Abstract
BACKGROUND AND PURPOSE
8-Nitroguanosine 3′,5′-cyclic monophosphate (8-nitro-cGMP), formed nitric oxide (NO)-dependently, is a physiological second messenger, yet little is known about its role in the pathophysiology of vascular diseases. To study the pharmacological activity of 8-nitro-cGMP in diabetic mice, we compared its effects on vascular reactivity of aortas from non-diabetic and diabetic mice.
EXPERIMENTAL APPROACH
Vascular tension recording was performed in thoracic aortic rings from wild-type (C57BL/6), non-diabetic db/+ and obese/diabetic db/db mice. Endothelial NO synthase (eNOS) uncoupling and superoxide were tested by Western blot and dihydroethidium fluorescence respectively.
KEY RESULTS
8-Nitro-cGMP, at concentrations up to 10 µM, enhanced phenylephrine-induced contractions in aortas from C57BL/6 and db/+ mice, but not from db/db mice. This enhancement was not observed with 8-bromo-cGMP. Pretreatment of aortas from C57BL/6 and db/+ mice with l-NAME (100 µM), superoxide dismutase (100 U·mL−1) or tiron (1 mM), abolished 8-nitro-cGMP-induced enhancement of the phenylephrine contraction. In 8-nitro-cGMP (10 µM)-treated C57BL/6 aortas, eNOS dimer/monomer ratio was significantly decreased and vascular superoxide production increased, suggesting that 8-nitro-cGMP-induced superoxide production via eNOS uncoupling may mediate the enhancement of the phenylephrine contraction. At higher concentrations (>10 µM), 8-nitro-cGMP produced relaxation of the phenylephrine-contracted aortas from C57BL/6, db/+ and db/db mice. The 8-nitro-cGMP-induced relaxation in db/db mouse aortas was found to be resistant to a phosphodiesterase 5 inhibitor, zaprinast (1 µM).
CONCLUSIONS AND IMPLICATIONS
The vasodilator effect of 8-nitro-cGMP may contribute to amelioration of the vascular endothelial dysfunction in diabetic mice, representing a novel pharmacological approach to prevent the complications associated with diabetes.
Keywords: 8-nitro-cGMP, vascular responses, aorta, db/db mouse, eNOS, superoxide anions
Introduction
Nitric oxide (NO) generated by endothelial nitric oxide synthase (eNOS) crucially determines vascular tone as well as vascular wall homeostasis. It is well established that NO stimulates cGMP production and induces subsequent signalling pathways in target cells. Recently, we discovered a nitrated derivative of cGMP, 8-nitro-cGMP, a novel second messenger that is formed nitric oxide synthase (NOS)-dependently in physiological systems and may be involved in redox activity and signal transduction by NO (Sawa et al., 2007). This has led us to investigate a possible role of 8-nitro-cGMP in vascular function.
NO availability may be attenuated in part by reaction with reactive oxygen species (ROS), particularly superoxide (·O2-) produced by oxidative stress in pathological conditions (Cai and Harrison, 2000; Vanhoutte et al., 2009). Increased oxidative stress in vessel walls is an important element in the development and progression of diabetes and its complications (Baynes and Thorpe, 1999). Therefore, ROS scavenging has been proposed as a therapeutic strategy to target oxidative stress in vascular disorders. In addition, it was reported that overexpression of superoxide dismutase (SOD)-1 in type 2 diabetic db/db mice attenuates several indices of renal injury, possibly by inducing a reduction of peroxynitrite generation by NO–superoxide interaction (DeRubertis et al., 2004).
Based on these findings, we hypothesized that 8-nitro-cGMP may play some role in the ROS-mediated pathophysiology of diabetic vascular dysfunction. To test this hypothesis, the present study was performed to examine the pharmacological activity of 8-nitro-cGMP in diabetic vessels, by comparing the effects of 8-nitro-cGMP on vascular responses of thoracic aortic rings from non-diabetic (C57BL/6 and db/+) and obese/diabetic (db/db) mice.
Methods
Animal experiments
Male BKS.Cg-Dock7m+/+Leprdb/J (db/db) mice, 13- to 14-week-old, age-matched littermates (db/+) and age-matched controls (C57BL/6) from The Jackson Laboratory (Bar Harbor, ME, USA) were used in this study. The animal protocol was approved by the Institutional Animal Care and Use Committee.
Aortic ring preparation and tension recording
The mice were killed by inhalation of diethyl ether and cervical dislocation and the thoracic aortas were excised immediately. Adventitial tissue was carefully removed. The aortic rings were mounted in a 3 mL organ bath chambers (MTOB-1, Labo Support, Osaka, Japan), filled with Krebs-Henseleit buffer (KHB, in mM: NaCl, 118.4; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 10), bubbled with 95% O2 and 5% CO2, and maintained at 37°C. The rings were connected to a force transducer to measure isometric tensions and recorded on a transducer data acquisition system (PowerLab, Chart v5, AD Instruments, Colorado Springs, CO, USA). Resting tension was set at 1.0 g and rings were allowed to equilibrate for 60 min. KHB was changed before and twice after each concentration–response curve. Cumulative concentration–response curves for acetylcholine (ACh; 1 nM to 100 µM), 8-nitro-cGMP and 8-bromo-cGMP (1 nM to 300 µM) were generated after contraction of rings with an α1-agonist, phenylephrine (Phe; 0.1 µM). All experiments for tension recording were conducted following induction of 20–30% of maximal Phe-induced contraction, to get a significant response to 8-nitro-cGMP or 8-bromo-cGMP. All inhibitors/scavengers used in this study were allowed to incubate with the preparation for 30 min before the construction of the concentration–response curve.
Detection of eNOS dimer disruption and superoxide production
For measurements of eNOS dimer/monomer ratio and in situ superoxide production, the isolated aortas were treated with 8-nitro-cGMP or 8-bromo-cGMP as follows: isolated mice aortas were removed, cleared of connective tissue and immersed in warm KHB (37°C) for equilibration for 90 min. Subsequently, vessel segments were incubated for 30 min in control KHB or in KHB containing 8-nitro-cGMP or 8-bromo-cGMP. The segments were then snap-frozen at −80°C for immunoblotting and dihydroethidium (DHE) staining.
