Abstract
The pro-inflammatory cytokine IL-1β has been shown to promote angiogenesis. It can have a neurotoxic or neuroprotective effect. Here, we have studied the expression of IL-1β in vivo and the effect of the IL-1 receptor antagonist on choroidal neovascularization (CNV) and retinal degeneration (RD). IL-1β expression significantly increased after laser injury (real time PCR) in C57BL/6 mice, in the C57BL/6 Cx3cr1−/− model of age-related macular degeneration (enzyme-linked immunoabsorbent assay), and in albino Wistar rats and albino BALB Cx3cr1+/+ and Cx3cr1−/− mice (enzyme-linked immunoabsorbent assay) after light injury. IL-1β was localized to Ly6G-positive, Iba1-negative infiltrating neutrophils in laser-induced CNV as determined by IHC. IL-1 receptor antagonist treatment significantly inhibited CNV but did not affect Iba1-positive macrophage recruitment to the injury site. IL-1β significantly increased endothelial cell outgrowth in aortic ring assay independently of vascular endothelial growth factor, suggesting a direct effect of IL-1β on choroidal endothelial cell proliferation. Inhibition of IL-1β in light- and laser-induced RD models did not alter photoreceptor degeneration in Wistar rats, C57BL/6 mice, or RD-prone Cx3cr1−/− mice. Our results suggest that IL-1β inhibition might represent a valuable and safe alternative to inhibition of vascular endothelial growth factor in the control of CNV in the context of concomitant photoreceptor degeneration as observed in age-related macular degeneration.
The IL-1 family of cytokines plays a key role in the initiation of acute inflammatory responses.1 IL-1β interacts with IL-1 receptor I (IL-1RI), composed of IL-1R and IL-1R accessory protein subunits. IL-1 receptor antagonist (IL-1Ra) competes with IL-1β for its binding site.1 IL-1β is a potent inflammatory mediator with chemotactic2 and angiogenic3,4 properties. It is a neurotoxic mediator in ischemic brain injury5 but can attenuate glutamate neurotoxicity in the retina6 and protect against light-induced or hereditary photoreceptor degeneration.7,8
Age-related macular degeneration (AMD) is the leading cause of vision loss in elderly persons in industrialized countries.9 Its most prominent pathologic features are photoreceptor degeneration and choroidal neovascularization (CNV).10 In AMD, IL-1β is secreted by retinal pigment epithelium (RPE) cells and CD68+ cells in choroidal neovascular membranes11 and is therefore a possible pro-angiogenic and neuroprotective or neurotoxic mediator in AMD.
IL-1Ra is clinically used to treat juvenile idiopathic arthritis12; it has been shown to inhibit neurotoxicity in ischemia in animal models.13 Intravitreal human IL-1Ra injections have been shown to efficiently inhibit CNV in a rat model.14 To analyze the possible implication of IL-1β in CNV and retinal degeneration, we studied the expression of IL-1β in laser-induced CNV in mice and in light-induced retinal degeneration in rats and mice. We localized IL-1β expression by IHC and inhibited IL-1β activity with IL-1Ra supplementation.
Materials and Methods
Animals
Ten-week-old Wistar rats and C57B6j wild-type mice were purchased from the Janvier Breeding Center (Le Genest-St-Isle, France). Cx3cr1−/− C57B6j mice were backcrossed for six generations into the BALB/c background (Janvier Breeding Center) to obtain the Cx3cr1−/− BALB/c strain and kept in specific pathogen-free conditions with food and water available ad libitum and housed in a 12/12 hours light/dark (100 to 500 lux) cycle. Animal experiments were approved by the Institutional Animal Care and Use Committee.
Laser Coagulation
Ten-week-old C57BL/6 mice were anesthetized with an intramuscular injection of ketamine (50 mg/kg) and xylazine (10 mg/kg). Their pupils were fully dilated with 1% tropicamide. Coverslips positioned on the mouse cornea were used as a contact glass. Four laser coagulations were performed four to five disk diameters away from the papillae with an Argon laser (532 nm) mounted on a slit lamp (400 mW, 50 milliseconds, and 50 μm). Mice were treated with daily subcutaneous injections of PBS or human recombinant IL-1Ra (Kineret; Biovitrum, Stockholm, Sweden) at 1 mg/d/kg until sacrifice.
Choroidal Flatmounts, IHC, and CNV Quantifications
Eyes were enucleated, fixed in 4% paraformaldehyde for 15 minutes at room temperature and sectioned at the limbus; the cornea and lens were discarded (for CNV quantification, mice were perfused with fluorescein dextran 106 before enucleation). Retinal and RPE/choroidal flatmounts were stained according to previously described standard immunohistochemical procedures.15 The primary antibodies and lectins used were Bandeirae simplicifolia lectin (Sigma-Aldrich, Saint Quentin Fallavier, France), goat anti-mouse IL-1β (R&D Systems, Lille, France), rabbit polyclonal anti–IL-1RI (Santa Cruz Biotechnology Inc., Heidelberg, Germany), rabbit polyclonal anti-Iba1 (Wako, Neuss, Germany), rat anti-mouse Ly6G (Miltenyi Biotec, Paris, France), and goat anti-human Collagen IV (R&D Systems). The corresponding Alexa secondary antibodies (Molecular Probes, Leiden, The Netherlands) were used to show the primary antibodies, and flatmounts were counterstained with DAPI. The choroids and retinas were radially incised, flatmounted, and viewed with the same fluorescence microscope. Flatmounts were viewed with a fluorescence microscope (DM5500B; Leica, Nanterre, France). All immunostaining was repeated at least three times, and stains omitting the primary antibody served as negative controls. Ly6G-positive and Iba1-positive cells were counted on stained whole RPE/choroidal flatmounts up to the ciliary body and on the outer segment side of the retina. The surface covered by perfused CNV was measured on photographs of fluorescein dextran 106 perfused choroidal flatmounts with ImageJ analysis software (surface covered by fluorescein staining). The average CNV size was calculated per eye.
