Abstract
Purpose
To determine whether MRI in combination with an intravascular contrast agent is sensitive to pharmacologically-induced vasodilation and vasoconstriction in the rat kidney.
Materials and methods
R2 imaging was performed in 25 Sprague Dawley rats at 3 tesla in the presence of ferumoxytol, an ultrasmall superparamagnetic iron oxide (USPIO) agent with a long plasma half-life. R2 changes were measured following manipulation of blood volume by intravenous administration of adenosine, a short-acting vasodilator, or NG-nitro-l-arginine methyl ester (l-NAME), a long-acting nitric oxide synthase inhibitor with known vasoconstrictive effects. As a control, R2 responses to adenosine and l-NAME were also examined in the absence of ferumoxytol.
Results
In the presence of ferumoxytol, adenosine induced a significant increase in R2, while l-NAME produced a reduction, although the latter was not statistically significant. Control experiments revealed small R2 changes in the absence of ferumoxytol. An incidental finding was that the cross-sectional area of the kidney also varied dynamically with adenosine and l-NAME.
Conclusion
Our results suggest that ferumoxytol-enhanced R2 imaging is sensitive to adenosine-induced vasodilation. The responses to l-NAME, however, were not statistically significant. The variations in kidney size and the R2 changes in the absence of ferumoxytol may reflect alterations in the volume of the renal tubules.
Keywords: MRI, kidney, blood volume, USPIO, renal tubules
INTRODUCTION
There is increasing interest in noninvasive assessment of hemodynamic responses to various pharmacological and physiological maneuvers in the kidney. While blood oxygen level dependent (BOLD) MRI (1,2) has proven to be a valuable tool for monitoring tissue oxygenation, it does not distinguish between changes in oxygen supply via blood flow and alterations in oxygen consumption. Techniques to measure renal blood flow are being explored, including arterial spin labeling and dynamic contrast-enhanced perfusion. However, they are limited by various practical issues, such as motion sensitivity and repeatability. Monitoring changes in blood volume with the use of intravascular contrast agents is potentially more accessible (3,4). This report describes a preliminary study to investigate the sensitivity of MRI in combination with ferumoxytol, an ultrasmall superparamagnetic iron oxide (USPIO) agent, to pharmacologically-induced vasodilation and vasoconstriction. Experiments were conducted on Sprague Dawley rats, and blood volume was manipulated by intravenous administration of adenosine or NG-nitro-l-arginine methyl ester (l-NAME).
Adenosine is known to stimulate a variety of vascular receptors in the kidney, but evidence suggests that when administered as an intravenous infusion its net effect is vasodilation (5). It was chosen for this study because its duration of action is very short (6). It could therefore be switched on and off repeatedly, enabling each animal to serve as its own control.
l-NAME is a synthetic nitric oxide synthase inhibitor, which blocks production of nitric oxide (NO) by the endothelium. Nitric oxide is a soluble gas that is continuously synthesized within endothelial cells from the amino acid l-arginine by the enzymatic action of nitric oxide synthase (7). It fulfils many important physiological functions in vessels, including relaxation of vascular tone and inhibition of inflammation, thrombosis and smooth muscle hyperplasia (8). By blocking synthesis of nitric oxide, administration of l-NAME in healthy subjects results in vasoconstriction. l-NAME is commonly used in BOLD MRI studies to assess basal bioavailability of nitric oxide in various disease states (9,10). In the present study, experiments were performed on young healthy rats to determine whether l-NAME-induced vasoconstriction could be detected by ferumoxytol-enhanced MRI.
USPIO particles such as ferumoxytol do not readily extravasate and are not filtered at the glomerulus, but instead are phagocytosed by macrophages of the reticuloendothelial system. Since uptake is slow, they remain in the blood pool for an extended period of time, making them potentially useful candidates for monitoring dynamic changes in blood volume. They have large magnetic dipole moments and therefore increase both R2 (= 1/T2) and R2* (= 1/T2*). However, R2 provides different information from R2* in the presence of an intravascular contrast agent; while the value of R2* is dominated by contributions from large vessels, R2 is relatively more sensitive to small vessels (11). We chose to measure R2 in this study to provide sensitivity to microvasculature and to minimize susceptibility artifacts. R2 imaging was performed using a Carr-Purcell-Meiboom-Gill (CPMG) multiple spin echo sequence in the presence of ferumoxytol. Images were acquired with and without adenosine, and, in separate groups of animals, before and after l-NAME. As a control, R2 responses to adenosine and l-NAME were also measured in the absence of ferumoxytol.
MATERIALS AND METHODS
Animals
All animal handling and experiments were conducted under a protocol approved by the local Institutional Animal Care and Use Committee (IACUC) and in accordance with animal welfare regulations. Experiments were performed on 25 male Sprague Dawley rats (weight: 245 – 385g, age: 7 – 12 weeks), which were purchased from Harlan Bioproducts for Science (Indianapolis, IN) and housed at 25°C with a 12-hour light/dark cycle and free access to food and water. All procedures were conducted under anesthesia using ketamine (60 – 100mg/kg i.p., Abbott Laboratories, North Chicago, IL) and Inactin (thiobutabarbital sodium, 100 mg/kg i.p., Sigma-Aldrich, St. Louis, MO). A catheter was placed in the femoral vein for administration of vasoactive drugs and/or contrast agent. In two animals the femoral artery was also catheterized to monitor mean arterial pressure (MAP). In six rats the bladder was pierced and an additional catheter inserted to allow continuous drainage of urine. This procedure was added to the protocol later in the study following evidence of urine retention in some of the earlier experiments. All catheters consisted of PE-50 tubing (Braintree Scientific, Braintree, MA) and were secured with 4-0 silk suture. Eleven rats were used to investigate the effects of adenosine (group I), and fourteen were used in the l-NAME studies (group II). The protocols followed in these two main groups and their subgroups are summarized in Table 1 and described in more detail below. At the end of each experiment the animal was euthanized by intravenous injection of 0.5 – 1.0 mL Sleepaway (26% sodium pentobarbital, 7.8% isopropyl alcohol, Fort Dodge Animal Health, Fort Dodge, IA).
Table 1.
Summary of the protocols used in the various groups of animals
| Group | N | adenosine | l-NAME | ferumoxytol | blood pressure |
imaging plane | temporal resolution |
|
|---|---|---|---|---|---|---|---|---|
| I | a | 6 | • | • | oblique axial | 9 min 39 sec | ||
| b | 2 | • | oblique axial | 9 min 39 sec | ||||
| c | 1 | • | coronal | 9 min 39 sec | ||||
| d | 2 | • | • | oblique axial | 1 min 6 sec | |||
| II | a | 6 | • | oblique axial | 9 min 39 sec | |||
| b | 4 | • | • | oblique axial | 9 min 39 sec | |||
| c | 4 | • | oblique axial | 9 min 39 sec | ||||
Ferumoxytol
Ferumoxytol (AMAG Pharmaceuticals, Cambridge, MA) is a colloidal solution of USPIO particles, each having a nonstoichiometric magnetite core of about 6.8 nm in diameter. Covering the core is a semisynthetic carbohydrate coating of polyglucose sorbitol carboxymethylether, which gives the particle an overall size of 17 – 31 nm in solution. The coating isolates the bioactive iron from plasma components, allowing for safe bolus administration, and resulting in a long plasma half-life of 1.5 – 3 hours in rats and about 15 hours in humans. Ferumoxytol has been approved for human use in the United States by the Food and Drug Administration for the treatment of iron deficiency anemia in adults with chronic kidney disease. In the present study, ferumoxytol was administered by bolus injection into the femoral vein at a dose of 8mg Fe/kg.
