Abstract
Deficiency in the serine protease inhibitor LEKTI is the etiological origin of Netherton syndrome. The principal morbidities of the disease are stratum corneum detachment and chronic inflammation. We show that the membrane protease, matriptase, initiates Netherton syndrome in a LEKTI-deficient mouse model by premature activation of a pro-kallikrein-related cascade. Auto-activation of pro-inflammatory and stratum corneum detachment-associated pro-kallikrein-related peptidases was either low or undetectable, but they were efficiently activated by matriptase. Ablation of matriptase from LEKTI-deficient mice dampened inflammation, eliminated aberrant protease activity, prevented stratum corneum detachment, and improved epidermal barrier function. The study uncovers a pathogenic matriptase-pro-kallikrein pathway that could be operative in several human skin and inflammatory diseases.
Keywords: asthma, atopic dermatitis, autoimmunity, inflammatory signaling, proteolytic pathway
The mammalian epidermis provides an essential permeability barrier that limits fluid loss to the surrounding environment and prevents bacteria, fungi, toxic compounds, and allergens from entering the body. The epidermis is a stratified epithelium, in which epidermal keratinocytes arise from proliferating basal cells and move outwards through a series of distinct differentiation events to form the stratum corneum, a two-compartment structure that consists of a lipid-enriched extracellular matrix, in which an interlocking meshwork of terminally differentiated corneocytes are embedded. Epidermal differentiation culminates with the shedding (desquamation) of the outermost layer of corneocytes, which is essential to control the thickness of the stratum corneum. Desquamation is accomplished by the physical degradation of adhesions between corneocytes (corneodesmosomes) in a tightly regulated proteolytic process. Members of the kallikrein subfamily of trypsin-like serine proteases, including kallikrein-related peptidase 5 and kallikrein-related peptidase 7, have been implicated in the degradation of corneodesmosomal proteins during desquamation 1-9.
Spatial dysregulation of epidermal kallikrein proteolytic activity can have serious pathological consequences. A notable example of this is Netherton syndrome, a congenital human disorder characterized by excessive proteolytic degradation of corneodesmosomes and cadherins, which leads to premature stratum corneum separation at the granular-transitional layer boundary 4,10-13. This causes widespread stratum corneum loss, direct exposure of living layers of the epidermis to the external environment, dehydration, hypothermia, severe skin inflammation, susceptibility to bacterial infections, failure to gain weight, and high postnatal mortality. Systemic allergic manifestations, such as asthma, atopic dermatitis, and food allergies are other hallmarks of the syndrome 14-16.
Netherton syndrome is caused by homozygosity or compound heterozygosity for an assortment of mutations in the SPINK5 gene 8,17 that encodes the lympho-epithelial Kazal-type-related inhibitor (LEKTI) 18, which consists of 15 Kazal-type serine protease inhibitory domains (D1-D15) 13. LEKTI is synthesized as a polyprotein that is processed by furin and is secreted as a number of bioactive fragments that contain one or more functional inhibitory domains 7. Importantly, the inhibition of the major epidermal kallikreins by LEKTI is pH-dependent, being efficient at neutral pH, but inefficient at acidic pH 7,19. This observation has led to the formulation of the current four-step hypothesis for the regulation of epidermal desquamation: 1) Epidermal pro-kallikreins are synthesized in the granular layer and are proteolytically converted to their active forms. 2) The activated epidermal kallikreins are prevented from prematurely degrading corneodesmosomes in the granular layer and the lower stratum corneum by the formation of inhibitory complexes with LEKTI. 3) The acidic pH of the upper epidermis dissociates the LEKTI-kallikrein complexes. 4) The inhibitor-free epidermal kallikreins degrade corneodesmosomes, causing desquamation of the uppermost layer of the stratum corneum. From this model, it follows that the absence of LEKTI allows epidermal kallikreins to degrade corneodesmosomes at the granular-transitional layer boundary where their initial activation takes place, thereby causing shedding of the entire stratum corneum and loss of the epidermal barrier. The increased epidermal kallikrein activity in the lower layers of the epidermis may also trigger an inflammatory cascade through the activation of a protease activated receptor (PAR)-2-NFκB pathway 20, and further impair barrier formation by degrading lipid hydrolases that manufacture stratum corneum-specific lipids 21. The molecular mechanisms that initiate the proteolytic activation of epidermal pro-kallikreins in the granular layer are not well understood. Pro-kallikrein-related peptidase 5 has been shown to undergo auto-activation after extended periods (> 5 h) of incubation at 37 °C, and an epidermal pro-kallikrein activation cascade with kallikrein-related peptidase 5 at the apex and involving pro-kallikrein-related peptidase 7, therefore, has been proposed to mediate physiological desquamation 22. This cascade could also be responsible for pro-kallikrein activation in the granular layer in Netherton syndrome or, alternatively, pro-kallikreins could be activated by other proteases expressed in the granular layer.
Matriptase is a transmembrane serine protease that is very unusual for a trypsin-like serine protease in that it undergoes efficient autoactivation, and therefore has the capacity to initiate proteolytic cascade reactions 23,24. In this respect, matriptase is similar to the closely related enteropeptidase, which is the physiological initiator of the digestive enzyme activation cascade 25. This prompted us to investigate the possible contribution of matriptase activation of pro-kallikreins to the aberrant proteolytic activity associated with Netherton syndrome. By using a biochemical approach, as well as taking advantage of the faithful replication of all the key features of Netherton syndrome in LEKTI-deficient mice 4-6,20, we now show that matriptase is an efficient activator of epidermal pro-kallikreins that co-localize with LEKTI at the granular-transitional layer boundary where epidermal separation takes place in Netherton syndrome. Furthermore, we show that all the central manifestations of Netherton syndrome in LEKTI-deficient mice, such as aberrant proteolytic activity in the lower epidermis, corneodesmosome fragility, stratum corneum loss, and skin inflammation, are dependent on the epidermal expression of matriptase. The study expands the molecular understanding of epidermal desquamation and its deregulation in Netherton syndrome, and it defines a matriptasepro-kallikrein activation pathway that may be operative in a variety of physiological and pathological processes.
Results
Matriptase co-localizes with LEKTI at the site of epidermal separation in Netherton syndrome
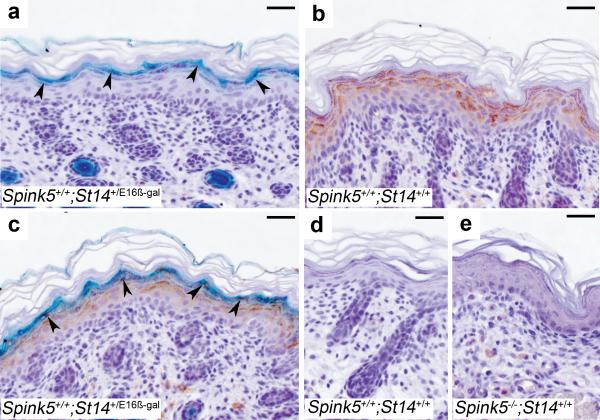
We first determined the localization of LEKTI and matriptase in normal mouse epidermis by combining LEKTI immunostaining with X-gal staining of sections from a mouse strain (St14+/E16β-gal) 26 that carries one wildtype St14 allele and one allele where the St14 exons that encode the serine protease domain of matriptase were replaced by a promoterless β-galactosidase marker gene (Figure 1). This analysis showed that matriptase was expressed in the suprabasal epidermis with the highest level of expression in the granular and transitional cell layers (Figure 1a) and displayed a conspicuous co-localization with LEKTI (Figure 1b and c). The specificity of the detection procedure was demonstrated by the absence of β-galactosidase activity in the epidermis of wildtype mice (Figure 1d), and by the lack of immunoreactivity in Spink5-/- epidermis (Figure 1e).
