Abstract
Semaphorins were originally identified as axon guidance cues involved in the development of the nervous system. In recent years, it is emerging that they also participate in various biological systems, including physiological and pathological processes. In this review, we primarily focus on our cumulative findings for the role of semaphorins and their receptors in the regulation of the immune system, while also summarizing recent progress in the context of cardiovascular system.
Keywords: Semaphorin, immune cell interactions, cardiac morphogenesis, plexins
1. Introduction
Cumulative findings indicate that the nervous and immune systems have considerable overlap and links.1) For example, some axon guidance molecules, such as slits and ephrins, have been shown to regulate immune cell migration.2,3) In addition, T-cell-antigen-presenting cell contact sites, the so-called ‘immunological synapse’, are structurally similar to the ‘neurological synapse’ that connects pairs of neurons. These shared molecules and interactions play critical roles in inducing proper immune responses.
Semaphorins were named for properties that are analogous to the system of flags and lights that is used in rail and maritime communication. They were initially identified as repulsive axon guidance factors that direct neuronal axons to their appropriate targets.4) To date, more than 20 types of semaphorins have been identified, and they have diverse functions in many physiological processes, including cardiogenesis,5) angiogenesis,6) vasculogenesis,7) tumor metastasis,8) osteoclastogenesis,9) and immune regulation.10) We isolated the cDNA of Sema4D through the search for CD40 (an immune co-stimulatory molecule)-inducible genes by subtractive PCR-cloning. In addition, we isolated the cDNA of Sema6D from the cDNA library of the heart by PCR-cloning using degenerative primers. The identification of Sema4D and Sema6D has clarified the important roles of the semaphorin family in the immune and cardiac systems, respectively. Here, we review the pleiotropic functions of semaphorins, primarily focusing on their roles in immune responses and cardiac morphogenesis.
2. Semaphorins and their receptors
Semaphorins are secreted and membrane-associated proteins that are characterized by a conserved extracellular amino-terminal ‘Sema’ domain. Based on their C-terminal structures, this diverse group of proteins has been further divided into eight subclasses.11) Semaphorins in classes I (invertebrate) and IV–VII are membrane-associated, whereas those in classes II (invertebrate), III, and VIII (virally encoded) are secreted. Two groups of proteins, plexins and neuropilins (NPs), have been identified as the primary semaphorin receptors.12–14) Most membrane-bound semaphorins directly bind plexins, whereas class III semaphorins require NPs as obligate coreceptors. However, increasing evidence has shown that semaphorin receptor usage is more complex than previously thought.15) For example, Sema3E signals independently of NPs through plexin-D1,7) while Sema7A uses integrins to exert its functions in both the nervous and immune systems.16,17) In addition, two molecules unrelated to plexins and NPs, CD7218) and T-cell immunoglobulin and mucin domain containing protein 2 (TIM-2),19) functionally interact with Sema4D and Sema4A, respectively, in the immune system (Fig. 1 ).
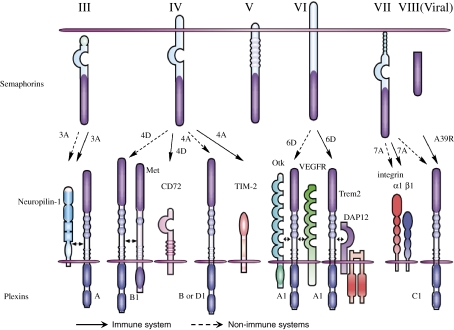
Figure 1.
Representative semaphorins and their receptors. Among the eight subclasses of semaphorins, class I and II semaphorins are found in invertebrates (not shown in figure) and class III–VII are vertebrate semaphorins. Classes II and III and viral semaphorins are secreted, whereas class IV–VI are transmembrane. Class VII represents GPI-anchored proteins. Sema3A directly binds NP-1 and associates with plexin-A1, resulting in inducing plexin-A-mediated signals. Although Sema4D binds to plexin-B1 in neurons, plexin-B1 couples with Met in epithelial cells and induces Sema4D-mediated cell outgrowth. In the immune system, Sema4D binds to CD72, which promotes B cell and DC activation. Sema4A recognizes plexin-B and D1 in the non-immune systems but uses TIM-2 as a receptor for T cell activation in the immune system. During cardiogenesis, plexin-A1 associates with OTK or VEGFR2 at distinct sites. However, plexin-A1 forms a receptor complex with TREM-2-DAP12, which is critical for DC activation and osteoclastogenesis. Sema7A has two types of receptors: α1β1 integrin for macrophage activation and plexin-C1 for inhibition of cell adhesion. Viral semaphorin A39R also recognizes plexin-C1 and modulates dendritic cell function.