Adenosine Studies
Adenosine has a plasma half-life of less than 10 seconds (6), resulting in a rapid onset and short duration of action. It stimulates a variety of vascular receptors, including A2 receptors, which induce vasodilation, and A1 receptors, which cause vasoconstriction. Although both types of receptors are present in the kidney, evidence suggests that, when administered as an intravenous infusion, the net effect of adenosine on the kidney is vasodilation (5).
In this study, adenosine was formulated from 200 mg/kg adenosine hemisulfate salt (Sigma-Aldrich, St Louis, MO), which was measured on an electronic laboratory balance (Ohaus Adventurer AR2140, Ohaus Corp., Pine Brook, NJ) and dissolved in 10 mL saline. An infusion pump (Genie Plus, Kent Scientific, Litchfield, CT) was used to infuse the dissolved adenosine into the femoral vein at a rate of 500 µg/kg/min (0.025 mL/min). The infusion was alternately switched on and off between image acquisitions to confirm reproducibility of the response.
In six rats (group I-a), the effect of adenosine was examined in the presence of ferumoxytol. As a control measure, the response was also recorded in the absence of contrast agent. Three image sets were acquired prior to ferumoxytol administration, with adenosine infusion alternately off, then on and then off again. Following injection of ferumoxytol the protocol was repeated. The infusion pump was switched on and off between alternate acquisitions until a further five image sets had been collected.
To investigate further the effects of adenosine in the absence of ferumoxytol, an additional five rats were studied (see Table 1). In two of these animals (group I-b), the pre-contrast imaging protocol was repeated to confirm earlier results. In another animal (group I-c) the kidneys were imaged in a different plane. In the remaining two animals (group I-d), imaging was performed with higher temporal resolution to observe the time scale of the responses. Mean arterial pressure was recorded simultaneously using a pressure transducer system (Kent Scientific, Litchfield, CT) connected to a catheter in the femoral artery.
l-NAME Studies
l-NAME (Sigma-Aldrich, St. Louis, MO) was administered into the femoral vein as a 10mg/kg bolus. Due to its long duration of action (> 2 hours) (12), comparisons were made among groups (see Table 1). Six rats received ferumoxytol only (group II-a), and four received ferumoxytol followed by l-NAME (group II-b). Comparison between these groups provided a means to distinguish the effect of l-NAME from that of gradual elimination of ferumoxytol from the blood pool. Another four rats were used as a control group and received l-NAME only (group II-c). In all experiments, baseline images were acquired prior to injection of l-NAME or ferumoxytol.
Image Acquisition
Imaging was performed on a whole-body 3 tesla Twinspeed system (GE Healthcare, Waukesha, WI) using a transmit/receive extremity coil. The animal was placed on a cushion at the center of the coil in a right lateral decubitus position. Following localizer acquisitions, a single slice was selected for R2 imaging. An oblique axial plane through both kidneys was chosen in all experiments except one, for which a coronal plane was used (see Table 1). R2 imaging was performed using a CPMG multiple spin echo sequence with refocusing pulses of 180°. All animals except those in group I-d were imaged using the following parameters: 16 echoes, 4 averages, field of view (FOV) = 8 × 4 cm2, slice thickness = 3 mm, nominal matrix size = 256 × 192, receiver bandwidth (BW) = 62.5 kHz, repetition time (TR) = 1500 ms, minimum echo time (TE) = 7 ms and echo spacing = 7 ms, giving an in-plane resolution of 0.3 × 0.4 mm2 and an acquisition time of 9 min 39 sec. The two animals in group I-d were imaged at higher temporal resolution using the following parameters: 8 echoes, 1 average, FOV = 6.5 × 3.25 cm2, slice thickness = 4 mm, nominal matrix size = 192 × 128, BW = 15.6 kHz, TR = 1000 ms, minimum TE = 10 ms and echo spacing = 10 ms, giving a similar in-plane resolution (0.3 × 0.5 mm2) and an acquisition time of 1 min 6 sec.
Image Processing
Images were analyzed offline using customized routines in Matlab (The MathWorks, Inc, Natick, MA). R2 values were calculated by fitting the intensity data as a function of TE to a monoexponential decay using a nonlinear least-squares Levenberg-Marquardt algorithm. In cases of rapid signal decay, the data were truncated where the intensity fell below twice the noise level (13). R2 maps were generated by applying the fitting procedure pixel by pixel. In addition, R2 estimates for various tissues were obtained by fitting the mean intensities in selected regions of interest (ROIs). Measurements were made in three anatomic zones: the cortex, the outer stripe of the outer medulla (OSOM), and the inner stripe of the outer medulla (ISOM). These zones were identified on the MR images by comparison with micro-CT studies (14), and three non-overlapping ROIs were drawn in each. R2 measurements were not made in the papilla (inner medulla) because of partial volume averaging with the renal pelvis in some acquisitions. A single kidney was analyzed in each animal. In all cases but one the right kidney was chosen since the animal’s positioning made it less subject to motion artifacts. In one rat, however, (group I-a) the right kidney exhibited a malformation, characterized by an enlarged pelvis, and analysis was performed instead on the left kidney.
Following the observation of dynamic variations in kidney size during the experiments, the R2 maps were analyzed retrospectively to estimate the cross-sectional area of the kidney. Manual segmentation was applied to each R2 map to separate the kidney from surrounding tissues. The renal pelvis was included in the kidney area since it was difficult to delineate from the papilla in some acquisitions.
Statistical Analysis
Statistical calculations were performed using the Statistical Toolbox in Matlab. The effects of adenosine and l-NAME were analyzed using 2-tailed Student t-tests. Paired t-tests were used in cases where each animal served as its own control, and unpaired t-tests were employed when comparisons were made between groups. A p-value of less than 0.05 is considered significant, and below 0.01 is regarded as highly significant. The abbreviations SD and SEM are used to denote the standard deviation and standard error in the mean respectively.
