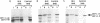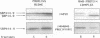Abstract
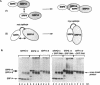
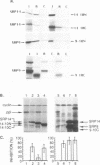
The targeting of nascent polypeptide chains to the endoplasmic reticulum is mediated by a cytoplasmic ribonucleoprotein, the signal recognition particle (SRP). The 9 kD (SRP9) and the 14 kD (SRP14) subunits of SRP are required to confer elongation arrest activity to the particle. SRP9 and SRP14 form a heterodimer which specifically binds to SRP RNA. We have constructed cDNAs that encode single polypeptide chains comprising SRP9 and SRP14 sequences in the two possible permutations linked by a 17 amino acid peptide. We found that both fusion proteins specifically bound to SRP RNA as monomeric molecules folded into a heterodimer-like structure. Our results corroborate the previous hypothesis that the authentic heterodimer binds to SRP RNA in equimolar ratio. In addition, both fusion proteins conferred elongation arrest activity to SRP(-9/14), which lacks this function, and one fusion protein could functionally replace the heterodimer in the translocation assay. Thus, the normal N-and C-termini of both proteins have no essential role in folding, RNA-binding and in mediating the biological activities. The possibility to express the heterodimeric complex as a single polypeptide chain facilitates the analysis of its functions and its structure in vivo and in vitro.
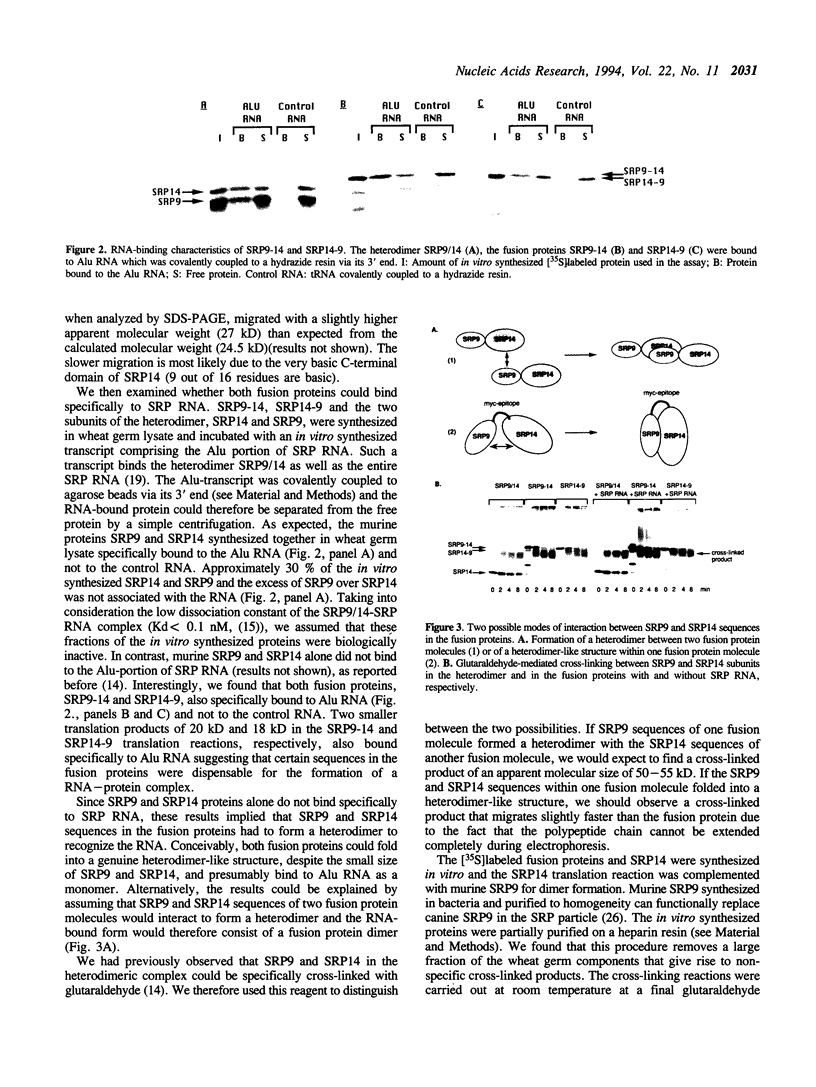
Full text
PDF


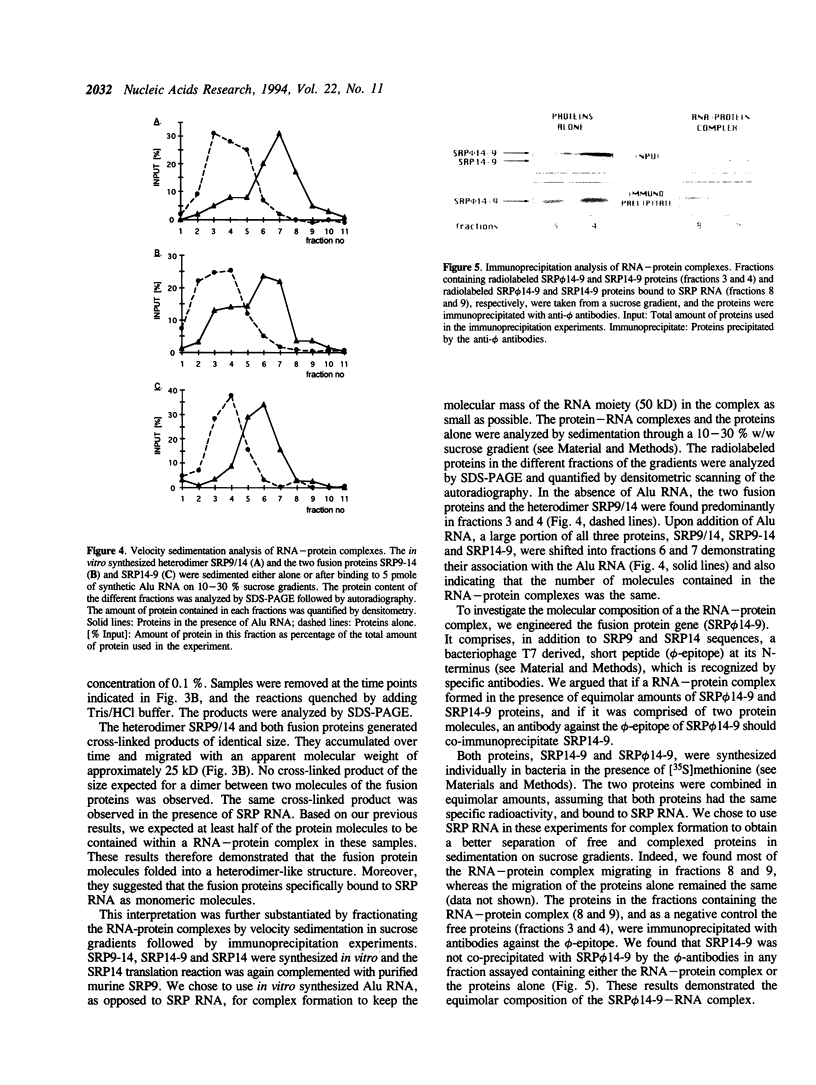

Images in this article
Selected References
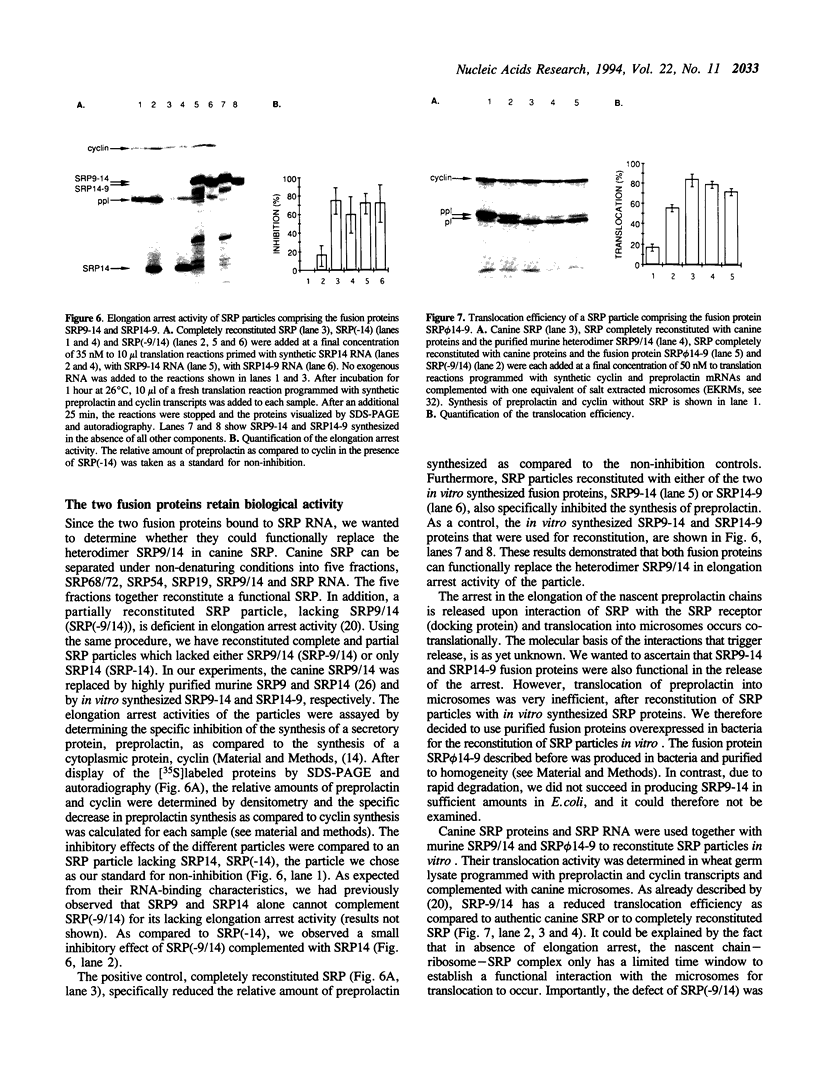
These references are in PubMed. This may not be the complete list of references from this article.