Plexins are canonical semaphorin receptors that have large cytoplasmic domains. In the nervous system, plexin-mediated signals have been shown to exert diverse neural functions by regulating GTPase activities and cytoplasmic/receptor-type protein kinases.20) These signals are also involved in integrin-mediated attachment.21–23) Of note, plexins can associate with different co-receptors in distinct tissues to allow semaphorins to exert pleiotropic functions. For instance, plexin-A1 is associated with the tyrosine kinase receptors off-track (OTK) and vascular endothelial growth factor receptor 2 (VEGFR2) in heart morphogenesis.24) In another context, plexin-A1 forms a receptor complex with triggering receptor expressed on myeloid cell (TREM)-2/DNAX-activating protein 12 (DAP12) during osteoclastogenesis.9) Furthermore, plexin-B1 has been shown to associate with the receptor tyrosine kinases Met and ErbB2, inducing invasive growth of epithelial cells.25,26) These observations provide insight into the diversity of semaphorin functions (Fig. 1).
3. Immune semaphorins
(1). Sema4D: a semaphorin involved in B cell/dendritic cell (DC) activation (Fig. 2a).
Sema4D, also known as CD100, is the first semaphorin protein for which immunoregulatory functions were identified. In the immune system, abundant expression of Sema4D is observed in resting T cells. The basal expression of Sema4D in B cells and DCs is very low, but protein levels are considerably higher following cellular activation.18,27) Sema4D promotes B cell activation in the context of proliferation and antibody production. Plexin-B1 and CD72 have been identified as Sema4D receptors in the nervous and immune systems, respectively.14,18,22) CD72 contains two immunoreceptor tyrosine-based inhibitory motifs (ITIM) in its cytoplasmic domain,28) and is known to function as a negative regulator of B cells through the recruitment of the tyrosine phosphatase SHP-1 to its phosphorylated ITIM.18) Ligation of Sema4D to CD72 induces dissociation of SHP-1, resulting in B cell activation (Fig. 2a ). Consistently, Sema4D-deficient mice display impaired antibody production, implicating Sema4D in B cell activation. In addition to its involvement in B-cell responses, Sema4D is involved in T cell activation by promoting the activation and maturation of DCs. In fact, Sema4D-deficient mice display impaired generation of antigen-specific T cells.18,27) Although Sema4D is a transmembrane protein, its extracellular region is proteolytically cleaved from the surface of activated lymphocytes in a metalloprotease-dependent process.29) Sema4D is also cleaved from the surface of platelets by the metalloprotease ADAM17.30) Elevated levels of soluble Sema4D protein are detectable in the culture supernatants of activated lymphocytes and in the sera of immunized and autoimmune mice.29)
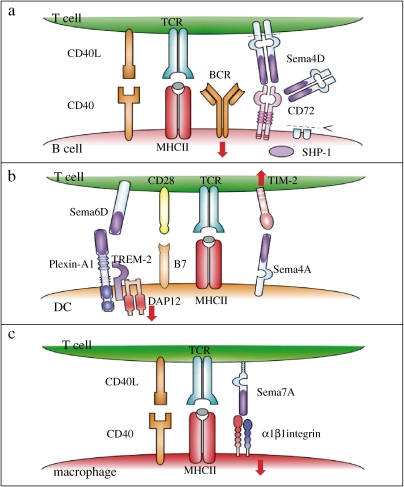
Figure 2.
Semaphorins in immune cell interactions. Semaphorins are involved in various phases of immune cell responses. (a) During T-cell-mediated B-cell activation, engagement of CD72 by Sema4D induces dephosphorylation of CD72 and dissociation from SHP-1, which results in enhancement of BCR signals. Sema4D can also be cleaved proteolytically and function as a soluble form in an autocrine/paracrine manner. (b) Sema6D on T cells can activate DCs through plexin-A1. Sema4A on DCs binds to TIM-2 and activates T cells. (c) T-cell-mediated inflammatory responses require antigen–MHC class II–TCR interaction and CD40L–CD40 signals. However, the interaction between Sema7A and α1β1 integrin is also critical for activation of inflammatory cells such as macrophages.
(2). Sema4A: a semaphorin involved in both T cell activation and differentiation.