RESULTS
Adenosine Studies
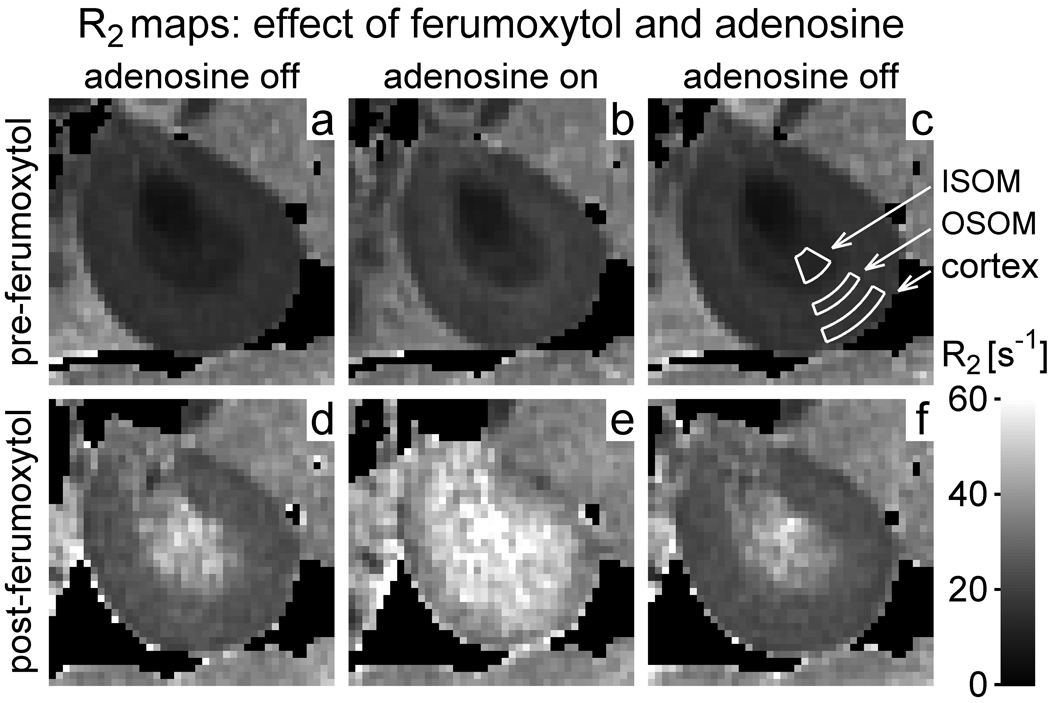
Figure 1 shows R2 maps from a typical rat in group I-a, which received adenosine and ferumoxytol. Maps a – f correspond to acquisitions 1 – 6 respectively. The first three acquisitions (top row) constitute the control experiment, in which the effect of adenosine was examined prior to ferumoxytol administration. The later acquisitions (bottom row) were performed after contrast injection, and were used to investigate the response to adenosine in the presence of ferumoxytol. Note that at baseline (map a) R2 exhibits regional variation, being lower in ISOM than in the OSOM and cortex, and lowest in the papilla. R2 increases dramatically when ferumoxytol is injected (d), although the elevation is greater in the ISOM and papilla than in the OSOM and cortex. A further increase in R2 is observed in all regions during adenosine infusion (e), and the effect is reversed when adenosine is subsequently switched off (f).
Figure 1.
R2 maps of the kidney from a representative rat in group I-a (ferumoxytol and adenosine). Maps a – f correspond to acquisitions 1 – 6 respectively. Map (c) shows example ROIs in the inner stripe of the outer medulla (ISOM), outer stripe of the outer medulla (OSOM) and cortex. The grayscale (see bar at lower right) extends from R2 = 0s−1 (black) to 60s−1 (white). Pixels with insufficient signal to perform the fitting procedure have been made black. Note that R2 increases with ferumoxytol (d) and increases further with adenosine (e). Even before contrast administration, however, R2 increases slightly with adenosine (b). The physical size of the kidney also appears to decrease in the presence of adenosine (b and e).
Results of the control experiment (top row) suggest that adenosine produces a slight increase in R2 even in the absence of ferumoxytol (b). The physical size of the kidney also appears to vary dynamically over the course of the experiment, decreasing slightly in the presence of adenosine (b and e) and increasing again when the infusion is stopped.
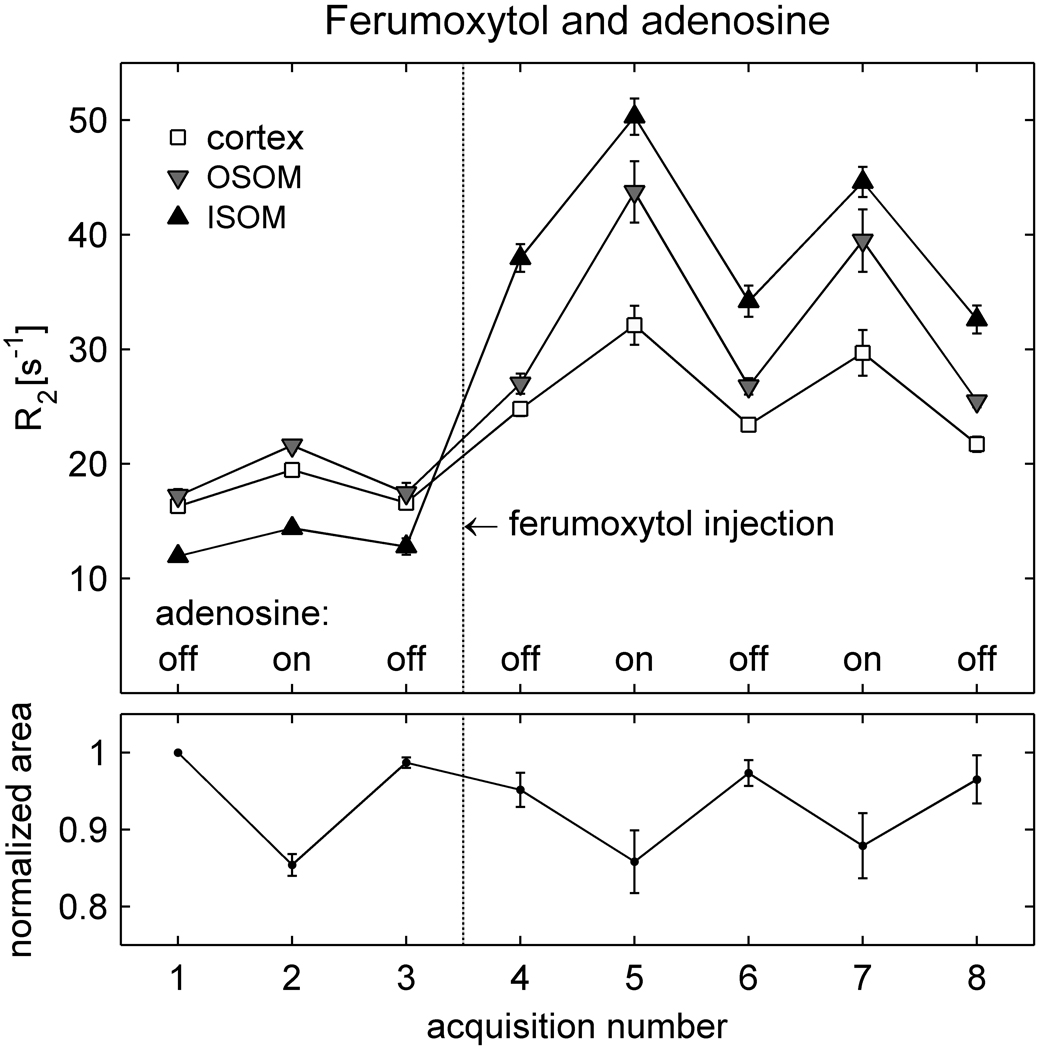
Figure 2 presents the results from group I-a averaged over all animals. At baseline (acquisition 1), R2 is lower in the ISOM than in the OSOM and cortex, as observed in Figure 1. R2 increases in all anatomic regions following ferumoxytol administration (acquisition 4), although the change is greater in the ISOM than in the OSOM and cortex. In the presence of ferumoxytol, a further increase in R2 is observed with adenosine infusion (acquisition 5). The response is greater in the ISOM and OSOM than in the cortex. Turning the adenosine infusion alternately off and on again (acquisitions 6 – 8) confirms that the results are reproducible, except for a slight reduction in R2 over time that probably reflects gradual elimination of ferumoxytol from the blood pool.