Figure 1. Matriptase and LEKTI co-localize at the site of epidermal separation in Netherton syndrome.
X-gal staining (cyan) (a), LEKTI immunostaining (brown) (b), and combined X-gal staining and immunohistochemistry (c) of epidermal sections from mice carrying a β-galactosidase-tagged St14 allele. Matriptase and LEKTI demonstrate co-localization at the granular-transitional layer (arrowheads in a and c) where premature corneodesmosome degradation causes epidermal separation in Netherton syndrome. Staining specificity is shown by the absence of X-gal staining in an epidermal section from a wildtype mouse (d) and lack of LEKTI immunoreactivity in a control section from a Spink5-/- mouse (e). Size bars all frames, 50 μm.
Matriptase is an activator of epidermal pro-kallikreins responsible for the development of Netherton syndrome
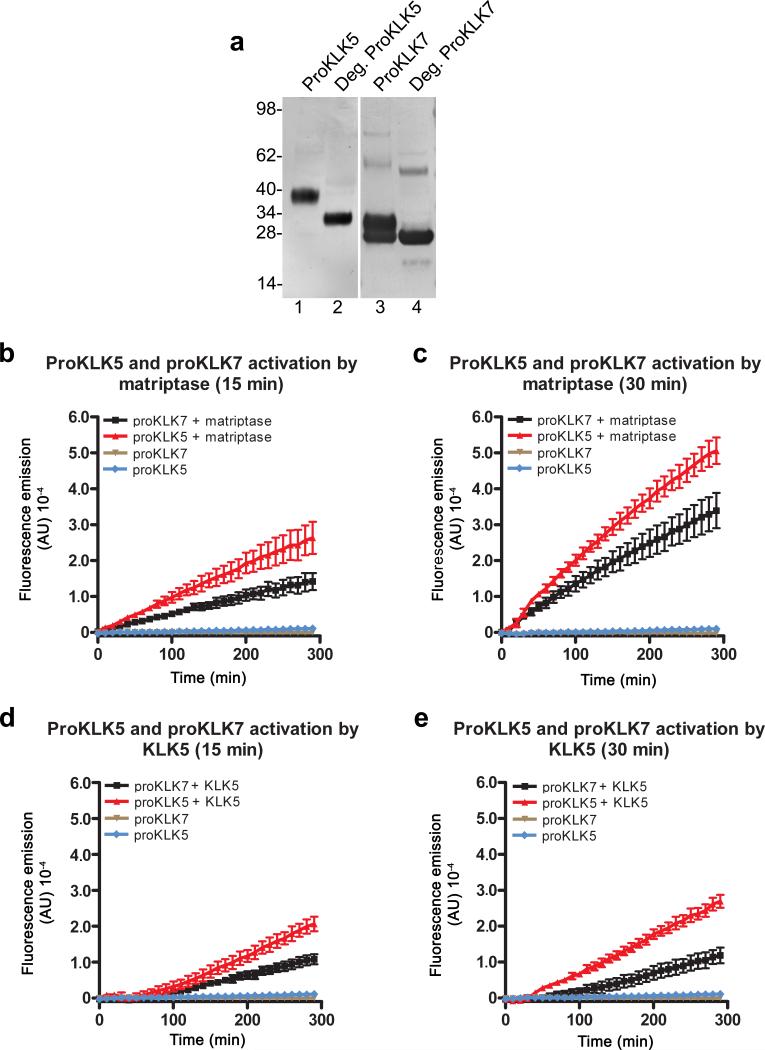
Epidermal kallikreins are all synthesized as inactive pro-enzymes that require proteolytic activation by cleavage after a basic amino acid residue (Arg or Lys) that is located in a highly conserved activation motif preceding the catalytic domain. The ability of matriptase to undergo efficient auto-activation, its previously established capacity to initiate proteolytic cascades 27,28, its specific cleavage of proteins after Arg or Lys residues, its co-expression with LEKTI in epidermis (Figure 1), and its unaltered expression in LEKTI-deficient suprabasal epidermis (Supplementary Figure 1) made matriptase a prime candidate for the activation of epidermal pro-kallikreins and the generation of aberrant epidermal kallikrein activity in the absence of LEKTI. To test this hypothesis, we expressed His-tagged versions the two major epidermal pro-kallikreins, pro-kallikrein-related peptidase 5 and pro-kallikrein-related peptidase 7 in HEK-293T cells, and purified them from serum-free conditioned medium using metal chelation affinity chromatography (Figure 2a). Recombinant pro-kallikrein-related peptidase 5 appeared as a single band with a molecular weight of 40 kDa (Figure 2a, lane 1) and with the expected molecular weight of 32 kDa after deglycosylation (Figure 2a, lane 2). Prokallikrein-related peptidase 7 appeared as two bands: one with a molecular weight of 28 kDa, expected for unglycosylated pro-kallikrein-related peptidase 7 and a second higher molecular weight 34 kDa band (Figure 2a, lane 3). Only the 28 kDa band was retained after pretreatment of the preparation with a combination of N-Glycosidase F, O-glycosidase, and α-2 (3,6,8,9) neuraminidase, showing that the two bands represent different glycosylation forms of pro-kallikrein-related peptidase 7 (Figure 2a, lane 4). In agreement with previous studies 22, the incubation of 50 nM pro-kallikrein-related peptidase 5 or pro-kallikrein-related peptidase 7 for 15 or 30 min at 37 °C resulted in very low kallikrein activity, as determined by the ability to hydrolyze the kallikrein-selective fluorogenic substrate Boc-Val-Pro-Arg-AMC (Figure 2b and c, brown and blue lines). However, incubation of either of the two pro-kallikrein-related peptidases with catalytic amounts of matriptase (2.5 nM, twenty-fold lower concentration) under identical reaction conditions resulted in the time-dependent conversion of both pro-kallikrein 5 and 7 to their proteolytically active forms capable of hydrolyzing the fluorogenic substrate (Figure 2b and c, red lines). As previously described 22, kallikrein-related peptidase 5 displayed pro-kallikrein-related peptidase 5 and pro-kallikrein-related peptidase 7 activating activity (Figure 2d and e) under identical reaction conditions. Taken together, the data show that the auto-activating serine protease, matriptase, is capable of activating epidermal pro-kallikreins, which do not undergo efficient auto-activation. Once converted to its active form, kallikrein-related peptidase 5 is capable of proteolytically activating pro-kallikrein-related peptidase 5 and pro-kallikrein-related peptidase 7, in line with the previously proposed role of the protease in the propagation of an epidermal prokallikrein-related peptidase cascade. Prostasin is a GPI-anchored serine protease that is proteolytically activated by matriptase during terminal epidermal differentiation and is only found as a pro-enzyme in matriptase-deficient human and rodent epidermis 27,29. Since prostasin was a poor activator of pro-kallikrein-related peptidase 5 and prokallikrein-related peptidase 7, even when incubated in up to a 50-fold molar excess (Supplementary Figure 2), we concluded that it does not serve as an activator of epidermal pro-kallikreins in normal or LEKTI-deficient epidermis.
Figure 2. Activation of epidermal pro-kallikreins by matriptase.