Sema4A is another class IV semaphorin. In the immune system, Sema4A is constitutively expressed on DCs.19) The expression of Sema4A is also detectable in activated T cells and T helper type 1 (Th1)-polarized cells. DC-derived Sema4A and T cell-derived Sema4A play different roles during the course of T cell-mediated immunity; in particular, DC-derived Sema4A is crucial for antigen-specific T cell priming, whereas T cell-derived Sema4A is involved in helper T cell differentiation31) (Fig. 2b). Indeed, the critical involvement of Sema4A in the differentiation of helper T cells has been demonstrated by the phenotypes of Sema4A-deficient mice. Sema4A deficient mice show impaired responses to heat-killed Propionibacterium acnes, a Th1-inducing agent. In contrast, these mice show enhanced T helper type 2 (Th2) responses against infection of Nippostrongylus brasiliensis, a Th2-inducing intestinal nematode.31) Furthermore, Sema4A-deficient mice on a Th2-prone BALB/c background spontaneously develop atopic dermatitis (T.M. unpublished data), indicating that Sema4A is involved in the regulation of Th1/Th2 development. Several lines of evidence support a role of TIM-2, which is induced on activated T cells, as a functional receptor for Sema4A.19) The expression of TIM-2 is preferentially upregulated on T cells during Th2 differentiation. Administration of recombinant TIM-2 protein suppresses the development of experimental autoimmune encephalomyelitis (EAE) is suitable mice immunized with proteolipid protein-derived peptide by inhibiting the generation of Th1 cells.32) Furthermore, TIM-2-deficient mice show exacerbated lung inflammation accompanied by dysregulated Th2 responses.33) It thus appears that Sema4A-TIM-2 interactions negatively regulate Th2 responses. However, there are some phenotypic differences between Sema4A-deficient and TIM-2-deficient mice. For example, T cells from TIM-2-deficient mice but not from Sema4A-deficient mice show enhanced basal proliferation. This observation raises the possibility that Sema4A and/or TIM-2 have other binding partners. Indeed, T cells express members of the plexin-B family and plexin-D1, both of which have Sema4A-binding activities.34)
(3). Sema6D and plexin-A1: an interaction involved in the T cell-dendritic cell interface and osteoclastogenesis.
Plexins are the canonical/primary semaphorin receptors during the development of the nervous and cardiovascular systems. Class III semaphorins bind a receptor complex formed by plexin-A1 and NP-1. Additionally, plexin-A1 serves as a direct binding receptor for the class VI semaphorins, Sema6C and Sema6D.24,35) In the immune system, plexin-A1 is specifically expressed by DCs.36) The function of plexin-A1 in DCs has been shown using RNA interference and by analysis of plexin-A1-deficient mice.9,36) ‘Knockdown’ of plexin-A1 in DCs by short hairpin RNA impairs their ability to activate T cells in vitro and in vivo. In addition, plexin-A1-deficient DCs are poor at stimulating antigen-specific T cells.36) In vivo, plexin-A1-deficient mice show impaired T cell-priming.9) These observations indicate that plexin-A1 expression in DCs is required for the initial activation and efficient generation of antigen-specific T cells. It is worthy of note that plexin-A1-deficient mice develop osteopetrosis as a result of decreased bone resorption due to defective osteoclastogenesis.9) Sema6D was suggested to be a ligand for plexin-A1. Indeed, recombinant Sema6D protein binds to and activates DCs, and this activity is profoundly attenuated in plexin-A1-deficient DCs. These observations suggest that Sema6D-expression on T cells is involved in DC-activation. The expression of Sema6D is also observed in osteoclasts. Recombinant Sema6D protein enhances in vitro osteoclastogenesis, suggesting that Sema6D and plexin-A1 might function in osteoclastogenesis in an osteoclast-autonomous manner. Plexin-A1 forms a receptor complex with TREM-2 and the adaptor molecule DAP12 in DCs and osteoclasts.9) Interestingly, DAP12-deficient mice show impaired T cell responses and develop osteopetrosis,37) and genetic mutations in human DAP12 or TREM-2 result in a bone fracture syndrome called Nasu-Hakola disease,38) supporting the idea that plexin-A1 physiologically associates with the TREM-2/DAP12 complex.
(4). Sema7A: a semaphorin involved in inflammatory responses.
Sema7A, also known as CD108, is a membrane-associated GPI-anchored protein.39) In the nervous system, Sema7A has been shown to promote olfactory bulb axon outgrowth and is required for the proper formation of the lateral olfactory tract during embryonic development.16) Plexin-C1 was initially identified as a receptor for Sema7A.14) However, in its Sema domain, Sema7A contains an arginine–glycine–aspartate sequence that is a well conserved integrin-binding motif, and it exerts its function through β1 integrin, not through plexin-C1.16) In the immune system, the expression of Sema7A is induced on activated T cells, and it is involved in T cell-mediated inflammatory immune responses.17) Recombinant Sema7A protein stimulates monocytes/macrophages through α1β1 integrin, also known as very late antigen-1, inducing the production of proinflammatory cytokines17) (Fig. 2c). Consistently, Sema7A-deficient mice are resistant to the development of inflammation, including hapten-induced contact hypersensitivity and experimental autoimmune EAE.17) These observations indicate that interactions between Sema7A and α1β1 integrin are crucial for T cell-mediated macrophage activation at sites of inflammation. Plexin-C1 is also expressed in macrophages; however, recombinant Sema7A protein induces normal production of proinflammatory cytokines in plexin-C1-deficient macrophages (unpublished data). Therefore, at least for T cell–macrophage interactions, α1β1 integrin but not plexin-C1 seems to be the predominant receptor for Sema7A, implying that integrin-mediated signaling is a common mechanism for Sema7A functions in both the nervous and immune systems.
(5). Sema3A and plexin-A4: a semaphorin and semaphorin receptor required for negative regulation of T cell responses.
