Abstract
The hypervariable region 1 (HVR1) comprising the first 27 aa of E2 glycoprotein is a target for neutralizing antibodies against hepatitis C virus (HCV), but the mechanisms of this neutralization in the cell-culture-infectious genotype 2a strain JFH1 HCV virus (HCVcc) system are unknown. Two rabbit polyclonal sera, R1020 and R140, recognizing the HVR1 of the genotype 1a isolates H77c and Glasgow (Gla), respectively, and a Gla HVR1-specific mouse mAb AP213 have been described previously. However, attempts to generate of antibodies to the JFH1 HVR1 were unsuccessful. Therefore, this study produced chimeric JFH1 HCVcc viruses harbouring the H77c or Gla HVR1 to assess the reactivity of antibodies to this region and their effects on virus infectivity. The inter-genotypic HVR1 swap did not significantly affect virus infectivity. The genotype 1a HVR1-specific antibodies neutralized chimeric viruses in an isolate-dependent manner, underlining the role of HVR1 in HCV infection. The neutralizing antibodies reacted mainly with the C-terminal portion of HVR1, and detailed mapping identified A17, F20 and Q21 in the Gla HVR1 sequence and T21 (and possibly L20) in the corresponding H77c sequence as key epitope residues for AP213 and R140, and R1020, respectively. Importantly, none of the antibodies inhibited in vitro binding of viral envelope glycoproteins to the best-characterized HCV receptor, CD81, or to the glycosaminoglycan attachment factors. However, the HVR1 antibodies were capable of post-attachment neutralization. Overall, this study emphasizes the role of HVR1 in HCVcc entry and provides new tools to study this region further in the context of complete virions.
INTRODUCTION
Hepatitis C virus (HCV) is a major cause of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. Genetic variability, a common feature of RNA viruses, is a major hindrance in developing effective treatments or vaccines to fight HCV. Indeed, HCV isolates are classified into seven distinct genotypes differing at the nucleotide level by around 30 % and each divided into numerous subtypes. Moreover, within a single individual, the virus exists as a constantly evolving quasispecies (Bukh et al., 1995; Pawlotsky, 2003; Simmonds, 1995).
HCV, a member of the family Flaviviridae, has a positive-sense RNA encoding a polyprotein, which is cleaved by cellular and viral proteases into structural and non-structural proteins (reviewed by Moradpour et al., 2007). The glycoproteins E1 and E2, which exhibit a high degree of variability, are responsible for virus cell attachment and entry. The retroviral HCV pseudoparticle (HCVpp) system (Bartosch et al., 2003b; Hsu et al., 2003) and more recently the cell-culture-infectious HCV (HCVcc) system (Lindenbach et al., 2005; Wakita et al., 2005; Zhong et al., 2005) have shown that HCV entry into target cells is a complex process involving virus first binding to low-specificity receptors such as glycosaminoglycans (Barth et al., 2003) and the low-density lipoprotein receptor (Agnello et al., 1999). This is followed by sequential interaction with specific host factors such as CD81, scavenger receptor class B type I (SR-BI), claudin-1, 6 or 9, and occludin, leading to entry via the endocytic route (Evans et al., 2007; Liu et al., 2009; Meertens et al., 2008; Pileri et al., 1998; Ploss et al., 2009; Scarselli et al., 2002; Zheng et al., 2007).
