Abstract
In the tear fluid the outermost part facing the tear–air interface is composed of lipids preventing evaporation of the tears. Phospholipid transfer protein (PLTP) mediates phospholipid transfer processes between serum lipoproteins and is also a normal component of human tears. To study whether PLTP plays any functional role in tear fluid we investigated PLTP-deficient mice, applying functional and morphologic analyses under normal housing and experimentally induced dry eye conditions. Aqueous tear fluid production, corneal epithelial morphology, barrier function, and occludin expression were assessed. In mice with a full deficiency of functional PLTP enhanced corneal epithelial damage, increased corneal permeability to carboxyfluorescein, and decreased corneal epithelial occludin expression were shown. These pathologic signs were worsened by experimentally induced dry eye both in wild-type and PLTP knock-out mice. Deficiency in the production of tear PLTP in mice is accompanied by corneal epithelial damage, a feature that is typical in human dry eye syndrome (DES). To complement animal experiments we collected tear fluid from human dry eye patients as well as healthy control subjects. Increased tear fluid PLTP activity was observed among DES patients. In conclusion, the presence of PLTP in tear fluid appears to be essential for maintaining a healthy and functional ocular surface. Increased PLTP activity in human tear fluid in DES patients suggests an ocular surface protective role for this lipid transfer protein.
Dry eye syndrome (DES) is a multifactorial disease of the ocular surface. DES is accompanied by tear film instability,1 increased osmolarity,2,3 inflammation of the ocular surface,4 and abnormalities in tear fluid components, especially lipids.5 Together these may lead to ocular surface damage.6 The conventional structure of the tear film consists of three layers: a superficial lipid layer, an aqueous middle layer, and a precorneal mucin layer.7,8 It appears, however, that the two latter compartments form a somewhat homogenous gel-like and mucin-enriched fluid.9,10 The superficial lipid layer is a complex mixture of polar and nonpolar lipids arranged into a layered structure. Based on the hydrophobic effect it has been suggested that the polar phospholipids are aligned adjacent to the aqueous-mucin layer and externally to this a layer composed of nonpolar lipids, such as cholesteryl esters and triglycerides, face the tear–air interface.8,11,12 The chemical composition and comprehensive lipid analysis of tear fluid have yet to be revealed. It is, however, clear that an imbalance of lipid layer in the tear fluid is involved intimately in the pathogenesis of DES.
Similarly to cellular membranes13 the tear fluid lipid layer is by no means a static lipid membrane. A diminished amount of certain lipids increases the evaporation rate and excess lipids cause thickening and packing difficulties in the air–tear interface leading to formation of lipid aggregates in the tear fluid. Because of the properties of lipids it is quite likely that lipid aggregates may contaminate the mucins as well as even corneal epithelial cells, rendering these to become less wettable. Accordingly, an active protein-mediated transport mechanism is needed. Glasgow and collaborators14 previously showed that tear fluid lipocalin is able to bind lipids in the tear fluid and prevent contamination of the ocular surface. Yet, we previously showed that lipocalin does not actually possess lipid transport activity and that other such proteins are actually found in the tear fluid.15,16
We earlier showed that high-active form of phospholipid transfer protein (PLTP), a glycoprotein with phospholipid transfer activity, is secreted mainly from the lacrimal gland17 and is a normal component of the human tear fluid.16 In plasma PLTP transfers different phospholipid species, but not neutral lipids, between lipoprotein particles,18 and has an important role in high-density lipoprotein metabolism.19 The human lung tissue, where PLTP is found at an air–water environment (ie, similar to the tear fluid), displays high levels of PLTP compared with other tissues. PLTP is highly expressed in alveolar type II cells, and, notably, is induced during hypoxia and in emphysema,20 suggesting novel surface protective properties of this molecule. Recently, we elucidated the mechanism of PLTP-facilitated phospholipid transfer21 and very recently showed that PLTP interacts with human tear fluid mucins.17 These findings together suggest that PLTP may play a role in the tear film lipid homeostasis by preventing ocular surface damage and maintaining the anterior lipid layer. Disturbances in the secretion of functional PLTP may affect the stability and/or function of the tear fluid anterior lipid layer.
Here, we show by using a PLTP knock-out (KO) mouse model that in mouse tear fluid the absence of PLTP leads to similar corneal morphologic changes that typically are seen in DES. Human subjects suffering from DES show increased levels of PLTP activity. Our data suggest that at least in mice PLTP is part of the machinery that is important for maintaining the delicate homeostasis between corneal epithelial cells and normal tear fluid and in part in the prevention of DES.