Sema3A was the first semaphorin identified in vertebrates. Sema3A directly binds to NP-1, inducing the activation of plexin-A proteins and the transduction of axon repulsive signals. Several lines of evidence suggest that Sema3A also functions in the immune system (Fig. 1, Table 1 ). The expression of Sema3A is detected in activated DCs, T cells, and some tumor cells. Sema3A inhibits spontaneous monocyte migration in vitro. In addition, Sema3A suppresses T cell proliferation by inhibiting actin cytoskeletal reorganization and downregulating MAPK signaling.40) Furthermore, Sema3A-deficient T cells exhibit enhanced in vitro proliferative responses to anti-CD3 antibodies.41) These observations suggest that Sema3A serves as a negative regulator of T cells. Similar to other plexin-A proteins, plexin-A4 forms a receptor complex with NP-1 to transduce class III semaphorin-mediated signaling or directly binds to Sema6A.42) Plexin-A4-deficient T cells exhibit hyper-proliferation and enhanced TCR signals upon anti-CD3 stimulation. Furthermore, plexin-A4-deficient mice show enhanced T cell priming and exacerbated T cell-mediated immune responses such as EAE.41) Therefore, plexin-A4 might interact with Sema3A in the immune system and this interaction might negatively regulate T cell responses. However, it remains unclear how plexin-A4 negatively regulates T cells and whether other semaphorins are relevant to plexin-A4-mediated immune responses.
Table 1.
Immune semaphorins, their receptors and diseases
| Semaphorins/ receptors |
Expression in the immune system |
Binding partner | Immunological activities | Related-neurological diseases |
|---|---|---|---|---|
| Sema3A | N.D. | Plexin A proteins Neuropilin-1 |
Inhibition of monocyte migration Inhibition of T-cell activation |
Alzheimer’s disease Atopic dermatitis |
| Sema4A | Dendritic cells Acticated-T cells Th1 cells |
Plexin B proteins Plexin-D1 TIM-2 |
T-cell activation Promotion of Th1-differentiation |
EAE Atopic dermatitis |
| Sema4D | T cells Activated-B cells Dendritic cells |
Plexin-B1 CD72 |
B-cell activation DC-activation |
EAE HAM |
| Sema5A | N.D. (Oligodendrocytes) |
N.D. | N.D. | Parkinson’s disease |
| Sema6D | T cells B cells NK cells |
Plexin-A1 | DC-activation | |
| Sema7A | Acticated-T cells | Plexin-C1 Integrin α1β1 |
Monocyte/macrophage-activation | Contact hypersensitivity EAE |
| Neuropilin-1 | T cells Treg cells |
Class III semaphorins | Alzheimer’s disease | |
| Plexin-A1 | Dendritic cells (Osteoclasts) |
Class VI semaphorins | DC-activation | EAE |
| Plexin-A4 | T cells Dendritic cells Macrophages |
Class VI semaphorins | Inhibition of T-cell activation | EAE |
| Plexin-B1 | Class IV semaphorins | |||
| TIM-2 | Acticated-T cells Th2 cells |
Sema4A | T-activation | EAE Airway atopy |
| CD72 | B cells (Dendritic cells) |
Sema4D | B-cell activation DC-activation |
|
| Integrin α1β1 | Monocytes Macrophages |
Sema7A | Monocyte/macrophage-activation | EAE |
(6). NP-1: a marker for regulatory T cells.
As described above, NP-1 was originally identified as a cell surface glycoprotein that acts as a class III semaphorin receptor. NP-1 is also known as human DC-specific antigen (blood DC antigen)-4, a specific plasmacytoid DC marker in humans, and was assigned CD304. In the immune system, the expression of NP-1 was observed in DCs and T cells.43) NP-1 is thought to be involved in the initiation of primary immune responses through a homophilic interaction at contact sites between T cells and DCs. In addition, NP-1 has been identified as a specific marker for CD4+CD25+ regulatory T (Treg) cells.44) Indeed, NP-1 is a member of the group of forkhead box P3 (Foxp3)-inducible genes, which also includes CD25, glucocorticoid-induced tumor necrosis factor receptor-related protein, and cytotoxic T-lymphocyte antigen-4 (CTLA-4).44) More recently, it has been suggested that NP-1 in Treg cells contributes to the long contact between Treg cells and DCs compared with the shorter contact between naive T cells and DCs. Treg cells made stable contacts with DCs that preceded the contact of naive T cells with DCs; this might lead to the inhibition of T-cell activation at steady state.45) The finding that Treg cells are endowed with the ability to have long interactions with DCs mediated by NP-1 supports the idea that NP-1 might contribute to physical interactions between T cells and DCs.
4. Semaphorins in cardiac morphogenesis
Cumulative findings also indicate the significance of semaphorins in the development of other organ systems, such as the cardiovascular system, in which some semaphorins control the migration of endothelial cells, cardiac myocytes, or their precursors.24,34,46,47)
(1). Sema6D-Plexin-A1 in cardiac morphogenesis.