(a) Recombinant pro-kallikrein-related peptidase 5 (ProKLK5) (lanes 1 and 2) and pro-kallikrein-related peptidase 7 (proKLK7) (lanes 3 and 4) with C-terminal His tags were analyzed by Western blot using poly-histidine antibodies. Pro-kallikrein-related peptidase 5 appears as a single band with the predicted molecular weight of 40 kDa prior to deglycosylation (lane 1) and 32 kDa after deglycosylation (lane 2). Pro-kallikrein-related peptidase 7 appears as two bands of 28 and 34 kDa prior to deglycosylation (lane 3), and as a single band with the predicted 28 kDa molecular weight after deglycosylation (lane 4). Molecular weight standards (kDa) at left. b and c. Pro-kallikrein-related peptidase 5 and pro-kallikrein-related peptidase 7 (50 nM) were incubated for 15 min (b) or 30 min (c) with and without 2.5 nM matriptase. Fluorescence development was recorded after addition of a fluorogenic kallikrein-selective peptide. Reactions containing pro-kallikreins alone displayed minimal hydrolytic activity (brown and blue lines), compared to reactions containing pro-kallikreins and matriptase (red and black lines). d and e. Identical activation assays were performed substituting 2.5 nM matriptase with 2.5 nM kallkrein-related peptidase 5, demonstrating that pro-kallikrein-related peptidase 5, once converted to kallikrein-related peptidase 5 can activate pro-kallikrein-related peptidase 5 and pro-kallikrein-related peptidase 7. The contribution of matriptase in b and c and kallikrein-related peptidase 5 in d and e to fluorescence emission was subtracted for clarity. Experiments were performed in triplicate. Data are shown as mean fluorescence ± standard error of the mean.
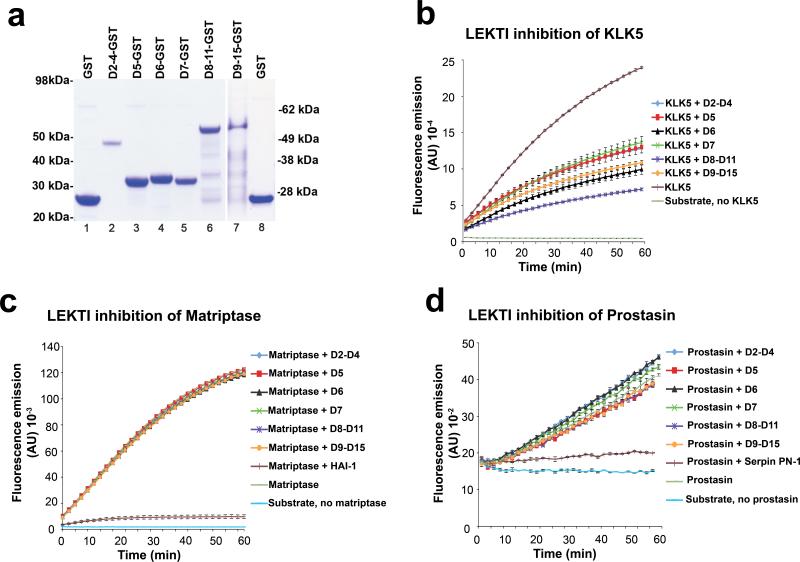
The co-localization of matriptase and LEKTI at the site of stratum corneum separation in Netherton syndrome suggested that not just kallikreins, but also matriptase could be a direct target for LEKTI 30 and that increased matriptase proteolytic activity also could contribute to excess corneodesmosome degradation in Netherton syndrome, independent of pro-kallikrein activation. To investigate this notion, we next tested the capacity of LEKTI to inhibit matriptase in a purified system (Figure 3). All functionally active inhibitory domains of LEKTI (domains 2-15) were generated as six recombinant GST fusion proteins using an E. coli strain that is permissive for disulfide bond formation and were purified by affinity chromatography as described previously (Figure 3a). GST alone did not display inhibitory activity towards any of the proteases tested (data not shown). Each of the six recombinant LEKTI-GST fusion proteins, however, was biologically active, as demonstrated by their ability to inhibit recombinant kallikrein-related peptidase 5 in a fluorogenic peptide hydrolysis assay (Figure 3b). However, none of the six LEKTI-GST fusion proteins demonstrated any inhibitory activity towards matriptase in a similar assay when tested in up to 800-fold molar excess of the protease, whereas the activity of the membrane protease could be completely inhibited by its cognate inhibitor, hepatocyte growth factor activator inhibitor-1 (HAI-1) (Figure 3c). We next incubated purified recombinant prostasin with the six recombinant LEKTI-GST fusion proteins and performed a fluorogenic peptide substrate assay to determine if prostasin was susceptible to LEKTI inhibition. Whereas the cognate inhibitor of prostasin, protease nexin-1 (PN-1), 31 completely abolished peptide substrate hydrolysis, none of the six recombinant LEKTI-GST fusion proteins displayed any detectable inhibitory activity towards this protease (Figure 3d). Taken together, these data show that neither matriptase nor prostasin are direct inhibitory targets for LEKTI.
Figure 3. Matriptase and prostasin are not inhibitory targets for LEKTI.
(a) Recombinant GST (lanes 1 and 8) and LEKTI-GST fusion proteins containing LEKTI domains 2-4 (lane 2), domain 5 (lane 3), domain 6 (lane 4), domain 7 (lane 5), domains 8-11 (lane 6), and domains 9-15 (lane 7) were generated in E. coli, purified by glutathione affinity chromatography, and analyzed by SDS/PAGE followed by Coomassie brilliant blue staining. Molecular weight standards are indicated at left and right. The purified LEKTI-GST fusion proteins were incubated with recombinant kallikrein-related peptidase 5 (KLK5) (b), recombinant matriptase (c), and recombinant prostasin (d). The fluorogenic kallikrein-selective peptide Boc-Val-Pro-Arg-AMC (b), or matriptase/prostasin-selective peptide Boc-Glu-Ala-Arg-AMC (c and d) were added and fluorescence development was recorded over time. The experiments were performed in triplicate and data are shown as mean fluorescence ± standard error of the mean. All LEKTI proteins display inhibitory activity towards kallikrein-related peptidase 5, but no inhibitory activity towards either matriptase or its downstream target, prostasin. Matriptase and prostasin are efficiently inhibited by their cognate inhibitors, hepatocyte growth factor activator inhibitor-1 (HAI-1) and protease nexin-1 (PN-1), respectively.