Figure 2.
Averaged results (mean ± SEM) from the animals in group I-a (N = 6), which received adenosine and ferumoxytol. The upper graph shows mean R2 values in the cortex, OSOM and ISOM over the course of the experiment. Adenosine infusion was switched on and off between successive acquisitions, both prior to ferumoxytol administration (acquisitions 1 – 3) and after ferumoxytol administration (acquisitions 4 – 8). The lower graph shows the area of the kidney, normalized to its value in acquisition 1, and averaged over all animals. The results for acquisition 6 – 8 were averaged over only 5 animals, since one rat was withdrawn from the scanner after acquisition 5 due to respiratory distress. As in Figure 1, R2 increases with ferumoxytol administration (acquisition 4) and increases further with adenosine (5 and 7). Adenosine also produces a slight increase in R2 in the absence of ferumoxytol (acquisition 2) and causes a reduction in the cross-sectional area of the kidney (acquisitions 2, 5 and 7).
Results of the control experiment, performed prior to ferumoxytol administration, show that adenosine produces small increases in R2 even in the absence of contrast agent (acquisition 2), corroborating the observation in Figure 1. However, the post-contrast R2 responses to adenosine are significantly larger than the pre-contrast responses (Table 2).
Table 2.
R2 responses to adenosine in the absence and presence of ferumoxytol (group I-a, N = 6)
| Response to adenosine | ΔR2 in cortex [s−1] | ΔR2 in OSOM [s−1] | ΔR2 in ISOM [s−1] |
|---|---|---|---|
| Pre-contrast response1 | 3.0 ± 0.7** (p = 0.007) |
4.3 ± 0.8** (p = 0.004) |
2.0 ± 0.7* (p = 0.03) |
| Post-contrast response1 | 8.2 ± 1.8** (p = 0.006) |
17.1 ± 3.0** (p = 0.002) |
14.2 ± 1.2** (p < 0.0001) |
| Difference2 | 5.2 ± 1.5* (p = 0.02) |
12.8 ± 2.4** (p = 0.003) |
12.1 ± 0.9** (p < 0.0001) |
Pre-contrast response = (acquisition 2) – mean [(acquisition 1) and (acquisition 3)]; Post-contrast response = (acquisition 5) – mean [(acquisition 4) and (acquisition 6)]; refer to Figure 2 for acquisition timing
Difference = (post-contrast response) – (pre-contrast response)
significant (p < 0.05),
highly significant (p < 0.01)
All results expressed as mean ± SEM
The lower graph of Figure 2 displays the cross-sectional area of the kidney, normalized to its value in the first acquisition, and averaged over all animals in the group. Note that the area decreases during adenosine infusion (acquisitions 2, 5 and 7) and recovers when adenosine is turned off. Further experiments were performed to confirm this finding and elucidate its origin. The pre-contrast portion of the protocol was repeated in another two animals (group I-b), who received adenosine only. Over both groups (I-a and I-b, N = 8), the change in area during adenosine infusion was −13.4 ± 1.1% (mean ± SEM), which was highly significant (Table 3). To investigate the possibility that the change may simply have been an artifact of through-plane displacement, an additional animal was imaged in a coronal plane (group I-c). No noticeable cranio-caudal displacement was observed in this rat and the cross-sectional area of the kidney in the coronal plane changed by −7.0%. Although coronal imaging was performed in only one animal, these observations provide evidence that the decrease in cross-sectional area reflects a real reduction in the physical size of the kidney.
Table 3.
R2 and kidney size responses to adenosine in the absence of ferumoxytol (pooled results of groups I-a and I-b, N = 8)
| Adenosine results in absence of ferumoxytol |
ΔR2 in cortex [s−1] | ΔR2 in OSOM [s−1] | ΔR2 in ISOM [s−1] | Δarea |
|---|---|---|---|---|
| Response to adenosine1 | 2.8 ± 0.5** (p = 0.0010) |
4.0 ± 0.7** (p = 0.0005) |
2.0 ± 0.5** (p = 0.005) |
−13.4 ± 1.1%** (p < 0.0001) |
| Correlation between ΔR2 and Δarea | r = −0.67 (p = 0.07) |
r = −0.66 (p = 0.07) |
r = −0.41 (p = 0.3) |
Response to adenosine = (acquisition 2) – mean [(acquisition 1) and (acquisition 3)]; refer to Figure 2 for acquisition timing
highly significant (p < 0.01)
All results expressed as mean ± SEM
Over groups I-a and I-b, both the size changes and the R2 responses to adenosine in the absence of ferumoxytol were highly significant (Table 3). However, the correlations between them did not reach statistical significance. This may simply reflect a tight clustering of both the R2 responses and the size changes, since it is more difficult to establish a statistically significant correlation if the variables concerned do not display a large range of values.
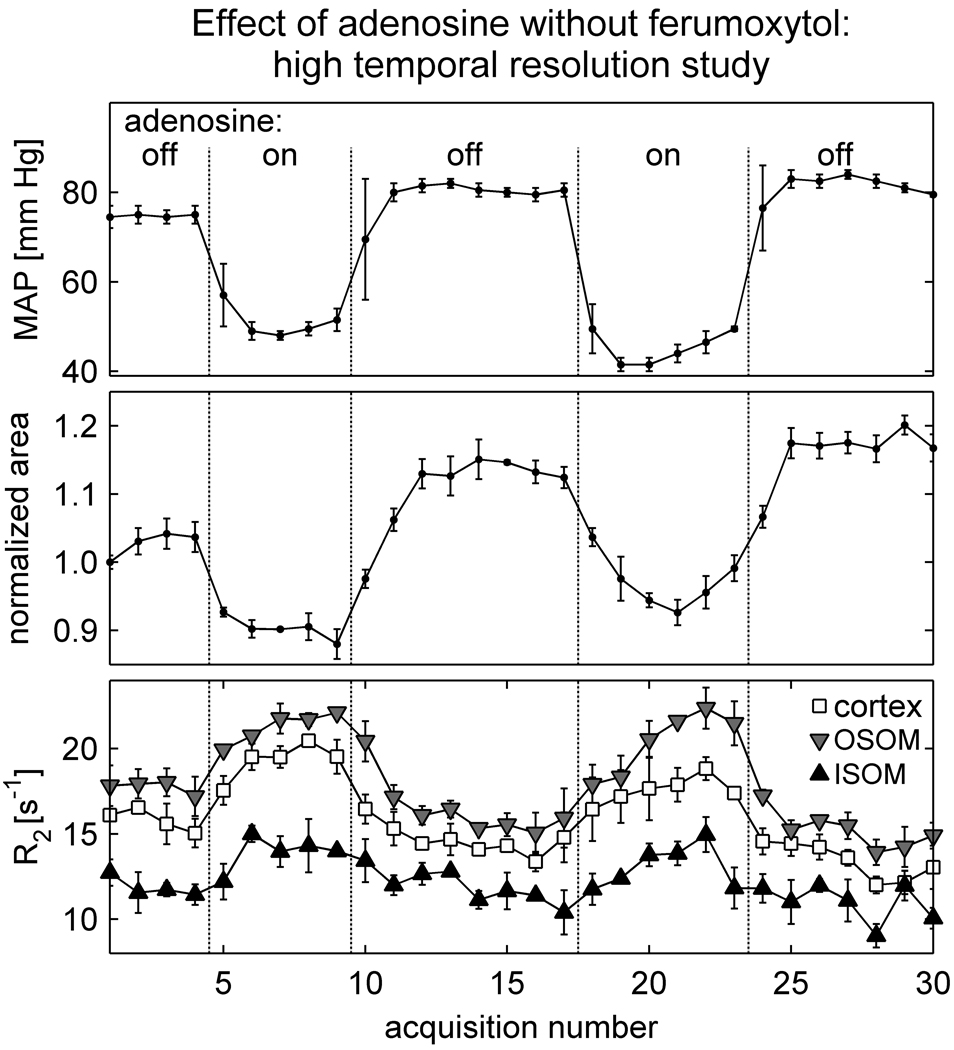
To investigate whether the kidney size changes and R2 responses were correlated in time, another two animals were imaged with higher temporal resolution (group I-d). Results from one of the animals are shown in Figure 3. Note that the blood pressure response to adenosine is rapid (< 1 minute). The kidney size changes almost as rapidly (within about 2 minutes), and R2 varies inversely, but in synchrony, with kidney size. In addition to the adenosine-related changes, there is a gradual drift in both R2 and kidney size over time. At the completion of the experiment, it was observed that the animal had not voided and its bladder was distended. To investigate whether ureteral pressure was responsible for the drift in R2 and kidney size over time, the experiment was repeated in a second animal following bladder catheterization. The results from the second animal were consistent with those of the first; the kidney size closely tracked the blood pressure changes, and R2 varied inversely, but in synchrony, with the size changes. However, the second animal also exhibited a drift in R2 and kidney size over time, indicating that ureteral pressure was not entirely responsible for the drift.
