R.N., O’Hearn T.M., Lai M.M., Sadun A.A. Linezolid-induced optic neuropathy: a mitochondrial disorder? British Journal of Ophthalmology. 2007;91:111–115. doi: 10.1136/bjo.2006.102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazin E., Soodyall H., Jalonen P., Lindholm E., Stoneking M., Gyllensten U. Mitochondrial mutation rate revisited: hot spots and polymorphism. Nature Genetics. 1998;18:109–110. doi: 10.1038/ng0298-109. [DOI] [PubMed] [Google Scholar]
- Ji Y.L., Jia X.Y., Li S.Q., Xiao X.S., Guo X.M., Zhang Q.J. Evaluation of the X-linked modifier loci for Leber hereditary optic neuropathy with the G11778A mutation in Chinese. Molecular Vision. 2010;16:416–424. [PMC free article] [PubMed] [Google Scholar]
- Ji Y.L., Zhang A.M., Jia X.Y., Zhang Y.P., Xiao X.S., Li S.Q. Mitochondrial DNA haplogroups M7b1’2 and M8a affect clinical expression of Leber hereditary optic neuropathy in chinese families with the m.11778G→A mutation. American Journal of Human Genetics. 2008;83:760–768. doi: 10.1016/j.ajhg.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns D.R., Heher K.L., Miller N.R., Smith K.H. Leber’s hereditary optic neuropathy. Clinical manifestations of the 14484 mutation. Archives of Ophthalmology. 1993;111:495–498. doi: 10.1001/archopht.1993.01090040087038. [DOI] [PubMed] [Google Scholar]
- Johns D.R., Neufeld M.J. Cytochrome c oxidase mutations in Leber hereditary optic neuropathy. Biochemical and Biophysical Research Communications. 1993;196:810–815. doi: 10.1006/bbrc.1993.2321. [DOI] [PubMed] [Google Scholar]
- Johns D.R., Neufeld M.J., Park R.D. An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochemical and Biophysical Research Communications. 1992;187:1551–1557. doi: 10.1016/0006-291x(92)90479-5. [DOI] [PubMed] [Google Scholar]
- Johns D.R., Smith K.H., Miller N.R., Sulewski M.E., Bias W.B. Identical twins who are discordant for Leber’s hereditary optic neuropathy. Archives of Ophthalmology. 1993;111:1491–1494. doi: 10.1001/archopht.1993.01090110057023. [DOI] [PubMed] [Google Scholar]
- Ju W.K., Kim K.Y., Angert M., Duong-Polk K.X., Lindsey J.D., Ellisman M.H. Memantine blocks mitochondrial OPA1 and cytochrome c release and subsequent apoptotic cell death in glaucomatous retina. Investigative Ophthalmology & Visual Science. 2009;50:707–716. doi: 10.1167/iovs.08-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W.K., Kim K.Y., Duong-Polk K.X., Lindsey J.D., Ellisman M.H., Weinreb R.N. Increased optic atrophy type 1 expression protects retinal ganglion cells in a mouse model of glaucoma. Molecular Vision. 2010;16:1331–1342. [PMC free article] [PubMed] [Google Scholar]
- Ju W.K., Kim K.Y., Lindsey J.D., Angert M., Duong-Polk K.X., Scott R.T. Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Investigative Ophthalmology & Visual Science. 2008;49:4903–4911. doi: 10.1167/iovs.07-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W.K., Kim K.Y., Lindsey J.D., Angert M., Patel A., Scott R.T. Elevated hydrostatic pressure triggers release of OPA1 and cytochrome C, and induces apoptotic cell death in differentiated RGC-5 cells. Molecular Vision. 2009;15:120–134. [PMC free article] [PubMed] [Google Scholar]
- Ju W.K., Lindsey J.D., Angert M., Patel A., Weinreb R.N. Glutamate receptor activation triggers OPA1 release and induces apoptotic cell death in ischemic rat retina. Molecular Vision. 2008;14:2629–2638. [PMC free article] [PubMed] [Google Scholar]
- Jun A.S., Brown M.D., Wallace D.C. A mitochondrial DNA mutation at nucleotide pair 14459 of the NADH dehydrogenase subunit 6 gene associated with maternally inherited Leber hereditary optic neuropathy and dystonia. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:6206–6210. doi: 10.1073/pnas.91.13.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei S., Chen-Kuo-Chang M., Cazevieille C., Lenaers G., Olichon A., Belenguer P. Expression of the Opa1 mitochondrial protein in retinal ganglion cells: its downregulation causes aggregation of the mitochondrial network. Investigative Ophthalmology & Visual Science. 2005;46:4288–4294. doi: 10.1167/iovs.03-1407. [DOI] [PubMed] [Google Scholar]
- Katz B.J., Zhao Y., Warner J.E.A., Tong Z.Z., Yang Z.L., Zhang K. A family with X-linked optic atrophy linked to the OPA2 locus Xp11.4-Xp11.2. American Journal of Medical Genetics: Part A. 2006;140A:2207–2211. doi: 10.1002/ajmg.a.31455. [DOI] [PubMed] [Google Scholar]
- Kaukonen J., Juselius J.K., Tiranti V., Kyttala A., Zeviani M., Comi G.P. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science. 2000;289:782–785. doi: 10.1126/science.289.5480.782. [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Herbst K., Sander B., Milea D. Selective wavelength pupillometry in Leber hereditary optic neuropathy. Clinical and Experimental Ophthalmology. 2010;38:322–324. doi: 10.1111/j.1442-9071.2010.02212.x. [DOI] [PubMed] [Google Scholar]
- Kellar-Wood H., Robertson N., Govan G.G., Compston D.A., Harding A.E. Leber’s hereditary optic neuropathy mitochondrial DNA mutations in multiple sclerosis. Annals of Neurology. 1994;36:109–112. doi: 10.1002/ana.410360121. [DOI] [PubMed] [Google Scholar]
- Kerrison J.B., Arnould V.J., Sallum J.M.F., Vagefi M.R., Barmada M.M., Li Y.Y. Genetic heterogeneity of dominant optic atrophy, Kjer type – identification of a second locus on chromosome 18q12.2-12.3. Archives of Ophthalmology. 1999;117:805–810. doi: 10.1001/archopht.117.6.805. [DOI] [PubMed] [Google Scholar]
- Kerrison J.B., Miller N.R., Hsu F., Beaty T.H., Maumenee I.H., Smith K.H. A case-control study of tobacco and alcohol consumption in Leber hereditary optic neuropathy. American Journal of Ophthalmology. 2000;130:803–812. doi: 10.1016/s0002-9394(00)00603-6. [DOI] [PubMed] [Google Scholar]
- Khrapko K. Two ways to make an mtDNA bottleneck. Nature Genetics. 2008;40:134–135. doi: 10.1038/ng0208-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Hwang J.M., Park S.S. Mitochondrial DNA C4171A/ND1 is a novel primary causative mutation of Leber’s hereditary optic neuropathy with a good prognosis. Annals of Neurology. 2002;51:630–634. doi: 10.1002/ana.10177. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Hwang J.M. Stratus OCT in dominant optic atrophy: features differentiating it from glaucoma. Journal of Glaucoma. 2007;16:655–658. doi: 10.1097/IJG.0b013e31804d23aa. [DOI] [PubMed] [Google Scholar]
- Kim U., Hwang J.M. Early stage ethambutol optic neuropathy: retinal nerve fiber layer and optical coherence tomography. European Journal of Ophthalmology. 2009;19:466–469. doi: 10.1177/112067210901900323. [DOI] [PubMed] [Google Scholar]
- Kirkman M.A., Korsten A., Leonhardt M., Dimitriadis K., De Coo I.F., Klopstock T. Quality of life in patients with leber hereditary optic neuropathy. Investigative Ophthalmology & Visual Science. 2009;50:3112–3115. doi: 10.1167/iovs.08-3166. [DOI] [PubMed] [Google Scholar]
- Kirkman M.A., Yu-Wai-Man P., Korsten A., Leonhardt M., Dimitriadis K., De Coo I.F. Gene-environment interactions in Leber hereditary optic neuropathy. Brain. 2009;132:2317–2326. doi: 10.1093/brain/awp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer B. Infantile optic atrophy with dominant transmission. Danish Medical Bulletin. 1956;3:135–141. [PubMed] [Google Scholar]
- Kjer B., Eiberg H., Kjer P., Rosenberg T. Dominant optic atrophy mapped to chromosome 3q region. II. Clinical and epidemiological aspects. Acta Ophthalmologica Scandinavica. 1996;74:3–7. doi: 10.1111/j.1600-0420.1996.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Kjer P. Infantile optic atrophy with dominant mode of inheritance: a clinical and genetic study of 19 Danish families. Acta Ophthalmologica. 1959:1–146. [PubMed] [Google Scholar]
- Kline L.B., Glaser J.S. Dominant optic atrophy – clinical profile. Archives of Ophthalmology. 1979;97:1680–1686. doi: 10.1001/archopht.1979.01020020248013. [DOI] [PubMed] [Google Scholar]
- Klivenyi P., Karg E., Rozsa C., Horvath R., Komoly S., Nemeth I. alpha-tocopherol/lipid ratio in blood is decreased in patients with Leber’s hereditary optic neuropathy and asymptomatic carriers of the 11778 mtDNA mutation. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:359–362. doi: 10.1136/jnnp.70.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobbert C., Apps R., Bechmann I., Lanciego J.L., Mey J., Thanos S. Current concepts in neuroanatomical tracing. Progress in Neurobiology. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Koch-Henriksen N., Sorensen P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurology. 2010;9:520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- Kovacs G.G., Hoftberger R., Horvath R., Barsi P., Komoly S., Lassmann H. Neuropathology of white matter disease in Leber’s hereditary optic neuropathy. Brain. 2005;128:35–41. doi: 10.1093/brain/awh310. [DOI] [PubMed] [Google Scholar]
- Kumaramanickavel G., Aung T., Sripriya S., Waseem N., Okada K., Seah S.K.L. Lack of association of IVS8+4 C/T and IVS8+32 T/C polymorphisms in the OPA1 gene with normal tension glaucoma in patients from Singapore, India and Japan. Investigative Ophthalmology & Visual Science. 2005;46:3811. [Google Scholar]