Ablation of matriptase eliminates aberrant epidermal protease activity caused by LEKTI deficiency
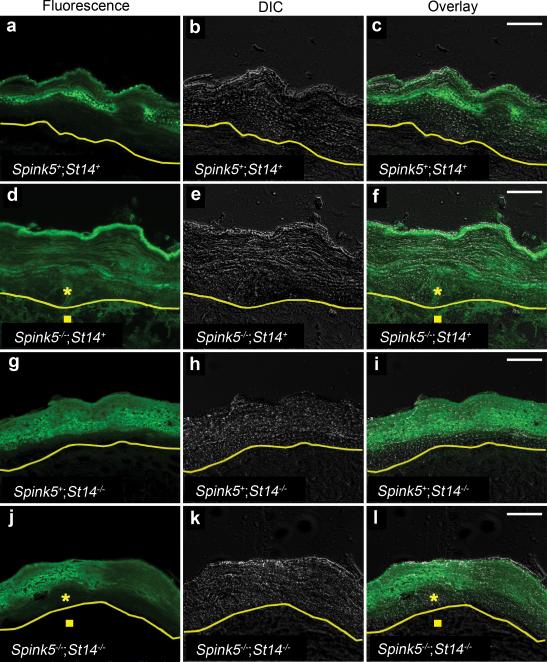
The efficient activation of epidermal pro-kallikreins by matriptase, when combined with the specific expression of the protease at the granular-transitional cell layer boundary, and the previously established ability of matriptase to undergo auto-activation all suggested that matriptase could be the initiator of a “run-away” epidermal kallikrein cascade in Netherton syndrome. To further investigate this notion, we took advantage of a previously developed LEKTI-deficient Spink5-/- mouse strain, which emulate central features of Netherton syndrome, including stratum corneum separation at the granular-transitional layer boundary, loss of epidermal barrier function, and severe skin inflammation 4-6,20. A key feature of LEKTI-deficient epidermis is the aberrant proteolytic activity in the lower layers of the epidermis that can be revealed by in situ zymography 4,11. We, therefore, first studied serine protease activity in the epidermis of normal and LEKTI-deficient skin in situ by overlaying skin sections with a quenched fluorescent casein substrate (Figure 4). As described in previous studies, caseinolytic activity was predominantly confined to the upper stratum corneum of LEKTI-sufficient skin (Figure 4a-c) and the majority of this caseinolytic activity was blocked by pre-incubation of the epidermal sections with recombinant LEKTI (Supplementary Figure 3). As also described previously, LEKTI-deficient epidermis displayed strong caseinolytic activity throughout the epidermis (Figure 4d-f, compare with a-c). A marked increase in caseinolytic activity was also observed in the underlying dermis of LEKTI-deficient mice (Figure 4d-f and data not shown). This increase in dermal proteolytic activity after LEKTI ablation has not been described previously, but was reproducible in multiple independent experiments. It likely reflects the influx of protease-laden inflammatory cells into the dermis and basal epidermal layers caused by stratum corneum loss as well as hyperactivation of a PAR-2 inflammatory pathway by epidermal kallikreins 20 (see below). Caseinolytic activity in matriptase-deficient epidermis was similar in location and intensity to normal epidermis, demonstrating that epidermal kallikrein activation and activity in the upper layer of the stratum corneum is retained in the absence of matriptase (Figure 4g-i). Interestingly, however, the genetic elimination of matriptase completely abolished the aberrant caseinolytic activity that was caused by LEKTI deficiency in both the lower epidermal layers as well as in the dermis (Figure 4j-l, compare to d-f). Indeed, the sites of expression of caseinolytic activity of LEKTI/matriptase double-deficient epidermis were identical to that of normal and matriptase-deficient epidermis by being restricted to the upper layers of the stratum corneum (compare Figure 4j-l with a-c and g-i).
Figure 4. Matriptase ablation eliminates aberrant in situ proteolytic activity caused by LEKTI-deficiency.
In situ zymography using a fluorescence-quenched casein overlay of sections of skin from newborn LEKTI- and matriptase-sufficient (Spink5+;St14+) (a-c), LEKTI-deficient (Spink5-/-;St14+) (d-f), matriptase-deficient (Spink5+;St14-/-) (g-i), and combined LEKTI- and matriptase-deficient (Spink5-/-;St14-/-) (j-l) mice. a, d, g, and j are fluorescence microscopy, b, e, h, and k are differential interference contrast (DIC), and c, f, i, and l are merged fluorescence and differential interference contrast images. The yellow line indicates the border between the epidermis and dermis as determined from DIC images. Increased proteolytic activity in lower layers of epidermis (star) and dermis (square) in d-f is eliminated by the ablation of matriptase (j-l). Size bars, 20 μm.
Matriptase elimination prevents spontaneous stratum corneum loss, restores keratohyalin granules, and improves the barrier function of LEKTI-deficient epidermis
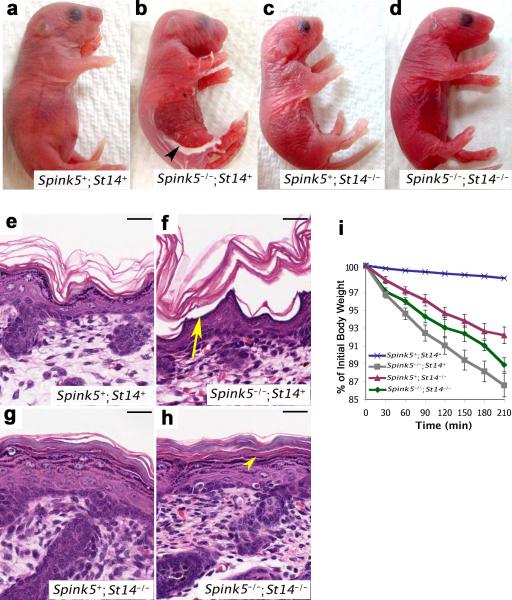
As previously described, the epidermis of newborn LEKTI-deficient mice mimicked Netherton syndrome epidermis by the extensive loss of the stratum corneum (compare Figure 5a and b). Compatible with a key role of matriptase in premature pro-kallikrein activation, the ablation of matriptase from LEKTI-deficient epidermis prevented spontaneous stratum corneum loss (compare Figure 5b and d), and LEKTI and matriptase double-deficient epidermis was outwardly indistinguishable from matriptase-deficient epidermis (compare Figure 5c and d). Stratum corneum loss in LEKTI-deficient skin was caused by separation of the epidermis at the granular-transitional cell layer boundary, and lead to the direct exposure of the living layers of the epidermis to the external environment (compare Figure 5e and f). However, epidermal separation at the granular-transitional cell layer boundary no longer was observed in LEKTI and matriptase double-deficient epidermis (compare Figure 5f and h), which was histologically indistinguishable from matriptase-deficient epidermis (compare Figure 5g and h). Elimination of matriptase from LEKTI-deficient epidermis also restored keratohyalin granules in the upper epidermis (compare Figure 5f and h) that contained profilaggrin, as determined by immunofluorescence (Supplementary Figure 4a-d). Furthermore, loss of matriptase prevented the accelerated processing of profilaggrin that is characteristic of LEKTI-deficient human and mouse epidermis, thus further supporting matriptase acting upstream of epidermal kallikreins (Supplementary Figure 4e and f). In addition to improving both the outward and histological appearance of the skin, the elimination of matriptase also led to a functional improvement of LEKTI-deficient epidermis in an established assay of inward-out epidermal barrier function (Figure 5i). Thus, although loss of matriptase by itself impairs epidermal barrier function by weakening epidermal tight junctions 32 and by preventing calpain/caspase-14/bleomycin-mediated profilaggrin/filaggrin processing 33-35, the ablation of the serine protease markedly improved, rather than further deteriorated, the barrier function of LEKTI-deficient epidermis. Taken together, the data show that the expression of these key features of Netherton syndrome in the LEKTI-deficient mouse model is matriptase-dependent.
Figure 5. Matriptase ablation prevents stratum corneum loss and improves the barrier function of LEKTI-deficient epidermis.
Outward (a-d) and microscopic (e-h) appearance of epidermis from newborn LEKTI- and matriptase-sufficient (Spink5+;St14+) (a and e), LEKTI-deficient (Spink5-/-;St14+) (b and f), matriptase-deficient (Spink5+;St14-/-) (c and g), and combined LEKTI- and matriptase-deficient (Spink5-/-;St14-/-) (d and h) mice. LEKTI-deficiency causes extensive loss of stratum corneum (arrowhead in b), which is caused by epidermal separation at the granular-transitional cell layer junction (arrow in f) and leads to exposure of the granular layer to the external environment. The condition is dependent on the expression of matriptase, as combined LEKTI- and matriptase-deficient mice do not display stratum corneum loss or epidermal separation (arrowhead in h). Size bars, 100 μm. i. Transepidermal water loss rates in matriptase-sufficient (blue line, N=177), LEKTI-deficient (grey line, N=11), matriptase-deficient (red line, N=10), and combined LEKTI- and matriptase-deficient (green line, N=5) mice at 37 °C. Data are shown as mean ± standard error of the mean. Loss of matriptase improves barrier function of LEKTI-deficient epidermis.