For immunoblotting, homogenates of thoracic aorta were prepared from the frozen segments in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% Triton X-100 and protease inhibitors. Protein concentrations were determined using BCA protein assay kit (Pierce, Rockford, IL, USA). Aortic eNOS dimer and monomer were separated, using low-temperature SDS-PAGE followed by Western blot analysis, as described in our previous study (Yamamoto et al., 2007a). In brief, protein extracts were mixed with fivefold SDS sample buffer (0.31 M Tris-HCl, pH 6.8, 10% SDS, 50% glycerol, 0.5 M dithiothreitol and 0.03% bromophenol blue) at 0°C. Electrophoresis was performed in a cold room (4°C). eNOS dimer and monomer were detected with anti-eNOS antibody (1:2000, BD Biosciences, Lexington, KY, USA). The immunoreactivity was detected by enhanced chemiluminescence method (ECL; GE Healthcare Bioscience, Piscataway, NJ, USA) and quantified using a luminescence image analyser LAS-4000mini and image analysis software Multi Gauge Ver.3.11 (Fuji Film, Tokyo, Japan).
In situ superoxide production was determined in vessel cryosections with the oxidative fluorescent dye DHE, as previously described (Yamamoto et al., 2007b; Nakamura et al., 2009). Cryosections (8 µm in thickness; Leica, Weltzar, Germany) from the aortas treated with 8-nitro-cGMP or 8-bromo-cGMP were incubated with DHE (5 µM) at 37°C for 30 min and then viewed by fluorescent microscopy (Nikon, Eclips, Tokyo, Japan). For each slide, at least five images from different sections of the slide were captured, and average staining intensity was calculated using image analysis software (Lumina Vision version 2.2, Mitani-Corp., Fukui, Japan).
Data analysis
Relaxation responses are expressed as a percentage reversal of the Phe-induced contraction. Responses are plotted graphically as means from at least four separate experiments with vertical bars representing SEM. Curves were fitted to all the data by non-linear regression using Prism (GraphPad Software, San Diego, CA, USA) to calculate Emax and EC50 values. EC50 values were used to compare the relaxant effects of the cGMP analogues. A t-test was used to assess the significance of differences between EC50 values. A P-value <0.05 was taken to indicate a statistically significant difference. Statistical analysis was performed using Prism software.
Materials
8-Nitro-cGMP and 8-bromo-cGMP were synthesized as described previously (Sawa et al., 2007). Phenylephrine hydrochloride (Phe), ACh, a NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME), a superoxide scavenger SOD (from bovine erythrocytes), a SOD mimetic tiron (4,5-dihydroxy-1,3-benzene-disulphonic acid) (Mohazzab et al., 1994), a cGMP-specific phosphodiesterase (PDE) inhibitor zaprinast and DHE were obtained from Wako Pure Chemical Industries (Osaka, Japan). Iberiotoxin was purchased from Peptide Institute Inc. (Osaka, Japan). Preparations of all stock solutions and their subsequent dilution were made using distilled water. Exceptions to this were zaprinast and DHE, which were dissolved in DMSO (0.1% final concentration). As a control, some aortas were treated with DMSO (0.1%) alone and no changes were observed.
Results
Effects of 8-nitro-cGMP on Phe-induced contraction in C57BL/6 mouse aorta
8-Nitro-cGMP by itself did not induce any significant change in basal tone of thoracic aortic rings from wild-type C57BL/6 mice (not shown). However, in the aortas contracted with the α-adrenoceptor agonist Phe (0.1 µM), 8-nitro-cGMP induced dose-dependent changes in isometric tension. As shown in Figure 1A, the cumulative addition of 0.001–100 µM 8-nitro-cGMP produced a biphasic effect: at concentrations up to 10 µM the increase in tension of the Phe-contracted aortas was statistically significant, and at higher than 10 µM the contracted rings were dose-dependently relaxed. A cell-permeable cGMP analogue, 8-bromo-cGMP induced only dose-dependent relaxation in the aortas contracted with Phe (not shown).
Figure 1.
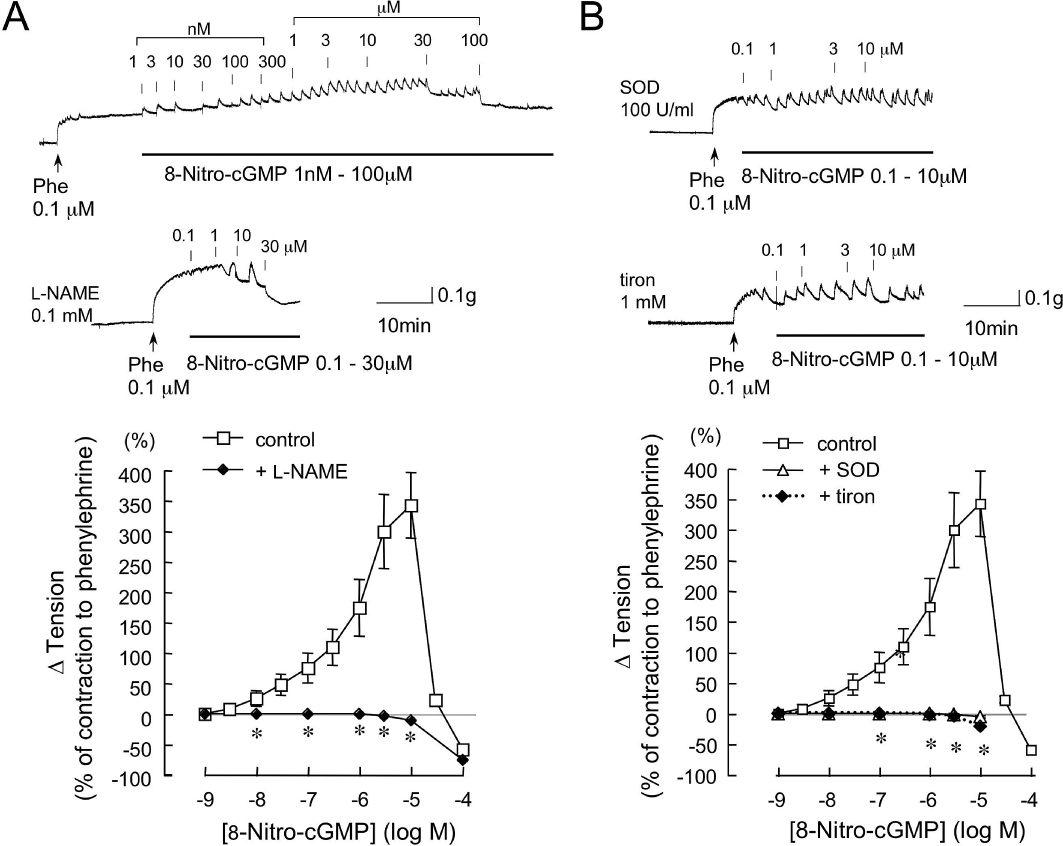
Effects of l-NAME, SOD and tiron on 8-nitro-cGMP-induced enhancement of contraction to phenylephrine (Phe) in aortic rings from C57BL/6 mouse. Original tracings and summarized data of vascular responses to cumulatively administered 8-nitro-cGMP in the Phe-contracted aorta. Vertical lines in each trace indicate administration of 8-nitro-cGMP. 8-Nitro-cGMP concentration-dependently produced a biphasic effect: an initial enhancement of contraction to Phe, followed by a relaxation. l-NAME (0.1 mM, A, lower trace), SOD (100 U·mL−1, B, upper trace) or tiron (1 mM, B, lower trace) was added 30 min before the addition of Phe (0.1 µM). l-NAME, SOD and tiron each abolished the enhancement of the contraction to Phe. Changes in vascular tension to 8-nitro-cGMP are expressed as % of the Phe-induced contraction. Each point represents the mean ± SEM (n = 4–6). *P < 0.05 versus control.