- Kyriakouli D.S., Boesch P., Taylor R.W., Lightowlers R.N. Progress and prospects: gene therapy for mitochondrial DNA disease. Gene Therapy. 2008;15:1017–1023. doi: 10.1038/gt.2008.91. [DOI] [PubMed] [Google Scholar]
- La Morgia C., Achilli A., Iommarini L., Barboni P., Pala M., Olivieri A. Rare mtDNA variants in Leber hereditary optic neuropathy families with recurrence of myoclonus. Neurology. 2008;70:762–770. doi: 10.1212/01.wnl.0000295505.74234.d0. [DOI] [PubMed] [Google Scholar]
- La Morgia C., Ross-Cisneros F.N., Sadun A.A., Hannibal J., Munarini A., Mantovani V. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain. 2010;133:2426–2438. doi: 10.1093/brain/awq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamminen T., Majander A., Juvonen V., Wikstrom M., Aula P., Nikoskelainen E. A mitochondrial mutation at nt 9101 in the ATP synthase 6 gene associated with deficient oxidative phosphorylation in a family with Leber hereditary optic neuroretinopathy. American Journal of Human Genetics. 1995;56:1238–1240. [letter] [PMC free article] [PubMed] [Google Scholar]
- Landrum J.T., Bone R.A. Lutein, zeaxanthin, and the macular pigment. Archives of Biochemistry and Biophysics. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- Larsson N.G., Andersen O., Holme E., Oldfors A., Wahlstrom J. Leber’s hereditary optic neuropathy and complex I deficiency in muscle. Annals of Neurology. 1991;30:701–708. doi: 10.1002/ana.410300511. [DOI] [PubMed] [Google Scholar]
- Lascaratos G., Ji D., Wood J.P.M., Osborne N.N. Visible light affects mitochondrial function and induces neuronal death in retinal cell cultures. Vision Research. 2007;47:1191–1201. doi: 10.1016/j.visres.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Lee E.J., Kim S.J., Choung H.K., Kim J.H., Yu Y.S. Incidence and clinical features of ethambutol-induced optic neuropathy in Korea. Journal of Neuro-Ophthalmology. 2008;28:269–277. doi: 10.1097/WNO.0b013e31818e3c6b. [DOI] [PubMed] [Google Scholar]
- Lenaers G., Reynier P., ElAchouri G., Soukkarieh C., Olichon A., Belenguer P. OPA1 functions in mitochondria and dysfunctions in optic nerve. International Journal of Biochemistry & Cell Biology. 2009;41:1866–1874. doi: 10.1016/j.biocel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Leo-Kottler B., Luberichs J., Besch D., Christ-Adler M., Fauser S. Leber’s hereditary optic neuropathy: clinical and molecular genetic results in a patient with a point mutation at np T11253C (isoleucine to threonine) in the ND4 gene and spontaneous recovery. Graefes Archive for Clinical & Experimental Ophthalmology. 2002;240:758–764. doi: 10.1007/s00417-002-0494-7. [DOI] [PubMed] [Google Scholar]
- Lessell S. Nutritional amblyopia. Journal of Neuro-Ophthalmology. 1998;18:106–111. [PubMed] [Google Scholar]
- Leung C.K.S., Weinreb R.N. Experimental detection of retinal ganglion cell damage in vivo. Experimental Eye Research. 2009;88:831–836. doi: 10.1016/j.exer.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Li C.H., Cheng Y.W., Liao P.L., Yang Y.T., Kang J.J. Chloramphenicol causes mitochondrial stress, decreases ATP biosynthesis, induces matrix metalloproteinase-13 expression, and solid-tumor cell invasion. Toxicological Sciences. 2010;116:140–150. doi: 10.1093/toxsci/kfq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby R.T., Gould D.B., Anderson M.G., John S.W.M. Complex genetics of glaucoma susceptibility. Annual Review of Genomics and Human Genetics. 2005;6:15–44. doi: 10.1146/annurev.genom.6.080604.162209. [DOI] [PubMed] [Google Scholar]
- Lightowlers R.N., Chinnery P.F., Turnbull D.M., Howell N. Mammalian mitochondrial genetics: heredity, heteroplasmy and disease. Trends in Genetics. 1997;13:450–455. doi: 10.1016/s0168-9525(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Liguori M. A phenotypic variation of dominant optic atrophy and deafness (ADOAD) due to a novel OPA1 mutation. Journal of Neurology. 2008;255:127–129. doi: 10.1007/s00415-008-0571-x. [DOI] [PubMed] [Google Scholar]
- Lodi R., Carelli V., Cortelli P., Iotti S., Valentino M.L., Barboni P. Phosphorus MR spectroscopy shows a tissue specific in vivo distribution of biochemical expression of the G3460A mutation in Leber’s hereditary optic neuropathy. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:805–807. doi: 10.1136/jnnp.72.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi R., Montagna P., Cortelli P., Iotti S., Cevoli S., Carelli V. ’Secondary’ 4216/ND1 and 13708/ND5 Leber’s hereditary optic neuropathy mitochondrial DNA mutations do not further impair in vivo mitochondrial oxidative metabolism when associated with the 11778/ND4 mitochondrial DNA mutation. Brain. 2000;123:1896–1902. doi: 10.1093/brain/123.9.1896. [DOI] [PubMed] [Google Scholar]
- Lodi R., Taylor D.J., Tabrizi S.J., Kumar S., Sweeney M., Wood N.W. In vivo skeletal muscle mitochondrial function in Leber’s hereditary optic neuropathy assessed by P-31 magnetic resonance spectroscopy. Annals of Neurology. 1997;42:573–579. doi: 10.1002/ana.410420407. [DOI] [PubMed] [Google Scholar]
- Lodi R., Tonon C., Valentino M.L., Iotti S., Clementi V., Malucelli E. Deficit of in vivo mitochondrial ATP production in OPA1-related dominant optic atrophy. Annals of Neurology. 2004;56:719–723. doi: 10.1002/ana.20278. [DOI] [PubMed] [Google Scholar]
- Loiseau D., Chevrollier A., Verny C., Guillet V., Gueguen N., de Crescenzo M.A.P. Mitochondrial coupling defect in Charcot-Marie-Tooth type 2A disease. Annals of Neurology. 2007;61:315–323. doi: 10.1002/ana.21086. [DOI] [PubMed] [Google Scholar]
- Longley M.J., Clark S., Man C.Y.W., Hudson G., Durham S.E., Taylor R.W. Mutant POLG2 disrupts DNA polymerase gamma subunits and causes progressive external ophthalmoplegia. American Journal of Human Genetics. 2006;78:1026–1034. doi: 10.1086/504303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland B., Wang C.R., Yonekawa H., Hermel E., Lindahl K.F. Maternally transmitted histocompatibility antigen of mice – a hydrophobic peptide of a mitochondrially encoded protein. Cell. 1990;60:971–980. doi: 10.1016/0092-8674(90)90345-f. [DOI] [PubMed] [Google Scholar]
- Luca C.C., Lam B.L., Moraes C.T. Erythromycin as a potential precipitating agent in the onset of Leber’s hereditary optic neuropathy. Mitochondrion. 2004;4:31–36. doi: 10.1016/j.mito.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Maagaard A., Holberg-Petersen M., Kvittingen E.A., Sandvik L., Bruun J.N. Depletion of mitochondrial DNA copies/cell in peripheral blood mononuclear cells in HIV-1-infected treatment-naive patients. HIV Medicine. 2006;7:53–58. doi: 10.1111/j.1468-1293.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- Mabuchi F., Tang S., Kashiwagi K., Yamagata Z., Iijima H., Tsukahara S. The OPA1 gene polymorphism is associated with normal tension and high tension glaucoma. American Journal of Ophthalmology. 2007;143:125–130. doi: 10.1016/j.ajo.2006.09.028. [DOI] [PubMed] [Google Scholar]
- MacKenzie J.A., Payne R.M. Mitochondrial protein import and human health and disease. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2007;1772:509–523. doi: 10.1016/j.bbadis.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D., Howell N. A variant of Leber hereditary optic neuropathy characterized by recovery of vision and by an unusual mitochondrial genetic etiology. American Journal of Human Genetics. 1992;51:1218–1228. [PMC free article] [PubMed] [Google Scholar]
- Mackey D.A., Buttery R.G. Leber hereditary optic neuropathy in Australia. Australian and New Zealand Journal of Ophthalmology. 1992;20:177–184. doi: 10.1111/j.1442-9071.1992.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Mackey D.A., Fingert J.H., Luzhansky J.Z., McCluskey P.J., Howell N., Hall A.J.H. Leber’s hereditary optic neuropathy triggered by antiretroviral therapy for human immunodeficiency virus. Eye. 2003;17:312–317. doi: 10.1038/sj.eye.6700362. [DOI] [PubMed] [Google Scholar]
- Mackey D.A., Oostra R.J., Rosenberg T., Nikoskelainen E., Bronte-Stewart J., Poulton J. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. American Journal of Human Genetics. 1996;59:481–485. [PMC free article] [PubMed] [Google Scholar]
- Mackey D.A., Trounce I. Genetics: optic nerve genetics-more than meets the eye. Nature Reviews Neurology. 2010;6:357–358. doi: 10.1038/nrneurol.2010.77. [DOI] [PubMed] [Google Scholar]
- Mahad D.J., Ziabreva I., Campbell G., Lax N., White K., Hanson P.S. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132:1161–1174. doi: 10.1093/brain/awp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D.J., Ziabreva I., Lassmann H., Turnbull D.M. Mitochondria and primary oligodendrocyte dysfunction in multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:101. [Google Scholar]
- Majander A., Finel M., Savontaus M.L., Nikoskelainen E., Wikstrom M. Catalytic activity of complex I in cell lines that possess replacement mutations in the ND genes in Leber’s hereditary optic neuropathy. European Journal of Biochemistry. 1996;239:201–207. doi: 10.1111/j.1432-1033.1996.0201u.x. [DOI] [PubMed] [Google Scholar]