Material and Methods
Materials
Transdermal scopolamine patches were obtained from Novartis (Scopoderm; Novartis International AG, Basel, Switzerland). Carboxyfluorescein (CF) 0.3% was from Holles Laboratories (Cohasset, MA). Schirmer paper was purchased form Zabby's (New Delhi, India). Polyclonal rabbit anti-occludin was from Zymed (San Francisco, CA). mAb59 IgG and rabbit polyclonal (R290 IgG) antibodies against human PLTP were produced as described earlier.22 Secondary antibodies, goat anti-mouse IgG horseradish peroxidase conjugate and goat anti-rabbit IgG horseradish peroxidase conjugate were purchased from Bio-Rad Laboratories (Hercules, CA). Alexa 488–conjugated goat anti-rabbit immunoglobulin was purchased from Molecular Probes (Eugene, OR). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animals
C57BL/6 wild-type (WT) mice (Charles River, Wilmington, MA) and C57BL/6 PLTP KO mice23 were bred in Taconic Europe Facilities (Ejby, Denmark) and then transferred to the National Public Health Institute (Helsinki, Finland) Animal Care Centre for further breeding. The mice were housed in a room under a controlled temperature (23°C ± 1°C) and light cycle, with free access to water and standard mouse chow (no. 2018, 18% protein, 5% fat; Harlan Teklad Global Diets, Blackthorn, UK). All of the animal experiments were conducted under the National Public Health Institute guidelines (license KTL 2004-02) for the humane treatment of laboratory animals. All procedures in the study protocol adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Human Subjects
This part of the study was performed according to the principles of the Declaration of Helsinki, and was approved by the Ethics Committee of the Hospital District of Southwest Finland. Informed consent was obtained from each subject. Tear fluid was collected from dry eye patients (n = 7; age, 28–66 years; 2 men and 5 women) and healthy subjects (n = 4; age, 32–38 years; 2 men and 2 women). Except for typical dry eye symptoms and findings, the clinical investigation of the subjects before collection of tear fluid showed no signs of ocular inflammation or allergy.
Methods
Blood Samples
Blood samples were collected from vena saphena magna into sterile heparin-containing tubes. The samples were kept at room temperature for 1 hour and centrifuged at 3000 × g for 15 minutes. The plasma was separated and stored at −70°C.
Induction of Experimentally Induced Dry Eye with Cholinergic Receptor Blockade
Induction of experimentally induced dry eye (EIDE) was achieved by cholinergic receptor blockade as described by Dursun et al.24 Briefly, transdermal scopolamine patches were applied by cutting the patches into four pieces, wrapping them around the depilated midtail, and securing them with cellophane tape. Patches were reapplied on days 0, 2, 4, and 6.
Corneal and Eyelid Histopathology
Eyes and eyelids from WT and PLTP KO mice with and without EIDE were surgically excised, fixed in 10% formalin, and embedded in paraffin. Sections (6 μm) were prepared and stained with H&E and PAS. Sections from three different eyes in each group were examined and photographed with a Nikon Eclipse TE2000-E (Nikon Corp., Tokyo, Japan) microscope equipped with a Nikon Digital Sight DS-5MC camera.
The corneal epithelial damage was assessed as described previously.25 In brief, the number of detaching apical cells was counted from a full microscope field-of-vision from three separate tissue sections, by two independent masked observers (N.L.S. and J.M.H.), using a ×20 objective. Three mice were evaluated in each treatment group.
Western Blot Analysis
Plasma was collected as described earlier and tear fluid samples were collected in a glass microcapillary from the lateral canthus of WT and PLTP KO mice. Plasma and tears (1 μL) were run on 12.5% SDS–polyacrylamide gel electrophoresis and electrically transferred to a nitrocellulose membrane (400 mA for 45 minutes) using 0.1 mol/L Tris with 0.192 mol/L glycine in 20% methanol as a transfer buffer. The membrane was blocked with milk powder (5% wt/vol in Tris-buffered saline, 0.05% Tween, pH 7.5, for 1 hour at room temperature). The membranes were subjected to electrochemiluminescence detection for PLTP using mAb59.
Measurement of Aqueous Tear Production
Tear production was measured before and 7 days after induction of EIDE. A Schirmer paper was cut in half, held with forceps, and applied to the ocular surface in the lateral canthus for 60 seconds. Wetting of the paper was measured in millimeters.
Permeability to CF
CF (1 μL of a 0.3% solution) was applied to the ocular surface. After 10 minutes the mice were sacrificed with high-dose carbon dioxide. Corneas without scleral rims were excised, rinsed twice with 200 μL balanced salt solution, weighed, and placed in 100 μL balanced salt solution. The solution containing the corneal tissue was protected from light and gently mixed for 90 minutes. The CF concentration in the incubation fluid was measured (excitation, 490 nm; emission, 535 nm) using a Wallac 1420 Victor2 Multilabel Counter (Beckman Coulter, Fullerton, CA). The results are reported as fluorescence units per milligram of corneal tissue.
Occludin IHC
Sections (5 μm) of formalin-fixed, paraffin-embedded, whole eyes sections from WT, PLTP KO, and EIDE-induced WT and PLTP KO mice were mounted on chromium-gelatin–treated slides. The sections were first soaked in xylene to remove paraffin and then rehydrated in graded alcohol series (100% to 70%). To recover antigenicity we pretreated the sections for 12 minutes at 37°C with pepsin (0.5% wt/vol) containing 0.1 mol/L HCl. The sections were permeabilized and blocked with 0.1 mol/L Na2HPO4 phosphate buffer containing 0.2% Triton X-100 and 10% normal goat serum for 60 minutes and labeled overnight in a moist chamber with polyclonal rabbit anti-occludin primary antibody (dilution, 1:25) diluted in 0.1 mol/L phosphate buffer containing 0.1% Triton X-100 and 2.5% normal goat serum. Tissues without primary antibody served as negative controls. Sections were rinsed two times (30 minutes each) in phosphate buffer and labeled for 1 hour with Alexa 488–conjugated goat anti-rabbit immunoglobulin. The stained sections were rinsed three times (30 minutes each) in phosphate buffer and mounted with Aquamount (BDH Chemicals, Poole, UK) and coverslips were applied. The sections were examined under fluorescent illumination (excitation, 460–500 nm; emission, 510–560 nm) by a Nikon Eclipse TE2000-E microscope equipped with a Nikon Digital Sight DS-5MC camera. The images were evaluated and quantitated further by Adobe Photoshop CS5 (San Jose, CA).