Microvascular Sprouting from Aortic Explants
Aortae from adult C57BL/6 were cut into 1-mm-thick rings and covered with 30 μL of Matrigel (BD Biosciences, Le Pont de Claix, France) in 24-well tissue cultures plates. Aortic rings were cultured for 3 days in Dulbecco's Modified Eagle's Medium (Invitrogen, Cergy Pontoise, France) containing 10% fetal calf serum, 1% penicillin/streptomycin, and 0.2% fungizone. Explants were exposed to IL-1β (5 ng/mL; R&D Systems), IL-1Ra (10 mg/mL), and soluble vascular endothelial growth factor receptor 1 (VEGFR1s; 150 ng/mL; R&D Systems) from day 3 to day 7 of culture. Photographs of individual explants were taken every day, and the surface covered by the aortic ring and the vascular sprouts was measured daily from day 3 to day 7. The surface of each individual aortic ring and pre-incubation sprouts at day 3 was subtracted from the surface at day 4 to calculate the vascular sprouting that occurred in the presence of the ligands and controls.
Reverse Transcription and Real-Time PCR
Total RNA was isolated with NucleoSpin RNA II Kit (Macherey-Nagel, Hoerdt, France). Single-stranded cDNA was synthesized from total RNA (pretreated with DNaseI amplification grade) with the use of oligo(dT) as primer and superscript reverse transcriptase (Invitrogen, Cergy Pontoise, France). Subsequent real-time PCR was performed with the use of cDNA, qPCR SuperMix-UDG Platinum SYBR Green (Invitrogen), and the following primers (0.5 pmol/μL): mm (mouse) actin sense: 5′-AAGGCCAACCGTGAAAAGAT-3′; mm actin antisense: 5′-GTGGTACGACCAGAGGCATAC-3′; mm IL-1β sense: 5′-CATGGAATCCGTGTCTTCCT-3′; mm IL-1β antisense: 5′-GAGCTGTCTGCTCATTCACG-3′; mm VEGF sense: 5′-GTGAGCCAGGCTGCAGGAAG-3′; mm VEGF antisense: 5′-GAATGCGTCTGCCGGAGTCT-3′; rn (rat) actin sense: 5′-AAAGAAAGGGTGTAAAACGCAG-3′; rn actin antisense: 5′-AAAGACCTCTATGCCAACACAG-3′; rn IL-1β sense: 5′-GGAACCCGTGTCTTCCTAAA-3′; and rn IL-1β antisense: 5′-CTGACTTGGCAGAGGACAAA-3′.
PCR reactions were performed in 40 cycles of 15 seconds at 95°C, 45 seconds at 60°C. Product was not generated in control reactions in which reverse transcriptase was omitted during cDNA synthesis.
Light-Induced Degeneration
Ten-week-old rats and 8-week-old Cx3cr1−/− mice were adapted to darkness for 12 hours, and pupils were fully dilated with 1% atropin (Novartis, Rueil Malmaison, France). Animals were then exposed to green LED light (4500 lux) for 12 hours and subsequently kept in cyclic 12 hours/12 hours normal animal facility conditions. Control and light-exposed rats and mice were treated with daily subcutaneous injections of PBS or IL-1Ra (Kineret; Biovitrum) at 1 mg/d/kg until sacrifice and a volume of 5 μL (rats) or 2 μL (mice) of IL-1Ra at 150 mg/mL was injected intravitreally at day 0 and day 3 after illumination.
Protein Analysis
Eyes were enucleated and sectioned at the limbus, and the cornea and lens were discarded. The complex retina/RPE/choroid/sclera was placed in 150 μL of PBS 1× supplemented with a protease inhibitor cocktail (Calbiochem, Fontenay-sous-Bois, France) followed by homogenization with a plastic pestle. The lysate were cleared of debris by centrifugation at 2000 rcf for 10 minutes at 4°C. Total protein content of supernatant was determined by commercial assay (Bradford kit, Eragny-sur-Oise, France). Supernatant IL-1β level was determined with a sandwich enzyme-linked immunoabsorbent assay, according to the manufacturer's instructions (R&D Systems) and normalized for total protein.
Histology
For histology, eyes were fixed in 0.5% glutaraldehyde, 4% paraformaldehyde PBS for 2 hours, dehydrated, and mounted in HistoResin. Oriented sections (5 μm), crossing the center of the laser injury (CNV model) or inferior pole, optic nerve, and superior pole (light-induced model), were cut and stained with toluidine blue. Rows of nuclei in the outer nuclear layer were counted at different distances from the injury site (CNV model) or optic nerve (light-induced model).
Statistical Analysis
GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA) was used for data analysis and graphic representation. All values are reported as mean ± SEM. Statistical analysis was performed by one-way and two-way analysis of variance followed by post hoc Bonferroni's test for comparison among means. P < 0.05 was considered statistically significant.