The relaxation induced by 8-nitro-cGMP at higher than 10 µM in the aortas from C57BL/6 mouse was decreased by 25% after pretreatment of the aortas with a BK-type Ca2+-activated K+ channel blocker, iberiotoxin (100 nM) (results not illustrated).
Inhibitory effects of l -NAME and superoxide scavenger on 8-nitro-cGMP-induced enhancement of contraction to Phe in C57BL/6 mouse aorta
We have previously reported that mechanical removal of the endothelium in SD rat carotid artery significantly attenuates the 8-nitro-cGMP-enhanced contraction to Phe (Sawa et al., 2007). To determine the involvement of eNOS in 8-nitro-cGMP-induced enhancement of contraction to Phe in mouse aortas, we examined the effects of l-NAME, an inhibitor of NOS, on the 8-nitro-cGMP-induced enhancement. As shown in Figure 1A, pretreatment of aortas with 100 µM l-NAME abolished 8-nitro-cGMP-induced enhancement of the contraction to Phe.
Our previous studies had demonstrated superoxide formation in response to 8-nitro-cGMP by use of electron spin resonance spectroscopy (Sawa et al., 2007). To further investigate the involvement of superoxide in the 8-nitro-cGMP-enhanced contractile responses, we examined the effects of the ROS scavenger, SOD and a SOD mimetic, tiron, on this response to 8-nitro-cGMP. In our preliminary experiments with aortas from C57BL/6 mice, Phe-induced contraction was attenuated by SOD (100 U·mL−1) or tiron (1 mM) (to 18% of contraction to Phe). As shown in Figure 1B, pretreatment of aortas from C57BL/6 mice with 100 U·mL−1 SOD abolished 8-nitro-cGMP-induced enhancement of the contraction to Phe. The potency of 1 mM tiron in the aortas was similar to that of SOD.
Involvement of eNOS uncoupling in 8-nitro-cGMP-enhanced contraction
To assess the possibility that 8-nitro-cGMP disrupts the eNOS dimer in aortas from C57BL/6 mice, we used low-temperature SDS-PAGE Western blots to characterize the dimer and monomer of eNOS in the aortic segments incubated with buffer alone, 8-nitro-cGMP or 8-bromo-cGMP at 10 µM for 30 min respectively. As shown in Figure 2A, the 8-nitro-cGMP-treated aortas were found to have a significant decrease in eNOS dimer/monomer ratio as assessed by the relative band density of the expected 280 KDa (dimer) and 140 KDa (monomer) immunoreactive bands. SDS-PAGE revealed that there was no difference in total eNOS expression in each aorta.
Figure 2.
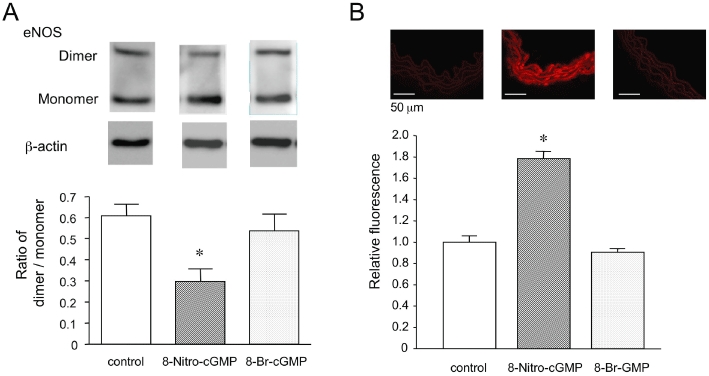
Disruption of eNOS dimer and superoxide production by 8-nitro-cGMP in aortas from C57BL/6 mouse. (A) Representative Western blots and densitometric analysis for eNOS dimer (280 KDa) and monomer (140 KDa) in C57BL/6 mouse aortic segments treated with vehicle, 8-nitro-cGMP or 8-bromo-cGMP. Each eNOS density was normalized to β-actin (45 KDa). (B) Representative fluorescent photomicrographs and quantitative analysis of DHE-labelled microscopic sections of C57BL/6 mouse aortic segments incubated with vehicle, 8-nitro-cGMP or 8-bromo-cGMP. Each value represents the mean ± SEM (n = 3–4). *P < 0.05 versus control and 8-bromo-cGMP.