Tear Fluid Osmolarity and Samples
Tear fluid osmolarity (mOsm/L) in human subjects was measured with a Tearlab osmometer (Ocusense, Inc., San Diego, CA). Tear fluid samples (3 to 5 μL) subsequently were collected from the lower conjunctival sac using Blaubrand intramark 5-μL micropipets (Brand GMBH, Wertheim, Germany), causing minimal conjunctival irritation. The samples immediately were cooled and stored at −18°C until analyzed.
Preparation of Phosphatidylcholine Liposomes for PLTP Activity Measurement
To prepare phosphatidylcholine liposomes, 10 μmol of egg phosphatidylcholine (Sigma-Aldrich), 20 μL (corresponding to 1 μCi) of [14C] dipalmitoyl-phosphatidylcholine, and 100 nmol of butylhydroxytoluene antioxidant (stock 1 nmol/μL in chloroform), were pipetted into glass tubes on ice. Organic solvent was evaporated under N2 until dry and the mixture was lyophilized for 30 minutes. Onto the dry lipid film 1 mL of PLTP buffer (10 mmol/L Tris–HCl, 150 mmol/L NaCl, and 1 mmol/L EDTA, pH 7.4) was added and vortexed vigorously to solubilize lipid material. The sample was sonicated for 3 × 5 minutes in an ice bath. After each 5-minute sonication, the tubes rested 1 minute on ice, and then sonication was continued, avoiding foaming. The sonicated material was transferred into an Eppendorf (Hamburg, Germany) tube and centrifuged for 10 minutes at 15,000 rpm at room temperature to pellet particulate material and titanium residual released from the sonicator probe. The opalescent liposomes were transferred under N2 flow to a new 1-mL Eppendorf tube and kept at +4°C wrapped in aluminum foil. By using this procedure, radioactivity counting of 15 μL of this substrate produces about 15,000 cpm (16,300 dpm; counting efficiency, 92%).
PLTP-Facilitated Phospholipid Transfer Activity
PLTP-facilitated phospholipid transfer activity was measured as follows: after solutions were pipetted to an Eppendorf tube: i) high-density lipoprotein3, (density range, 1.125 to 1.21 g/mL), free of PLTP activity, 250 μg as protein; ii) Tris-buffer (10 mmol/L Tris–HCl, 154 mmol/L NaCl, and 1 mmol/L EDTA, pH 7.4); iii) phosphatidylcholine liposomes, 15 μL; and iv) sample (1:10 diluted plasma or tear fluid, 10 μL). The final assay volume was 400 μL. The following tubes and controls were included: blank tube (buffer as a sample without PLTP), tube for total radioactivity per assay (total counts in 15 μL), and a control sample (frozen plasma or serum in 0.5-mL aliquots stored at −70°C). Each of the earlier-described assay tubes was run in duplicate. The tubes were incubated at 37°C for 45 minutes in a water bath with light shaking. After incubation, the tubes were placed on ice and the reaction was stopped by adding 300 μL of stop-mix solution containing 7.2 g NaCl and 10.5 g MnCl2 in 224 mL of distilled H2O and 8 mL Heparin (5000 IU/mL; Lövens, Ballerup, Denmark). After addition of the stop-mix solution, the tubes were vortexed vigorously for 10 seconds, kept for 10 minutes at room temperature, and then centrifuged for 10 minutes at 15,000 rpm. After centrifugation, 500 μL of supernatant was used for radioactivity counting. As a control with a normal PLTP activity (5000−7000 nmol/h/mL), fresh plasma or serum was collected and stored at −70°C in 0.5-mL aliquots. PLTP activity of this control was monitored in each assay series (in the beginning and at the end of the sample series). As a low PLTP activity control, serum or plasma with a low activity (∼2500 nmol/mL/h) was used. This control was stored under the same conditions as the normal control.
Statistics
The Student's t-test or the Mann-Whitney U-test were used where appropriate for between-group statistical comparisons.
Results
PLTP Is Present in Plasma and in Tear Fluid in WT Mice
Blood samples were drawn from WT and PLTP KO mice. High immunoreactivity for PLTP was seen in the plasma of WT mice but was totally absent in the plasma of PLTP KO mice (Figure 1). Pooled tear fluid samples of 3 eyes derived from both normal and PLTP KO groups then were analyzed using Western blot with anti-PLTP mAb59 antibody. Normal C57BL/6 mice showed strong immunoreactivity at the apparent molecular weight of approximately 80 kDa, at the predicted molecular weight of PLTP. In the tear fluid of PLTP KO mice no immunoreactive PLTP was detected (data not shown).