The E2 glycoprotein is the main target for the antiviral humoral immune response. Its variability is mostly confined to the so-called hypervariable regions (HVRs) (Hijikata et al., 1991; Troesch et al., 2006; Weiner et al., 1991), which may differ by up to 80 % among HCV genotypes and even among subtypes of the same genotype. HVR1, which encompasses the first 27 aa of E2 (residues 384–410 of the viral polyprotein) contains an immunodominant epitope. Antibodies to HVR1 neutralize virus infection, but the action of this immune pressure on top of the inherent genetic instability of the virus rapidly selects resistant variants (Farci et al., 2000; Kurosaki et al., 1993; Rosa et al., 1996; Shimizu et al., 1994; Weiner et al., 1992; Zibert et al., 1995). Therefore, antibodies to HVR1 are typically isolate specific. In contrast, conformational epitopes on E2 are less prone to variation and are able to elicit more broadly neutralizing antibodies in patients (Johansson et al., 2007; Keck et al., 2007; Law et al., 2008; Mancini et al., 2009; Owsianka et al., 2008; Perotti et al., 2008; Schofield et al., 2005). Interestingly, the efficiency of neutralization by antibodies targeting regions outside HVR1 is altered by interplay between HVR1, SR-BI and high-density lipoproteins (Bartosch et al., 2005; Dreux et al., 2006; Voisset et al., 2005), emphasizing the role of HVR1 as an immunological decoy. Nevertheless, HVR1 also plays a role in HCV entry, possibly mediated by an interaction with SR-BI (Scarselli et al., 2002) and/or glycosaminoglycans (Barth et al., 2003). Deletion of HVR1 in HCVpp (Bartosch et al., 2003c) or HCVcc (Bankwitz et al., 2010) systems, or in virus produced in chimpanzee (Forns et al., 2000b), results in a significant loss of infectivity. In addition, anti-HVR1 antibodies have been shown to be protective in chimpanzees (Farci et al., 1996). Consistent with a role in entry, some features of HVR1, such as its overall conformation and the position of basic residues, are conserved among isolates (Callens et al., 2005; Penin et al., 2001).
Understanding the mechanisms of antibody-mediated neutralization is crucial to generate vaccine candidates. Whilst the mechanisms of cross-reactive neutralizing antibodies are beginning to be elucidated, the mode of action of more common isolate-specific neutralizing antibodies, mainly targeting HVR1, is not well characterized. Importantly, there are few data about anti-HVR1 antibody neutralization in the newly described HCVcc system. Therefore, in this study, the mechanism of anti-HVR1 antibody neutralization of HCVcc was investigated using a group of three antibodies in the HCVcc system, and neutralizing epitopes within HVR1 were characterized.
RESULTS
Generation of anti-HVR1 mAb and polyclonal sera
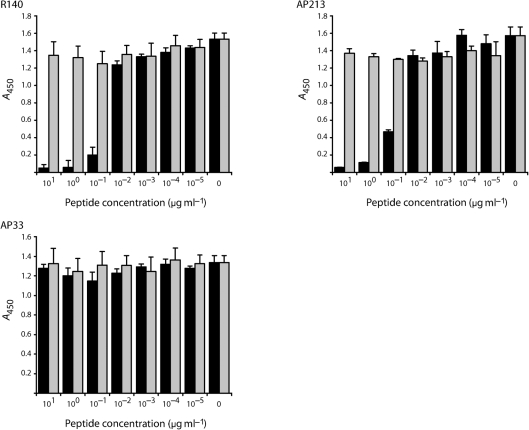
Two polyclonal antisera, R1020 and R140, were generated by immunization of rabbits with peptides representing the HVR1 of two closely related genotype 1a strains, H77c and Glasgow (Gla), respectively. Furthermore, mAb AP213 was raised in mice immunized with a recombinant form of HCV strain Gla E1E2 (Patel et al., 2000). A peptide competition assay indicated that both R140 and mAb AP213 were HVR1 specific (Fig. 1). Pre-incubation of these antibodies with peptide 1084.B representing Gla HVR1 (but not the control peptide 2060.1) prevented recognition of Galanthus nivalis antigen (GNA)-captured Gla E1E2 in a dose-dependent manner. As expected, neither of the peptides inhibited recognition of Gla E1E2 by mAb AP33, a broadly reactive mAb whose epitope is located immediately downstream of HVR1 (Owsianka et al., 2001; Tarr et al., 2006). The specificity of R1020 to strain H77c HVR1 has been demonstrated previously (Owsianka et al., 2005).
Fig. 1.
Peptide competition assay. mAbs AP33 and AP213 and the rabbit antiserum R140 were incubated with a range of concentrations of HVR1-derived (1084.B; filled bars) or irrelevant (2060.1; shaded bars) peptide. The reactivity of the peptide/antibody mixture for H77c-GlaHVR1 E1E2 (see Methods) was then tested by GNA capture ELISA.
Anti-HVR1 antibodies neutralize the infectivity of chimeric HCVcc
HVR1 is a target for neutralizing antibodies in natural infections (Weiner et al., 1992; Zibert et al., 1995) and many anti-HVR1 antibodies are able to neutralize HCV infections in vitro and in vivo, as demonstrated in the HCVpp and chimpanzee models (Bartosch et al., 2003a; Farci et al., 1996; Hsu et al., 2003). Despite several attempts, we were unable to raise antibodies against the strain JFH1 HCVcc HVR1. We showed previously that R1020 was able to neutralize infection of cells by HCVpp incorporating H77c E1E2 (Owsianka et al., 2005). Similarly, here we found that the AP213 and R140 antibodies neutralized the infectivity of HCVpp incorporating H77c E1E2 with Gla HVR1 (see Methods and Supplementary Table S1, available in JGV Online). We set out to characterize these antibodies further using the HCVcc system.