Results
IL-1β Is Induced in Laser-Induced Neovascularization and Localizes to the Injury Site
IL-1β is known for its pro-angiogenic properties.3,4 It is secreted by CD68+ cells in choroidal neovascular membranes in AMD.11 Its inhibition has been shown to prevent laser-induced subretinal neovascularization in rats, but its induction, expression pattern, and effect on inflammation in the laser model has not been analyzed.14,16 We first analyzed IL-1β expression at different time points in laser-induced CNV in the retina (Figure 1A) and choroid (Figure 1B) of C57BL/6 mice. A strong induction of IL-1β mRNA is observed as early as 1 hour in the retina (55-fold) and at 6 hours (35-fold) and 20 hours (18-fold) in the choroid. To a lesser extent, a tendency for vascular endothelial growth factor (VEGF) mRNA induction was detected in the retina at 1 hour (Figure 1C) but not in the choroid (Figure 1D; control, n = 4; 1 hour, n = 8; 6 hours, n = 7; 20 hours, n = 5; 72 hours, n = 4 retinas/choroids). To identify IL-1β–expressing cells, we performed IHC of IL-1β (green staining; Figure 1, E, I, and M), the neutrophil marker Ly6G (Figure 1, F, J, and N), and the monocyte/macrophage marker Iba1 (Figure 1, G, K, and O) on choroidal/RPE flatmounts of uninjured control eyes (Figure 1, E–H) and 10 hours (Figure 1, I–L) and 72 hours (Figure 1, M–P) after laser impact. Uninjured control eyes did not stain positive for IL-1β, Ly6G, or Iba1 (Figure 1, E–H). At 10 hours after the injury small IL-1β–positive cells are visible at the site of effect (Figure 1I) (n = 4 eyes). Triple labeling at 10 hours with the use of the neutrophil marker Ly6G (red fluorescence; Figure 1J) and the monocyte/macrophage marker Iba1 (blue fluorescence; Figure 1K) shows that the IL-1β–positive cells are Ly6G positive and Iba1 negative (Figure 1L merge). Most of the detected IL-1β–positive cells were Ly6G positive. Seventy-two hours after the impact IL-1β (Figure 1M) and Ly6G (Figure 1N) were faint and diffuse, whereas numerous Iba1-positive cells were detected at the injury site (blue staining; Figure 1, O and P merge). IHC at 10 hours and 72 hours, omitting the primary antibodies, resulted in no staining (data not shown) (n = 4 eyes per time point).
Figure 1.
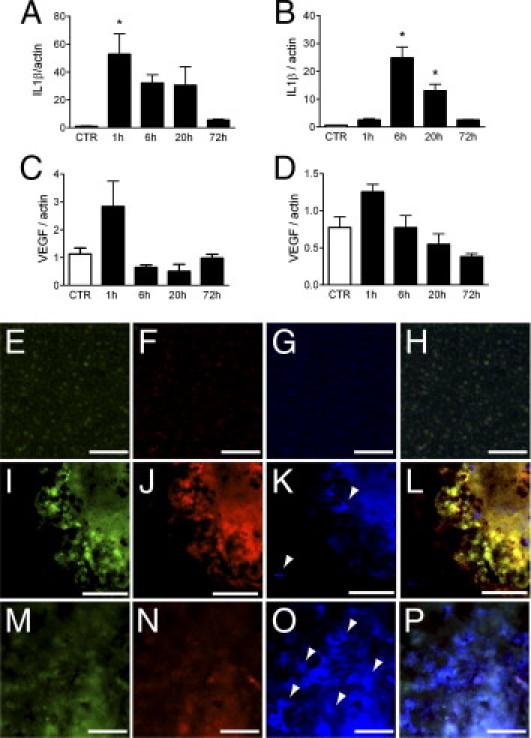
IL-1β expression in laser-induced neovascularization. IL-1β real-time PCR in retina (A) and choroid (B) and VEGF real-time PCR in retina (C) and choroid (D) at different time points in laser-induced CNV in the eye cup of C57BL/6 mice (control n = 4 eyes, 1 hour n = 8 eyes, 6 hours n = 7 eyes, 20 hours n = 5 eyes, 72 hours n = 4 eyes; one-way analysis of variance *P = 0.03 (A and B). Representative micrographs of IHC of uninjured (E–H), 10 hours (I–L), and 72 hours (M–P) of laser-injured choroid/RPE flatmounts (n = 4 mice per time point). IL-1β staining (green staining E, I, and M), Ly6G staining (red staining F, J, and N), Iba1 staining (blue staining G, K, and O) merged photographs (H, L, and P). Scale bars = 50 μm (E–P). Arrowheads: Iba1-positive cells (K and O).
IL-1Ra Inhibits CNV Independently of Macrophage Recruitment
To evaluate the effect of IL-1β induction on CNV we treated one group of experimental animals with subcutaneous saline injections (n = 4 mice) and one group with subcutaneous injections of 1 mg/kg/day recombinant human IL-1Ra (n = 5 mice). At day 14 after laser injury the CNV was visualized on isolectin-stained (red staining), fluorescein dextran perfused (green/yellow staining) RPE/choroidal flatmounts of saline- (Figure 2A) and IL-1Ra–treated mice (Figure 2B). Mature, perfused CNV (presenting a tubular structure with a lumen) was quantified as fluorescein-positive surface (Figure 2C). IL-1Ra–treated animals showed a 60% reduction in CNV. Initial lesion size at 10 hours after laser impact, identified by the loss of the RPE cell layer, was quantified on choroidal/RPE flatmounts of saline- and IL-1Ra–treated mice and showed no significant difference (Figure 2D), excluding a possible initial difference of the laser injury in the two groups.
Figure 2.