To visualize vascular superoxide production in response to 8-nitro-cGMP, we performed DHE fluorescence staining of frozen section aortas. As shown in Figure 2B, incubation of the aortas with 8-nitro-cGMP at 10 µM for 30 min significantly increased superoxide production, compared with 8-bromo-cGMP.
Vascular reactivity in diabetic mouse aorta
An important role of ROS contributing to the impaired regulation of arteriolar tone has recently received considerable attention in type 2 diabetes. Thus, we investigated the effects of 8-nitro-cGMP on isometric tension of aortas from type 2 diabetic db/db mice. The db/db mice at 13–14 weeks of age weighed more than their age-matched db/+ mice (db/db group: 48.85 ± 1.14 g; db/+ group: 29.5 ± 0.9 g; n = 10–12) with higher levels of blood glucose and plasma insulin (Dong et al., 2010).
Contractility of thoracic aortas to high K+ solution was not significantly different in db/db and db/+ mice (db/db group: 0.215 ± 0.007 g; db/+ group: 0.235 ± 0.009 g; n = 10–12). By contrast, the force generated in response to Phe (0.1 µM) was markedly greater in db/db aortas (Figure 3A). ACh-induced relaxations of db/db aortas contracted with Phe (0.1 µM) were significantly reduced compared with their respective controls (Figure 3B; db/db: Emax= 76.1 ± 4.6%, EC50= 105.6 nM; db/+: Emax= 90.1 ± 1.8%, EC50= 39.5 nM; n = 6). The endothelium-independent vasodilator response to an exogenous NO donor, sodium nitroprusside, was not different in db/db and db/+ mice (data not shown).
Figure 3.
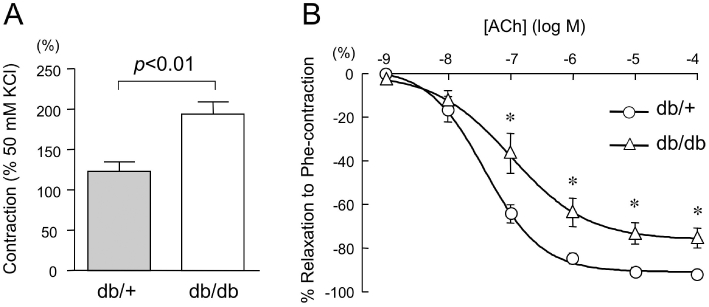
Vascular reactivity in aortas from db/+ and db/db mice. (A) Phe (0.1 µM)-induced contraction of thoracic aortic rings from db/+ and db/db mice. Data are expressed as % of the tension induced by 50 mM KCl. (B) Cumulative concentration–relaxation curves to acetylcholine (ACh) of Phe-contracted aortas from db/+ and db/db mice (n = 6 for each group). Relaxations are expressed as a percentage reversal of the contraction induced by Phe (1 µM). Data are shown as mean ± SEM. *P < 0.02 versus db/+.
Effects of 8-nitro-cGMP on Phe-induced contraction in diabetic mouse aorta
Typical responses of individual db/db and db/+ mouse aortas are shown in Figure 4A and B; the averaged concentration–response relationships from these experiments for both aortic rings are summarized in Figure 4C and D. In db/+ mice, the cumulative addition of 0.001–300 µM 8-nitro-cGMP produced a biphasic effect: at concentrations up to 10 µM the increase in tension of the Phe-contracted aortas was statistically significant, and at higher than 10 µM the contracted aortas were dose-dependently relaxed (Figure 4C). This enhancement of vasoconstriction by 8-nitro-cGMP was not observed in db/db mice, but only the relaxation was observed. The 8-bromo-cGMP-induced dose-dependent relaxation of the Phe-contracted rings was not significantly different in db/+ and db/db mice (Figure 4D). As shown in Figure 4A and B, 8-nitro-cGMP evoked a more rapid relaxant response than 8-bromo-cGMP in each mouse aorta.
Figure 4.
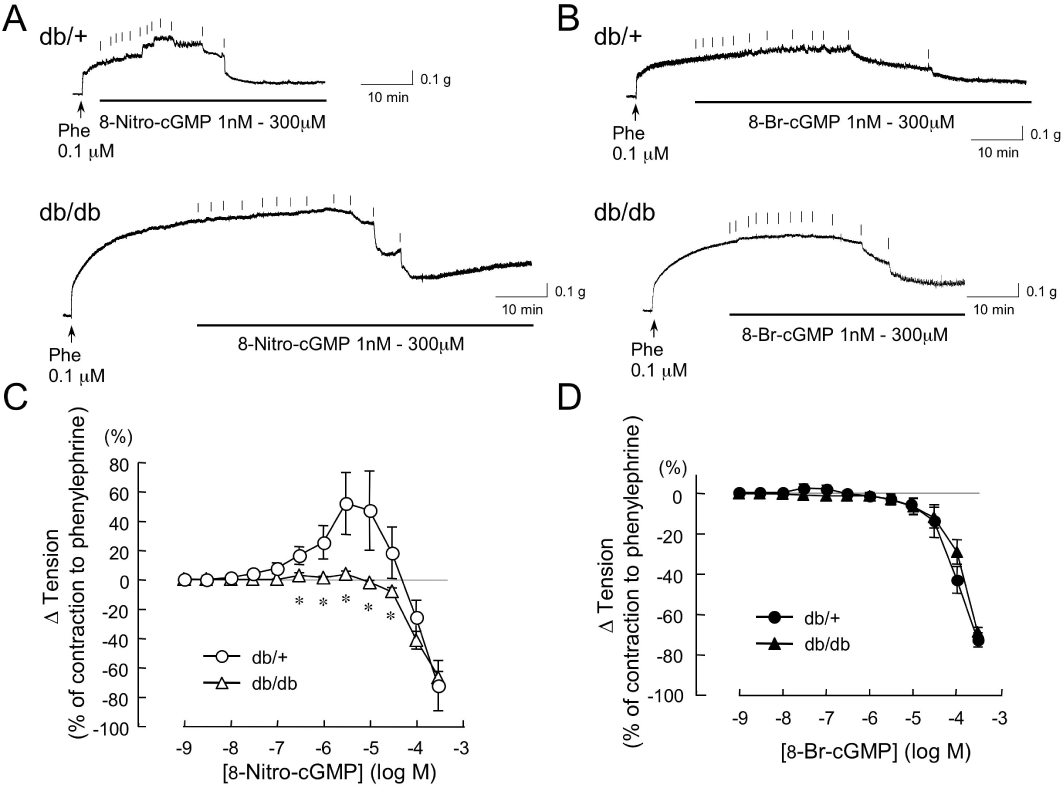
Effects of 8-nitro-cGMP on the contraction to Phe in the aortas from db/+ and db/db mice. Original tracings (A and B) and summarized data (C and D) of vascular responses to cumulatively administered 8-nitro-cGMP and 8-bromo-cGMP in the Phe-contracted aortas from db/+ and db/db mice. Vertical lines in each trace indicate administration of 8-nitro-cGMP (A) or 8-bromo-cGMP (B). Changes in vascular tension to 8-nitro-cGMP (C) and 8-bromo-cGMP (D) are expressed as % of the Phe (0.1 µM)-induced contraction. Each point represents the mean ± SEM (n = 5–9 for each group). *P < 0.05 versus db/+.