One of the best characterized roles of a semaphorin in cardiac development is that of Sema6D, which belongs to the class VI transmembrane-type semaphorin subfamily. In mice, the expression of Sema6D is first detected in the cardiac crescent and neural fold of E9 embryos.24) The roles of Sema6D in the developing heart have been revealed by a series of studies using the chick embryo system. Inoculation of transfected cells that release a large amount of soluble Sema6D into cultured chick embryos at Hamberger and Hamilton (HH) stage 9 results in enhanced looping of the cardiac tube and enlargement of the ventricular region. In ovo inoculation of Sema6D producing cells or recombinant soluble Sema6D into HH stage 29 embryos results in an expanded ventricular cavity with a thin myocardial layer and an enlarged endocardial cushion. In contrast, RNAi-mediated knockdown of Sema6D inhibits looping of the cardiac tube.24) In this context, Sema6D signals are largely mediated through Plexin-A1, which is also expressed in the embryonic heart. Indeed, RNAi-mediated knockdown of Plexin-A1 or expression of truncated Plexin-A1 resulted in shrunken ventricles.24) Therefore, the Sema6D-Plexin-A1 axis is critically involved in the dynamic remodeling of the cardiac tube and formation of the ventricle and endocardial cushion.
(2). Sema6D exerts distinct biological activities in endothelial cells in different regions of the cardiac tube.
Sema6D inhibits the migration of outgrowing cells from the ventricle segment. In contrast, Sema6D promotes the migration of outgrowing cells from the conotruncal and atrio-ventricular valve segments, which later fuse to form the endocardial cushion. These biological activities of Sema6D appear to be mediated through Plexin-A1, as they are abrogated by RNAi-mediated knockdown of Plexin-A1 or expression of truncated Plexin-A1 in endothelial cells from the ventricle or conotruncal segments.24) Interestingly, we have demonstrated that Plexin-A1 forms a complex with OTK in the endothelial cells of the ventricular region of the cardiac tube, whereas it forms a complex with VEGFR2 in endothelial cells of the conotruncal segments. Further, the Sema6D-induced migration of endothelial cells is suppressed both by RNAi against Plexin-A1 and by RNAi against VEGFR2.24) This differential association of Plexin-A1 with additional receptor components allows Sema6D to exert distinct biological activities in adjacent regions, which is critical for complex cardiac morphogenesis.
(3). Reverse signaling of Sema6D in the cardiac ventricle.
Among the semaphorin family members, class VI semaphorins are unique because of their relatively long cytoplasmic regions, which may imply the existence of reverse signaling. Indeed, it has been reported that the Sema6A cytoplasmic region can associate with two molecules,48) Abl kinase and Enabled (Ena), a member of the Ena/VASP family. Abl kinase and Ena are known to play opposing roles downstream of drosophila Robo, an axon guidance receptor for Slit.49) Ena has also been implicated in reverse signaling of drosophila Sema1a.50) Upon binding to the ectodomain of Plexin-A1, Abl kinase is recruited to the cytoplasmic tail of Sema6D and activated, resulting in phosphorylation of Ena and dissociation from Sema6D.46) In the case of fate-mapping studies using myocardial cells, cells carrying defects in Sema6D reverse signaling arrest in the compact layer, while expression of constitutively active Abl kinase enhances the migration of cells from the compact layer to the trabeculae.46) Thus, class VI semaphorins transduce reverse signals through their cytoplasmic domains to regulate biological output.
5. Semaphorin-mediated signals in endothelial cell migration
As described above, the class III semaphorins exert pleiotropic functions through NP-1/Plexin-A receptor complexes, which regulate small GTPases and integrins.21,51,52) However, the molecular mechanisms underlying this regulation/integration have remained unclear.
We have determined that the FERM domain-containing guanine nucleotide exchange factor (GEF) FARP2 functions as an immediate downstream signal transducer of the Plexin-A1-NP-1 receptor complex in Sema3A-mediated repulsion of neuronal axons.23) Sema3A-induced dissociation of FARP2 from Plexin-A1 and activation of its Rac-GEF activity triggers a series of biochemical events, including Rac activation and the binding of Rnd1, a member of the Rho GTPase family, to Plexin-A1. This binding stimulates the GAP activity of Plexin-A1 for R-Ras, a member of the Ras GTPase family. The down-regulation of R-Ras leads to the cytoskeletal disassembly that is critical for Plexin-A1-mediated growth cone collapse.22) In parallel with this event, dissolved FARP2 competes with an isoform of type-1 phosphatidylinositol phosphate kinase, PIPKIγ661, for the FERM domain of talin. PIP2 generated by PIPKIγ661 in association with talin is important for the stability of integrin-mediated focal adhesions,53,54) and the inhibition of PIPKIγ661 kinase activity by FARP2 binding down-regulates integrin function. Thus, semaphorin-Plexin signaling is involved in cardiac morphogenesis by regulating cytoskeletal dynamics and cell-matrix interactions.