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
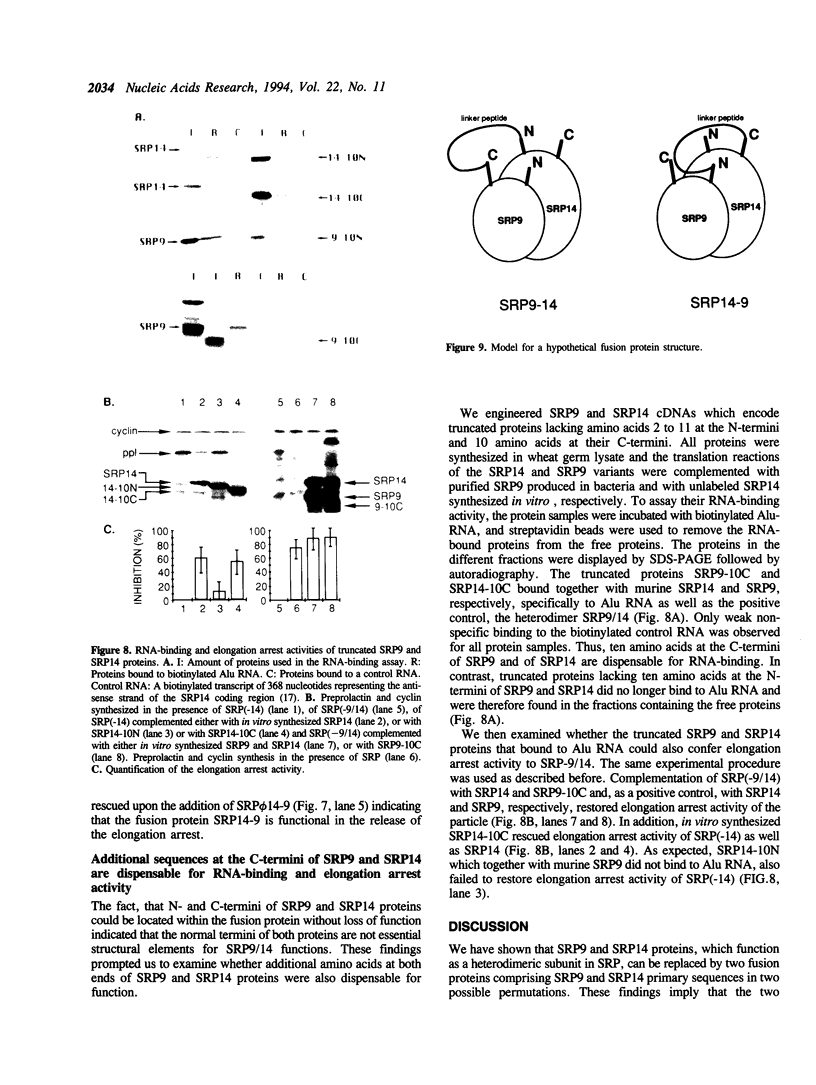
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R. Protein translocation across the endoplasmic reticulum: a tunnel with toll booths at entry and exit. Cell. 1993 Nov 19;75(4):589–592. doi: 10.1016/0092-8674(93)90476-7. [DOI] [PubMed] [Google Scholar]
- Glockshuber R., Malia M., Pfitzinger I., Plückthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990 Feb 13;29(6):1362–1367. doi: 10.1021/bi00458a002. [DOI] [PubMed] [Google Scholar]
- Janiak F., Walter P., Johnson A. E. Fluorescence-detected assembly of the signal recognition particle: binding of the two SRP protein heterodimers to SRP RNA is noncooperative. Biochemistry. 1992 Jun 30;31(25):5830–5840. doi: 10.1021/bi00140a019. [DOI] [PubMed] [Google Scholar]
- Larsen N., Zwieb C. SRP-RNA sequence alignment and secondary structure. Nucleic Acids Res. 1991 Jan 25;19(2):209–215. doi: 10.1093/nar/19.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H., Dobberstein B. Structure and function of signal recognition particle (SRP). Mol Biol Rep. 1993 Aug;18(2):143–147. doi: 10.1007/BF00986769. [DOI] [PubMed] [Google Scholar]
- Lütcke H., Prehn S., Ashford A. J., Remus M., Frank R., Dobberstein B. Assembly of the 68- and 72-kD proteins of signal recognition particle with 7S RNA. J Cell Biol. 1993 Jun;121(5):977–985. doi: 10.1083/jcb.121.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W. RNA recognition: a family matter? Cell. 1993 Jun 4;73(5):837–840. doi: 10.1016/0092-8674(93)90265-r. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T., Uhlenbeck O. C. Circularly permuted DNA, RNA and proteins--a review. Gene. 1993 Mar 30;125(2):111–114. doi: 10.1016/0378-1119(93)90317-v. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Strub K., Walter P. Human SRP RNA and E. coli 4.5S RNA contain a highly homologous structural domain. Cell. 1988 Oct 7;55(1):4–6. doi: 10.1016/0092-8674(88)90003-7. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Römisch K., Webb J., Lingelbach K., Gausepohl H., Dobberstein B. The 54-kD protein of signal recognition particle contains a methionine-rich RNA binding domain. J Cell Biol. 1990 Nov;111(5 Pt 1):1793–1802. doi: 10.1083/jcb.111.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger D., Brennwald P., Liao X., Wise J. A. Identification of RNA sequences and structural elements required for assembly of fission yeast SRP54 protein with signal recognition particle RNA. Mol Cell Biol. 1993 Mar;13(3):1353–1362. doi: 10.1128/mcb.13.