Inhibitory effects of l -NAME and a superoxide scavenger on 8-nitro-cGMP-induced enhancement of contraction to Phe in db/+ mouse aorta
As shown in Figure 5, pretreatment of aortic rings from db/+ mouse with 100 µM l-NAME abolished the 8-nitro-cGMP-induced enhancement of the contraction to Phe. Moreover, in the presence of l-NAME, the vasorelaxant responses of 8-nitro-cGMP (300 µM) in db/+ aorta were stronger than those in the absence of l-NAME in db/db aorta (Figure 5A; db/+: −93.7 ± 0.01% tension, Figure 4C; db/db: −66.9 ± 4.3%, P < 0.05).
Figure 5.
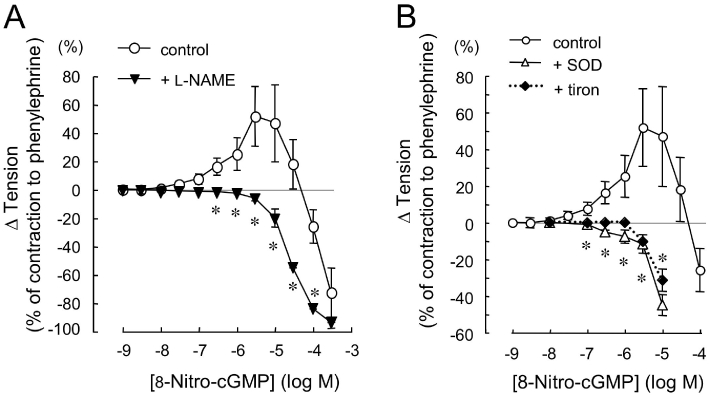
Effects of l-NAME, SOD and tiron on 8-nitro-cGMP-induced enhancement of the contraction to Phe in aortas from db/+ mice. l-NAME (0.1 mM, A), SOD (100 U·mL−1, B) or tiron (1 mM, B) was added 30 min before the addition of Phe. Changes in vascular tension to 8-nitro-cGMP are expressed as % of the Phe-induced contraction in db/+ mouse aorta. Each point represents the mean ± SEM (n = 4–6). *P < 0.05 versus control.
In agreement with the results with aortas from C57BL/6 mice, pretreatment of db/+ aortas with 100 U·mL−1 SOD abolished the 8-nitro-cGMP-induced enhancement of the contraction to Phe (Figure 5B). The potency of 1 mM tiron in aortas from db/+ mice was similar to that of SOD.
Indomethacin, a COX-1/2 inhibitor, did not affect the vascular responses elicited by 8-nitro-cGMP in both aortas from db/+ and db/db mice (data not shown). Moreover, l-NAME did not have a significant effect on the relaxation elicited by 8-nitro-cGMP in db/db aortas (data not shown).
Effects of a PDE5 inhibitor on 8-nitro-cGMP-induced relaxation of the contraction to Phe
A cGMP-specific PDE is responsible for degradation of cGMP in vascular tissues and thus the activity of PDE influences the vascular tone. Pretreatment for 30 min with zaprinast (1 µM), a selective cyclic nucleotide PDE5 inhibitor, did not affect the concentration–response curve for 8-nitro-cGMP-induced relaxation in aortas from db/db mice (Figure 6A). In contrast, the vasorelaxant responses of 8-bromo-cGMP were enhanced in the presence of zaprinast (Figure 6B), indicating that 8-bromo-cGMP is probably degraded into inactive 5′-GMP. As shown in Figure 6C, the effect of 100 µM 8-bromo-cGMP was significantly enhanced by the zaprinast pretreatment.
Figure 6.
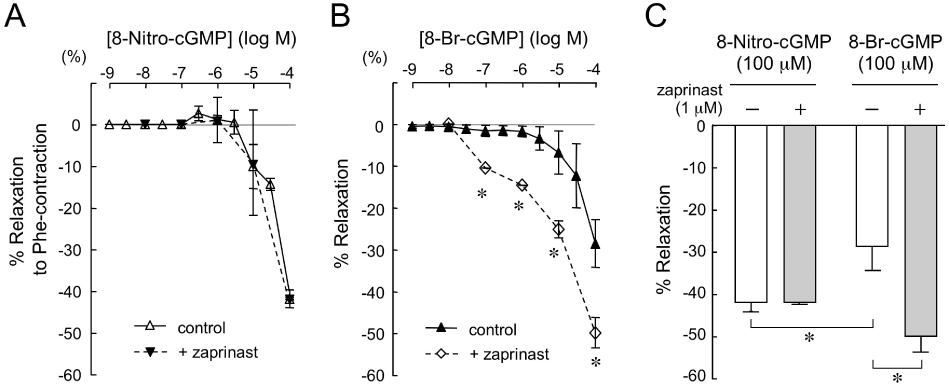
Effects of zaprinast on 8-nitro-cGMP or 8-bromo-cGMP-induced relaxation in db/db aorta contracted by Phe. Cumulative concentration–relaxation curves to 8-nitro-cGMP (A) or 8-bromo-cGMP (B) of the Phe-contracted aortas from db/db mice (n = 4–6 for each group). Zaprinast (1 µM) was added 30 min before the addition of Phe. (C) Comparison of the relaxation responses to 8-nitro-cGMP (100 µM) and 8-bromo-cGMP (100 µM) induced in the presence and absence of zaprinast in db/db aortic rings contracted by Phe. Data are shown as mean ± SEM. *P < 0.05 versus control.