6. Perspectives
Accumulating evidence reveals that several semaphorins and their receptors have multiple biological activities. These semaphorins form a family of immunoregulatory molecules, called ‘immune semaphorins’ (Fig. 3, Table 1). Indeed, the lack of semaphorin family proteins results in several immune disorders, including autoimmune diseases, allergy, and congenital bone disease. Lack of these proteins also causes unresponsiveness to physiological immune responses (Table 1). Therefore, semaphorin family proteins are at least involved in immunological homeostasis, based on sophisticated immune cell communication systems. Furthermore, the patterning and morphogenesis of the heart, as regulated by semaphorins and their receptors are discussed in this review. However, the molecular information available in this context remains fragmented. To understand heart development further, we should complete the definition of the molecular systems required for its formation, and then seek the points of integration between these systems.
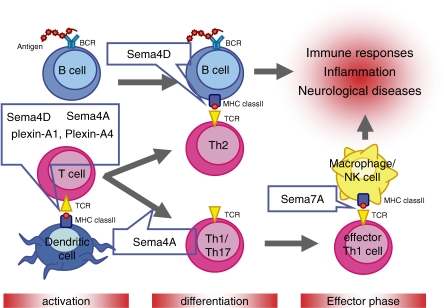
Figure 3.
Involvement of immune semaphorins in various phases of immune responses. In the initial phase of T cell immune responses, Sema4D and Sema6D expressed by T cells activate DCs through their receptors, CD72 and plexin-A1, respectively. Plexin-A1 is also involved as a negative regulator. Sema4A expressed on DCs is also involved in T cell priming through its binding partner, TIM-2, of which expression is induced on activated T cells. Sema4A is required for regulating the differentiation of Th cells. In contrast, Sema4D is upregulated on activated T cells and promotes humoral immune responses through the activation of B cells. Differentiated effector T cells express Sema7A on their cell surface, and interactions between T cell expressing Sema7A and macrophage expressing α1β1 integrin induce macrophage activation, resulting in the promotion of inflammatory responses. BCR, B cell receptor; MHC, major histocompatibility complex; TCR, T cell receptor.
Several important issues remain to be resolved. Although semaphorins function to regulate cell motility and morphology by activating plexins, most of the immunological studies of semaphorins have only focused on their costimulatory effects. However, it is plausible that semaphorins exert their functions by affecting the cytoskeleton. Further studies are required to clarify the role of semaphorins in cell-cell interactions, cell morphology, and dynamics, both in the immune system and cardiac morphogenesis. Finally, understanding of semaphorins and their receptors should allow pharmacological modulation of their functions, and may lead to the identification of potential therapeutic targets for several human diseases.
Acknowledgements
We thank Drs. M. Mizui and H. Takamatsu for preparing this manuscript.
Abbreviations
- NPs
neuropilins
- TIM-2
T-cell immunoglobulin and mucin domain containing protein 2
- OTK
tyrosine kinase receptors off-track
- VEGFR2
vascular endothelial growth factor receptor 2
- TREM-2
triggering receptor expressed on myeloid cell
- DAP12
DNAX-activating protein 12
- ITIM
immunoreceptor tyrosine-based inhibitory motifs (ITIM)
- Th1
T helper type 1
- Th2
T helper type 1
- EAE
experimental autoimmune encephalomyelitis
- Treg
regulatory T cells
- Foxp3
forkhead box P3
- CTLA-4
cytotoxic T-lymphocyte antigen-4 (CTLA-4)
- Ena
Enabled
- GEF
guanine nucleotide exchange factor
Reference
- 1).Steinman L. (2004) Elaborate interactions between the immune and nervous systems. Nat. Immunol. 5, 575–581 [DOI] [PubMed] [Google Scholar]
- 2).Wu J.Y., Feng L., Park H.T., Havlioglu N., Wen L., Tang H., et al. (2001) The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410, 948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Aasheim H.C., Delabie J., Finne E.F. (2005) Ephrin-A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood 105, 2869–2876 [DOI] [PubMed] [Google Scholar]
- 4).Kolodkin A.L., Matthes D.J., Goodman C.S. (1993) The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75, 1389–1399 [DOI] [PubMed] [Google Scholar]
- 5).Toyofuku T., Kikutani H. (2007) Semaphorin signaling during cardiac development. Adv. Exp. Med. Biol. 600, 109–117 [DOI] [PubMed] [Google Scholar]
- 6).Serini G., Maione F., Giraudo E., Bussolino F. (2009) Semaphorins and tumor angiogenesis. Angiogenesis 12, 187–193 [DOI] [PubMed] [Google Scholar]
- 7).Gu C., Yoshida Y., Livet J., Reimert D.V., Mann F., Merte J., et al. (2005) Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 307, 265–268 [DOI] [PubMed] [Google Scholar]