Figure 3.
Results from a representative rat in group I-d, demonstrating the timescale of the responses to adenosine in the absence of ferumoxytol. Imaging was performed with high temporal resolution (1 min 6 sec per acquisition). The points and error bars in each graph indicate respectively the midpoint and range of mean arterial pressure (upper graph), the mean and SD of three estimates of cross-sectional kidney area normalized to its baseline value (central graph), and the mean and SD of R2 over three ROIs in each anatomic zone (lower graph). Note that the blood pressure and kidney size decrease with adenosine, while R2 increases with adenosine in all regions. There is also a gradual drift in R2 and kidney size over time.
l-NAME Studies
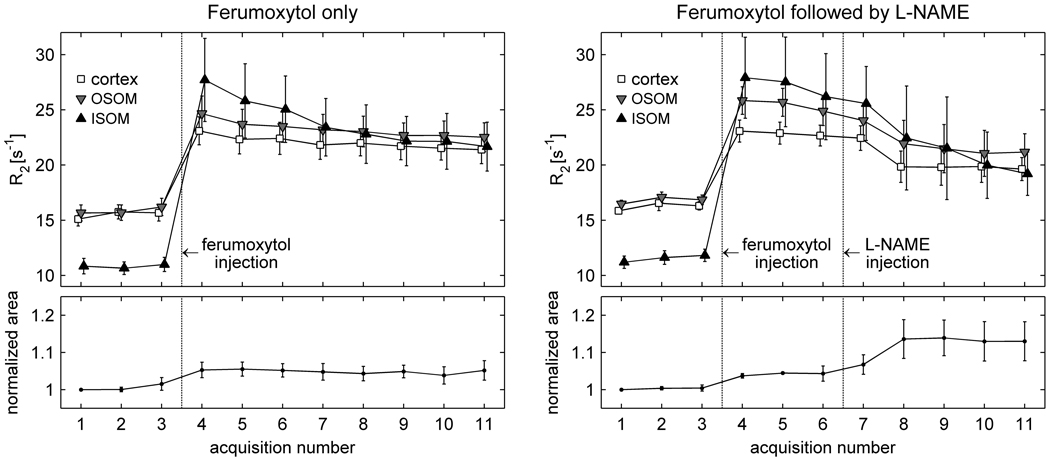
The effect of l-NAME in the presence of ferumoxytol is shown in Figure 4, which compares the results in group II-a (ferumoxytol only, N = 6) and group II-b (ferumoxytol followed by l-NAME, N = 4). The R2 values in all regions increase sharply with contrast administration. They then decline smoothly in the group receiving ferumoxytol only, suggesting gradual elimination of the contrast agent from the blood pool. In the group receiving l-NAME in the presence of ferumoxytol, a comparatively greater reduction is seen in R2 following l-NAME injection. Changes were also observed in the cross-sectional area of the kidney; a small increase occurred with contrast injection, and a second, slightly larger, increase following l-NAME administration.
Figure 4.
Averaged results (mean ± SEM) from the animals in group II-a (ferumoxytol only, N = 6) and group II-b (ferumoxytol followed by l-NAME, N = 4). The points corresponding to different regions in the upper graph have been slightly offset horizontally from each other so that the error bars do not overlay. In the left graph (ferumoxytol only) the results for acquisition 1 were averaged over only five animals, since one rat in that group received only two baseline acquisitions. A comparison of the graphs suggests that both R2 and kidney size change slightly following l-NAME, although the responses are not immediate.
A statistical comparison of groups II-a and II-b is presented in Table 4. The values shown represent changes in R2 and kidney size during the post-contrast period, i.e. from the time immediately following ferumoxytol administration until the end of the exam. The R2 reductions in group II-a, receiving ferumoxytol only, presumably reflect gradual elimination of contrast from the blood pool; those in group II-b, receiving l-NAME in the presence of ferumoxytol, are slightly greater, although the difference is not statistically significant. The area of the kidney also increases on average in the group receiving l-NAME, but the size change does not reach statistical significance.
Table 4.