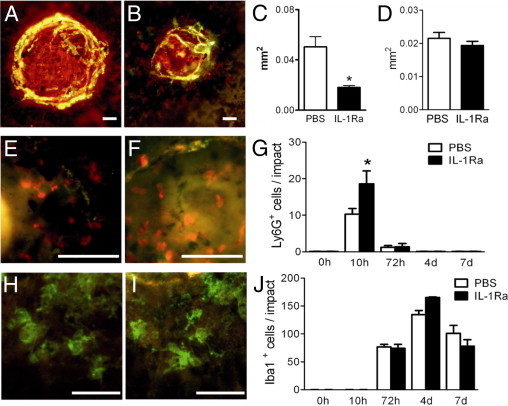
IL-1Ra inhibits CNV independently of macrophage recruitment. Photographs of isolectin (red) stained and fluorescein dextran perfused (green) RPE/choroidal flatmounts of PBS-treated (A; n = 4) and IL-1Ra–treated (B; n = 5) C57BL/6 mice 14 days after laser injury. Quantification of fluorescein-positive area (C; Mann-Whitney *P < 0.0001). Quantification of RPE lesion size of PBS- (n = 4 mice) and IL-1Ra– (n = 4 mice) treated mice at 10 hours after laser impact (D). Representative photographs of Ly6G- (red) and Iba1- (green) stained RPE/choroidal flatmounts of the border of the injured area of saline- (E and H; n = 3 mice/group) and IL-1Ra–treated (F and I; n = 3 mice/group) mice 10 hours (E and F) and 72 hours (H and I) after laser injury. Quantification of Ly6G- (G) and Iba1- (J) positive cells/impact at different time points (F; Mann-Whitney *P = 0.14). Scale bars = 50 μm (A, B, E, F, H, and I).
IL-1β has chemotactic properties and can alter macrophage recruitment to the granulomateous tissue that forms after injury.2 It might thereby indirectly influence angiogenesis. To test this hypothesis, we visualized neutrophils and macrophages/microglial cells 10 hours (Figure 2, E and F) and 72 hours (Figure 2, H and I) after laser injury, on Ly6G (red staining) and Iba1 (green staining) stained RPE/choroidal flatmounts of saline- (Figure 2, E and H; n = 3 mice) and IL-1Ra–treated (Figure 2, F and I; n = 3 mice) mice. Neutrophil (Figure 2G) and macrophage/microglial cell (Figure 2J) prevalence were quantified. Ly6G-positive neutrophils were significantly recruited to the injury site, reaching their maximum at 10 hours. Neutrophil recruitment was stronger in IL-1Ra–treated animals (Figure 2G; n = 3 to 4 mice per time point). IL-1Ra treatment did not alter Iba1-positive macrophage/microglial cell recruitment, which reached the maximum in both saline- and IL-1Ra–treated groups at 4 days (Figure 2J).
IL-1β Induces Vascular Sprouting in Aortic Rings
IL-1β exerts its activity by acting on the IL-1RI.1 It induces angiogenesis in the cornea in vivo,3 IL-6 expression,17 and microparticle release from vascular endothelial cells in vitro.18 IL-1RI is expressed on numerous cell types; notably, it is constitutively expressed on vascular endothelium.17 IL-1RI IHC on choroidal/RPE flatmounts of C57BL/6 mice 5 days after the injury shows IL-1RI–positive staining around the lesion site (Figure 3A) (n = 3 mice). Double labeling with the use of the vascular endothelial cell marker collagen IV (red fluorescence; Figure 3B) shows that IL-1RI is expressed in the vascular endothelium surrounding the lesion and in some unidentified cells (Figure 3C merge).
Figure 3.
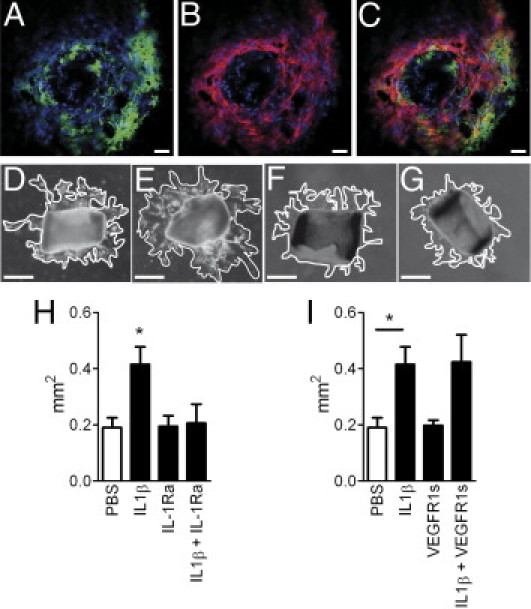
IL-1β induces vascular sprouting in aortic rings. Confocal microscopic image of IL-1RI (green staining A) and Collagen IV IHC (red staining B, merge C) of the laser-injury site at day 5 (n = 3 mice) of C57BL/6 mice. Photographs of aortic rings at day 4, 24 hours after incubation with control (D), IL-1β (E), IL-1Ra (F), or IL-1β and IL-1Ra (G). H: Measurements of the area of vascular sprouting in the presence of control (n = 6), IL-1β (n = 12), IL-1Ra (n = 6), or IL-1β and IL-1Ra (n = 6). I: Measurements of the area of vascular sprouting in the presence of control (n = 6), IL-1β (n = 12), soluble VEGFR1 (n = 5) or IL-1β and soluble VEGFR1 (n = 6). *P < 0.05 one-way analysis of variance. Scale bars: (A–C) = 50 μm; (D–G) = 350 μm.
To test the effect of IL-1β on vascular sprouting we used the aortic ring assay. Photographs at 4 days and 24 hours after ligand incubation show the vascular sprouting of PBS (Figure 3D), IL-1β (Figure 3E), IL-1Ra (Figure 3F), or IL-1β and IL-1Ra incubated aortic rings (Figure 3G). Measurements of the area covered by the vascular sprouts showed a significant increase of growth in IL-1β–exposed aortic rings after 24 hours of IL-1β exposure at 4 days that was inhibited by simultaneous IL-1Ra incubation (Figure 3H). Vascular sprouting remained significantly increased up to 6 days (72 hours of ligand exposure) before aortic rings started dying (data not shown). Simultaneous incubation of IL-1β with the soluble VEGFR1 did not significantly inhibit the effect of IL-1β incubation alone (Figure 3I).