Figure 1.
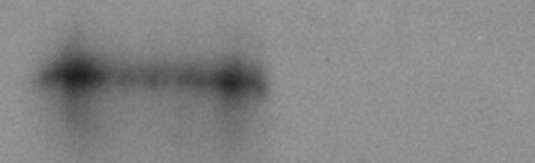
Western blot analysis of WT and PLTP KO mice plasma. Plasma collected from WT (left) and PLTP KO (right) mice were run on 12.5% SDS–polyacrylamide gel electrophoresis and electrically transferred to a nitrocellulose membrane. The membranes were subjected to electrochemiluminescence detection for PLTP using mAb59.
Aqueous Tear Production
The pharmacologic cholinergic receptor blockade with transdermal scopolamine patches leads to a significant reduction of aqueous tear production.24,25 PLTP KO and WT mice had similar tear production before induction of EIDE (2.1 ± 0.2 and 2.2 ± 0.2 mm, respectively). After 7 days of EIDE the tear productions were decreased significantly in WT and PLTP KO mice (0.2 ± 0.1 mm for both). No statistical difference in tear production in these two groups was detected after EIDE.
Corneal Histology
Mouse corneas were stained with H&E and PAS. WT mouse corneas showed no evidence of ocular surface disease (Figures 2A and 3A). In contrast, in PLTP KO mouse corneas, detaching apical cells were observed significantly more frequently (Figures 2B and 3B; P = 0.015). The number of these detaching apical cells was even higher in both EIDE-induced WT (Figures 2C and 3C) and PLTP KO (Figures 2D and 3D) mouse corneas (both P < 0.001).
Figure 2.
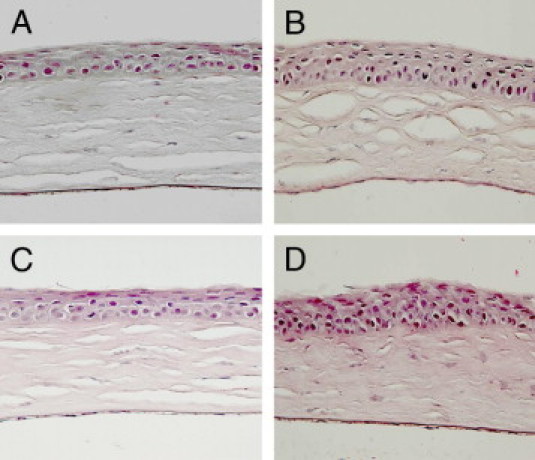
Histology of corneas of WT and PLTP KO mice. Histologic sections of paraffin-embedded corneas were stained with H&E. A: Normal cornea of untreated C57BL/6 mouse. B: Cornea of untreated PLTP KO mouse with increased number of detaching apical cells. C: Cornea of normal WT mouse after 7 days of treatment with scopolamine patch. D: After 7 days of treatment with scopolamine patch PLTP KO showed corneal erosion and hyperkeratinization. Original magnification, ×40.
Figure 3.
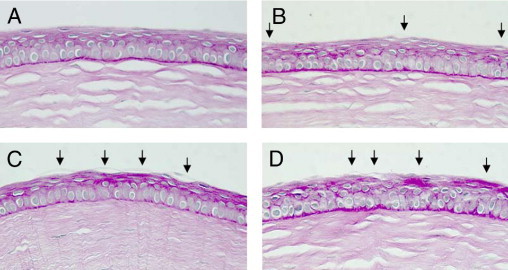
PAS staining of WT and PLTP KO mouse corneas. A: Normal cornea of WT C57BL/6 mouse. B: Cornea staining of untreated PLTP KO mouse with visible detaching superficial corneal epithelial cells. C: Cornea of normal WT mouse after 7 days of EIDE. D: After 7 days of EIDE, PLTP KO showed significant damage to the corneal epithelium. Arrows indicate detaching apical cells. Original magnification, ×40.
During the study period a total of 253 PLTP KO mice were housed in our laboratory. In this group, six (2.4%) spontaneous perforations were observed; in the same period of time WT C57BL/6 mice (n = 631) had no corneal perforations. We analyzed one of the perforated eyes by staining whole eye with H&E (Figure 4). The perforation was observed paracentrally and it had self-sealed by outpunching of the iris to the perforated ulcus. Stromal damage was evident and infiltration of lymphocytes to the ulcus area was seen. Adjacent epithelia was disorganized and thickened.
Figure 4.
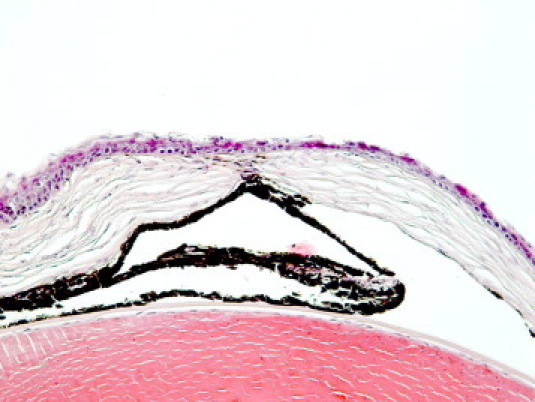
H&E staining of a spontaneously perforated PLTP KO mouse cornea. Original magnification, ×40.