- Majander A., Huoponen K., Savontaus M.L., Nikoskelainen E., Wikstrom M. Electron transfer properties of NADH:ubiquinone reductase in the ND1/3460 and the ND4/11778 mutations of the Leber hereditary optic neuroretinopathy (LHON) FEBS Letters. 1991;292:289–292. doi: 10.1016/0014-5793(91)80886-8. [DOI] [PubMed] [Google Scholar]
- Man P.Y., Griffiths P.G., Brown D.T., Howell N., Turnbull D.M., Chinnery P.F. The epidemiology of Leber hereditary optic neuropathy in the North East of England. American Journal of Human Genetics. 2003;72:333–339. doi: 10.1086/346066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi G., Fu J., Ojaimi J., Sadlock J.E., Kwong J.Q., Guy J. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nature Genetics. 2002;30:394–399. doi: 10.1038/ng851. [DOI] [PubMed] [Google Scholar]
- Mao P.Z., Reddy P.H. Is multiple sclerosis a mitochondrial disease? Biochimica et Biophysica Acta-Molecular Basis of Disease. 2010;1802:66–79. doi: 10.1016/j.bbadis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbank N.J., Craig J.E., Leek J.P., Toohey M., Churchill A.J., Markham A.F. Deletion of the OPA1 gene in a dominant optic atrophy family: evidence that haploinsufficiency is the cause of disease. Journal of Medical Genetics. 2002;39:e47. doi: 10.1136/jmg.39.8.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella, M., Seo, B.B., Thomas, B.B., Matsuno-Yagi, A., Yagi, T., 2010. Successful amelioration of mitochondrial optic neuropathy using the yeast NDI1 gene in a rat animal model. PLoS One, 5, e11472. [DOI] [PMC free article] [PubMed]
- Margulis L. Symbiosis and evolution. Scientific American. 1971;225:48–57. doi: 10.1038/scientificamerican0871-48. [DOI] [PubMed] [Google Scholar]
- Martin W., Muller M. The hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. [DOI] [PubMed] [Google Scholar]
- Martinelli P., Rugarli E.I. Emerging roles of mitochondrial proteases in neurodegeneration. Biochimica et Biophysica Acta-Bioenergetics. 2010;1797:1–10. doi: 10.1016/j.bbabio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Maruyama H., Morino H., Ito H., Izumi Y., Kato H., Watanabe Y. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- Mashima Y., Kigasawa K., Hasegawa H., Tani M., Oguchi Y. High incidence of pre-excitation syndrome in Japanese families with Leber’s hereditary optic neuropathy. Clinical Genetics. 1996;50:535–537. doi: 10.1111/j.1399-0004.1996.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Mashima Y., Kigasawa K., Wakakura M., Oguchi Y. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy? Journal of Neuro-Ophthalmology. 2000;20:166–170. doi: 10.1097/00041327-200020030-00006. [DOI] [PubMed] [Google Scholar]
- Mashima Y., Yamada K., Wakakura M., Kigasawa K., Kudoh J., Shimizu N. Spectrum of pathogenic mitochondrial DNA mutations and clinical features in Japanese families with Leber’s hereditary optic neuropathy. Current Eye Research. 1998;17:403–408. doi: 10.1080/02713689808951221. [DOI] [PubMed] [Google Scholar]
- Mayorov V., Biousse V., Newman N.J., Brown M.D. The role of the ND5 gene in LHON: characterization of a new, heteroplasmic LHON mutation. Annals of Neurology. 2005;58:807–811. doi: 10.1002/ana.20669. [DOI] [PubMed] [Google Scholar]
- McFarland R., Chinnery P.F., Blakely E.L., Schaefer A.M., Morris A.A.M., Foster S.M. Homoplasmy, heteroplasmy, and mitochondrial dystonia. Neurology. 2007;69:911–916. doi: 10.1212/01.wnl.0000267843.10977.4a. [DOI] [PubMed] [Google Scholar]
- McKinley S.H., Foroozan R. Optic neuropathy associated with linezolid treatment. Journal of Neuro-Ophthalmology. 2005;25:18–21. doi: 10.1097/00041327-200503000-00005. [DOI] [PubMed] [Google Scholar]
- Meire F., Delaey J.J., Debie S., Vanstaey M., Matton M.T. Dominant optic-nerve atrophy with progressive hearing-loss and chronic progressive external ophthalmoplegia (Cpeo) Ophthalmic Paediatrics and Genetics. 1985;5:91–97. doi: 10.3109/13816818509007861. [DOI] [PubMed] [Google Scholar]
- Meire F.M., Van Coster R., Cochaux P., Obermaier-Kusser B., Candaele C., Martin J.J. Neurological disorders in members of families with Leber’s hereditary optic neuropathy (LHON) caused by different mitochondrial mutations. Ophthalmic Genetics. 1995;16:119–126. doi: 10.3109/13816819509059971. [DOI] [PubMed] [Google Scholar]
- Melamud A., Kosmorsky G.S., Lee M.S. Ocular ethambutol toxicity. Mayo Clinic Proceedings. 2003;78:1409–1411. doi: 10.4065/78.11.1409. [DOI] [PubMed] [Google Scholar]
- Meyer E., Michaelides M., Tee L.J., Robson A.G., Rahman F., Pasha S. Nonsense mutation in TMEM126A causing autosomal recessive optic atrophy and auditory neuropathy. Molecular Vision. 2010;16:650–664. [PMC free article] [PubMed] [Google Scholar]
- Milea D., Sander B., Wegener M., Jensen H., Kjer B., Jorgensen T.M. Axonal loss occurs early in dominant optic atrophy. Acta Ophthalmologica. 2010;88:342–346. doi: 10.1111/j.1755-3768.2008.01469.x. [DOI] [PubMed] [Google Scholar]
- Miller J.W. Preliminary results of gene therapy for retinal degeneration. New England Journal of Medicine. 2008;358:2282–2284. doi: 10.1056/NEJMe0803081. [DOI] [PubMed] [Google Scholar]
- Mokranjac D., Bourenkov G., Hell K., Neupert W., Groll M. Structure and function of Tim14 and Tim16, the J and J-like components of the mitochondrial protein import motor. EMBO Journal. 2006;25:4675–4685. doi: 10.1038/sj.emboj.7601334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna P., Plazzi G., Cortelli P., Carelli V., Lugaresi E., Barboni P. Abnormal lactate after effort in healthy carriers of Leber’s hereditary optic neuropathy. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;58:640–641. doi: 10.1136/jnnp.58.5.640. [letter] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B.A., Gonzalez Aviles G.D., Larkins C.E., Hillman M.J., Caspary T. Mitochondrial retention of Opa1 is required for mouse embryogenesis. Mammalian Genome. 2010;21:350–360. doi: 10.1007/s00335-010-9272-8. [DOI] [PubMed] [Google Scholar]
- Morfini G.A., Burns M., Binder L.I., Kanaan N.M., LaPointe N., Bosco D.A. Axonal transport defects in neurodegenerative diseases. Journal of Neuroscience. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J.E. Circulation and axonal transport in the optic nerve. Eye. 2004;18:1089–1095. doi: 10.1038/sj.eye.6701574. [DOI] [PubMed] [Google Scholar]
- Nekhaeva E., Bodyak N.D., Kraytsberg Y., McGrath S.B., Van Orsouw N.J., Pluzhnikov A. Clonally expanded mtDNA point mutations are abundant in individual cells of human tissues. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5521–5526. doi: 10.1073/pnas.072670199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman N.J. Leber hereditary optic neuropathy: bad habits, bad vision. Brain. 2009;132:2306–2308. doi: 10.1093/brain/awp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman N.J., Biousse V. Hereditary optic neuropathies. Eye. 2004;18:1144–1160. doi: 10.1038/sj.eye.6701591. [DOI] [PubMed] [Google Scholar]
- Newman N.J., Biousse V., David R., Bhatti M.T., Hamilton S.R., Farris B.K. Prophylaxis for second eye involvement in leber hereditary optic neuropathy: an open-labeled, nonrandomized multicenter trial of topical brimonidine purite. American Journal of Ophthalmology. 2005;140:407–415. doi: 10.1016/j.ajo.2005.03.058. [DOI] [PubMed] [Google Scholar]
- Newman N.J., Torroni A., Brown M.D., Lott M.T., Fernandez M.M., Wallace D.C. Epidemic neuropathy in Cuba not associated with mitochondrial-DNA mutations found in Lebers hereditary optic neuropathy patients. American Journal of Ophthalmology. 1994;118:158–168. doi: 10.1016/s0002-9394(14)72895-8. [DOI] [PubMed] [Google Scholar]
- Nijtmans L.G.J., Ugalde C., van den Heuvel L.P., Smeitink J.A.M. Function and dysfunction of the oxidative phosphorylation system. Mitochondrial Function and Biogenesis. 2004;8:149–176. [Google Scholar]
- Nikoskelainen E., Sogg R.L., Rosenthal A.R., Friberg T.R., Dorfman L.J. The early phase in Leber hereditary optic atrophy. Archives of Ophthalmology. 1977;95:969–978. doi: 10.1001/archopht.1977.04450060055002. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen E.K. Clinical picture of LHON. Clinical Neuroscience. 1994;2:115–120. [Google Scholar]
- Nikoskelainen E.K., Huoponen K., Juvonen V., Lamminen T., Nummelin K., Savontaus M.L. Ophthalmologic findings in Leber hereditary optic neuropathy, with special reference to mtDNA mutations. Ophthalmology. 1996;103:504–514. doi: 10.1016/s0161-6420(96)30665-9. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen E.K., Marttila R.J., Huoponen K., Juvonen V., Lamminen T., Sonninen P. Leber’s “plus”: neurological abnormalities in patients with Leber’s hereditary optic neuropathy. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;59:160–164. doi: 10.1136/jnnp.59.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoskelainen E.K., Savontaus M.L., Huoponen K., Antila K., Hartiala J. Pre-excitation syndrome in Leber’s hereditary optic neuropathy. Lancet. 1994;344:857–858. doi: 10.1016/s0140-6736(94)92830-4. [DOI] [PubMed] [Google Scholar]