IL-1Ra Does Not Affect Photoreceptor Survival of Laser- and Light-Induced Injury
In AMD, CNV develops in the context of photoreceptor degeneration. IL-1β supplementation has been shown to inhibit photoreceptor degeneration in rat models of light-induced degeneration7 and to protect photoreceptor degeneration observed in Royal College of Surgeons rats.8 IL-1β inhibition might have a deleterious effect on retinal degeneration. Laser injury used to induce CNV also induces a local inflammatory reaction and retinal degeneration in the vicinity of the laser impact. To evaluate whether IL-1Ra influences the laser-induced photoreceptor degeneration, we treated laser-injured C57BL/6 mice with daily subcutaneous injections of saline (n = 3 mice) or IL-1Ra (n = 4 mice) and prepared histologic sections crossing the lesion site at day 14 (Figure 4, A and B, respectively). Quantification of rows of nuclei at increasing distances from the injury site showed no difference in photoreceptor cell loss in the vicinity of the laser impact (Figure 4C).
Figure 4.
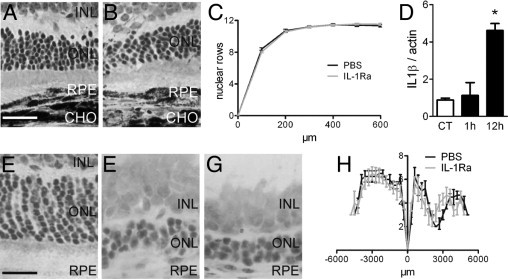
IL-1 and light- and laser-induced injury. Histology of the photoreceptor cell layer at 100 μm of the laser injury in PBS- (A; n = 3 mice) and IL-1R– (B; n = 4 mice) treated C57BL/6 mice. Quantification of number of rows of photoreceptor nuclei at increasing distance from the injury site in PBS- and IL-1Ra–treated animals (C). Real-time PCR of IL-1β expression in Wistar eye cup mRNA extracts (D; control n = 4, 1 hour n = 5, 12 hours n = 5). Normal light-raised Wistar rats (E); light-injured PBS-treated (F; n = 4) and light-injured IL-1Ra–treated (G; n = 6) eyes. Quantification of number of rows of photoreceptor nuclei at different distances from the optic nerve measured on oriented sections, crossing the inferior pole, optic nerve, and superior pole (H). *P = 0.0003. Scale bars: A and B = 50 μm; E–G = 20 μm. ONL, outer nuclear layer; INL, inner nuclear layer; RPE, retinal pigment epithelium; CHO, choroid; CT, control.
To analyze the effect of IL-1β inhibition in a non-neovascular context, we exposed Wistar rats to light-induced retinal degeneration and analyzed IL-1β expression by real-time PCR in eye cups (Figure 4D; control, n = 4 eyes; 1 hour, n = 5 eyes; 12 hours, n = 5 eyes). IL-1β transcript was significantly induced (five times) in the eye at 12 hours after the onset of light-induced stress. To evaluate the influence of the endogenously produced IL-1β on photoreceptor survival we injected Wistar rats with either PBS (n = 4 rats) or IL-1Ra (n = 6 rats) (subcutaneously 1 mg/kg/day and intravitrealy and 5 μL of a 150 mg/mL solution at day 1 and day 3 to ensure penetration of a possibly intact blood retinal barrier in the eye). Control animals that were raised under normal light conditions showed no signs of toxicity at this dosage (data not shown). At 7 days, histology of rats treated with control (Figure 4E), with light-exposed PBS (Figure 4F), and light-exposed IL-1Ra (Figure 4G) showed significant thinning of the outer nuclear layer in both groups. Quantification of rows of nuclei at different distances from the optic nerve measured from the inferior pole to the superior pole showed no differences in photoreceptor cell loss throughout the retina (Figure 4H).
IL-1Ra Does Not Affect Photoreceptor Survival in Degeneration-Prone Cx3cr1−/− Mice
We have previously shown, that the T280M polymorphism of CX3CR1 leads to dysfunctional CX3CR1 and is associated with AMD. Furthermore, Cx3cr1−/− mice develop spontaneous photoreceptor degeneration by the age of 18 months and exaggerated photoreceptor degeneration in light-induced models.19,20 Here, we analyzed the expression and effect of IL-1β in the Cx3cr1−/− model of AMD. First, we analyzed IL-1β expression in C57BL/6 Cx3cr1+/+ and C57BL/6 Cx3cr1−/− mice at 3 and 18 months of age (Figure 5A; n = 4 eyes/group). IL-1β protein was significantly increased in 18-month-old C57BL/6 Cx3cr1−/− mice compared with Cx3cr1+/+ mice. Similarly, 4500 lux light-exposed BALB Cx3cr1+/+ and BALB Cx3cr1−/− animals showed a significant increase of IL-1β protein 24 hours after onset of the light injury compared with normal light-raised Cx3cr1+/+ and Cx3cr1−/− mice (Figure 5B; n = 4 eyes/group). Interestingly, the basal level of IL-1β expression was more important in young C57BL/6 mice than in BALB mice. The increase of IL-1β protein levels in light-induced injury in BALB Cx3cr1+/+ and Cx3cr1−/− mice was not different from each other and comparable to 18-month-old C57BL/6 Cx3cr1−/− mice. To evaluate if IL-1β plays a role in photoreceptor degeneration in the context of CX3CR1 deficiency, we exposed 2-month-old BALB Cx3cr1−/− mice before significant spontaneous degeneration was observed to light-induced degeneration19 (Figure 5C). PBS (n = 4 mice) or IL-1Ra (n = 6 mice) were injected subcutaneously at 1 mg/kg/d and intravitrealy (2 μL of a 150-mg/mL solution) at day 1 and day 3 to ensure penetration of a possibly intact blood retinal barrier in the eye. Saline-treated controls (n = 6 mice; Figure 5D) degenerated similarly to IL-1Ra–treated Cx3cr1−/− mice (Figure 5E; n = 6 mice), and no significant difference could be observed throughout the retina (Figure 5F). Control Cx3cr1−/− animals that were raised under normal light conditions and treated with intravitreal and subcutaneous PBS or IL-1Ra showed no signs of toxicity at this dosage (Figure 5G).