Corneal Epithelial Permeability Is Increased Significantly in PLTP KO Mice
Increased corneal epithelial permeability to CF is a characteristic finding in dry eye. Corneal epithelial permeability to CF (molecular weight, 750 Da) was assessed in WT, PLTP KO, and EIDE WT and PLTP KO mice. Compared with WT mice, permeability to CF was increased significantly in PLTP KO mice and also in both EIDE mice groups (Figure 5).
Figure 5.
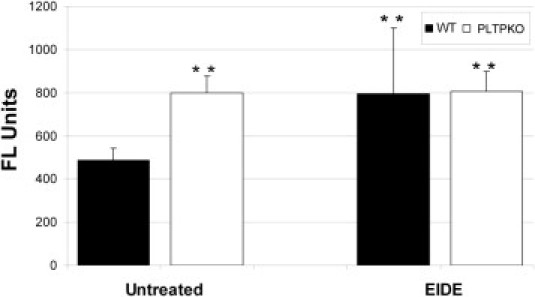
Corneal permeability to 750 Da CF in untreated and after 7 days of EIDE in WT (black bars) and PLTP KO (white bars) mice. **P < 0.01 compared with WT mice. The results are reported as fluorescence units/mg corneal tissue. FL units, units of fluorescein emission at 535 nm.
PLTP KO Mice Have Decreased Expression of the Tight Junction Protein Occludin
The normal cornea barrier function is maintained by tight junctions in the differentiated apical corneal epithelial cells. Increased occludin cleavage (ie, diminished occludin expression) has been reported in dry eye.25 Corneal endothelium showed strong immunoreactivity against polyclonal occludin antibody and this immunoreactivity was used to normalize occludin expression in the epithelium, that is, epithelial immunoreactivity was compared with endothelial immunoreactivity, which was considered to be constant in all studied mice groups. In WT mice occludin protein expression was seen in the endothelium as well as in the epithelium, but not in the corneal stroma (not shown). The expression pattern for occludin in PLTP KO mice was significantly different: endothelium showed bright immunofluorescence but epithelial expression was reduced significantly (not shown). Both EIDE mice showed decreased epithelial occludin protein expression (not shown). Quantitation of epithelial occludin protein expression confirmed these findings and showed a significantly decreased expression of occludin in PLTP KO mice as well as in EIDE PLTP KO mice compared with WT mice (P < 0.05; Figure 6). Although epithelial occludin expression was lower in EIDE WT mice compared with WT mice, this result was not significant (P = 0.11).
Figure 6.
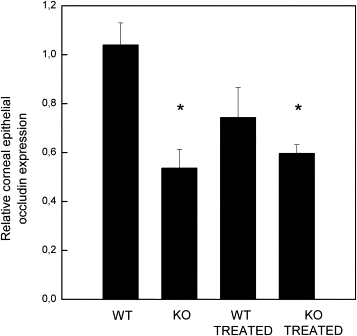
Corneal epithelial occludin expression in WT and PLTP KO mice. Compared with normal C57BL/6 mice PLTP KO mice showed significant reduction in occludin expression. Scopolamine blockade also reduces occludin expressions in PLTP KO mice and to a lesser extent in C57BL/6 mice. The measured epithelial anti-occludin fluorescence values were standardized by dividing epithelial fluorescence count with endothelial fluorescence values. *P < 0.05 compared with untreated WT C57BL/6 mice.
PLTP KO Mice Show Normal Histology of Eyelids
In several dry eye KO models, such as ACAT-1 null (ACAT-1−/−) mice, the dry eye symptoms arise from the atrophy of the palpebral glands.26 To verify that the observed dry eye induction was not caused by atrophy of the meibomian glands we examined the histology of WT and PLTP KO mice eyelids. The histologic analysis of PLTP KO mice eyelids was normal and the histology of meibomian glands was similar to those in WT mice (Figure 7).
Figure 7.
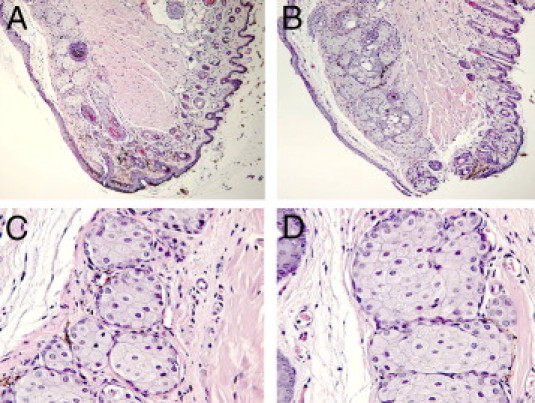
Histology of meibomian glands of WT C57BL/6 and PLTP KO mice. Tarsal sections of WT and PLTP KO C57BL/6 mice were prepared from eyelids and used for histologic examination (H&E staining). The mice were 8-week-old females. A: Structure of normal mouse lid (original magnification, ×10). B: Structure of PLTP KO mouse lid (original magnification, ×10). Structure of meibomian glands of normal (C) and PLTP KO (D) mice (original magnification, ×40).