Discussion
In the present study, we compared the effects of a novel nitrated derivative of cGMP, 8-nitro-cGMP, on vascular responses of aortas from non-diabetic and diabetic mice. Our data, showing that 8-nitro-cGMP induced enhancement of contraction to Phe and relaxation concentration-dependently in C57BL/6 and db/+ mice aortas (Figures 1A and 4A), are consistent with our previous study using normal rat carotid artery (Sawa et al., 2007). In our previous study we also showed that the 8-nitro-cGMP-induced enhancement of vascular reactivity to Phe in the rat carotid artery is endothelium-dependent as removal of endothelium significantly attenuated the 8-nitro-cGMP-induced hyperreactivity. In addition, it was found that 8-nitro-cGMP caused no enhancement of contraction to Phe in aortas from eNOS-deficient mice. In the present study, we have demonstrated that pretreatment of C57BL/6 or db/+ aortas with l-NAME significantly reduces the 8-nitro-cGMP-induced enhancement of the contraction to Phe (Figures 1A and 5A), suggesting the involvement of eNOS in this hyperreactivity response. Furthermore, we showed, for the first time, that the 8-nitro-cGMP-induced enhancement of the contraction to Phe was not apparent in a db/db mouse aorta (Figure 4A), in which the contraction to Phe was enhanced (Figure 3A) and the endothelium-dependent relaxation to ACh was impaired (Figure 3B).
The spontaneously diabetic db/db mice used in the present study developed severe obesity, typically representing type 2 diabetes, which is associated with a substantially increased risk of cardiovascular disease. It has been demonstrated that Phe-induced contraction is enhanced and ACh-induced relaxation is inhibited in mesenteric artery from db/db mice, indicating impaired endothelial function (Pannirselvam et al., 2002). Furthermore, Pannirselvam et al. (2003) have suggested that the cellular basis of endothelial dysfunction in the db/db mice may be due to an increased production of superoxide and decreased availability of tetrahydrobiopterin resulting in the uncoupling of eNOS. In the present study, using the db/db mouse aorta with increased vasocontractility to Phe, we observed that the 8-nitro-cGMP-induced enhancement of the contraction to Phe was absent in these aortas (Figure 4A and C). In contrast, vascular responses to depolarization with KCl (50 mM) were similar in C57BL/6, db/+ and db/db mice aortas. Thus, it is unlikely that a generalized difference in vascular responsiveness or changes in calcium-activated contractile mechanisms are involved in the lack of an augmented Phe contractile response by 8-nitro-cGMP in db/db mice.
Furthermore we demonstrated, by use of Western blot analysis following low-temperature SDS-PAGE, that eNOS protein dimers can be disrupted, at least in part, by 8-nitro-cGMP in aortic segments from C57BL/6 mouse (Figure 2A). Additionally, an increase in superoxide production was detected in the 8-nitro-cGMP-treated aortic segments by use of DHE staining (Figure 2B). In contrast, 8-bromo-cGMP did not disrupt either the eNOS dimer or superoxide production in the C57BL/6 mouse aorta. These biological data support the idea that 8-nitro-cGMP may induce superoxide production via eNOS uncoupling. Thus, superoxide production by 8-nitro-cGMP may be involved in the enhancement of the Phe-induced contraction.
We have previously shown that NADPH oxidase-induced superoxide production in the db/db mice aortas is greater than those in db/+ mice (Dong et al., 2010). It has also been suggested that eNOS exists in an uncoupled state and the nitrotyrosine level is higher in db/db mice aortas (Moien-Afshari et al., 2008). Taken together, these results indicate that 8-nitro-cGMP does not enhance Phe-induced contractions in the db/db aortas because of a constitutively higher level of oxidative stress in these animals (Dong et al., 2010) and uncoupled eNOS protein.
To determine the involvement of superoxide production in the 8-nitro-cGMP-induced enhancement of contraction to Phe, we tested the effects of the superoxide scavengers, SOD and tiron, on the 8-nitro-cGMP-induced enhancement of the contraction. Without contraction by Phe, 8-nitro-cGMP did not affect the basal tone of each aorta from non-diabetic and diabetic mice. We also found that SOD and tiron reduced the 8-nitro-cGMP-induced enhancement of contraction to Phe in C57BL/6 and db/+ mice aortas (Figures 1B and 5B), suggesting the involvement of superoxide.
As shown in Figure 3A, the contraction to Phe was enhanced in the vessels from db/db mice with endothelial dysfunction. We have also previously found that contraction to Phe is significantly enhanced in the aortas from eNOS-deficient mice and in the l-NAME-treated aortas from C57BL/6 mice, suggesting that eNOS is involved in the relaxation induced by α1-adrenoceptor stimulation (Figure 7). Furthermore, in studies using α1-blockers in rat aortas, it has been shown that NO can be released through stimulation of α1-adrenoceptors on the endothelial cells and inhibits the contraction to noradrenaline (Kaneko and Sunano, 1993), and that α1-adrenoceptors on the endothelial cells regulate angiogenesis (Ciccarelli et al., 2008). As shown in Figure 7, an increase in intracellular Ca2+ via α1-adrenoceptor stimulation, possibly in the endothelial cells, leads to activation of eNOS, which in turn may be uncoupled by the application of 8-nitro-cGMP and produce superoxide anion as we have previously suggested (Sawa et al., 2007).
Figure 7.