Figure 5.
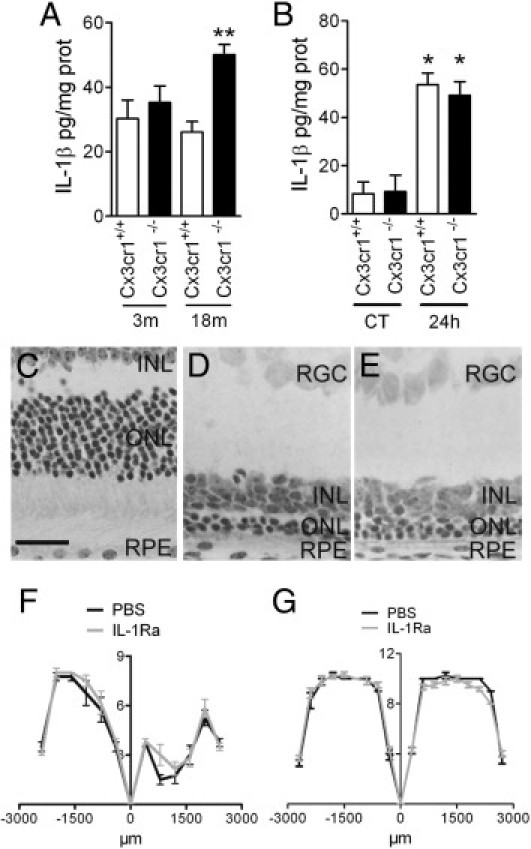
IL-1β and Cx3cr1−/− AMD model and light-induced injury Cx3cr1−/−mice. IL-1β enzyme-linked immunoabsorbent assay of eye cups of 3- and 18-month-old C57BL/6 Cx3cr1+/+ and C57BL/6 Cx3cr1−/− animals (A; n = 5 to 6). IL-1β enzyme-linked immunoabsorbent assay of eye cups of control and light-exposed 8-week-old BALB Cx3cr1+/+ (wild-type) and BALB Cx3cr1−/− eye cups (B; n = 4 eyes per group). Normal light-raised BALB Cx3cr1−/− (C); light-injured PBS-treated BALB Cx3cr1−/− (D; n = 4), and light-injured IL-1Ra–treated BALB Cx3cr1−/− (E; n = 6) eyes. Quantification of number of rows of photoreceptor nuclei at different distances from the optic nerve on oriented sections of PBS- and IL-treated light-injured 2-month-old BALB Cx3cr1−/− mice (F). Quantification of number of rows of photoreceptor nuclei at different distances from the optic nerve on oriented sections of PBS- and IL-1Ra–treated control BALB Cx3cr1−/− mice (G). *P < 0.001; **P = 0.0055. Scale bar = 25 μm. CT, control; ONL, outer nuclear layer; INL, inner nuclear layer; RPE, retinal pigment epithelium; RGC, retinal ganglion cells.
Discussion
We demonstrate that IL-1β expression rapidly increases in laser- and light-induced injury. The expression in laser-induced injury shows rapid induction that lasts for approximately 2 days (Figure 1). IHC at 10 hours localizes IL-1β mainly to infiltrating neutrophils, as shown by a positive Ly6G co-staining (specific for neutrophils and not expressed on monocytes21) and negative Iba1 co-staining (specifically expressed on macrophages and microglial cells22) (Figure 1). The identity of IL-1β–expressing cells was further confirmed by their multilobulated nuclei (data not shown) and is corroborated by the IL-1β expression kinetics that coincide with early neutrophil recruitment in CNV.23 Most IL-1β–expressing cells in our experiments were Ly6G-positive neutrophils, and the contribution of Iba1-positive macrophages/microglia was negligible. Nevertheless, occasional IL-1β staining was also detected in RPE cells adjacent to the laser injury. Activated polymorphonuclear neutrophils have been shown to express IL-1β,24 and our data show that these cells are the predominant source of IL-1β after laser injury in the mouse. IL-1β is expressed in CD68+ cells in human neovascular membranes that were interpreted to be macrophages.11 Although macrophages can express CD68, it has been shown that the main CD68-expressing cells in the injured central nervous system are neutrophils.25 It is possible that neutrophils participate in IL-1β in AMD. Studies that use more specific markers of neutrophils and macrophages are needed to determine the cell types expressing IL-1β in the human disease.
IL-1β inhibition by recombinant IL-1Ra significantly reduced the development of subretinal neovascularization in the mouse model of laser-induced subretinal neovascularization (Figure 2). Our results corroborate an earlier report that IL-1Ra inhibits neovascularization in a rat model.14,16
Neutrophils and macrophages promote CNV23,26–28; IL-1Ra has been shown to inhibit macrophage recruitment to the atherosclerotic plaque in a mouse model.2 We therefore tested the influence of IL-1Ra treatment on neutrophil and macrophage recruitment to the laser-injury site as a possible mechanism of CNV inhibition (Figure 2). Interestingly, IL-1Ra treatment significantly increased the number of Ly6G-positive neutrophils at 10 hours, suggesting a possible IL-1β–dependent negative feedback in neutrophil recruitment. No inhibition of macrophage/microglial cell recruitment was observed in IL-1Ra–treated mice after laser injury. Therefore, IL-1Ra–dependent inhibition of CNV is unlikely to be mediated by an inhibitory effect on the macrophage recruitment.