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. Elongation arrest is not a prerequisite for secretory protein translocation across the microsomal membrane. J Cell Biol. 1985 Jun;100(6):1913–1921. doi: 10.1083/jcb.100.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub K., Moss J., Walter P. Binding sites of the 9- and 14-kilodalton heterodimeric protein subunit of the signal recognition particle (SRP) are contained exclusively in the Alu domain of SRP RNA and contain a sequence motif that is conserved in evolution. Mol Cell Biol. 1991 Aug;11(8):3949–3959. doi: 10.1128/mcb.11.8.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub K., Walter P. Assembly of the Alu domain of the signal recognition particle (SRP): dimerization of the two protein components is required for efficient binding to SRP RNA. Mol Cell Biol. 1990 Feb;10(2):777–784. doi: 10.1128/mcb.10.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub K., Walter P. Isolation of a cDNA clone of the 14-kDa subunit of the signal recognition particle by cross-hybridization of differently primed polymerase chain reactions. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9747–9751. doi: 10.1073/pnas.86.24.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Ullu E., Tschudi C. Alu sequences are processed 7SL RNA genes. Nature. 1984 Nov 8;312(5990):171–172. doi: 10.1038/312171a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Disassembly and reconstitution of signal recognition particle. Cell. 1983 Sep;34(2):525–533. doi: 10.1016/0092-8674(83)90385-9. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Wood H., Luirink J., Tollervey D. Evolutionary conserved nucleotides within the E.coli 4.5S RNA are required for association with P48 in vitro and for optimal function in vivo. Nucleic Acids Res. 1992 Nov 25;20(22):5919–5925. doi: 10.1093/nar/20.22.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf D., Bernstein H. D., Johnson A. E., Walter P. The methionine-rich domain of the 54 kd protein subunit of the signal recognition particle contains an RNA binding site and can be crosslinked to a signal sequence. EMBO J. 1990 Dec;9(13):4511–4517. doi: 10.1002/j.1460-2075.1990.tb07902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C. Recognition of a tetranucleotide loop of signal recognition particle RNA by protein SRP19. J Biol Chem. 1992 Aug 5;267(22):15650–15656. [PubMed] [Google Scholar]