Loss of matriptase restores corneodesmosome integrity to LEKTI-deficient epidermis
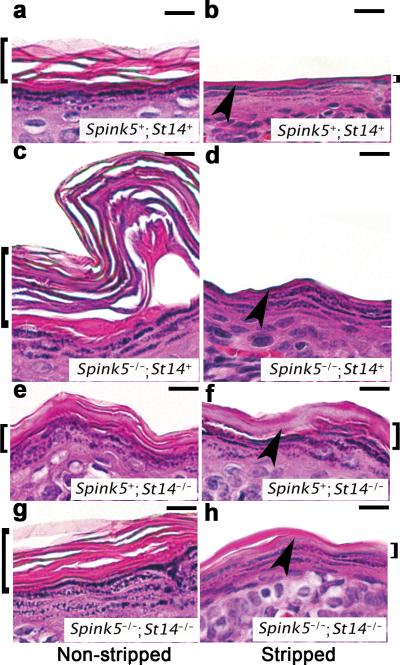
Corneodesmosome integrity can be assessed by a tape stripping procedure in which adhesive-coated discs are repeatedly applied to the same area of the skin, which then is subjected to histological analysis 36,37. Subjection of LEKTI-sufficient epidermis to repeated tape stripping led to the removal of most of the stratum corneum (Figure 6a and b). However, a layer of stratum corneum remained, and the granular layer was never exposed (Figure 6b). In contrast, when LEKTI-deficient epidermis was subjected to the identical procedure, the entire stratum corneum was removed, and living keratinocytes in the granular layer of the epidermis were directly exposed to the external environment, confirming that corneodesmosome integrity in the lower layers is compromised by the loss of LEKTI (Figure 6c and d). As expected, tape stripping of matriptase-deficient epidermis did not lead to complete stratum corneum removal (Figure 6e and f). Interestingly, however, the lower stratum corneum also remained intact in LEKTI-deficient epidermis from which matriptase had also been ablated (Figure 6g and h, compare with a and b, e and f), demonstrating that corneodesmosome integrity was restored in lower levels of the epidermis, thus confirming that matriptase ablation eliminates the aberrant protease activity in LEKTI-deficient epidermis. These data suggest that kallikrein proteolytic activity resulting from auto-activation of kallikrein-related peptidase 5 and kallikrein-related peptidase 5 activation of kallikrein-related peptidase 7 is not sufficient to cause premature desquamation of LEKTI-deficient epidermis.
Figure 6. Loss of matriptase restores the functional integrity of corneodesmosomes at the granular-transitional layer boundary.
Microscopic appearance of epidermis from newborn LEKTI- and matriptase-sufficient (Spink5+;St14+) (a and b), LEKTI-deficient (Spink5-/-;St14+) (c and d), matriptase-deficient (Spink5+;St14-/-) (e and f), and combined LEKTI- and matriptase-deficient (Spink5-/-;St14-/-) (g and h) mice not subjected (a, c, e, and g) or subjected (b, d, f, and h) to repeated tape-stripping. H & E staining. Size bars, 100 μm. Arrowhead indicates granular-transitional cell layer boundary, and brackets to the left and right side indicate the stratum corneum. Complete stratum corneum removal with exposure of the granular layer caused by loss of LEKTI is prevented by the elimination of matriptase.
Matriptase ablation prevents kallikrein hyperactivity-mediated inflammation caused by LEKTI-deficiency
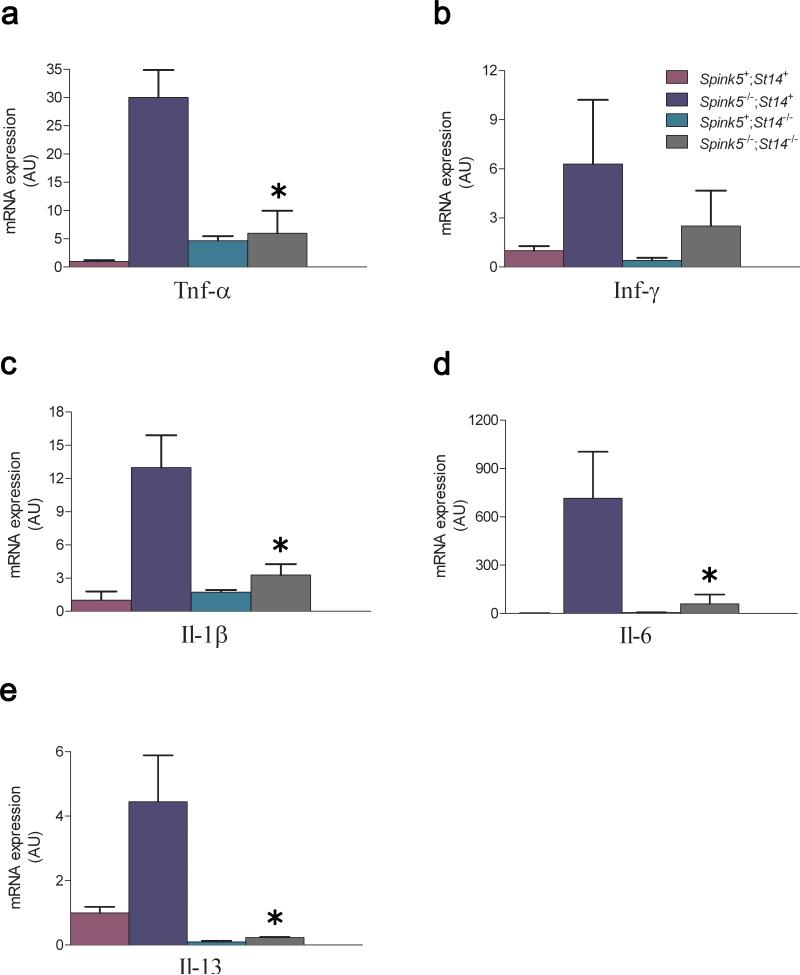
Recent studies have shown that in addition to premature stratum corneum shedding, the absence of LEKTI also causes the activation of pro-inflammatory pathways by kallikrein related peptidase 5-mediated activation of a PAR-2-nuclear factor κB axis 20 in the granular layer of the epidermis. The dependence of matriptase for aberrant protease activity in both LEKTI-deficient dermis and epidermis (Figure 4) led us to investigate if the activation of this kallikrein-PAR-2-nuclear factor κB pro-inflammatory pathway was also dependent on the expression of matriptase. Specifically, we determined the mRNA levels of tumor necrosis factor-α, interferon-γ, interleukin (Il)-1β, and Il-6 expression in LEKTI-deficient skin that was either sufficient or deficient in matriptase (Figure 7). In agreement with previous studies of Netherton syndrome and LEKTI-deficient mouse skin, the loss of LEKTI dramatically increased the expression of all four cytokines. Interestingly, the absence of matriptase greatly reduced inflammatory cytokine expression in LEKTI-deficient skin, with levels of the four cytokines being comparable to that of normal or matriptase-deficient skin. We also examined the Netherton allergy/atopy-associated Th2 and Th17 cytokines Il-4, Il-13 and Il-17, in LEKTI-deficient skin that was either sufficient or deficient in matriptase (Figure 7). Interestingly, the absence of matriptase greatly reduced Il-13 expression in LEKTI-deficient skin, with levels of Il-13 being comparable to that of normal or matriptase-deficient skin. Il-4 and Il-17 mRNA expression was undetectable in epidermis of mice of any genotype (data not shown).
Figure 7. Inflammation in LEKTI-deficient skin is matriptase-dependent.
Tumor necrosis factor-α (a), interferon-γ (b), Il-1β (c), Il-6 (d), and Il-13 (e) mRNA levels were determined in newborn LEKTI- and matriptase-sufficient (Spink5+;St14+) (purple), and littermate LEKTI-deficient (Spink5-/-;St14+) (blue), matriptase-deficient (Spink5+;St14-/-) (green), and combined LEKTI- and matriptase-deficient (Spink5-/-;St14-/-) (grey) mice by qPCR. At least three mice were analyzed for each genotype. The data are shown as mean fluorescence ± standard error of the mean. *P < 0.05, Mann-Whitney U test, two-tailed.