R2 and kidney size responses to l-NAME in the presence of ferumoxytol (comparison of group II-a, N = 6, and group II-b, N = 4)
| Changes during post-contrast period1 |
ΔR2 in cortex [s−1] | ΔR2 in OSOM [s−1] | ΔR2 in ISOM [s−1] | Δarea |
|---|---|---|---|---|
| Group II-a (ferumoxytol only: effect of contrast elimination) | −1.2 ± 0.5 (p = 0.07) |
−1.3 ± 0.7 (p = 0.12) |
−4.3 ± 2.1 (p = 0.10) |
−0.8 ± 1.5 % (p = 0.6) |
| Group II-b (injection of l-NAME in presence of ferumoxytol) | −3.1 ± 0.9* (p = 0.04) |
−4.4 ± 1.1* (p = 0.03) |
−7.6 ± 1.5* (p = 0.02) |
8.8 ± 4.7 % (p = 0.16) |
| Difference between means2 | −2.0 (p = 0.12) |
−3.0 (p = 0.07) |
−3.3 (p = 0.2) |
9.6 % (p = 0.13) |
Changes during post-contrast period = mean [(acquisition 10) and (acquisition 11)] – mean [(acquisition 4) to (acquisition 6)]; refer to Figure 4 for acquisition timing
Difference = (mean of group II-b) – (mean of group II-a); p-value does not assume equal variances
significant (p < 0.05)
Results expressed as mean ± SEM
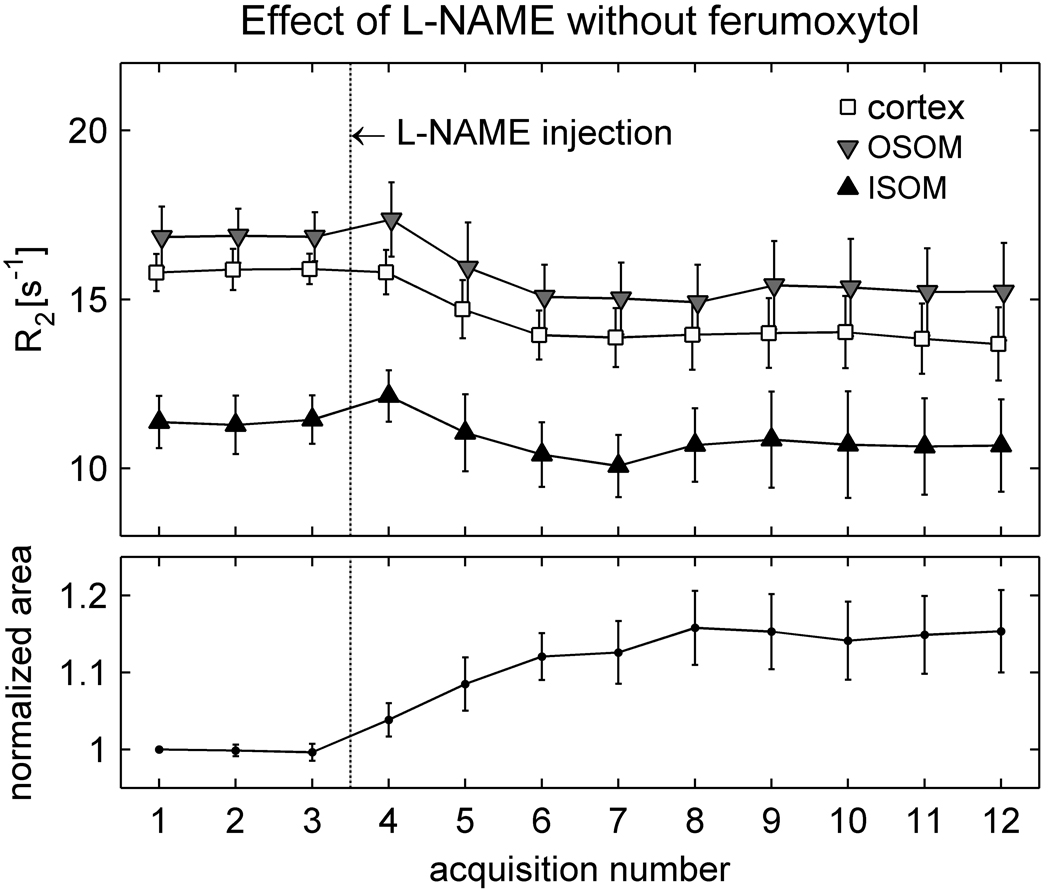
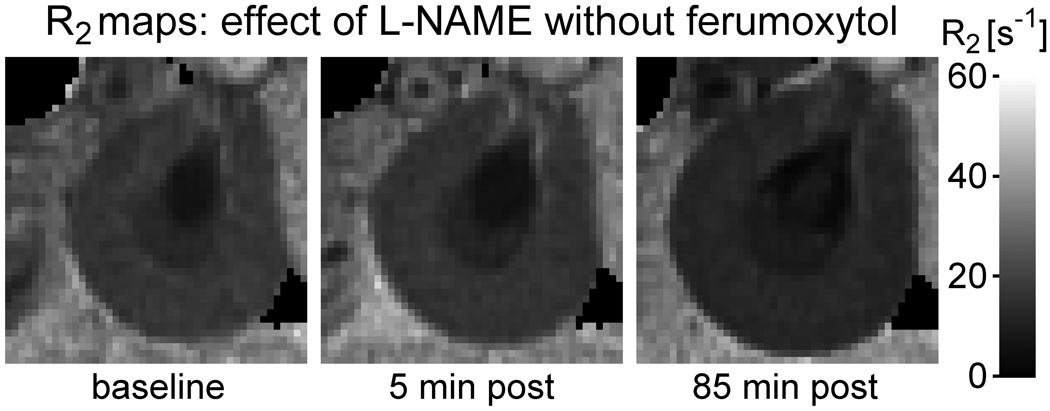
Results for the control group that received l-NAME only (group II-c) are presented in Figure 5. Note that even in the absence of ferumoxytol a slight decline in R2 is observed following l-NAME administration, and it is accompanied by a small increase in kidney size. The changes did not reach statistical significance (Table 5), which probably reflects the small number of animals studied and the relatively large inter-subject variation. The correlations between ΔR2 and size change, however, were statistically significant in all anatomic regions. This suggests that there may be a relationship between size change and ΔR2 in the absence of ferumoxytol, even though the overall magnitude of the response to l-NAME varied among the animals. Figure 6 shows R2 maps from the animal that exhibited the greatest response; a clear enlargement of the kidney occurs after l-NAME administration and is accompanied by a visible reduction in R2.
Figure 5.
Averaged results (mean ± SEM) from the control animals in group II-c (N = 4), which received l-NAME in the absence of ferumoxytol. The points corresponding to different regions in the upper graph have been slightly offset horizontally from each other so that the error bars do not overlay. Following l-NAME injection, R2 decreases on average in all regions while the kidney area increases. As in Figure 4, however, the responses are not immediate.
Table 5.
R2 and kidney size responses to l-NAME in the absence of ferumoxytol (group II-c, N = 4)
|
l-NAME results in absence of ferumoxytol |
ΔR2 in cortex [s−1] | ΔR2 in OSOM [s−1] | ΔR2 in ISOM [s−1] | Δarea |
| Response to l-NAME 1 | −1.9 ± 0.7 (p = 0.08) |
−1.9 ± 0.7 (p = 0.06) |
−1.0 ± 0.9 (p = 0.3) |
14 ± 5 % (p = 0.05) |
| Correlation between ΔR2 and Δarea | r = −0.974* (p = 0.03) |
r = −0.988* (p = 0.012) |
r = −0.965* (p = 0.03) |
Response to l-NAME = mean [(acquisition 7) and (acquisition 8)] – mean [(acquisition 1) to (acquisition 3)]; refer to Figure 5 for acquisition timing
significant (p < 0.05)
All results expressed as mean ± SEM
Figure 6.
R2 maps of the kidney from a rat in group II-c, which received l-NAME in the absence of ferumoxytol. The grayscale is identical to that used in Figure 1. Note that the kidney enlarges and R2 decreases following l-NAME administration.
To investigate whether increased ureteral pressure was responsible for the enlargement of the kidney in the l-NAME studies, bladder catheterization was performed in the later experiments. These included two rats from group II-a, two rats from group II-b and one rat from group II-c. The normalized change in cross-sectional area of the kidney was calculated for each rat between baseline (prior to l-NAME or ferumoxytol injection) and the last two acquisitions. For groups II-a / II-b / II-c respectively the changes were 6 ± 6 % / 21 ± 4 % / 15 ± 13 % without catheterization (mean ± SD) versus −0.05 ± 0.1 % / 4 ± 4 % / 15 % with catheterization. Thus the bladder catheterization did not eliminate the size increases, although it seems, on average, to have moderated them. The effect of catheterization was found to be not significant by two-way analysis of variance (p = 0.11).