- Nishigaki Y., Marti R., Copeland W.C., Hirano M. Site-speciflic somatic mitochondrial DNA point mutations in patients with thymidine phosphorylase deficiency. Journal of Clinical Investigation. 2003;111:1913–1921. doi: 10.1172/JCI17828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino I., Spinazzola A., Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–692. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- Nochez Y., Arsene S., Gueguen N., Chevrollier A., Ferre M., Guillet V. Acute and late-onset optic atrophy due to a novel OPA1 mutation leading to a mitochondrial coupling defect. Molecular Vision. 2009;15:598–608. [PMC free article] [PubMed] [Google Scholar]
- Nolan D., Mallal S. Complications associated with NRTI therapy: update on clinical features, and possible pathogenic mechanisms. Antiviral Therapy. 2004;9:849–863. [PubMed] [Google Scholar]
- Olichon A., Guillou E., Delettre C., Landes T., Arnaune-Pelloquin L., Emorine L.J. Mitochondrial dynamics and disease, OPA1. Biochimica et Biophysica Acta-Molecular Cell Research. 2006;1763:500–509. doi: 10.1016/j.bbamcr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Olichon A., Landes T., Arnaune-Pelloquin L., Emorine L.J., Mils V., Guichet A. Effects of OPA1 mutations on mitochondrial morphology and apoptosis: relevance to ADOA pathogenesis. Journal of Cellular Physiology. 2007;211:423–430. doi: 10.1002/jcp.20950. [DOI] [PubMed] [Google Scholar]
- Oostra R.J., Kemp S., Bolhuis P.A., Bleeker-Wagemakers E.M. No evidence for ’skewed’ inactivation of the X-chromosome as cause of Leber’s hereditary optic neuropathy in female carriers. Human Genetics. 1996;97:500–505. doi: 10.1007/BF02267075. [DOI] [PubMed] [Google Scholar]
- Oostra R.J., Van Galen M.J., Bolhuis P.A., Bleeker-Wagemakers E.M., Van den Bogert C. The mitochondrial DNA mutation ND6∗14,484C associated with leber hereditary optic neuropathy, leads to deficiency of complex I of the respiratory chain. Biochemical and Biophysical Research Communications. 1995;215:1001–1005. doi: 10.1006/bbrc.1995.2563. [DOI] [PubMed] [Google Scholar]
- Osborne N.N., Li G.Y., Ji D., Mortiboys H.J., Jackson S. Light affects mitochondria to cause apoptosis to cultured cells: possible relevance to ganglion cell death in certain optic neuropathies. Journal of Neurochemistry. 2008;105:2013–2028. doi: 10.1111/j.1471-4159.2008.05320.x. [DOI] [PubMed] [Google Scholar]
- Ostojic J., Jancic J., Kozic D., Semnic R., Koprivsek K., Prvulovic M. Brain white matter 1 H MRS in Leber optic neuropathy mutation carriers. Acta Neurologica Belgica. 2009;109:305–309. [PubMed] [Google Scholar]
- Palace J. Multiple sclerosis associated with Leber’s hereditary optic neuropathy. Journal of the Neurological Sciences. 2009;286:24–27. doi: 10.1016/j.jns.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Pareyson D., Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurology. 2009;8:654–667. doi: 10.1016/S1474-4422(09)70110-3. [DOI] [PubMed] [Google Scholar]
- Parker W.D., Jr., Oley C.A., Parks J.K. A defect in mitochondrial electron-transport activity (NADH-coenzyme Q oxidoreductase) in Leber’s hereditary optic neuropathy. New England Journal of Medicine. 1989;320:1331–1333. doi: 10.1056/NEJM198905183202007. [DOI] [PubMed] [Google Scholar]
- Parsons T.J., Muniec D.S., Sullivan K., Woodyatt N., AllistonGreiner R., Wilson M.R. A high observed substitution rate in the human mitochondrial DNA control region. Nature Genetics. 1997;15:363–368. doi: 10.1038/ng0497-363. [DOI] [PubMed] [Google Scholar]
- Payne M., Yang Z.L., Katz B.J., Warner J.E.A., Weight C.J., Zhao Y. Dominant optic atrophy, sensorineural hearing loss, ptosis, and ophthalmoplegia: a syndrome caused by a missense mutation in OPA1. American Journal of Ophthalmology. 2004;138:749–755. doi: 10.1016/j.ajo.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Pegoraro E., Carelli V., Zeviani M., Cortelli P., Montagna P., Barboni P. X-inactivation patterns in female Leber’s hereditary optic neuropathy patients do not support a strong X-linked determinant. American Journal of Medical Genetics. 1996;61:356–362. doi: 10.1002/(SICI)1096-8628(19960202)61:4<356::AID-AJMG10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Pei W.H., Kratz L.E., Bernardini I., Sood R., Yokogawa T., Dorward H. A model of Costeff Syndrome reveals metabolic and protective functions of mitochondrial OPA3. Development. 2010;137:2587–2596. doi: 10.1242/dev.043745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L., Scorrano L. A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death and Differentiation. 2007;14:1275–1284. doi: 10.1038/sj.cdd.4402145. [DOI] [PubMed] [Google Scholar]
- Pello R., Martin M.A., Carelli V., Nijtmans L.G., Achilli A., Pala M. Mitochondrial DNA background modulates the assembly kinetics of OXPHOS complexes in a cellular model of mitochondrial disease. Human Molecular Genetics. 2008;17:4001–4011. doi: 10.1093/hmg/ddn303. [DOI] [PubMed] [Google Scholar]
- Perales-Clemente, E., Fernandez-Silva, P., Acin-Perez, R., Perez-Martos, A., Enriquez, J.A., 2010. Allotopic expression of mitochondrial-encoded genes in mammals: achieved goal, undemonstrated mechanism or impossible task? Nucleic Acids Research, Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Perkins G., Renken C., Martone M.E., Young S.J., Ellisman M. Electron tomography of neuronal mitochondria: three-dimensional structure and organization of cristae and membrane contacts. Journal of Structural Biology. 1997;119:260–272. doi: 10.1006/jsbi.1997.3885. [DOI] [PubMed] [Google Scholar]
- Pesch U.E.A., Fries J.E., Bette S., Kalbacher H., Wissinger B., Alexander C. OPA1, the disease gene for autosomal dominant optic atrophy, is specifically expressed in ganglion cells and intrinsic neurons of the retina. Investigative Ophthalmology & Visual Science. 2004;45:4217–4225. doi: 10.1167/iovs.03-1261. [DOI] [PubMed] [Google Scholar]
- Pesch U.E.A., Leo-Kottler B., Mayor S., Jurklies B., Kellner U., Apfelstedt-Sylla E. OPA1 mutations in patients with autosomal dominant optic atrophy and evidence for semi-dominant inheritance. Human Molecular Genetics. 2001;10:1359–1368. doi: 10.1093/hmg/10.13.1359. [DOI] [PubMed] [Google Scholar]
- Pfeffer G., Cote H.C.F., Montaner J.S., Li C.C., Jitratkosol M., Mezei M.M. Ophthalmoplegia and ptosis: mitochondrial toxicity in patients receiving HIV therapy. Neurology. 2009;73:71–72. doi: 10.1212/WNL.0b013e3181aae814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phasukkijwatana N., Kunhapan B., Stankovich J., Chuenkongkaew W.L., Thomson R., Thornton T. Genome-wide linkage scan and association study of PARL to the expression of LHON families in Thailand. Human Genetics. 2010;128:39–49. doi: 10.1007/s00439-010-0821-8. [DOI] [PubMed] [Google Scholar]
- Pich S., Bach D., Briones P., Liesa M., Camps M., Testar X. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Human Molecular Genetics. 2005;14:1405–1415. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- Pommer R., Schoeler S., Mawrin C., Szibor R., Kirches E. The G11778A LHON mutation does not enhance ethambutol cytotoxicity in a cybrid model. Clinical Neuropathology. 2008;27:414–423. doi: 10.5414/npp27414. [DOI] [PubMed] [Google Scholar]
- Ponjavic V., Andreasson S., Tranebjaerg L., Lubs H.A. Full-field electroretinograms in a family with Mohr–Tranebjaerg syndrome. Acta Ophthalmologica Scandinavica. 1996;74:632–635. doi: 10.1111/j.1600-0420.1996.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Powell B., Toomes C., Scott S., Yeung A., Marchbank N., Spry P. Polymorphisms in OPA1 are associated with normal tension glaucoma. Molecular Vision. 2003;9:460–464. [PubMed] [Google Scholar]
- Pradhan M., Sharp D., Best S., Vincent A., Vaphiades M. Drug-induced optic neuropathy-TB or Not TB. Survey of Ophthalmology. 2010;55:378–385. doi: 10.1016/j.survophthal.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Puomila A., Hamalainen P., Kivioja S., Savontaus M.L., Koivumaki S., Huoponen K. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. European Journal of Human Genetics. 2007;15:1079–1089. doi: 10.1038/sj.ejhg.5201828. [DOI] [PubMed] [Google Scholar]
- Puomila A., Huoponen K., Mantyjarvi M., Hamalainen P., Paananen R., Sankila E.M. Dominant optic atrophy: correlation between clinical and molecular genetic studies. Acta Ophthalmologica Scandinavica. 2005;83:337–346. doi: 10.1111/j.1600-0420.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- Purohit S.S., Tomsak R.L. Nutritional deficiency amblyopia or Leber’s hereditary optic neuropathy? Neuro-Ophthalmology. 1997;18:111–116. [Google Scholar]
- Qi X.P., Lewin A.S., Hauswirth W.W., Guy J. Suppression of complex I gene expression induces optic neuropathy. Annals of Neurology. 2003;53:198–205. doi: 10.1002/ana.10426. [DOI] [PubMed] [Google Scholar]
- Qi X.P., Lewin A.S., Sun L., Hauswirth W.W., Guy J. SOD2 gene transfer protects against optic neuropathy induced by deficiency of complex I. Annals of Neurology. 2004;56:182–191. doi: 10.1002/ana.20175. [DOI] [PubMed] [Google Scholar]
- Qi X.P., Sun L., Hauswirth W.W., Lewin A.S., Guy J. Use of mitochondrial antioxidant defenses for rescue of cells with a Leber hereditary optic neuropathy-causing mutation. Archives of Ophthalmology. 2007;125:268–272. doi: 10.1001/archopht.125.2.268. [DOI] [PubMed] [Google Scholar]