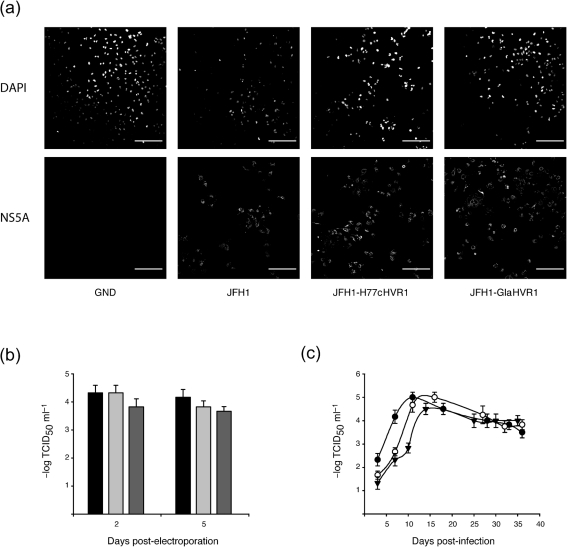
Complete envelope proteins from the Gla strain form non-functional aggregates (Patel et al., 2000). Therefore, we generated chimeric JFH1 cDNA in which its HVR1-encoding region was swapped for the corresponding H77c or Gla sequence (JFH1-H77cHVR1 or JFH1-GlaHVR1, respectively) to study the effect of the R1020 or AP213 and R140 antibodies on HCVcc infection. The viral NS5A protein was detected in Huh7 cells electroporated with RNA derived from constructs representing the wild-type (WT) JFH1 or the JFH1 HVR1 chimeras, but not from the replication-deficient GND mutant (Wakita et al., 2005), indicating that the chimeras were replication competent (Fig. 2a). Moreover, after electroporation, the infectious virus titres released by both chimeras into cell culture medium at 2 or 5 days post-electroporation were comparable to those for WT JFH1 (Fig. 2b). Next, we monitored the spread of infection of the WT and chimeric viruses (released from the electroporated cells) by infecting naïve cells. Huh7 cells infected at an m.o.i. of 0.005 with different viruses were passaged over a period of 5 weeks. Measurement of the infectious virus yield in the medium at each passage (Fig. 2c) showed that the chimeric viruses spread efficiently to naïve cells, albeit with a slight delay compared with WT JFH1. Importantly, after seven passages, no mutation was found in the envelope-encoding region of these infectious chimeric viruses. Moreover, electroporated cell lysates were tested by ELISA for reactivity with anti-HVR1 antibodies. As expected, R1020 serum specifically reacted with JFH1-H77cHVR1 lysates, and R140 and AP213 antibodies specifically recognized JFH1-GlaHVR1 lysates, whilst AP33 mAb reacted with both chimeric constructs as well as with WT JFH1 E2 (data not shown).
Fig. 2.
Chimeric HCVcc with genotype 1a HVR1 is infectious. (a) JFH1 chimeras were shown to replicate in Huh7 cells. Cells were fixed at 48 h after electroporation with the different constructs, stained for nuclei (DAPI; top row) and for NS5A (bottom row), and analysed by confocal microscopy. Bars, 200 μm. (b) Infectivity of the cell supernatant, assessed at 2 or 5 days after electroporation with JFH1 chimeras. Filled bars, WT JFH1; light grey bars, JFH1-H77cHVR1; dark grey bars, JFH1-GlaHVR1. (c) Production of infectious chimeric virus after infection of naïve Huh7 cells. Naïve cells infected at a low m.o.i. with supernatant from electroporated cells were serially passaged and infectious virus yields were measured in the cell supernatant at each passage by TCID50 assay. •, WT JFH1; ○, JFH1-H77cHVR1; ▾, JFH1-GlaHVR1.