Tear Hyperosmolarity Increases PLTP Activity in Human DES Patients
Tear fluid hyperosmolarity is considered a universal finding in DES patients.3 Accordingly, we measured the tear fluid osmolarity for 7 DES patients and 4 healthy controls (Figure 8). Dry eye patients showed significantly higher tear fluid osmolarity compared with healthy controls (302 to 309 versus 320 to 336 mOsm/L; P < 0.001). After the osmolarity measurements a tear fluid sample of approximately 5 μL was collected from the lower conjunctival sac and PLTP activity was assessed from these samples. In DES patients PLTP activity was found to be significantly higher (8873 ± 2118 μmol/mL/h; range, 6276–12,545 μmol/mL/h) compared with healthy controls (4180 ± 1490 μmol/mL/h; range, 2492–6220 μmol/mL/h) (P = 0.003).
Figure 8.
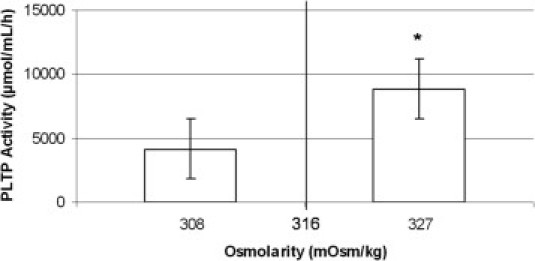
Assessment of tear fluid PLTP activity in human DES patients and healthy controls. DES patients showed significantly increased tear fluid osmolarity (P < 0.001) with a concomitant increases in tear fluid PLTP activity (*P = 0.003).
Discussion
We report here that mice deficient in PLTP show characteristic DES findings. The PLTP KO mice showed morphologic changes in corneal epithelium, increased corneal permeability to CF, and cleavage of corneal epithelial occludin. The tear fluid production in PLTP KO mice was constant and comparable with that shown in wild-type littermates. Also, the eyelid and meibomian gland morphologies were normal. During our experimental follow-up time 2.4% of PLTP KO mice suffered spontaneous perforation of an eye, whereas the wild-type animals grown under the same conditions had no eye problems. Finally, our preliminary studies showed increased tear fluid PLTP activity in human DES patients compared with healthy controls, suggesting a protective role for PLTP in human beings also.
Dry eye symptoms detectable in PLTP-deficient mice were compared with the dry eye mouse model described by Dursun et al,24 in which tear fluid production is attenuated by the cholinergic blockade with scopolamine. This approach was chosen because it does not cause anatomic changes in lacrimal or palpebral structures. Compared with the cholinergic blockade, the PLTP KO mice showed similar corneal changes, increased corneal permeability, and cleavage of epithelial occludin. Furthermore, the cholinergic blockade reduced tear production as described earlier.24,25 We also treated the PLTP KO mice with scopolamine to see whether the effects are additive. PLTP KO with EIDE did not induce further corneal permeability or occludin cleavage. This could be explained by the two models separately causing maximal permeability to CF with the concentrations used. The activation of matrix metalloproteases, especially matrix metalloprotease-9, which primarily are responsible for occludin cleavage in corneal epithelium,27 seems to be involved with the same outcome both in PLTP KO animals and with EIDE treatment.
Several animal models that mimic human dry eye have been introduced and they have been important tools for investigating the multiple factors that have been implicated in the pathogenesis of DES. Yet, several of the present DES models resemble tear deficiency or Sjögren syndrome–like autoimmune disease because they diminish aqueous tear production via mechanical,28 autoimmune,29,30 or chemical24 effect on the lacrimal gland. Because the tear lipid film imbalance and the evaporative DES are common diseases of the eye especially among elderly citizens, a model that carefully mimics this condition is of utmost importance. Mechanical cauterization of meibomian gland orifices presented by Gilbard et al31 and other lid surgery techniques definitely affect tear lipid film composition, but the normal appearance of palpebral aperture is easily hampered. Different mouse models of meibomian gland dysfunction (including aplasia, hypoplasia, and atrophy) have been generated through mutant and transgenic techniques.32 These models definitely have their advantages; however, such a total absence of tear lipids is rare in human DES. Also, the tear lipid film composition seems to differ from direct meibomian secretion.33 Few animal models introduced for blepharitis34 show reduction in tear fluid lipid production associated with dry eye symptoms, but also in these models the normal anatomic appearance of either lid or eye surface typically is altered. In addition, the blepharitis itself induces ocular surface irritation and alters the immunologic profile of the ocular surface, which is an important factor in the development of DES.