- Qi X.P., Sun L., Lewin A.S., Hauswirth W.W., Guy J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Investigative Ophthalmology & Visual Science. 2007;48:1–10. doi: 10.1167/iovs.06-0789. [DOI] [PubMed] [Google Scholar]
- Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros P.A., Torres R.J., Salomao S., Berezovsky A., Carelli V., Sherman J. Colour vision defects in asymptomatic carriers of the Leber’s hereditary optic neuropathy (LHON) mtDNA 11778 mutation from a large Brazilian LHON pedigree: a case-control study. British Journal of Ophthalmology. 2006;90:150–153. doi: 10.1136/bjo.2005.074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha S., Robinson B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends in Biochemical Sciences. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Ramos CdVF, Bellusci C., Savini G., Carbonelli M., Berezovsky A., Tamaki C. Association of optic disc size with development and prognosis of Leber’s hereditary optic neuropathy. Investigative Ophthalmology & Visual Science. 2009;50:1666–1674. doi: 10.1167/iovs.08-2695. [DOI] [PubMed] [Google Scholar]
- Ray K., Mookherjee S. Molecular complexity of primary open angle glaucoma: current concepts. Journal of Genetics. 2009;88:451–467. doi: 10.1007/s12041-009-0065-3. [DOI] [PubMed] [Google Scholar]
- Reid E. Science in motion: common molecular pathological themes emerge in the hereditary spastic paraplegias. Journal of Medical Genetics. 2003;40:81–86. doi: 10.1136/jmg.40.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynier P., Amati-Bonneau P., Verny C., Olichon A., Simard G., Guichet A. OPA3 gene mutations responsible for autosomal dominant optic atrophy and cataract. Journal of Medical Genetics. 2004:41. doi: 10.1136/jmg.2003.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T., Child A., Hitchings R., Brice G., Miller L., Coca-Prados M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Richardson C., Smith T., Schaefer A., Turnbull D., Griffiths P. Ocular motility findings in chronic progressive external ophthalmoplegia. Eye. 2005;19:258–263. doi: 10.1038/sj.eye.6701488. [DOI] [PubMed] [Google Scholar]
- Rizzo J.F., Lessell S. Tobacco amblyopia. American Journal of Ophthalmology. 1993;116:84–87. doi: 10.1016/s0002-9394(14)71749-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez M.C., MacDonald J.R., Mahoney D.J., Parise G., Beal M.F., Tarnopolsky M.A. Beneficial effects of creatine, CoQ(10), and lipoic acid in mitochondrial disorders. Muscle & Nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- Rotig A., de Lonlay P., Chretien D., Foury F., Koenig M., Sidi D. Frataxin gene expansion causes aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. European Journal of Human Genetics. 1998;6:C304. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- Rotig A., deLonlay P., Chretien D., Foury F., Koenig M., Sidi D. Aconitase and mitochondrial iron–sulphur protein deficiency in Friedreich ataxia. Nature Genetics. 1997;17:215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- Rotig A., Sidi D., Munnich A., Rustin P. Molecular insights into Friedreich’s ataxia and antioxidant-based therapies. Trends in Molecular Medicine. 2002;8:221–224. doi: 10.1016/s1471-4914(02)02330-4. [DOI] [PubMed] [Google Scholar]
- Rouault T.A., Tong W.H. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nature Reviews Molecular Cell Biology. 2005;6:345–351. doi: 10.1038/nrm1620. [DOI] [PubMed] [Google Scholar]
- Rucker J.C., Hamilton S.R., Bardenstein D., Isada C.M., Lee M.S. Linezolid-associated toxic optic neuropathy. Neurology. 2006;66:595–598. doi: 10.1212/01.wnl.0000201313.24970.b8. [DOI] [PubMed] [Google Scholar]
- Rustin P., Bonnet D., Rotig A., Munnich A., Sidi D. Idebenone treatment in Friedreich patients: one-year-long randomized placebo-controlled trial. Neurology. 2004;62:524–525. doi: 10.1212/wnl.62.3.524. [DOI] [PubMed] [Google Scholar]
- Rustin P., von Kleist-Retzow J.C., Chantrel-Groussard K., Sidi D., Munnich A., Rotig A. Effect of idebenone on cardiomyopathy in Friedreich’s ataxia: a preliminary study. Lancet. 1999;354:477–479. doi: 10.1016/S0140-6736(99)01341-0. [DOI] [PubMed] [Google Scholar]
- Ryu S.W., Jeong H.J., Choi M., Karbowski M., Choi C. Optic atrophy 3 as a protein of the mitochondrial outer membrane induces mitochondrial fragmentation. Cellular and Molecular Life Sciences. 2010;67:2839–2850. doi: 10.1007/s00018-010-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacai P.Y., Salomao S.R., Carelli V., Pereira J.M., Belfort R., Jr., Sadun A.A. Visual evoked potentials findings in non-affected subjects from a large Brazilian pedigree of 11778 Leber’s hereditary optic neuropathy. Documenta Ophthalmologica. 2010;121:147–154. doi: 10.1007/s10633-010-9241-2. [DOI] [PubMed] [Google Scholar]
- Sacconi S., Trevisson E., Salviati L., Ayme S., Rigal O., Redondo A.G. Coenzyme Q(10) is frequently reduced in muscle of patients with mitochondrial myopathy. Neuromuscular Disorders. 2010;20:44–48. doi: 10.1016/j.nmd.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Sadun A.A., Carelli V., Salomao S.R., Berezovsky A., Quiros P.A., Sadun F. Extensive investigation of a large Brazilian pedigree of 11778/haplogroup J Leber hereditary optic neuropathy. American Journal of Ophthalmology. 2003;136:231–238. doi: 10.1016/s0002-9394(03)00099-0. [DOI] [PubMed] [Google Scholar]
- Sadun A.A., Martone J.F., Mucimendoza R., Reyes L., Dubois L., Silva J.C. Epidemic optic neuropathy in Cuba – eye findings. Archives of Ophthalmology. 1994;112:691–699. doi: 10.1001/archopht.1994.01090170139037. [DOI] [PubMed] [Google Scholar]
- Sadun A.A., Salomao S.R., Berezovsky A., Sadun F., Denegri A.M., Quiros P.A. Subclinical carriers and conversions in Leber hereditary optic neuropathy: a prospective psychophysical study. Transactions of American Ophthalmological Society. 2006;104:51–61. [PMC free article] [PubMed] [Google Scholar]
- Sadun A.A., Win P.H., Ross-Cisneros F.N., Walker S.O., Carelli V. Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc. 2000;98:223–232. discussion 232–5. [PMC free article] [PubMed] [Google Scholar]
- Saijo T., Hayashi K., Yamada H., Wakakura M. Linezolid-induced optic neuropathy. American Journal of Ophthalmology. 2005;139:1114–1116. doi: 10.1016/j.ajo.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Sala G., Trombin F., Beretta S., Tremolizzo L., Presutto P., Montopoli M. Antioxidants partially restore glutamate transport defect in Leber hereditary optic neuropathy cybrids. Journal of Neuroscience Research. 2008;86:3331–3337. doi: 10.1002/jnr.21773. [DOI] [PubMed] [Google Scholar]
- Salinas S., Proukakis C., Crosby A., Warner T.T. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurology. 2008;7:1127–1138. doi: 10.1016/S1474-4422(08)70258-8. [DOI] [PubMed] [Google Scholar]
- Salomao S.R., Berezovsky A., Andrade R.E., Belfort R., Carelli V., Sadun A.A. Visual electrophysiologic findings in patients from an extensive Brazilian family with Leber’s hereditary optic neuropathy – visual electrophysiology in LHON. Documenta Ophthalmologica. 2004;108:147–155. doi: 10.1023/b:doop.0000036829.37053.31. [DOI] [PubMed] [Google Scholar]
- Samuels D.C. Mitochondrial AZT metabolism. IUBMB Life. 2006;58:403–408. doi: 10.1080/15216540600791571. [DOI] [PubMed] [Google Scholar]
- Sanchez R.N., Smith A.J., Carelli V., Sadun A.A., Keltner J.L. Leber hereditary optic neuropathy possibly triggered by exposure to tire fire. Journal of Neuro-Ophthalmology. 2006;26:268–272. doi: 10.1097/01.wno.0000249320.27110.ab. [DOI] [PubMed] [Google Scholar]
- Sapey E., Burdon M.A., Nightingale S. Evidence of active demyelination in a man with Leber’s hereditary optic neuropathy mtDNA 14484 genotype. Neuro-Ophthalmology. 2001;26:119–126. [Google Scholar]
- Sato M., Kawase K., Yamamoto T., Dubo S., Shink E.E., Si E. Lack of association between normal-tension glaucoma and intron 8 polymorphisms in the gene causing autosomal dominant optic atrophy, OPA1, in Japan. Investigative Ophthalmology & Visual Science. 2003;44:1124. [Google Scholar]
- Savini G., Barboni P., Valentino M.L., Montagna P., Cortelli P., De Negri A.M. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations. Ophthalmology. 2005;112:127–131. doi: 10.1016/j.ophtha.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Schaefer A.M., McFarland R., Blakely E.L., He L., Whittaker R.G., Taylor R.W. Prevalence of mitochondrial DNA disease in adults. Annals of Neurology. 2008;63:35–39. doi: 10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- Schimpf S., Schaich S., Wissinger B. Activation of cryptic splice sites is a frequent splicing defect mechanism caused by mutations in exon and intron sequences of the OPA1 gene. Human Genetics. 2006;118:767–771. doi: 10.1007/s00439-005-0096-7. [DOI] [PubMed] [Google Scholar]
- Schmucker S., Puccio H. Understanding the molecular mechanisms of Friedreich’s ataxia to develop therapeutic approaches. Human Molecular Genetics. 2010;19:R103–R110. doi: 10.1093/hmg/ddq165. [DOI] [PubMed] [Google Scholar]