DISCUSSION
In the absence of ferumoxytol, R2 was highest in the OSOM and decreased towards the papilla, consistent with known differences in water content (3,15). Administration of ferumoxytol elevated R2 greatly in all regions, confirming the strong T2 relaxivity of the agent. The magnitude of the rise, however, exhibited regional variation, being lower in the cortex and increasing progressively towards the papilla. This variation agreed with previous observations (3), and probably reflects the net effect of regional differences in vascular volume fraction (14), hematocrit (16,17) and vessel size distribution (11), as well as a concentration gradient caused by water transport between the descending and ascending vasa recta (3). The dependence on vascular volume fraction arises from the fact that ferumoxytol does not readily extravasate and is not filtered by the glomeruli. Hematocrit also plays a role since ferumoxytol is confined to the plasma compartment of the vascular space and does not enter red blood cells. In addition, vessel size distribution is relevant since R2 is more sensitive to small vessels (11). All these factors exhibit regional variation within the kidney (14,16,17), and likely contribute to the observed differences in R2 elevation following ferumoxytol administration. Another mechanism thought to be involved is water transport associated with the corticopapillary osmotic gradient (3); motion of water out of the descending vasa recta and into the ascending vasa recta would produce an increase in the plasma concentration of ferumoxytol with depth in the medulla.
In the presence of ferumoxytol, subsequent infusion of adenosine resulted in a further R2 increase in all regions of the kidney, suggesting that ferumoxytol-enhanced MRI is indeed sensitive to adenosine-induced vasodilation. The magnitude of the increase was lower in the cortex than in the outer medulla (OSOM and ISOM). Since R2 is believed to vary linearly with dilation-based increases in vascular volume fraction over the physiologically relevant range (11), this observation suggests that less vasodilation occurs in the cortex than the outer medulla. A possible explanation could be competition in the cortex between A2 adenosine receptors, which mediate vasodilation, and A1 receptors, which induce vasoconstriction in the afferent arterioles (5). In interpreting these results it is important to bear in mind that R2 has relatively greater sensitivity to small vessels (11). The effect of adenosine on large vessels would be better studied by imaging R2* and may be of interest for future investigations.
Bolus injection of l-NAME in the presence of ferumoxytol caused a small reduction in R2, which was not statistically significant. Although it is possible that a larger number of animals may yield a statistically significant response, our present results have not demonstrated sensitivity of ferumoxytol-enhanced MRI to l-NAME-induced vasoconstriction.
Small R2 variations were also observed in the absence of ferumoxytol; a slight increase was found during adenosine infusion, while a non-significant decrease was recorded following l-NAME injection. These trends cannot be explained in terms of blood volume changes, since unenhanced blood has low R2; vasodilation would therefore be expected to reduce R2 in the absence of ferumoxytol, while vasoconstriction should increase it. Neither can they be explained in terms of oxygenation changes, since deoxygenated blood has higher magnetic susceptibility than well oxygenated blood (1), and adenosine would be expected to increase oxygenation while l-NAME should reduce it.
Other physiological mechanisms must therefore be responsible for the R2 variations in the absence of ferumoxytol, and we hypothesize that they involve changes in the volume of the renal tubules. This hypothesis would explain the concomitant variations in kidney size. Since filtrate in the tubules has low R2, an expansion of the tubular volume would increase kidney size and reduce R2. This is consistent with the l-NAME results, which exhibited a negative correlation between the kidney size and R2 changes in the absence of ferumoxytol. Similarly, a reduction in tubular volume would reduce kidney size and increase R2, as observed with adenosine.
A review of the literature gives support to the above hypothesis. It was shown during the 1960s and 1970s that the kidney is a distensible organ whose size varies dynamically in response to physiological and pharmacological interventions (18–24). Evidence suggested that the variations were attributable to changes in the volume of the renal tubules; diuretics were found to produce an enlargement of the kidney (19–23), as did ureteral obstruction (23), while reductions in perfusion pressure caused the kidney to shrink (23,24). It seems plausible that the kidney size changes observed in the present study were due to variations in tubular volume, associated primarily with changes in urine flow. l-NAME is known to cause diuresis (25), due at least in part to its effects on systemic blood pressure. Adenosine, on the other hand, reduces glomerular filtration rate (GFR) and urine flow (26,27) through a mechanism believed to involve vasoconstriction of the afferent arterioles, via A1 receptors, in conjunction with vasodilation of the efferent arterioles, via A2 receptors (28). These effects could account in large part for the observed kidney size and R2 variations in the absence of ferumoxytol. Other factors may also have affected tubular volume in our experiments, including bladder filling in some of the animals, which could have increased ureteral pressure. The volume of injected fluids may also have increased urine output, and any reduction in the animals’ body temperature over the course of the MRI scan may have further contributed to pressure diuresis via peripheral vasoconstriction. The resulting alterations in tubular volume may be a confounding factor in the detection of renal blood volume changes by means of R2 measurements in the presence of ferumoxytol, which was the original focus of the study. The magnitude of the effect, however, could be species dependent (22,29).
One of the interesting implications of our results is that tubular volume changes may also be a confounding factor for BOLD MRI, especially in rodents. BOLD MRI relies on measurements of R2*, which can be decomposed into two terms: the transverse relaxation rate R2 and a susceptibility-weighted contribution R2′. Since deoxyhemoglobin is paramagnetic, variations in the oxygenation level of the blood will alter its magnetic susceptibility, causing changes in R2′. It is typically assumed that changes in R2* are entirely attributable to variations in blood oxygenation, mediated by the term R2′. The present study, however, demonstrates that interventions such as adenosine and l-NAME administration may have effects on R2 that cannot be explained by variations in blood volume or oxygenation, but more likely reflect changes in the volume of the renal tubules.
This may explain an observation from a recent BOLD study on rats (10), which reported an initial increase in medullary R2* in response to l-NAME infusion, followed by a subsequent reduction before the end of the infusion period. The initial R2* increase correlated with changes in mean arterial pressure, blood flow and medullary pO2, as measured by invasive probes. However the subsequent R2* decrease showed no such correlations. A plausible explanation for the delayed reduction in R2* may be a gradual increase in tubular volume, which would have lowered R2, thereby reducing R2*. To test this hypothesis, a retrospective analysis of kidney size was performed on data from rats in the BOLD study (10) that received an infusion of 10 mg/kg l-NAME over 30 minutes. The cross-sectional area of the kidney on oblique axial images was found to increase with respect to baseline by 5.3 ± 3.5 % (mean ± SD) at 18 minutes and 12.8 ± 5.7 % at 30 minutes (p = 0.014 and p = 0.003 respectively). This is consistent with the hypothesis that the delayed reduction in R2* may be due to increased tubular volume.
In view of the potentially confounding effects of R2 in BOLD studies, it is possible that a more reliable surrogate of medullary oxygenation may be provided by R2′, which would require measurements of both R2 and R2*. This has previously been suggested by Yang et al. (30), who measured R2 and R2* responses to furosemide in rat kidneys.