IL-1β exerts its activity by acting on the IL-1RI.1 Vascular endothelium, which constitutively expresses IL-1RI,17 releases IL-617 and microparticles18 on IL-1β stimulation. We show that collagen IV–expressing vascular endothelial cells in CNV express IL-1RI in vivo (Figure 3). To evaluate a direct effect of IL-1β on vascular sprouting independently of inflammatory recruitment, we submitted aortic rings to IL-1β in vitro (Figure 3). We show that IL-1β significantly induces vascular sprouting of aortic rings after 24 hours of IL-1β exposure. This difference was maintained for up to 72 hours of IL-1β incubation. A previous study has shown an inhibitory effect of IL-1β on vascular sprouting in the aortic ring assay at 10 days.29 In our study, aortic rings became unviable after 7 days in culture, and we could therefore not corroborate or contradict these findings. IL-1β has been suggested to induce angiogenesis via a sustained VEGF induction in fibroblasts.30 In our in vivo experiments we did not detect a sustained VEGF induction after the IL-1β induction in laser-injured eyes. Furthermore, vascular sprouting of aortic rings simultaneously incubated with IL-1β and the soluble VEGFR1 did not differ from rings incubated only with IL-1β, suggesting that the pro-angiogenic effect is independent of VEGF. Previous studies have shown that IL-1β directly induces IL-617 and microparticle release from the vascular endothelium31 and that IL-1β inhibitor CK112 and CK116 inhibit human umbilical vein endothelial cell proliferation.16 The pro-angiogenic effect of IL-1β we observed in vivo and in vitro might therefore be a direct effect of endothelial cell proliferation.
In AMD, subretinal neovascularization occurs simultaneously with retinal degeneration. To make anti–IL-1β therapy a viable option, the influence of IL-1β on photoreceptors has to be evaluated in vivo. Previous reports have shown that IL-1β develops neurotoxic properties in the brain.5,13,32 However, in the retina IL-1β was shown to inhibit ganglion cell death,6 and IL-1β substitution saved photoreceptors in light-induced models7 and genetic retinal degeneration.8 Here, we show that IL-1β is significantly induced in the light-induced model in albino rats (Figure 4D) and in albino mice (Figure 5B). To test the effect of IL-1β inhibition on retinal degeneration, we treated the laser-injured C57BL/6 mice and light-exposed rats with IL-1Ra at dosages that were sufficient to significantly inhibit CNV in mice (see Figure 2) and rats.14 Interestingly, IL-1Ra at a dose that significantly inhibits CNV does not exacerbate photoreceptor degeneration in vivo (Figure 4).
We have previously shown that a dysfunctional CX3CR1 is associated with AMD. Cx3cr1−/− mice develop spontaneous subretinal macrophage accumulation that is associated with photoreceptor degeneration by the age of 18 months.19 Similarly, these mice develop exaggerated photoreceptor degeneration in light-induced models.20 We analyzed the expression and effect of IL-1β in the Cx3cr1−/− model of AMD. IL-1β significantly increases with age in Cx3cr1−/− animals. This increase coincides with the observed subretinal inflammation in these animals19 and might reflect IL-1β produced by subretinal inflammatory cells. In light-induced injury to BALB Cx3cr1+/+ and Cx3cr1−/− mice, IL-1β was rapidly induced, similar to the rat and to laser-induced CNV. As shown in the mouse CNV model and rat models (Figure 4), IL-1Ra substitution in control and light-exposed Cx3cr1−/− mice in dosages sufficient to significantly inhibit CNV had no deleterious effect on photoreceptor survival (Figure 5). Although exogenous IL-1β substitution has been shown to protect photoreceptor degeneration in a light-induced model,7 its inhibition does not exacerbate degeneration in wild-type mice (CNV model) or light-exposed rats or in degeneration-prone Cx3cr1−/− mice. The apparent contradiction of IL-1β–mediated photoreceptor protection and the absence of an exacerbating effect of IL-1Ra might be explained by different IL-1β concentrations. Although the neuroprotective effect of LaVail et al7 and Whiteley et al8 are found at concentrations of 0.5 to 5 μg of IL-1β per eye, the maximum IL-1β concentration we measured with age- and light-induced injury correspond to approximately 50 pg/eye. The endogeneously produced amount of IL-1β might therefore be well below neuroprotective concentrations and therefore have no effect on photoreceptor survival.
In summary, IL-1β is induced by laser- and light-induced injury. We confirm that IL-1Ra substitution efficiently inhibits the development of CNV in the mouse and show that it has no deleterious effect on photoreceptor survival. Taken together, our data suggest that IL-1Ra is a safe alternative for the treatment of neovascular AMD.
Acknowledgments
We thank Christopher Brent Murray for critical review of the manuscript and Bernadette Lescure and Nadège Brunel for technical assistance.
Footnotes
Supported by grants from INSERM, ANR “blanc” (AO5120DD), European Grant “Innochem” (LSHB-CT-2005-518167), ANR Maladies Neurologiques et Psychiatriques (ANR-08-MNPS-003) and ERC starting grant (ERC-2007 St.G. 210345). F.S. is a recipient of a contract “Interface” from Assistance Publique-Hopitaux de Paris.
S.L., W.R., M.H., and S.C. contributed equally to this work.