- Schon E.A., DiMauro S., Hirano M., Gilkerson R.W. Therapeutic prospects for mitochondrial disease. Trends in Molecular Medicine. 2010;16:268–276. doi: 10.1016/j.molmed.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzer M., Alward W.L., Feldman F., Cashwell L.F., Wilensky J., Geijssen H.C. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. American Journal of Ophthalmology. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- Scruggs E.R., Naylor A.J.D. Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology. 2008;82:83–88. doi: 10.1159/000134943. [DOI] [PubMed] [Google Scholar]
- Seo J.H., Hwang J.M., Park S.S. Comparison of retinal nerve fibre layers between 11778 and 14484 mutations in Leber’s hereditary optic neuropathy. Eye (London) 2009 doi: 10.1038/eye.2009.36. [DOI] [PubMed] [Google Scholar]
- Seo J.H., Hwang J.M., Park S.S. Antituberculosis medication as a possible epigenetic factor of Leber’s hereditary optic neuropathy. Clinical and Experimental Ophthalmology. 2010;38:363–366. doi: 10.1111/j.1442-9071.2010.02240.x. [DOI] [PubMed] [Google Scholar]
- Shah V.A., Randhawa S., Mizen T., Lee A.G., Foroozan R. You’re too old for that. Survey of Ophthalmology. 2008;53:403–410. doi: 10.1016/j.survophthal.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Shahrestani P., Leung H.T., Le P.K., Pak W.L., Tse S., Ocorr K. Heterozygous mutation of Drosophila Opa1 causes the development of multiple organ abnormalities in an age-dependent and organ-specific manner. Plos One. 2009;4 doi: 10.1371/journal.pone.0006867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh S., Ta C., Basham A.A., Mansour S. Leber hereditary optic neuropathy associated with antiretroviral therapy for human immunodeficiency virus infection. American Journal of Ophthalmology. 2001;131:143–145. doi: 10.1016/s0002-9394(00)00716-9. [DOI] [PubMed] [Google Scholar]
- Shankar S.P., Fingert J.H., Carelli V., Valentino M.L., King T.M., Daiger S.P. Evidence for a novel x-linked modifier locus for leber hereditary optic neuropathy. Ophthalmic Genetics. 2008;29:17–24. doi: 10.1080/13816810701867607. [DOI] [PubMed] [Google Scholar]
- Shi P., Gal J., Kwinter D.M., Liu X.Y., Zhu H.N. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2010;1802:45–51. doi: 10.1016/j.bbadis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Mori N., Kishi M., Sugata H., Tsuda A., Kubota N. A novel mutation in the OPA1 gene in a Japanese patient with optic atrophy. American Journal of Ophthalmology. 2003;135:256–257. doi: 10.1016/s0002-9394(02)01929-3. [DOI] [PubMed] [Google Scholar]
- Shindler K.S., Ventura E., Rex T.S., Elliott P., Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Investigative Ophthalmology & Visual Science. 2007;48:3602–3609. doi: 10.1167/iovs.07-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorr N., Christenbury J.D., Goldberg R.A. Management of ptosis in chronic progressive external ophthalmoplegia. Ophthal Plast Reconstr Surg. 1987;3:141–145. doi: 10.1097/00002341-198703030-00005. [DOI] [PubMed] [Google Scholar]
- Shoubridge E.A., Karpati G., Hastings K.E.M. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal-muscle fiber segments in mitochondrial disease. Cell. 1990;62:43–49. doi: 10.1016/0092-8674(90)90238-a. [DOI] [PubMed] [Google Scholar]
- Shy M.E., Garbern J.Y., Kamholz J. Hereditary motor and sensory neuropathies: a biological perspective. Lancet Neurology. 2002;1:110–118. doi: 10.1016/s1474-4422(02)00042-x. [DOI] [PubMed] [Google Scholar]
- Sigal I.A., Ethier C.R. Biomechanics of the optic nerve head. Experimental Eye Research. 2009;88:799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Sigal I.A., Flanagan J.G., Tertinegg I., Ethier C.R. 3D morphometry of the human optic nerve head. Experimental Eye Research. 2010;90:70–80. doi: 10.1016/j.exer.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Siguroardottir S., Helgason A., Gulcher J.R., Stefansson K., Donnelly P. The mutation rate in the human mtDNA control region. American Journal of Human Genetics. 2000;66:1599–1609. doi: 10.1086/302902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinnider L.F., Ghadially F.N. Chloramphenicol-induced mitochondrial and ultrastructural changes in hematopoietic cells. Archives of Pathology & Laboratory Medicine. 1976;100:601–605. [PubMed] [Google Scholar]
- Smith K.H., Johns D.R., Heher K.L., Miller N.R. Heteroplasmy in Leber’s hereditary optic neuropathy. Archives of Ophthalmology. 1993;111:1486–1490. doi: 10.1001/archopht.1993.01090110052022. [DOI] [PubMed] [Google Scholar]
- Smith P.R., Cooper J.M., Govan G.G., Harding A.E., Schapira A.H. Platelet mitochondrial function in Leber’s hereditary optic neuropathy. Journal of the Neurological Sciences. 1994;122:80–83. doi: 10.1016/0022-510x(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Smith U., Smith D.S., Yunis A.A. Chloramphenicol-related changes in mitochondrial ultrastructure. Journal of Cell Science. 1970;7:501–521. doi: 10.1242/jcs.7.2.501. [DOI] [PubMed] [Google Scholar]
- Spelbrink J.N., Li F.Y., Tiranti V., Nikali K., Yuan Q.P., Tariq M. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene LF-like protein localized in mitochondria. Nature Genetics. 2001;28:223–231. doi: 10.1038/90058. [DOI] [PubMed] [Google Scholar]
- Spinazzi M., Cazzola S., Bortolozzi M., Baracca A., Loro E., Casarin A. A novel deletion in the GTPase domain of OPA1 causes defects in mitochondrial morphology and distribution, but not in function. Human Molecular Genetics. 2008;17:3291–3302. doi: 10.1093/hmg/ddn225. [DOI] [PubMed] [Google Scholar]
- Spinazzola A., Invernizzi F., Carrara F., Lamantea E., Donati A., DiRocco M. Clinical and molecular features of mitochondrial DNA depletion syndromes. Journal of Inherited Metabolic Disease. 2009;32:143–158. doi: 10.1007/s10545-008-1038-z. [DOI] [PubMed] [Google Scholar]
- Spinazzola A., Zeviani M. Mitochondrial diseases: a cross-talk between mitochondrial and nuclear genomes. In: Espinos C.F.V.P.F., editor. vol. 652. 2009. pp. 69–84. (Inherited Neuromuscular Diseases: Translation from Pathomechanisms to Therapies). [DOI] [PubMed] [Google Scholar]
- Spruijt L., Kolbach D.N., de Coo R.F., Plomp A.S., Bauer N.J., Smeets H.J. Influence of mutation type on clinical expression of Leber hereditary optic neuropathy. American Journal of Ophthalmology. 2006;141:676–682. doi: 10.1016/j.ajo.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Spruijt L., Smeets H.J., Hendrickx A., Bettink-Remeijer M.W., Maat-Kievit A., Schoonderwoerd K.C. A MELAS-associated ND1 mutation causing Leber hereditary optic neuropathy and spastic dystonia. Archives of Neurology. 2007;64:890–893. doi: 10.1001/archneur.64.6.890. [DOI] [PubMed] [Google Scholar]
- Sripriya S., Nirmaladevi J., George R., Hemamalini A., Baskaran M., Prema R. OPTN gene: profile of patients with glaucoma from India. Molecular Vision. 2006;12:816–820. [PubMed] [Google Scholar]
- Stemmler T.L., Lesuisse E., Pain D., Dancis A. Frataxin and mitochondrial FeS cluster biogenesis. Journal of Biological Chemistry. 2010;285:26737–26743. doi: 10.1074/jbc.R110.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.D., Hudson G., Yu-Wai-Man P., Blakeley E.L., He L., Horvath R. Opa1 in multiple mitochondrial DNA deletion disorders. Neurology. 2008;71:1829–1831. doi: 10.1212/01.wnl.0000335931.54095.0a. [DOI] [PubMed] [Google Scholar]
- Stone E.M., Newman N.J., Miller N.R., Johns D.R., Lott M.T., Wallace D.C. Visual recovery in patients with Leber’s hereditary optic neuropathy and the 11778 mutation. Journal of Clinical Neuro-Ophthalmology. 1992;12:10–14. [PubMed] [Google Scholar]
- Suen D.F., Norris K.L., Youle R.J. Mitochondrial dynamics and apoptosis. Genes & Development. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaka E., Ohde H., Shinoda K., Mashima Y. Woman with atypical unilateral Leber’s hereditary optic neuropathy with visual improvement. Clinical & Experimental Ophthalmology. 2007;35:868–870. doi: 10.1111/j.1442-9071.2007.01628.x. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Sparman M., Sritanaudomchai H., Ma H., Clepper L., Woodward J. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman L.S., Bisker E.R., Sackel D.J., Long D.A., Jr., Galetta K.M., Ratchford J.N. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Annals of Neurology. 2010;67:749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Le P.K., Tse S., Wallace D.C., Huang T.S. Heterozygous mutation of Opa1 in Drosophila shortens lifespan mediated through increased reactive oxygen species production. Plos One. 2009;4 doi: 10.1371/journal.pone.0004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnopolsky M.A. The mitochondrial cocktail: rationale for combined nutraceutical therapy in mitochondrial cytopathies. Advanced Drug Delivery Reviews. 2008;60:1561–1567. doi: 10.1016/j.addr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky M.A., Baker S.K., Myint T., Maxner C.E., Robitaille J., Robinson B.H. Clinical variability in maternally inherited leber hereditary optic neuropathy with the G14459A mutation. American Journal of Medical Genetics: Part A. 2004;124:372–376. doi: 10.1002/ajmg.a.20449. [DOI] [PubMed] [Google Scholar]