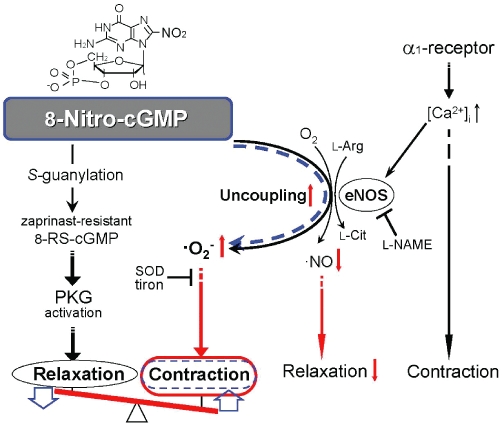
Hypothetical scheme for the mechanism that mediates the vascular tone induced by 8-nitro-cGMP. Stimulation of α1-adrenoceptors by Phe increases intracellular Ca2+ concentrations ([Ca2+]i), leading to contraction of vascular smooth muscle cells. In vascular endothelial cells, concomitantly, eNOS is activated by the increase in [Ca2+]i induced by α1-adrenoceptor stimulation to produce NO and induce vascular relaxation via PKG activation. In the non-diabetic mouse aortas, 8-nitro-cGMP applied extracellularly may induce uncoupling (black line) of the eNOS activated by the Phe-induced increase in [Ca2+]i, and may produce superoxide (·O2−), resulting in enhancement of the Phe-induced contraction. In addition, not only PKG activation via 8-nitro-cGMP itself, but also 8-RS-cGMP, which is formed by protein S-guanylation and is resistant to PDE5, may induce a potentially strong PKG activation and then vasorelaxation. The rank order of potency for the effect of 8-nitro-cGMP at lower concentrations up to 10 µM is contraction > relaxation. However, the rank order reverses at higher concentrations of 8-nitro-cGMP. In the diabetic mouse aorta with eNOS uncoupling (red arrow), the relaxation via NO generation is depressed (red arrow) and superoxide is produced (red arrow), and thus the amplitude of the contraction induced by Phe is increased (red oval and seesaw-like balance). 8-Nitro-cGMP, applied in the diabetic aorta, does not induce eNOS uncoupling (broken blue line) and thus does not enhance Phe-contraction (blue oval), but induces only relaxation via PKG activation. The relaxant effect of 8-nitro-cGMP on the enhancement of vasoconstriction might be beneficial in compensating for excess oxidative stress (blue arrows), such as endothelial dysfunction of diabetic mice.
8-Nitro-cGMP not only enhanced the contraction to Phe in db/+ mouse aortas but also induced relaxation in Phe-contracted aortas from both db/+ and db/db mice (Figure 4A). Compared with 8-bromo-cGMP (100 µM)-induced relaxation, 8-nitro-cGMP (100 µM) induced a more rapid (Figure 4B) and larger (Figure 6C) relaxation response in the Phe-contracted aortas from C57BL/6, db/+ and db/db mice. In a previous study, by use of Western blot analysis, it was demonstrated that both 8-nitro-cGMP and 8-bromo-cGMP have strong protein kinase G (PKG)-activating potential, as shown by vasodilator-stimulated phosphoprotein phosphorylation in human uterine smooth muscle cells, which was inhibited by a PKG-specific inhibitor Rp-8-CPT-cGMPS [8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphorothioate, Rp isomer] (Sawa et al., 2007). Phosphorylation of several target proteins by PKG in vascular smooth muscle cells leads to a decrease in cytosolic Ca2+ and phosphorylated myosin, resulting in vascular relaxation (Lincoln and Cornwell, 1993; Feil et al., 2003).
To address the potentially different relaxant effects of 8-nitro-cGMP and 8-bromo-cGMP, we compared their relaxation responses in the Phe-contracted aortas in the presence of a selective PDE5 inhibitor, zaprinast (Kukovetz et al., 1979; Komas et al., 1991; McMahon et al., 1993). We observed that zaprinast significantly potentiated the relaxant effect of 8-bromo-cGMP, but not that of 8-nitro-cGMP, in the Phe-contracted aortas from db/db mouse, in which the 8-nitro-cGMP-induced enhancement of contraction to Phe was absent (Figure 6). Previously, it has been demonstrated that 8-nitro-cGMP reacts readily with the nucleophilic cysteine sulphhydryls (Cys-SH) of intracellular proteins and peptides, such as glutathione, to form adducts, 8-thioalkoxy-cGMP (8-RS-cGMP), via so-called S-guanylation (Figure 7) (Sawa et al., 2003; 2007;). Because 8-RS-cGMP may still have PKG activity and be resistant to the effects of PDE5, the relaxant response to 8-nitro-cGMP might be more resilient than that to 8-bromo-cGMP and not affected by the PDE5 inhibitor. Furthermore, in in vitro experiments with the recombinant PKG protein, we have found that PKG is highly sensitive to kinase activation by 8-nitro-cGMP (unpublished data). On the other hand, in our previous study we found that the membrane permeability of 8-nitro-cGMP in cultured cells is much less than that of 8-bromo-cGMP (Sawa et al., 2007). Nevertheless, once 8-nitro-cGMP penetrates the cell membrane, intracellular 8-nitro-cGMP may have higher PKG activity than that of 8-bromo-cGMP for the reason described above.
Our data showing that 8-nitro-cGMP-induced relaxation in the Phe-contracted aorta from the non-diabetic mouse was enhanced in the presence of l-NAME (Figures 1A and 5A), are consistent with results from our previous study obtained in denuded rat carotid artery and eNOS-deficient mouse aorta (Sawa et al., 2007). Recently, it was shown that superoxide production via eNOS uncoupling in rat aortic rings exposed to endothelin-1 is reduced by eNOS inhibition with l-NAME treatment and by endothelium denudation (Romero et al., 2009). In our experiments with non-diabetic mice aortas (Figures 1A and 5A), inhibition of eNOS by l-NAME might lead to a reduction of superoxide generation via 8-nitro-cGMP-induced eNOS uncoupling, resulting in the suppression of 8-nitro-cGMP-induced contraction and thus an increase in relaxation. Whereas, in db/db mice aortas, with a constitutively high level of oxidative stress and eNOS protein in an uncoupled state (Moien-Afshari et al., 2008; Dong et al., 2010), as described above, 8-nitro-cGMP induced only a relaxation of the Phe-induced contraction, and l-NAME had no additive effect on the relaxation.