- 8).Capparuccia L., Tamagnone L. (2009) Semaphorin signaling in cancer cells and in cells of the tumor microenvironment—two sides of a coin. J. Cell Sci. 122, 1723–1736 [DOI] [PubMed] [Google Scholar]
- 9).Takegahara N., Takamatsu H., Toyofuku T., Tsujimura T., Okuno T., Yukawa K., et al. (2006) Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat. Cell Biol. 8, 615–622 [DOI] [PubMed] [Google Scholar]
- 10).Suzuki K., Kumanogoh A., Kikutani H. (2008) Semaphorins and their receptors in immune cell interactions. Nat. Immunol. 9, 17–23 [DOI] [PubMed] [Google Scholar]
- 11).Semaphorin Nomenclature Committee (1999) Unified nomenclature for the semaphorins/collapsins. Cell 97, 551–552 [DOI] [PubMed] [Google Scholar]
- 12).Winberg M.L., Noordermeer J.N., Tamagnone L., Comoglio P.M., Spriggs M.K., Tessier-Lavigne M., et al. (1998) Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 95, 903–916 [DOI] [PubMed] [Google Scholar]
- 13).Takahashi T., Fournier A., Nakamura F., Wang L.H., Murakami Y., Kalb R.G., et al. (1999) Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99, 59–69 [DOI] [PubMed] [Google Scholar]
- 14).Tamagnone L., Artigiani S., Chen H., He Z., Ming G.I., Song H., et al. (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71–80 [DOI] [PubMed] [Google Scholar]
- 15).Zhou Y., Gunput R.A., Pasterkamp R.J. (2008) Semaphorin signaling: progress made and promises ahead. Trends Biochem. Sci. 33, 161–170 [DOI] [PubMed] [Google Scholar]
- 16).Pasterkamp R.J., Peschon J.J., Spriggs M.K., Kolodkin A.L. (2003) Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424, 398–405 [DOI] [PubMed] [Google Scholar]
- 17).Suzuki K., Okuno T., Yamamoto M., Pasterkamp R.J., Takegahara N., Takamatsu H., et al. (2007) Semaphorin 7A initiates T-cell-mediated inflammatory responses through α1β1 integrin. Nature 446, 680–684 [DOI] [PubMed] [Google Scholar]
- 18).Kumanogoh A., Watanabe C., Lee I., Wang X., Shi W., Araki H., et al. (2000) Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity 13, 621–631 [DOI] [PubMed] [Google Scholar]
- 19).Kumanogoh A., Marukawa S., Suzuki K., Takegahara N., Watanabe C., Ch’ng E., et al. (2002) Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 419, 629–633 [DOI] [PubMed] [Google Scholar]
- 20).Kruger R.P., Aurandt J., Guan K.L. (2005) Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 6, 789–800 [DOI] [PubMed] [Google Scholar]
- 21).Serini G., Valdembri D., Zanivan S., Morterra G., Burkhardt C., Caccavari F., et al. (2003) Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424, 391–397 [DOI] [PubMed] [Google Scholar]
- 22).Oinuma I., Ishikawa Y., Katoh H., Negishi M. (2004) The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 305, 862–865 [DOI] [PubMed] [Google Scholar]
- 23).Toyofuku T., Yoshida J., Sugimoto T., Zhang H., Kumanogoh A., Hori M., et al. (2005) FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat. Neurosci. 8, 1712–1719 [DOI] [PubMed] [Google Scholar]
- 24).Toyofuku T., Zhang H., Kumanogoh A., Takegahara N., Suto F., Kamei J., et al. (2004) Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 18, 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Giordano S., Corso S., Conrotto P., Artigiani S., Gilestro G., Barberis D., et al. (2002) The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat. Cell Biol. 4, 720–724 [DOI] [PubMed] [Google Scholar]
- 26).Swiercz J.M., Worzfeld T., Offermanns S. (2008) ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J. Biol. Chem. 283, 1893–1901 [DOI] [PubMed] [Google Scholar]
- 27).Shi W., Kumanogoh A., Watanabe C., Uchida J., Wang X., Yasui T., et al. (2000) The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity 13, 633–642 [DOI] [PubMed] [Google Scholar]
- 28).Pan C., Baumgarth N., Parnes J.R. (1999) CD72-deficient mice reveal nonredundant roles of CD72 in B cell development and activation. Immunity 11, 495–506 [DOI] [PubMed] [Google Scholar]
- 29).Wang X., Kumanogoh A., Watanabe C., Shi W., Yoshida K., Kikutani H. (2001) Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood 97, 3498–3504 [DOI] [PubMed] [Google Scholar]
- 30).Zhu L., Bergmeier W., Wu J., Jiang H., Stalker T.J., Cieslak M., et al. (2007) Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc. Natl. Acad. Sci. USA 104, 1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Kumanogoh A., Shikina T., Suzuki K., Uematsu S., Yukawa K., Kashiwamura S., et al. (2005) Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity 22, 305–316 [DOI] [PubMed] [Google Scholar]