- Taylor R.W., Jobling M.S., Turnbull D.M., Chinnery P.F. Frequency of rare mitochondrial DNA mutations in patients with suspected Leber’s hereditary optic neuropathy. Journal of Medical Genetics. 2003:40. doi: 10.1136/jmg.40.7.e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.W., Schaefer A.M., Barron M.J., McFarland R., Turnbull D.M. The diagnosis of mitochondrial muscle disease. Neuromuscular Disorders. 2004;14:237–245. doi: 10.1016/j.nmd.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Taylor R.W., Turnbull D.M. Mitochondrial DNA mutations in human disease. Nature Reviews Genetics. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharaphan P., Chuenkongkaew W.L., Luangtrakool K., Sanpachudayan T., Suktitipat B., Suphavilai R. Mitochondrial DNA haplogroup distribution in pedigrees of Southeast Asian G11778A Leber hereditary optic neuropathy. Journal of Neuro-Ophthalmology. 2006;26:264–267. doi: 10.1097/01.wno.0000249318.88991.c4. [DOI] [PubMed] [Google Scholar]
- Thomas D.Y., Wilkie D. Inhibition of mitochondrial synthesis in yeast by erythromycin – cytoplasmic and nuclear factors controlling resistance. Genetical Research. 1968;11:33–41. doi: 10.1017/s0016672300011174. [DOI] [PubMed] [Google Scholar]
- Tok J.B.H., Bi L.R. Aminoglycoside and its derivatives as ligands to target the ribosome. Current Topics in Medicinal Chemistry. 2003;3:1001–1019. doi: 10.2174/1568026033452131. [DOI] [PubMed] [Google Scholar]
- Tonon C., Lodi R. Idebenone in Friedreich’s ataxia. Expert Opinion on Pharmacotherapy. 2008;9:2327–2337. doi: 10.1517/14656566.9.13.2327. [DOI] [PubMed] [Google Scholar]
- Tonska K., Kodron A., Bartnik E. Genotype-phenotype correlations in Leber hereditary optic neuropathy. Biochimica et Biophysica Acta-Bioenergetics. 2010;1797:1119–1123. doi: 10.1016/j.bbabio.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Toomes C., Marchbank N.J., Mackey D.A., Craig J.E., Newbury-Ecob R.A., Bennett C.P. Spectrum, frequency and penetrance of OPA1 mutations in dominant optic atrophy. Human Molecular Genetics. 2001;10:1369–1378. doi: 10.1093/hmg/10.13.1369. [DOI] [PubMed] [Google Scholar]
- Torroni A., Wallace D.C. Mitochondrial DNA variation in human populations and implications for detection of mitochondrial DNA mutations of pathological significance. Journal of Bioenergetics and Biomembranes. 1994;26:261–271. doi: 10.1007/BF00763098. [DOI] [PubMed] [Google Scholar]
- Touitou V., Johnson M.A., Miller N.R., Guo Y., Bernstein D.A., Bernstein S.L. Sustained neuroprotection after a single intravitreal injection of PGJ2 in a rodent model of NAION. Neuro-Ophthalmology. 2010;34:271–272. [Google Scholar]
- Tranebjaerg L. Deafness-dystonia-optic neuronopathy syndrome. In: Pagon R.A., Bird T.C., Dolan C.R., Stephens K., editors. GeneReviews. University of Washington, Seattle; Seattle (WA): 2003. [Google Scholar]
- Tranebjaerg L., Jensen P.K., Van Ghelue M., Vnencak-Jones C.L., Sund S., Elgjo K. Neuronal cell death in the visual cortex is a prominent feature of the X-linked recessive mitochondrial deafness-dystonia syndrome caused by mutations in the TIMM8a gene. Ophthalmic Genetics. 2001;22:207–223. doi: 10.1076/opge.22.4.207.2220. [DOI] [PubMed] [Google Scholar]
- Tranebjaerg L., Schwartz C., Eriksen H., Andreasson S., Ponjavic V., Dahl A. A new X-linked recessive deafness syndrome with blindness, dystonia, fractures, and mental deficiency is linked to Xq22. Journal of Medical Genetics. 1995;32:257–263. doi: 10.1136/jmg.32.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranebjaerg L., van Ghelue M., Nilssen O., Hodes M.E., Dlouhy S.R., Farlow M.R. Jensen syndrome is allelic to Mohr–Tranebjaerg syndrome and both are caused by stop mutations in the DDP gene. American Journal of Human Genetics. 1997;61:2043. [Google Scholar]
- Trobe J.D., Glaser J.S., Cassady J., Herschler J., Anderson D.R. Nonglaucomatous excavation of the optic disk. Archives of Ophthalmology. 1980;98:1046–1050. doi: 10.1001/archopht.1980.01020031036004. [DOI] [PubMed] [Google Scholar]
- Tsao K., Aitken P.A., Johns D.R. Smoking as an aetiological factor in a pedigree with Leber’s hereditary optic neuropathy. British Journal of Ophthalmology. 1999;83:577–581. doi: 10.1136/bjo.83.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoulis C., Engelsen B.A., Telstad W., Aasly J., Zeviani M., Winterthun S. The spectrum of clinical disease caused by the A467T and W748SPOLG mutations: a study of 26 cases. Brain. 2006;129:1685–1692. doi: 10.1093/brain/awl097. [DOI] [PubMed] [Google Scholar]
- Ujike H., Tanabe Y., Takehisa Y., Hayabara T., Kuroda S. A family with X-linked dystonia-deafness syndrome with a novel mutation of the DDP gene. Archives of Neurology. 2001;58:1004–1007. doi: 10.1001/archneur.58.6.1004. [DOI] [PubMed] [Google Scholar]
- Valentino M.L., Avoni P., Barboni P., Pallotti F., Rengo C., Torroni A. Mitochondrial DNA nucleotide changes C14482G and C14482A in the ND6 gene are pathogenic for Leber’s hereditary optic neuropathy. Annals of Neurology. 2002;51:774–778. doi: 10.1002/ana.10193. [DOI] [PubMed] [Google Scholar]
- Valentino M.L., Barboni P., Ghelli A., Bucchi L., Rengo C., Achilli A. The ND1 gene of complex I is a mutational hot spot for Leber’s hereditary optic neuropathy. Annals of Neurology. 2004;56:631–641. doi: 10.1002/ana.20236. [DOI] [PubMed] [Google Scholar]
- Van Goethem G., Dermaut B., Lofgren A., Martin J.J., Van Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nature Genetics. 2001;28:211–212. doi: 10.1038/90034. [DOI] [PubMed] [Google Scholar]
- Vanjaarsveld H., Kuyl J.M., Alberts D.W. Exposure of rats to low concentration of cigarette-smoke increases myocardial sensitivity to ischemia reperfusion. Basic Research in Cardiology. 1992;87:393–399. doi: 10.1007/BF00796524. [DOI] [PubMed] [Google Scholar]
- Vanopdenbosch L., Dubois B., D’Hooghe M.B., Meire F., Carton H. Mitochondrial mutations of Leber’s hereditary optic neuropathy: a risk factor for multiple sclerosis. Journal of Neurology. 2000;247:535–543. doi: 10.1007/s004150070153. [DOI] [PubMed] [Google Scholar]
- Venegas-Francke P., Fruns-Quintana M., Oporto-Caroca M. Bilateral optic neuritis due to chloramphenicol. Revista De Neurologia. 2000;31:699–700. [PubMed] [Google Scholar]
- Vergani L., Martinuzzi A., Carelli V., Cortelli P., Montagna P., Schievano G. MtDNA mutations associated with Leber’s hereditary optic neuropathy: studies on cytoplasmic hybrid (cybrid) cells. Biochemical and Biophysical Research Communications. 1995;210:880–888. doi: 10.1006/bbrc.1995.1740. [DOI] [PubMed] [Google Scholar]
- Verny C., Loiseau D., Scherer C., Lejeune P., Chevrollier A., Gueguen N. Multiple sclerosis-like disorder in OPA1-related autosomal dominant optic atrophy. Neurology. 2008;70:1152–1153. doi: 10.1212/01.wnl.0000289194.89359.a1. [DOI] [PubMed] [Google Scholar]
- Votruba M., Fitzke F.W., Holder G.E., Carter A., Bhattacharya S.S., Moore A.T. Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Archives of Ophthalmology. 1998;116:351–358. doi: 10.1001/archopht.116.3.351. [DOI] [PubMed] [Google Scholar]
- Votruba M., Moore A.T., Bhattacharya S.S. Clinical features, molecular genetics, and pathophysiology of dominant optic atrophy. Journal of Medical Genetics. 1998;35:793–800. doi: 10.1136/jmg.35.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votruba M., Thiselton D., Bhattacharya S.S. Optic disc morphology of patients with OPA1 autosomal dominant optic atrophy. British Journal of Ophthalmology. 2003;87:48–53. doi: 10.1136/bjo.87.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakakura M., Yokoe J. Evidence for preserved direct pupillary light response in Lebers hereditary optic neuropathy. British Journal of Ophthalmology. 1995;79:442–446. doi: 10.1136/bjo.79.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D.C., Singh G., Lott M.T., Hodge J.A., Schurr T.G., Lezza A.M. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Walsh M.K., Quigley H.A. In vivo time-lapse fluorescence imaging of individual retinal ganglion cells in mice. Journal of Neuroscience Methods. 2008;169:214–221. doi: 10.1016/j.jneumeth.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Michikawa Y., Mallidis C., Bai Y., Woodhouse L., Yarasheski K.E. Muscle-specific mutations accumulate with aging in critical human mtDNA control sites for replication. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4022–4027. doi: 10.1073/pnas.061013598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanrooij S., Luoma P., van Goethem G., van Broeckhoven C., Suomalainen A., Spelbrink J.N. Twinkle and POLG defects enhance age-dependent accumulation of mutations in the control region of mtDNA. Nucleic Acids Research. 2004;32:3053–3064. doi: 10.1093/nar/gkh634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham H.R., Koster J., van Roermund C.W.T., Mooyer P.A.W., Wanders R.J.A., Leonard J.V. A lethal defect of mitochondrial and peroxisomal fission. New England Journal of Medicine. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- Weisschuh N., Neumann D., Wolf C., Wissinger B., Gramer E. Prevalence of myocilin and optineurin sequence variants in German normal tension glaucoma patients. Molecular Vision. 2005;11:284–287. [PubMed] [Google Scholar]
- Wheeler L., WoldeMussie E., Lai R. Role of alpha-2 agonists in neuroprotection. Survey of Ophthalmology. 2003;48:S47–S51. doi: 10.1016/s0039-6257(03)00004-3. [DOI] [PubMed] [Google Scholar]