In summary, this study shows that the absence of LEKTI at the granular transitional cell boundary leads to a run-away kallikrein proteolytic cascade, which is initiated by and strictly dependent upon matriptase expression. This causes premature corneodesmosome degradation, loss of stratum corneum, and the activation of epidermal pro-inflammatory pathways characteristic of Netherton syndrome.
Discussion
Studies conducted over the last two decades have yielded new insights into the process of terminal epidermal differentiation of the mammalian epidermis, including the molecular mechanisms that regulate stratum corneum thickness through the desquamation of the uppermost epidermal layers 30. This research was pioneered by Egelrud and colleagues, who first described the abundant expression of a set of epidermis-specific kallikreins in the stratum corneum and demonstrated their capacity to degrade corneodesmosomal proteins: the key step of epidermal desquamation 1-3. A second decisive advance made by Hovnanian and colleagues was the identification of the deficiency of the serine protease inhibitor LEKTI as the cause of Netherton syndrome, a congenital debilitating disease characterized by premature shedding of the stratum corneum 13. Subsequent generation and analysis of LEKTI-deficient mouse models of Netherton syndrome, complemented by elegant biochemical studies, revealed that epidermal kallikreins are direct inhibitory targets for LEKTI 4-6. These studies also showed that the stability of LEKTI-kallikrein inhibitory complexes is pH-dependent, and that the complexes dissociate in the acidic environment of the upper stratum corneum, thereby restricting kallikrein activity and, thus, desquamation, to the uppermost layers of the stratum corneum 7. Epidermal kallikreins are synthesized as catalytically inactive zymogens, incapable of degrading corneodesmosomes in either normal or LEKTI-deficient Netherton syndrome epidermis. The current study expands these prior advances by showing that the auto-activating membrane protease, matriptase, has an essential role in the premature desquamation at the granular-transitional cell layer boundary by causing premature activation of epidermal pro-kallikreins. We also provide evidence that matriptase-dependent pro-kallikrein activation is at least partly responsible for immunological aspects of the syndrome by showing that the increased expression of key pro-inflammatory cytokines, including the Th2 cytokine, IL-13, in LEKTI-deficient epidermis, is also dependent on matriptase. In addition to gaining new insights into the pathogenesis of Netherton syndrome, our findings also suggest a novel strategy for treatment of the disease. Thus, several small and large molecule inhibitors with high specificity for matriptase currently are in pre-clinical development 38, which may be eminently suited to partially inhibit matriptase to restore homeostasis of LEKTI-deficient epidermis.
It remains to be established if the matriptase-pro-kallikrein cascade defined in this study contributes not only to the pathologic proteolysis in Netherton syndrome, but also to normal desquamation of homeostatic epidermis and other stratified epithelia. The impairment of desquamation of matriptase-deficient human and mouse epidermis 29,33 would argue in favor of this. Arguing against this, however, is our finding of relatively unaltered levels of in situ proteolytic activity in the upper layers of matriptase-deficient and combined LEKTI and matriptase-deficient stratum corneum. Prolonged incubation of pro-kallikrein-related peptidase 5 (> 5 h) at body temperature has been reported to cause auto-activation, and kallikrein-related peptidase 5 has the capacity of activate pro-kallikrein-related peptidase 7 at a slow rate 22. Furthermore, other kallikreins, including kallikrein-related peptidases 1, 6, 8, 13, and 14 are expressed in the epidermis. It is, therefore, likely that the micro-environment within the upper epidermis could favor prokallikrein auto-activation and a pro-kallikrein activation cascade, as proposed previously 9,22,39,40 and that desquamation, therefore, is only modestly impaired in the upper stratum corneum of matriptase-deficient epidermis 33. It is also important to note, however, that although matriptase-deficient and combined LEKTI and matriptase-deficient epidermis were indistinguishable in their outward and microscopic appearance, epidermal proteolytic activity, and corneodesmosome integrity, they were not exact phenocopies as regards the rate of transepidermal fluid loss. This indicates that either some low level of residual pro-kallikrein activation takes place at the granular-transitional layer (although this was undetectable by in situ zymography) or that there are additional, as yet undefined, molecular pathways (intrinsic to the skin or systemic) besides premature epidermal pro-kallikrein activity that contribute to Netherton syndrome pathogenesis. Besides providing novel molecular insights into the pathogenesis of Netherton syndrome, the current study may also be relevant for the understanding common epidermal/immune disorders. Loss-of-function mutations in the FLG gene encoding profilaggrin are extremely frequent in some populations, and homozygosity or heterozygosity for loss-of-function FLG alleles causes the common semi-dominant skin condition, ichthyosis vulgaris and is a predisposing factor in atopic eczema and asthma 41,42. Clearly, the level of epidermal filaggrin is critical to these diseases, and our demonstration that a matriptase-prokallikrein pathway regulates the rate of processing of profilaggrin to filaggrin suggests that genetic or environmentally-induced perturbations in the activity of this proteolytic pathway may represent additional predisposing factors for these common disorders. Furthermore, dysregulated kallikrein proteolytic activity has been proposed to be involved not only in skin and inflammatory diseases, but also in cancer 43,44. Matriptase is widely expressed in epithelial tissues and is also expressed by certain leukocyte populations, including monocytoid cells and mast cells 28,45,46. Notably, spatial dysregulation of matriptase suffices to initiate both chronic inflammation and epithelial neoplasia 47. It is therefore plausible, if not likely, that the matriptase-pro-kallikrein activation cascade identified in this study may be relevant to the pathogenesis of additional inflammatory, autoimmune, and proliferative diseases.
Supplementary Material
Acknowledgments
We thank Drs. Daniel Martin, Larry Fisher, and Larry Wahl for technical assistance. We thank Drs. J. Silvio Gutkind, and Mary Jo Danton for critically reviewing this manuscript. Support for this study was from the NIDCR Intramural Research Program.
Online Methods
Animals
Spink5 and St14 null mice, respectively, FVB/NJ and mixed 129/Ola/NIHBlackSwiss genetic backgrounds, have been reported previously 6,48. Spink5 and St14 double-deficient mice, and their littermate single-deficient and sufficient mice were generated by interbreeding. Animal experiments were approved by the NIDCR Institutional Animal Care and Use Committee.
X-gal staining and immunohistochemistry
X-gal staining and immunohistochemistry was performed as previously described 49,32 using anti-human LEKTI antibodies, which cross-react with mouse LEKTI (Zymed Laboratories, South San Francisco, CA), and anti-matriptase antibodies (R&D Systems, Minneapolis, MN).
Proteases and inhibitors
Kallikrein-related peptidases, matriptase, prostasin, HAI-1, and PN-1 were purchased from R&D Systems. Recombinant LEKTI fragments were generated as described 7. Full length pro-kallikrein-related peptidase 5 and -7 cDNA sequences were generated and furnished with 6-His C-terminal extensions by high-fidelity PCR using a mouse skin cDNA library and proKLK5f/proKLK5r and proKLK7f/proKLK7r primer sets, respectively (Supplementary Table 1). The PCR fragments were digested with EcoRI and BamHI and inserted into the eukaryotic expression vector pCEFL (a kind gift from J. Silvio Gutkind, NIDCR). The expression plasmids were transiently transfected into HEK-293T cells using Turbofect (Fermentas, Glen Burnie, MD), and recombinant pro-kallikrein-related peptidase 5 and 7 were purified from 72 h serum-free conditioned medium using Hi Bind resin chromatography, as recommended by the manufacturer (Novagen). Deglycosylation of pro-kallikrein-related peptidase 7 was performed using a mixture of N-Glycosidase F, O-Glycosidase and α-2 (3,6,8,9)-neuraminidase, as recommended by the manufacturer (Sigma-Aldrich, St Louis, MO).