We have reported previously that PLTP is found in normal human tear fluid in at least twofold higher concentration from that observed in plasma.16 Both apolipoproteins A-I and E that are capable of interacting with PLTP in plasma35 are absent in tear fluid, and therefore these protein–protein interactions are lacking.21 Nevertheless, PLTP is active in tear fluid.21 Very recently we showed that PLTP interacts with mucin in tear fluid, which can be a functionally important complex.17 Based on the current data we suggest that a possible explanation for the presence of PLTP in the tear fluid may be a scavenging property of PLTP: the lipid contamination from the anterior lipid layer of the tear film is directed to the ocular surface. Here, PLTP can remove the harmful overload of lipids and other lipophilic substances and transfer these to the lacrimal drainage system. It needs to be emphasized that PLTP can maximally bind and transfer 43 and 13 moles of phosphatidylcholine and cholesterol, respectively, per mole of PLTP,36 allowing for a very efficient lipid transfer activity. This mode of action is very similar to the one described for human tear lipocalin.14 In line with these findings human DES patients show significantly increased levels of PLTP activity in tears, suggesting an induction of PLTP activity in hyperosmolar conditions. However, it needs to be emphasized that at present we have no evidence that the absence of PLTP in the tear fluid per se causes DES. It also may be envisioned that PLTP may possess anti-inflammatory or other protective functions in the tear fluid. This is in line with the proposed pleiotropic effects of PLTP in plasma,37 as well as with the very high lipid binding and transfer potential of PLTP.36 We believe that the mouse PLTP KO model serves as a relevant model of human DES. The specific mechanism of PLTP in lipid transfer processes in tear, detailed lipid composition of tear fluid, and possible alterations in PLTP activity in DES patients will be important targets to investigate in more detail to define the exact role of PLTP in DES.
Acknowledgment
We thank Leena Kainulainen for experienced animal care.
Footnotes
Supported by the Finnish Eye Foundation (N.L.S., J.M.H.), The Finnish Eye and Tissue Bank Foundation (N.L.S., J.M.H.), the Finnish Foundation for Cardiovascular Research (M.J.), the Academy of Finland (J.M.H.), and the Sigrid Juselius Foundation (M.J., J.M.H.).
References
- 1.Goto E., Endo K., Suzuki A., Fujikura Y., Matsumoto Y., Tsubota K. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2003;44:533–539. doi: 10.1167/iovs.02-0170. [DOI] [PubMed] [Google Scholar]
- 2.Gilbard J.P., Farris R.L. Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch Ophthalmol. 1979;97:1642–1646. doi: 10.1001/archopht.1979.01020020210003. [DOI] [PubMed] [Google Scholar]
- 3.Tomlinson A., Khanal S., Ramaesh K., Diaper C., McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder S.C., Jones D., Ji Z., Afonso A., Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 5.Bron A.J., Tiffany J.M., Gouveia S.M., Yokoi N., Voon L.W. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Pakarinen M., Tervo T., Tarkkanen A. Tarsorraphy in the treatment of persistent corneal lesions. Acta Ophthalmol Suppl. 1987;182:69–73. doi: 10.1111/j.1755-3768.1987.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 7.Holly F.J. Formation and rupture of the tear film. Exp Eye Res. 1973;15:515–525. doi: 10.1016/0014-4835(73)90064-x. [DOI] [PubMed] [Google Scholar]
- 8.McCulley J.P., Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–88. [PMC free article] [PubMed] [Google Scholar]
- 9.Butovich I.A. The Meibomian puzzle: combining pieces together. Prog Retin Eye Res. 2009;28:483–498. doi: 10.1016/j.preteyeres.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H.B., Yamabayashi S., Ou B., Tanaka Y., Ohno S., Tsukahara S. Structure and composition of rat precorneal tear film: A study by an in vivo cryofixation. Invest Ophthalmol Vis Sci. 1997;38:381–387. [PubMed] [Google Scholar]
- 11.Greiner J.V., Glonek T., Korb D.R., Leahy C.D. Meibomian gland phospholipids. Curr Eye Res. 1996;15:371–375. doi: 10.3109/02713689608995827. [DOI] [PubMed] [Google Scholar]
- 12.McCulley J.P., Shine W.E. Meibomian secretions in chronic blepharitis. Adv Exp Med Biol. 1998;438:319–326. doi: 10.1007/978-1-4615-5359-5_45. [DOI] [PubMed] [Google Scholar]
- 13.Steinman R.M., Mellman I.S., Muller W.A., Cohn Z.A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasgow B.J., Marshall G., Gasymov O.K., Abduragimov A.R., Yusifov T.N., Knobler C.M. Tear lipocalins: potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999;40:3100–3107. [PubMed] [Google Scholar]
- 15.Saaren-Seppala H., Jauhiainen M., Tervo T.M., Redl B., Kinnunen P.K., Holopainen J.M. Interaction of purified tear lipocalin with lipid membranes. Invest Ophthalmol Vis Sci. 2005;46:3649–3656. doi: 10.1167/iovs.05-0176. [DOI] [PubMed] [Google Scholar]
- 16.Jauhiainen M., Setala N.L., Ehnholm C., Metso J., Tervo T.M., Eriksson O., Holopainen J.M. Phospholipid transfer protein is present in human tear fluid. Biochemistry. 2005;44:8111–8116. doi: 10.1021/bi050151k. [DOI] [PubMed] [Google Scholar]