- White K.E., Davies V.J., Hogan V.E., Piechota M.J., Nichols P.P., Turnbull D.M. OPA1 deficiency associated with increased autophagy in retinal ganglion cells in a murine model of dominant optic atrophy. Investigative Ophthalmology & Visual Science. 2009;50:2567–2571. doi: 10.1167/iovs.08-2913. [DOI] [PubMed] [Google Scholar]
- Wiggs J.L. Genetic etiologies of glaucoma. Archives of Ophthalmology. 2007;125:30–37. doi: 10.1001/archopht.125.1.30. [DOI] [PubMed] [Google Scholar]
- Wilkinson P.A., Crosby A.H., Turner C., Bradley L.J., Ginsberg L., Wood N.W. A clinical, genetic and biochemical study of SPG7 mutations in hereditary spastic paraplegia. Brain. 2004;127:973–980. doi: 10.1093/brain/awh125. [DOI] [PubMed] [Google Scholar]
- Williams P.A., Morgan J.E., Votruba M. Opa1 deficiency in a mouse model of dominant optic atrophy leads to retinal. Brain. 2010;133:2942–2951. doi: 10.1093/brain/awq218. [DOI] [PubMed] [Google Scholar]
- Wissinger B., Besch D., Baumann B., Fauser S., Christ-Adler M., Jurklies B. Mutation analysis of the ND6 gene in patients with Lebers hereditary optic neuropathy. Biochemical and Biophysical Research Communications. 1997;234:511–515. doi: 10.1006/bbrc.1997.6660. [DOI] [PubMed] [Google Scholar]
- Wolf C., Gramer E., Muller-Myhsok B., Pasutto F., Reinthal E., Wissinger B. Evaluation of nine candidate genes in patients with normal tension glaucoma: a case control study. BMC Medical Genetics. 2009:10. doi: 10.1186/1471-2350-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A., Cavelier L., Collins-Schramm H.E., Seldin M.F., McGrogan M., Savontaus M.L. Differentiation-specific effects of LHON mutations introduced into neuronal NT2 cells. Human Molecular Genetics. 2002;11:431–438. doi: 10.1093/hmg/11.4.431. [DOI] [PubMed] [Google Scholar]
- Woo S.J., Kim D.M., Kim J.Y., Park S.S., Ko H.S., Yoo T. Investigation of the association between OPA1 polymorphisms and normal-tension glaucoma in Korea. Journal of Glaucoma. 2004;13:492–495. doi: 10.1097/01.ijg.0000137870.25779.40. [DOI] [PubMed] [Google Scholar]
- Yaffe M.P., Stuurman N., Vale R.D. Mitochondrial positioning in fission yeast is driven by association with dynamic microtubules and mitotic spindle poles. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11424–11428. doi: 10.1073/pnas.1534703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Harrison C.A., Chuang G.C., Ballinger S.W. The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2007;621:61–74. doi: 10.1016/j.mrfmmm.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W.L., Jiao X.D., Hejtmancik J., Leske M., Hennis A., Nemesure B. Evaluation of the association between OPA1 polymorphisms and primary open-angle glaucoma in Barbados families. Molecular Vision. 2006;12:649–654. [PubMed] [Google Scholar]
- Yarosh W., Monserrate J., Tong J.J., Tse S., Le P.K., Nguyen K. The molecular mechanisms of OPA1-mediated optic atrophy in Drosophila model and prospects for antioxidant treatment. Plos Genetics. 2008;4 doi: 10.1371/journal.pgen.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M.Y., Kao S.H., Wang A.G., Wei Y.H. Increased 8-hydroxy-2′-deoxyguanosine in leukocyte DNA in Leber’s hereditary optic neuropathy. Investigative Ophthalmology & Visual Science. 2004;45:1688–1691. doi: 10.1167/iovs.03-0568. [DOI] [PubMed] [Google Scholar]
- Yen M.Y., Wang A.G., Chang W.L., Hsu W.M., Liu J.H., Wei Y.H. Leber’s hereditary optic neuropathy-the spectrum of mitochondrial DNA mutations in Chinese patients. Japanese Journal of Ophthalmology. 2002;46:45–51. doi: 10.1016/s0021-5155(01)00460-9. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Muneyuki E., Hisabori T. ATP synthase – a marvellous rotary engine of the cell. Nature Reviews Molecular Cell Biology. 2001;2:669–677. doi: 10.1038/35089509. [DOI] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Bateman D.E., Hudson G., Griffiths P.G., Chinnery P.F. Leber hereditary optic neuropathy presenting in a 75-year-old man. Journal of Neuroophthalmology. 2008;28:155. doi: 10.1097/WNO.0b013e3181772db4. [DOI] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Davies V.J., Piechota M.J., Cree L.M., Votruba M., Chinnery P.F. Secondary mtDNA defects do not cause optic nerve dysfunction in a mouse model of dominant optic atrophy. Investigative Ophthalmology & Visual Science. 2009;50:4561–4566. doi: 10.1167/iovs.09-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Griffiths P.G., Hudson G., Chinnery P.F. Inherited mitochondrial optic neuropathies. Journal of Medical Genetics. 2009;46:145–158. doi: 10.1136/jmg.2007.054270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Griffiths P.G., Burke A., Sellar P.W., Clarke M.P., Gnanaraj L. The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology. 2010;117:1538. doi: 10.1016/j.ophtha.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Griffiths P.G., Gorman G.S., Lourenco C.M., Wright A.F., Auer-Grumbach M. Multi-system neurological disease is common in patients with OPA1 mutations. Brain. 2010;133:771–786. doi: 10.1093/brain/awq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man, P., Shankar, S.P., Biousse, V., Miller, N.R., Bean, L.J.H., Coffee, B., et al., 2010c. Genetic screening for OPA1 and OPA3 mutations in patients with suspected inherited optic neuropathies. Ophthalmology, Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Yu-Wai-Man P., Sitarz K.S., Samuels D.C., Griffiths P.G., Reeve A.K., Bindoff L.A. OPA1 mutations cause cytochrome c oxidase deficiency due to loss of wild-type mtDNA molecules. Human Molecular Genetics. 2010;19:3043–3052. doi: 10.1093/hmg/ddq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Stewart J.D., Hudson G., Andrews R.M., Griffiths P.G., Birch M.K. OPA1 increases the risk of normal but not high tension glaucoma. Journal of Medical Genetics. 2010;47:120–125. doi: 10.1136/jmg.2009.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man, P., Trenell, M.I., Hollingsworth, K.G., Griffiths, P.G., Chinnery, P.F., 2010f. OPA1 mutations impair mitochondrial function in both pure and complicated dominant optic atrophy. Brain, Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Yunis A.A. Chloramphenicol toxicity – 25 years of research. American Journal of Medicine. 1989;87:N44–N48. [PubMed] [Google Scholar]
- Yunis A.A., Smith U.S. Reversible bone marrow suppression from chloramphenicol – a consequence of mitochondrial injury. Archives of Internal Medicine. 1970;126:272–275. [PubMed] [Google Scholar]
- Zanna C., Ghelli A., Porcelli A.M., Carelli V., Martinuzzi A., Rugolo M. Apoptotic cell death of cybrid cells bearing Leber’s hereditary optic neuropathy mutations is caspase independent. Apoptosis: From Signaling Pathways to Therapeutic Tools. 2003;1010:213–217. doi: 10.1196/annals.1299.037. [DOI] [PubMed] [Google Scholar]
- Zanna C., Ghelli A., Porcelli A.M., Karbowski M., Youle R.J., Schimpf S. OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain. 2008;131:352–367. doi: 10.1093/brain/awm335. [DOI] [PubMed] [Google Scholar]
- Zaveri M.S., Conger A., Salter A., Frohman T.C., Galetta S.L., Markowitz C.E. Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis. Archives of Neurology. 2008;65:924–928. doi: 10.1001/archneur.65.7.924. [DOI] [PubMed] [Google Scholar]
- Zeviani M. OPA1 mutations and mitochondrial DNA damage: keeping the magic circle in shape. Brain. 2008;131:314–317. doi: 10.1093/brain/awm339. [DOI] [PubMed] [Google Scholar]
- Zhadanov S.I., Atamanov V.V., Zhadanov N.I., Oleinikov O.V., Osipova L.P., Schurr T.G. A novel mtDNA ND6 gene mutation associated with LHON in a Caucasian family. Biochemical and Biophysical Research Communications. 2005;332:1115–1121. doi: 10.1016/j.bbrc.2005.05.059. [DOI] [PubMed] [Google Scholar]
- Zhang A.M., Jia X., Zhang Q., Yao Y.G. No association between the SNPs (rs3749446 and rs1402000) in the PARL gene and LHON in Chinese patients with m.11778G>A. Human Genetics. 2010;128:465–468. doi: 10.1007/s00439-010-0875-7. [DOI] [PubMed] [Google Scholar]
- Zhang X., Jones D., Gonzalez-Lima F. Mouse model of optic neuropathy caused by mitochondrial complex I dysfunction. Neuroscience Letters. 2002;326:97–100. doi: 10.1016/s0304-3940(02)00327-0. [DOI] [PubMed] [Google Scholar]
- Zhu G., Wu C.J., Zhao Y., Ashwell J.D. Optineurin negatively regulates TNF alpha-induced NF-kappa B activation by competing with NEMO for ubiquitinated RIP. Current Biology. 2007;17:1438–1443. doi: 10.1016/j.cub.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Zuchner S., De Jonghe P., Jordanova A., Claeys K.G., Guergueltcheva V., Cherninkova S. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Annals of Neurology. 2006;59:276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- Zuchner S., Mersiyanova I.V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E.L. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nature Genetics. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- Zuchner S., Vance J.M. Mechanisms of disease: a molecular genetic update on hereditary axonal neuropathies. Nature Clinical Practice Neurology. 2006;2:45–53. doi: 10.1038/ncpneuro0071. [DOI] [PubMed] [Google Scholar]