In conclusion, the original goal of this work was to determine whether USPIO-enhanced MRI is sensitive to blood volume changes in the rat kidney. Our results suggest that R2 imaging in the presence of ferumoxytol is sensitive to adenosine-induced vasodilation. The R2 responses to l-NAME, however, did not reach statistical significance. An incidental finding of the study was that R2 values in the absence of ferumoxytol also vary slightly with adenosine and l-NAME, and are accompanied by changes in the physical size of the kidney. These variations cannot be explained in terms of alterations in blood volume or oxygenation and may reflect changes in the volume of the renal tubules. This finding may have implications for BOLD MRI of the kidney, which relies on measurements of R2*, a quantity that depends on both R2 and a susceptibility-related term R2′.
Acknowledgments
Grant support: NIH DK-53221
REFERENCES
- 1.Prasad PV. Evaluation of intra-renal oxygenation by BOLD MRI. Nephron Clin Pract. 2006;103:c58–c65. doi: 10.1159/000090610. [DOI] [PubMed] [Google Scholar]
- 2.Li LP, Halter S, Prasad PV. Blood oxygen level-dependent MR imaging of the kidneys. Magn Reson Imaging Clin N Am. 2008;16:613–625. doi: 10.1016/j.mric.2008.07.008. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trillaud H, Degreze P, Combe C, Palussiere J, Chambon C, Grenier N. Evaluation of intrarenal distribution of ultrasmall superparamagnetic iron oxide particles by magnetic resonance imaging and modification by furosemide and water restriction. Invest Radiol. 1994;29:540–546. doi: 10.1097/00004424-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Trillaud H, Degreze P, Combe C, et al. USPIO-enhanced MR imaging of glycerol-induced acute renal failure in the rabbit. Magn Reson Imaging. 1995;13:233–240. doi: 10.1016/0730-725x(94)00114-i. [DOI] [PubMed] [Google Scholar]
- 5.Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther. 2005;315:1150–1157. doi: 10.1124/jpet.105.091017. [DOI] [PubMed] [Google Scholar]
- 6.Biaggioni I. Clinical and molecular pharmacologic characteristics of adenosine-induced vasodilation. Clin Pharmacol Ther. 2004;75:137–139. doi: 10.1016/j.clpt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 8.Cannon RO., 3rd Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 1998;44:1809–1819. [PubMed] [Google Scholar]
- 9.Li L, Storey P, Kim D, Li W, Prasad P. Kidneys in hypertensive rats show reduced response to nitric oxide synthase inhibition as evaluated by BOLD MRI. J Magn Reson Imaging. 2003;17:671–675. doi: 10.1002/jmri.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li LP, Ji L, Santos EA, Dunkle E, Pierchala L, Prasad P. Effect of nitric oxide synthase inhibition on intrarenal oxygenation as evaluated by blood oxygenation level-dependent magnetic resonance imaging. Invest Radiol. 2009;44:67–73. doi: 10.1097/RLI.0b013e3181900975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med. 1995;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- 12.Wang YX, Poon CI, Pang CC. Vascular pharmacodynamics of NG-nitro-L-arginine methyl ester in vitro and in vivo. J Pharmacol Exp Ther. 1993;267:1091–1099. [PubMed] [Google Scholar]
- 13.Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med. 1995;34:910–914. doi: 10.1002/mrm.1910340618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Sanz A, Rodriguez-Barbero A, Bentley MD, Ritman EL, Romero JC. Three-dimensional microcomputed tomography of renal vasculature in rats. Hypertension. 1998;31:440–444. doi: 10.1161/01.hyp.31.1.440. [DOI] [PubMed] [Google Scholar]
- 15.Saikia TC. Composition of the Renal Cortex and Medulla of Rats During Water Diuresis and Antidiuresis. Q J Exp Physiol Cogn Med Sci. 1965;50:146–157. doi: 10.1113/expphysiol.1965.sp001777. [DOI] [PubMed] [Google Scholar]
- 16.Pallone TL, Robertson CR, Jamison RL. Renal medullary microcirculation. Physiol Rev. 1990;70:885–920. doi: 10.1152/physrev.1990.70.3.885. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen SN. Intrarenal red cell and plasma volumes in the non-diuretic rat. Determination by means of 51Cr labelled red cells and 125I-gamma-M-immunoglobulin. Pflugers Arch. 1973;342:61–72. doi: 10.1007/BF00593250. [DOI] [PubMed] [Google Scholar]
- 18.Wolpert SM. Variation in Kidney Length During the Intravenous Pyelogram. Br J Radiol. 1965;38:100–103. doi: 10.1259/0007-1285-38-446-100. [DOI] [PubMed] [Google Scholar]
- 19.Finberg JP, Peart WS. Renal tubular flow dynamics during angiotensin diuresis in the rat. Br J Pharmacol. 1970;39:357–372. doi: 10.1111/j.1476-5381.1970.tb12899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorph S, Oigaard A. Renal distension in response to water-soluble contrast medium and various diuretics. Scand J Urol Nephrol. 1975;9:114–118. doi: 10.3109/00365597509180917. [DOI] [PubMed] [Google Scholar]
- 21.Dorph S, Sovak M, Talner LB, Rosen L. Why does kidney size change during I.V. urography? Invest Radiol. 1977;12:246–250. doi: 10.1097/00004424-197705000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hegedus V, Faarup P, Norgaard T, Lonholdt C. Volume changes in the rat renal cortex during urography. Br J Radiol. 1978;51:793–798. doi: 10.1259/0007-1285-51-610-793. [DOI] [PubMed] [Google Scholar]
- 23.Omvik P, Jr, Raeder M, Kiil F. Determinants of renal cortical volume. Am J Physiol. 1971;221:1560–1567. doi: 10.1152/ajplegacy.1971.221.6.1560. [DOI] [PubMed] [Google Scholar]
- 24.Collier RO, Swann HG. Relation of kidney size to blood pressure. Am J Physiol. 1971;220:488–491. doi: 10.1152/ajplegacy.1971.220.2.488. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Bowmer CJ, Yates MS. Diuretic effect of NG-nitro-L-arginine methyl ester in the rat. J Pharm Pharmacol. 1994;46:510–512. doi: 10.1111/j.2042-7158.1994.tb03838.x. [DOI] [PubMed] [Google Scholar]
- 26.Osswald H, Schmitz HJ, Kemper R. Renal action of adenosine: effect on renin secretion in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1978;303:95–99. doi: 10.1007/BF00496190. [DOI] [PubMed] [Google Scholar]
- 27.Elias AN, Wesley RC, Gordon IL, Pandian MR, Vaziri ND. Effects of adenosine infusion on renal function, plasma ANP and ADH concentrations and central hemodynamics in anesthetized pigs. Gen Pharmacol. 1997;28:429–433. doi: 10.1016/s0306-3623(96)00242-x. [DOI] [PubMed] [Google Scholar]
- 28.Bell TD, Welch WJ. Regulation of renal arteriolar tone by adenosine: novel role for type 2 receptors. Kidney Int. 2009;75:769–771. doi: 10.1038/ki.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorph S, Hegedus V, Palbol J. Kidney distension during IV urography in normal rats and in rats with artificial unilateral renal artery stenosis. Br J Radiol. 1979;52:461–463. doi: 10.1259/0007-1285-52-618-461. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Cao J, Wang X, Li X, Xu Y, Jiang X. Evaluation of renal oxygenation in rat by using R2′ at 3-T magnetic resonance: initial observation. Acad Radiol. 2008;15:912–918. doi: 10.1016/j.acra.2008.01.015. [DOI] [PubMed] [Google Scholar]