- 17.Setala N.L., Holopainen J.M., Metso J., Yohannes G., Hiidenhovi J., Andersson L.C., Eriksson O., Robciuc A., Jauhiainen M. Interaction of phospholipid transfer protein with human tear fluid mucins. J Lipid Res. 2010;51:3126–3134. doi: 10.1194/jlr.M006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huuskonen J., Ehnholm C. Phospholipid transfer protein in lipid metabolism. Curr Opin Lipidol. 2000;11:285–289. doi: 10.1097/00041433-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Tall A.R., Krumholz S., Olivecrona T., Deckelbaum R.J. Plasma phospholipid transfer protein enhances transfer and exchange of phospholipids between very low density lipoproteins and high density lipoproteins during lipolysis. J Lipid Res. 1985;26:842–851. [PubMed] [Google Scholar]
- 20.Jiang X.C., D'Armiento J., Mallampalli R.K., Mar J., Yan S.F., Lin M. Expression of plasma phospholipid transfer protein mRNA in normal and emphysematous lungs and regulation by hypoxia. J Biol Chem. 1998;273:15714–15718. doi: 10.1074/jbc.273.25.15714. [DOI] [PubMed] [Google Scholar]
- 21.Setala N.L., Holopainen J.M., Metso J., Wiedmer S.K., Yohannes G., Kinnunen P.K., Ehnholm C., Jauhiainen M. Interfacial and lipid transfer properties of human phospholipid transfer protein: implications for the transfer mechanism of phospholipids. Biochemistry. 2007;46:1312–1319. doi: 10.1021/bi0621866. [DOI] [PubMed] [Google Scholar]
- 22.Siggins S., Jauhiainen M., Olkkonen V.M., Tenhunen J., Ehnholm C. PLTP secreted by HepG2 cells resembles the high-activity PLTP form in human plasma. J Lipid Res. 2003;44:1698–1704. doi: 10.1194/jlr.M300059-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X.C., Bruce C., Mar J., Lin M., Ji Y., Francone O.L., Tall A.R. Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels. J Clin Invest. 1999;103:907–914. doi: 10.1172/JCI5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dursun D., Wang M., Monroy D., Li D.Q., Lokeshwar B.L., Stern M.E., Pflugfelder S.C. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- 25.Pflugfelder S.C., Farley W., Luo L., Chen L.Z., De Paiva C.S., Olmos L.C., Li D.Q., Fini M.E. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagyu H., Kitamine T., Osuga J., Tozawa R., Chen Z., Kaji Y., Oka T., Perrey S., Tamura Y., Ohashi K., Okazaki H., Yahagi N., Shionoiri F., Iizuka Y., Harada K., Shimano H., Yamashita H., Gotoda T., Yamada N., Ishibashi S. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J Biol Chem. 2000;275:21324–21330. doi: 10.1074/jbc.M002541200. [DOI] [PubMed] [Google Scholar]
- 27.Smith V.A., Rishmawi H., Hussein H., Easty D.L. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001;85:147–153. doi: 10.1136/bjo.85.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maitchouk D.Y., Beuerman R.W., Ohta T., Stern M., Varnell R.J. Tear production after unilateral removal of the main lacrimal gland in squirrel monkeys. Arch Ophthalmol. 2000;118:246–252. doi: 10.1001/archopht.118.2.246. [DOI] [PubMed] [Google Scholar]
- 29.Humphreys-Beher M.G., Hu Y., Nakagawa Y., Wang P.L., Purushotham K.R. Utilization of the non-obese diabetic (NOD) mouse as an animal model for the study of secondary Sjogren's syndrome. Adv Exp Med Biol. 1994;350:631–636. doi: 10.1007/978-1-4615-2417-5_105. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Z., Stevenson D., Schechter J.E., Mircheff A.K., Atkinson R., Trousdale M.D. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:25–32. doi: 10.1097/00003226-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Gilbard J.P., Rossi S.R., Heyda K.G. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 1989;96:1180–1186. doi: 10.1016/s0161-6420(89)32753-9. [DOI] [PubMed] [Google Scholar]
- 32.Barabino S., Dana M.R. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 2004;45:1641–1646. doi: 10.1167/iovs.03-1055. [DOI] [PubMed] [Google Scholar]
- 33.Butovich I.A. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008;49:3779–3789. doi: 10.1167/iovs.08-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan C.C., Gery I., Kohn L.D., Nussenblatt R.B., Mozes E., Singer D.S. Periocular inflammation in mice with experimental systemic lupus erythematosus: A new experimental blepharitis and its modulation. J Immunol. 1995;154:4830–4835. [PubMed] [Google Scholar]
- 35.Karkkainen M., Oka T., Olkkonen V.M., Metso J., Hattori H., Jauhiainen M., Ehnholm C. Isolation and partial characterization of the inactive and active forms of human plasma phospholipid transfer protein (PLTP) J Biol Chem. 2002;277:15413–15418. doi: 10.1074/jbc.M112247200. [DOI] [PubMed] [Google Scholar]
- 36.Nishida H.I., Nishida T. Phospholipid transfer protein mediates transfer of not only phosphatidylcholine but also cholesterol from phosphatidylcholine-cholesterol vesicles to high density lipoproteins. J Biol Chem. 1997;272:6959–6964. doi: 10.1074/jbc.272.11.6959. [DOI] [PubMed] [Google Scholar]
- 37.Albers J.J., Cheung M.C. Emerging roles for phospholipid transfer protein in lipid and lipoprotein metabolism. Curr Opin Lipidol. 2004;15:255–260. doi: 10.1097/00041433-200406000-00004. [DOI] [PubMed] [Google Scholar]