- 32).Chakravarti S., Sabatos C.A., Xiao S., Illes Z., Cha E.K., Sobel R.A., et al. (2005) Tim-2 regulates T helper type 2 responses and autoimmunity. J. Exp. Med. 202, 437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Rennert P.D., Ichimura T., Sizing I.D., Bailly V., Li Z., Rennard R., et al. (2006) T cell, Ig domain, mucin domain-2 gene-deficient mice reveal a novel mechanism for the regulation of Th2 immune responses and airway inflammation. J. Immunol. 177, 4311–4321 [DOI] [PubMed] [Google Scholar]
- 34).Toyofuku T., Yabuki M., Kamei J., Kamei M., Makino N., Kumanogoh A., et al. (2007) Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 26, 1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Yoshida Y., Han B., Mendelsohn M., Jessell T.M. (2006) PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron 52, 775–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Wong A.W., Brickey W.J., Taxman D.J., van Deventer H.W., Reed W., Gao J.X., et al. (2003) CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat. Immunol. 4, 891–898 [DOI] [PubMed] [Google Scholar]
- 37).Bakker A.B., Hoek R.M., Cerwenka A., Blom B., Lucian L., McNeil T., et al. (2000) DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity 13, 345–353 [DOI] [PubMed] [Google Scholar]
- 38).Paloneva J., Mandelin J., Kiialainen A., Bohling T., Prudlo J., Hakola P., et al. (2003) DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J. Exp. Med. 198, 669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Yamada A., Kubo K., Takeshita T., Harashima N., Kawano K., Mine T., et al. (1999) Molecular cloning of a glycosylphosphatidylinositol-anchored molecule CDw108. J. Immunol. 162, 4094–4100 [PubMed] [Google Scholar]
- 40).Catalano A., Caprari P., Moretti S., Faronato M., Tamagnone L., Procopio A. (2006) Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood 107, 3321–3329 [DOI] [PubMed] [Google Scholar]
- 41).Yamamoto M., Suzuki K., Okuno T., Ogata T., Takegahara N., Takamatsu H., et al. (2008) Plexin-A4 negatively regulates T lymphocyte responses. Int. Immunol. 20, 413–420 [DOI] [PubMed] [Google Scholar]
- 42).Suto F., Tsuboi M., Kamiya H., Mizuno H., Kiyama Y., Komai S., et al. (2007) Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 53, 535–547 [DOI] [PubMed] [Google Scholar]
- 43).Tordjman R., Lepelletier Y., Lemarchandel V., Cambot M., Gaulard P., Hermine O., et al. (2002) A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 3, 477–482 [DOI] [PubMed] [Google Scholar]
- 44).Bruder D., Probst-Kepper M., Westendorf A.M., Geffers R., Beissert S., Loser K., et al. (2004) Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol. 34, 623–630 [DOI] [PubMed] [Google Scholar]
- 45).Sarris M., Andersen K.G., Randow F., Mayr L., Betz A.G. (2008) Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28, 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Toyofuku T., Zhang H., Kumanogoh A., Takegahara N., Yabuki M., Harada K., et al. (2004) Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 6, 1204–1211 [DOI] [PubMed] [Google Scholar]
- 47).Toyofuku T., Yoshida J., Sugimoto T., Yamamoto M., Makino N., Takamatsu H., et al. (2008) Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev. Biol. 321, 251–262 [DOI] [PubMed] [Google Scholar]
- 48).Klostermann A., Lutz B., Gertler F., Behl C. (2000) The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J. Biol. Chem. 275, 39647–39653 [DOI] [PubMed] [Google Scholar]
- 49).Bashaw G.J., Kidd T., Murray D., Pawson T., Goodman C.S. (2000) Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101, 703–715 [DOI] [PubMed] [Google Scholar]
- 50).Godenschwege T.A., Hu H., Shan-Crofts X., Goodman C.S., Murphey R.K. (2002) Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat. Neurosci. 5, 1294–1301 [DOI] [PubMed] [Google Scholar]
- 51).Kuhn T.B., Brown M.D., Wilcox C.L., Raper J.A., Bamburg J.R. (1999) Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. J. Neurosci. 19, 1965–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Zanata S.M., Hovatta I., Rohm B., Puschel A.W. (2002) Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J. Neurosci. 22, 471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Di Paolo G., Pellegrini L., Letinic K., Cestra G., Zoncu R., Voronov S., et al. (2002) Recruitment and regulation of phosphatidylinositol phosphate kinase type 1γ by the FERM domain of talin. Nature 420, 85–89 [DOI] [PubMed] [Google Scholar]
- 54).Ling K., Doughman R.L., Firestone A.J., Bunce M.W., Anderson R.A. (2002) Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420, 89–93 [DOI] [PubMed] [Google Scholar]