Protease inhibition assays
Recombinant kallikrein-related peptidase 5 (25 nM final concentration) was mixed either with 90 nM recombinant LEKTI domains D2-D4, 830 nM D5, D6, and D7, 161 nM D8-D11 or with 7.3 nM of D9-D15 in 100 μl 0.1 mM Tris-HCl, pH 8.0, 200 mM NaCl at 37 °C . The fluorogenic substrate Boc-Val-Pro-Arg-AMC (Sigma-Aldrich) was then added to 0.16 μM final concentration, and fluorescence-release was measured using a Wallac plate reader (Perkin Elmer, Waltham, MA). Matriptase and prostasin inhibition assays were performed the same way using 1 nM recombinant matriptase or 156 nM of prostasin incubated with LEKTI fragments or 250 nM recombinant HAI-1 or 600 nM PN-1 in 50 mM Tris-HCl, pH 9.0, 50 mM NaCl, 0.01% Tween 20 and 0.1 μM of the fluorogenic substrate, Boc-Glu-Ala-Arg-AMC (R&D Systems). Control reactions included substitution of LEKTI fragments with 500 nM Glutathione S-transferase (GST).
Pro-kallikrein-related peptidase 5 and pro-kallikrein-related peptidase 7 activation assays
Recombinant pro-kallikrein-related peptidase 5 or pro-kallikrein-related peptidase 7 (both 50 nM) were incubated alone or with either 2.5 nM recombinant matriptase, 2.5 nM recombinant kallikrein-related peptidase 5 or 400 nM recombinant prostasin in 100 μl 50 mM Tris-HCl, pH 7.5, 200 mM NaCl at 37 °C for 15 or 30 min. The fluorogenic substrate Boc-Val-Pro-Arg-AMC (Sigma-Aldrich) was then added to 0.16 μM final concentration, and fluorescence release was measured as above. Identical assays were performed in parallel with either recombinant matriptase, kallikrein-related peptidase 5 or prostasin to assess cleavage of the fluorogenic substrates by these proteases.
In situ zymography
In situ zymography on frozen skin sections (5 μm) was done using BODIPY FL labeled denatured casein (EnzCheck Ultra Protease Assay Kit, Invitrogen, Carlsbad, CA), following the manufacturer's protocol. Protease activity was visualized using a IX81 inverted microscope equipped with a Fluoview1000 confocal scanning module (Olympus America Inc., Center Valley, PA). All images were acquired using a 60X water immersion objective, UPLSAPO N. A. 1.2 (Olympus America Inc.). A 488 nm laser was used as the illumination source. Green fluorescence images were acquired using a 505-605 nm bandpass filter. Differential interference contrast images were acquired in parallel. Image processing was performed using Imaris 6.2 (Bitplane, Saint Paul, MN). The final images were prepared with Adobe Photoshop CS.
Histological analysis
Whole litters of newborn to 48 h old pups were euthanized by decapitation, fixed for 24 h in 4 % paraformaldehyde (PFA) in PBS, processed into paraffin, sectioned into sagittal 3 μm sections, and stained with hematoxylin and eosin (h & e).
Trans-epidermal fluid loss assay and assessment of stratum corneum integrity
The trans-epidermal fluid loss assay and assessment of stratum corneum integrity was performed as described 33.
RNA preparation and real-time PCR
Total RNA was prepared by extraction in Trizol reagent (Invitrogen), as recommended by the manufacturer. Reverse transcription and PCR amplification were performed using the RETROscript™ Kit (Ambion Inc., Austin TX), as recommended by the manufacturer. First strand cDNA synthesis was performed using an Oligo dT primer. The primer pairs used for qPCR of Tumor necrosis factor-α, Interferon-γ, Il-1β, and Il-6 are listed in Supplementary Table 1. Annealing temperature for all primer sets was 60 °C. Expression levels were normalized to GAPDH and 18S RNA levels in each sample. Il-4, -13 and -17a mRNA levels were determined using the Qiagen QuantiTect Primer Assay, as recommended by the manufacturer (Qiagen, Valencia, CA).
Detection of profilaggrin/filaggrin by immunofluorescence
Frozen skin sections were cut (12 μm) onto silanated glass slides and fixed in methanol for 10 min at -20 °C. The slides were then washed in PBS and blocked in PBS with 10 % fetal bovine serum, 0.04 % saponin (blocking solution) for 1 h at room temperature. The slices were incubated for 1 h with the primary antibody in blocking solution, washed 3 × 10 min in PBS and then incubated with the appropriate secondary antibody for 1 h at room temperature. Rabbit-anti-filaggrin (Covance, Trenton, NJ) and rat-anti-integrin α6 integrin (BD Biosciences, San Jose, CA) were used as primary antibodies. Alexa-488 and -594 conjugated secondary antibodies were purchased from Invitrogen (San Diego, CA). The samples were then washed 3 × 10 min in PBS, mounted with Fluoromount-G (Southern Biotech, Birmingham, AL) and imaged by confocal microscopy. Images were acquired using a IX81 inverted microscope equipped with a Fluoview1000 confocal scanning module (Olympus America Inc., Center Valley, PA). All images were acquired using a 60X oil immersion objective, PlanApo N (Olympus America Inc.). For detection of Alexa-488 and -594 dyes, samples were sequentially illuminated with 488 and 561 nm lasers and the emitted light was collected through 505-550 nm and 585-685 nm bandpass filters, respectively. The final images were prepared with Adobe Photoshop CS.
Detection of profilaggrin/filaggrin by SDS/PAGE and Western blot
Proteins were extracted from whole newborn skins (dermis and epidermis) by homogenization in 8 M urea, 10 mM EDTA, 50 mM Tris-HCl, pH 8 and centrifuged at 27,000 × g for 30 min. The pellets were discarded and protein in the supernatants was resolved by SDS-PAGE on 4–12% NuPAGE BisTris gels (Invitrogen) under reducing conditions and either electroblotted onto PVDF membranes (Invitrogen) or stained with Coomassie brilliant blue. The PVDF membranes were blocked in 5 % fat-free milk and incubated with rabbit pro-filaggrin/filaggrin polyclonal antibodies (Covance, Madison, WI) followed by alkaline phosphatase-labeled goat anti-rabbit antibodies. Membranes were then developed using the NBT/BCIP substrate (Roche Diagnostics, Mannheim, GER).
Statistical analysis
The significance of difference between groups was determined by the Mann-Whitney U test (two-tailed).