References
- 1.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 2.Isoda K., Sawada S., Ishigami N., Matsuki T., Miyazaki K., Kusuhara M., Iwakura Y., Ohsuzu F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 3.BenEzra D., Hemo I., Maftzir G. In vivo angiogenic activity of interleukins. Arch Ophthalmol. 1990;108:573–576. doi: 10.1001/archopht.1990.01070060121061. [DOI] [PubMed] [Google Scholar]
- 4.Carmi Y., Voronov E., Dotan S., Lahat N., Rahat M.A., Fogel M., Huszar M., White M.R., Dinarello C.A., Apte R.N. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. 2009;183:4705–4714. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki Y., Matsuura N., Shozuhara H., Onodera H., Itoyama Y., Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]; discussion 681
- 6.Namekata K., Harada C., Guo X., Kikushima K., Kimura A., Fuse N., Mitamura Y., Kohyama K., Matsumoto Y., Tanaka K., Harada T. Interleukin-1 attenuates normal tension glaucoma-like retinal degeneration in EAAC1-deficient mice. Neurosci Lett. 2009;465:160–164. doi: 10.1016/j.neulet.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 7.LaVail M.M., Unoki K., Yasumura D., Matthes M.T., Yancopoulos G.D., Steinberg R.H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteley S.J., Klassen H., Coffey P.J., Young M.J. Photoreceptor rescue after low-dose intravitreal IL-1beta injection in the RCS rat. Exp Eye Res. 2001;73:557–568. doi: 10.1006/exer.2001.1066. [DOI] [PubMed] [Google Scholar]
- 9.Friedman D.S., O'Colmain B.J., Munoz B., Tomany S.C., McCarty C., de Jong P.T., Nemesure B., Mitchell P., Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 10.Sarks S.H. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh H., Takagi H., Takagi C., Suzuma K., Otani A., Ishida K., Matsumura M., Ogura Y., Honda Y. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1891–1898. [PubMed] [Google Scholar]
- 12.Pascual V., Allantaz F., Arce E., Punaro M., Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banwell V., Sena E.S., Macleod M.R. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis. 2009;18:269–276. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Olson J.L., Courtney R.J., Rouhani B., Mandava N., Dinarello C.A. Intravitreal anakinra inhibits choroidal neovascular membrane growth in a rat model. Ocul Immunol Inflamm. 2009;17:195–200. doi: 10.1080/09273940802710705. [DOI] [PubMed] [Google Scholar]
- 15.Checchin D., Sennlaub F., Levavasseur E., Leduc M., Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47:3595–3602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- 16.Zou Y., Xu X., Chiou G.C. Effect of interleukin-1 blockers, CK112, and CK116 on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro. J Ocul Pharmacol Ther. 2006;22:19–25. doi: 10.1089/jop.2006.22.19. [DOI] [PubMed] [Google Scholar]
- 17.Boraschi D., Rambaldi A., Sica A., Ghiara P., Colotta F., Wang J.M., de Rossi M., Zoia C., Remuzzi G., Bussolino F. Endothelial cells express the interleukin-1 receptor type I. Blood. 1991;78:1262–1267. [PubMed] [Google Scholar]
- 18.Leroyer A.S., Rautou P.E., Silvestre J.S., Castier Y., Leseche G., Devue C., Duriez M., Brandes R.P., Lutgens E., Tedgui A., Boulanger C.M. CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J Am Coll Cardiol. 2008;52:1302–1311. doi: 10.1016/j.jacc.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 19.Combadiere C., Feumi C., Raoul W., Keller N., Rodero M., Pezard A., Lavalette S., Houssier M., Jonet L., Picard E., Debre P., Sirinyan M., Deterre P., Ferroukhi T., Cohen S.Y., Chauvaud D., Jeanny J.C., Chemtob S., Behar-Cohen F., Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raoul W., Keller N., Rodero M., Behar-Cohen F., Sennlaub F., Combadiere C. Role of the chemokine receptor CX3CR1 in the mobilization of phagocytic retinal microglial cells. J Neuroimmunol. 2008;198:56–61. doi: 10.1016/j.jneuroim.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Daley J.M., Thomay A.A., Connolly M.D., Reichner J.S., Albina J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 22.Imai Y., Ibata I., Ito D., Ohsawa K., Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsumi-Miyahara C., Sonoda K.H., Egashira K., Ishibashi M., Qiao H., Oshima T., Murata T., Miyazaki M., Charo I.F., Hamano S., Ishibashi T. The relative contributions of each subset of ocular infiltrated cells in experimental choroidal neovascularization. Br J Ophthalmol. 2004;88:1217–1222. doi: 10.1136/bjo.2003.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malyak M., Smith M.F., Jr, Abel A.A., Arend W.P. Peripheral blood neutrophil production of interleukin-1 receptor antagonist and interleukin-1 beta. J Clin Immunol. 1994;14:20–30. doi: 10.1007/BF01541172. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto H., Kumon Y., Watanabe H., Ohnishi T., Shudou M., Ii C., Takahashi H., Imai Y., Tanaka J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J Neurosci Res. 2007;85:994–1009. doi: 10.1002/jnr.21198. [DOI] [PubMed] [Google Scholar]
- 26.Espinosa-Heidmann D.G., Suner I.J., Hernandez E.P., Monroy D., Csaky K.G., Cousins S.W. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai E., Anand A., Ambati B.K., van Rooijen N., Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J., Pham L., Zhang N., He S., Gamulescu M.A., Spee C., Ryan S.J., Hinton D.R. Neutrophils promote experimental choroidal neovascularization. Mol Vis. 2005;11:414–424. [PubMed] [Google Scholar]
- 29.Mountain D.J., Singh M., Singh K. Interleukin-1beta-mediated inhibition of the processes of angiogenesis in cardiac microvascular endothelial cells. Life Sci. 2008;82:1224–1230. doi: 10.1016/j.lfs.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kvanta A. Expression and regulation of vascular endothelial growth factor in choroidal fibroblasts. Curr Eye Res. 1995;14:1015–1020. doi: 10.3109/02713689508998523. [DOI] [PubMed] [Google Scholar]
- 31.Leroyer A.S., Ebrahimian T.G., Cochain C., Recalde A., Blanc-Brude O., Mees B., Vilar J., Tedgui A., Levy B.I., Chimini G., Boulanger C.M., Silvestre J.S. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–2817. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- 32.Cardona A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., Huang D., Kidd G., Dombrowski S., Dutta R., Lee J.C., Cook D.N., Jung S., Lira S.A., Littman D.R., Ransohoff R.M. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]