Furthermore, the 8-nitro-cGMP-induced relaxation was not inhibited by treatment with SOD or the non-catalytic tiron in the non-diabetic mice aortas (Figure 5B), but was decreased in the presence of a BK-type Ca2+-activated K+ channel blocker, iberiotoxin (100 nM), in the C57BL/6 mouse aortas. These results suggest the partial involvement of the BK-type Ca2+-activated K+ channels in the intracellular mechanism mediating relaxation; produced via 8-nitro-cGMP-induced activation of PKG.
Whether or to what extent changes in endogenous generation of 8-nitro-cGMP could contribute to impaired vascular reactivity in db/db mouse aorta still remain to be elucidated. Nevertheless, based on our present findings, 8-nitro-cGMP might be important not only in physiological functions but also in compensatory mechanisms for impaired vasodilatation induced by endothelial dysfunction, as depicted in Figure 7.
To summarize, we have demonstrated that 8-nitro-cGMP, applied acutely, induces bi-directional regulation of vascular responses, that is, enhancement of contraction and relaxation, in a dose-dependent manner in aortic rings from non-diabetic mice. In db/db mice with dysfunction of the vascular endothelial cells, only a vasorelaxation response that was resistant to a PDE5 inhibitor was induced by 8-nitro-cGMP. Therefore, the vasodilator effect of 8-nitro-cGMP might contribute to the amelioration of hypertension induced by vascular endothelial dysfunction, although further in vivo studies are needed to confirm this possibility. The effects of 8-nitro-cGMP might have important implications for therapeutic strategies aimed at restoring endothelial function.
Acknowledgments
This work was supported in part by Grants-in-Aid for scientific research from the Ministry of Education, Culture, Sports and Technology of Japan.
Glossary
Abbreviations
- eNOS
endothelial nitric oxide synthase
- l-NAME
Nω-nitro-l-arginine methyl ester
- 8-nitro-cGMP
8-nitroguanosine 3′,5′-cyclic monophosphate
- PDE
phosphodiesterase
- PKG
protein kinase G
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Conflict of interest
The authors have no conflicts of interest to declare.
Supporting Information
Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Ciccarelli M, Santulli G, Campanile A, Galasso G, Cervèro P, Altobelli GG, et al. Endothelial α1-adrenoceptors regulate neo-angiogenesis. Br J Pharmacol. 2008;153:936–946. doi: 10.1038/sj.bjp.0707637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis FR, Craven PA, Melhem MF, Salah EM. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004;53:762–768. doi: 10.2337/diabetes.53.3.762. [DOI] [PubMed] [Google Scholar]
- Dong YF, Liu L, Kataoka K, Nakamura T, Fukuda M, Tokutomi Y, et al. Aliskiren prevents cardiovascular complications and pancreatic injury in a mouse model of obesity and type 2 diabetes. Diabetologia. 2010;53:180–191. doi: 10.1007/s00125-009-1575-5. [DOI] [PubMed] [Google Scholar]
- Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res. 2003;93:907–916. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Sunano S. Involvement of alpha-adrenoceptors in the endothelium-dependent depression of noradrenaline-induced contraction in rat aorta. Eur J Pharmacol. 1993;240:195–200. doi: 10.1016/0014-2999(93)90898-r. [DOI] [PubMed] [Google Scholar]
- Komas N, Lugnier C, Stoclet JC. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br J Pharmacol. 1991;104:495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukovetz WR, Holzmann S, Wurm A, Poch G. Evidence for cyclic GMP-mediated relaxant effects of nitro-compounds in coronary smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:129–138. doi: 10.1007/BF00500277. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- McMahon TJ, Ignarro LJ, Kadowitz PJ. Influence of Zaprinast on vascular tone and vasodilator responses in the cat pulmonary vascular bed. J Appl Physiol. 1993;74:1704–1711. doi: 10.1152/jappl.1993.74.4.1704. [DOI] [PubMed] [Google Scholar]
- Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- Moien-Afshari F, Ghosh S, Elmi S, Rahman MM, Sallam N, Khazaei M, et al. Exercise restores endothelial function independently of weight loss or hyperglycaemic status in db/db mice. Diabetologia. 2008;51:1327–1337. doi: 10.1007/s00125-008-0996-x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Kataoka K, Fukuda M, Nako H, Tokutomi Y, Dong YF, et al. Critical role of apoptosis signal-regulating kinase 1 in aldosterone/salt-induced cardiac inflammation and fibrosis. Hypertension. 2009;54:544–551. doi: 10.1161/HYPERTENSIONAHA.109.135392. [DOI] [PubMed] [Google Scholar]
- Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol. 2002;136:255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol. 2003;140:701–706. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M, Jiménez R, Sánchez M, López-Sepúlveda R, Zarzuelo MJ, O'Valle F, et al. Quercetin inhibits vascular superoxide production induced by endothelin-1: role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis. 2009;202:58–67. doi: 10.1016/j.atherosclerosis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Sawa T, Akaike T, Ichimori K, Akuta T, Kaneko K, Nakayama H, et al. Superoxide generation mediated by 8-nitroguanosine, a highly redox-active nucleic acid derivative. Biochem Biophys Res Commun. 2003;311:300–306. doi: 10.1016/j.bbrc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Sawa T, Zaki MH, Okamoto T, Akuta T, Tokutomi Y, Kim-Mitsuyama S, et al. Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat Chem Biol. 2007;3:727–735. doi: 10.1038/nchembio.2007.33. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EHC, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto E, Kataoka K, Shintaku H, Yamashita T, Tokutomi Y, Dong YF, et al. Novel mechanism and role of angiotensin II induced vascular endothelial injury in hypertensive diastolic heart failure. Arterioscler Thromb Vasc Biol. 2007a;27:2569–2575. doi: 10.1161/ATVBAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- Yamamoto E, Yamashita T, Tanaka T, Kataoka K, Tokutomi Y, Lai ZF, et al. Pravastatin enhances beneficial effects of olmesartan on vascular injury of salt-sensitive hypertensive rats, via pleiotropic effects. Arterioscler Thromb Vasc Biol. 2007b;27:556–563. doi: 10.1161/01.ATV.0000254855.24394.f9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