References
- 1.Caubet C, et al. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004;122:1235–44. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- 2.Brattsand M, Egelrud T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J Biol Chem. 1999;274:30033–40. doi: 10.1074/jbc.274.42.30033. [DOI] [PubMed] [Google Scholar]
- 3.Hansson L, et al. Cloning, expression, and characterization of stratum corneum chymotryptic enzyme. A skin-specific human serine proteinase. J Biol Chem. 1994;269:19420–6. [PubMed] [Google Scholar]
- 4.Descargues P, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. doi: 10.1038/ng1493. [DOI] [PubMed] [Google Scholar]
- 5.Hewett DR, et al. Lethal, neonatal ichthyosis with increased proteolytic processing of filaggrin in a mouse model of Netherton syndrome. Hum Mol Genet. 2005;14:335–46. doi: 10.1093/hmg/ddi030. [DOI] [PubMed] [Google Scholar]
- 6.Yang T, et al. Epidermal detachment, desmosomal dissociation, and destabilization of corneodesmosin in Spink5-/- mice. Genes Dev. 2004;18:2354–8. doi: 10.1101/gad.1232104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deraison C, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol Biol Cell. 2007;18:3607–19. doi: 10.1091/mbc.E07-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachem JP, et al. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006;126:1609–21. doi: 10.1038/sj.jid.5700288. [DOI] [PubMed] [Google Scholar]
- 9.Stefansson K, Brattsand M, Ny A, Glas B, Egelrud T. Kallikrein-related peptidase 14 may be a major contributor to trypsin-like proteolytic activity in human stratum corneum. Biol Chem. 2006;387:761–8. doi: 10.1515/BC.2006.095. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki Y, Nomura J, Koyama J, Horii I. The role of proteases in stratum corneum: involvement in stratum corneum desquamation. Arch Dermatol Res. 1994;286:249–53. doi: 10.1007/BF00387596. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu N, et al. Elevated stratum corneum hydrolytic activity in Netherton syndrome suggests an inhibitory regulation of desquamation by SPINK5-derived peptides. J Invest Dermatol. 2002;118:436–43. doi: 10.1046/j.0022-202x.2001.01663.x. [DOI] [PubMed] [Google Scholar]
- 12.Descargues P, et al. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J Invest Dermatol. 2006;126:1622–32. doi: 10.1038/sj.jid.5700284. [DOI] [PubMed] [Google Scholar]
- 13.Chavanas S, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–2. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 14.Smith DL, Smith JG, Wong SW, deShazo RD. Netherton's syndrome. Br J Dermatol. 1995;133:153–4. doi: 10.1111/j.1365-2133.1995.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith DL, Smith JG, Wong SW, deShazo RD. Netherton's syndrome: a syndrome of elevated IgE and characteristic skin and hair findings. J Allergy Clin Immunol. 1995;95:116–23. doi: 10.1016/s0091-6749(95)70159-1. [DOI] [PubMed] [Google Scholar]
- 16.Bitoun E, et al. Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol. 2002;118:352–61. doi: 10.1046/j.1523-1747.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 17.Chavanas S, et al. Localization of the Netherton syndrome gene to chromosome 5q32, by linkage analysis and homozygosity mapping. Am J Hum Genet. 2000;66:914–21. doi: 10.1086/302824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magert HJ, et al. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J Biol Chem. 1999;274:21499–502. doi: 10.1074/jbc.274.31.21499. [DOI] [PubMed] [Google Scholar]
- 19.Schechter NM, et al. Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lympho-epithelial Kazal-type inhibitor (LEKTI). Biol Chem. 2005;386:1173–84. doi: 10.1515/BC.2005.134. [DOI] [PubMed] [Google Scholar]
- 20.Briot A, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–47. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hachem JP, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510–20. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- 22.Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005;124:198–203. doi: 10.1111/j.0022-202X.2004.23547.x. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi T, et al. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–42. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 24.Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 2000;275:36720–5. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- 25.Kitamoto Y, Yuan X, Wu Q, McCourt DW, Sadler JE. Enterokinase, the initiator of intestinal digestion, is a mosaic protease composed of a distinctive assortment of domains. Proc Natl Acad Sci U S A. 1994;91:7588–92. doi: 10.1073/pnas.91.16.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.List K, Szabo R, Molinolo A, Nielsen BS, Bugge TH. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. Am J Pathol. 2006;168:1513–25. doi: 10.2353/ajpath.2006.051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netzel-Arnett S, et al. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 2006;281:32941–5. doi: 10.1074/jbc.C600208200. [DOI] [PubMed] [Google Scholar]
- 28.Kilpatrick LM, et al. Initiation of plasminogen activation on the surface of monocytes expressing the type II transmembrane serine protease matriptase. Blood. 2006;108:2616–23. doi: 10.1182/blood-2006-02-001073. [DOI] [PubMed] [Google Scholar]
- 29.Alef T, et al. Ichthyosis, Follicular Atrophoderma, and Hypotrichosis Caused by Mutations in ST14 Is Associated with Impaired Profilaggrin Processing. J Invest Dermatol. 2009;129:862–869. doi: 10.1038/jid.2008.311. [DOI] [PubMed] [Google Scholar]
- 30.Ovaere P, Lippens S, Vandenabeele P, Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci. 2009 doi: 10.1016/j.tibs.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Chen LM, et al. Prostasin attenuates inducible nitric oxide synthase expression in lipopolysaccharide-induced urinary bladder inflammation. Am J Physiol Renal Physiol. 2006;291:F567–77. doi: 10.1152/ajprenal.00047.2006. [DOI] [PubMed] [Google Scholar]
- 32.List K, et al. Epithelial Integrity Is Maintained by a Matriptase-Dependent Proteolytic Pathway. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.List K, et al. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1. J Cell Biol. 2003;163:901–10. doi: 10.1083/jcb.200304161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamata Y, et al. Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated filaggrin into amino acids. J Biol Chem. 2009;284:12829–36. doi: 10.1074/jbc.M807908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denecker G, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–74. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- 36.Marttin E, Neelissen-Subnel MT, De Haan FH, Bodde HE. A critical comparison of methods to quantify stratum corneum removed by tape stripping. Skin Pharmacol. 1996;9:69–77. doi: 10.1159/000211392. [DOI] [PubMed] [Google Scholar]
- 37.Dreher F, et al. Colorimetric method for quantifying human Stratum corneum removed by adhesive-tape stripping. Acta Derm Venereol. 1998;78:186–9. doi: 10.1080/000155598441495. [DOI] [PubMed] [Google Scholar]
- 38.List K. Matriptase: a culprit in cancer? Future Oncol. 2009;5:97–104. doi: 10.2217/14796694.5.1.97. [DOI] [PubMed] [Google Scholar]
- 39.Borgono CA, et al. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem. 2007;282:3640–52. doi: 10.1074/jbc.M607567200. [DOI] [PubMed] [Google Scholar]
- 40.Kishi K, et al. Characterization of a membrane-bound arginine-specific serine protease from rat intestinal mucosa. J Biochem (Tokyo) 2001;130:425–30. doi: 10.1093/oxfordjournals.jbchem.a003002. [DOI] [PubMed] [Google Scholar]
- 41.Smith FJ, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–42. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- 42.Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122:1285–94. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramsay AJ, et al. Kallikrein-related peptidase 4 (KLK4) initiates intracellular signaling via protease-activated receptors (PARs). KLK4 and PAR-2 are co-expressed during prostate cancer progression. J Biol Chem. 2008;283:12293–304. doi: 10.1074/jbc.M709493200. [DOI] [PubMed] [Google Scholar]
- 44.Hollenberg MD, et al. Kallikreins and proteinase-mediated signaling: proteinase-activated receptors (PARs) and the pathophysiology of inflammatory diseases and cancer. Biol Chem. 2008;389:643–51. doi: 10.1515/BC.2008.077. [DOI] [PubMed] [Google Scholar]
- 45.Cheng MF, et al. Matriptase expression in the normal and neoplastic mast cells. Eur J Dermatol. 2007;17:375–80. doi: 10.1684/ejd.2007.0233. [DOI] [PubMed] [Google Scholar]
- 46.Oberst MD, et al. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem. 2003;51:1017–25. doi: 10.1177/002215540305100805. [DOI] [PubMed] [Google Scholar]
- 47.List K, et al. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–50. doi: 10.1101/gad.1300705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.List K, et al. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21:3765–79. doi: 10.1038/sj.onc.1205502. [DOI] [PubMed] [Google Scholar]
- 49.Szabo R, et al. Potent inhibition and global co-localization implicate the transmembrane Kunitz-type serine protease inhibitor hepatocyte growth factor activator inhibitor-2 in the regulation of epithelial matriptase activity. J Biol Chem. 2008;283:29495–504. doi: 10.1074/jbc.M801970200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.