Therefore, these chimeric HCVcc were suitable to study the neutralizing effect of our group of anti-HVR1 antibodies. mAb AP33, known to inhibit JFH1 infection (Dhillon et al., 2010; Iro et al., 2009; Tarr et al., 2006; Vieyres et al., 2009), served as a control and was able to neutralize WT and the chimeric HCVcc (Fig. 3). However, neutralization of the chimeric HCVcc was not as potent as for WT JFH1, with at least a tenfold increase in the IC50 value. R1020 IgGs neutralized JFH1-H77cHVR1 virus and to a lesser extent the heterologous JFH1-GlaHVR1 virus in a dose-dependent fashion. mAb AP213 and R140 IgGs neutralized only the JFH1-GlaHVR1 virus.
Fig. 3.
Anti-HVR1 antibodies neutralize chimeric HCVcc infection. (b) HCVcc harbouring the JFH1 (○), H77c (•) or Gla (▴) HVR1 were incubated with a range of concentrations of anti-HVR1 or control antibodies prior to infection of naïve Huh7 cells. At 2 days post-infection (p.i.), infectivity was evaluated by f.f.u. assay. The residual infectivity relative to infectivity in the absence of antibody is shown.
Stringent isolate specificity of anti-HVR1 antibodies
To gain further insights into the role of HVR1 in HCV infection, we set out to identify the amino acid residues critical for interaction with our anti-HVR1 antibodies. First, the reactivity of anti-HVR1 antibodies was assessed against a panel of 25 E1E2 constructs representing the six major genotypes of HCV, with non-redundant HVR1 sequences (Lavillette et al., 2005; Owsianka et al., 2005) (Fig. 4). Expression of 20 of these clones was validated by strong reaction with AP33 mAb in ELISA and these clones were therefore kept for further analysis. The AP213 and R140 antibodies reacted only with the Gla strain. Interestingly, residue Q21 was the only Gla-specific residue in this panel of HVR1 sequences and could account for the restricted isolate-specificity of the AP213 and R140 antibodies. In contrast, some cross-reactivity was observed for the R1020 antiserum, which weakly recognized some strains belonging to genotypes 1a and 2b. Interestingly, the presence of the L20 residue seemed to correlate with some epitope recognition as it was present in the H77c HVR1 as well as in four of the six clones weakly recognized by R1020 IgGs, but absent from all the negative clones. Only residue N8 was H77c specific, and this could explain the strong reactivity of the R1020 antiserum with this strain. Together, these data confirmed the isolate specificity of our anti-HVR1 antibodies.
Fig. 4.
Reactivity of anti-HVR1 antibodies with a panel of patient-derived E1E2 glycoproteins. A panel of E1E2 constructs (Lavillette et al., 2005; Owsianka et al., 2005) was expressed in HEK cells and the cell lysates analysed by GNA ELISA for their recognition by anti-HVR1 antibodies. HVR1 sequences were aligned with the H77c (top panel) or Gla (bottom panel) HVR1 sequence and identical residues are shaded in grey. Residues specific for the H77c or Gla HVR1 are boxed. Clones were classified into three groups depending on whether they were non-reactive (–), weakly reactive (+/−) or strongly reactive (+) with anti-HVR1 antibodies in the linear range of the assay.
AP213 mAb epitope mapping by phage display
We next used the phage display technique to map more precisely the epitope residues recognized by our antibodies. Because R1020 and R140 are polyclonal antibodies and this method is more suitable for mAbs, we restricted our analysis to mAb AP213. Following three rounds of affinity selection against mAb AP213, 32 peptide sequences were isolated (Fig. 5). Overall, the frequency of phenylalanine (F), lysine (K) and proline (P) residues present in the HVR1 sequence was significantly increased in the selected peptides compared with the original library (at least doubled), suggesting that they were indeed selected during panning. The sequences were arranged into three groups. In groups A (nine clones) and B (12 clones), a glutamine residue (Q) was strictly conserved, often preceded by a large aromatic residue [tyrosine (Y) or tryptophan (W) in group A and phenylalanine (F) in group B] and sometimes followed by a proline residue (four sequences). Finally, group C (11 clones) exhibited an ‘FGP’ motif in all but one clone, which resembled again the ‘FQP’ motif in the Gla HVR1. We also found an overall frequent repetition of basic residues [lysine (K), arginine (R) and histidine (H)], often aligning with K25 in HVR1. Although heterogeneous, these data suggest that the 20FQPXXK25 motif in HVR1 is a key element in mAb AP213 binding.
Fig. 5.
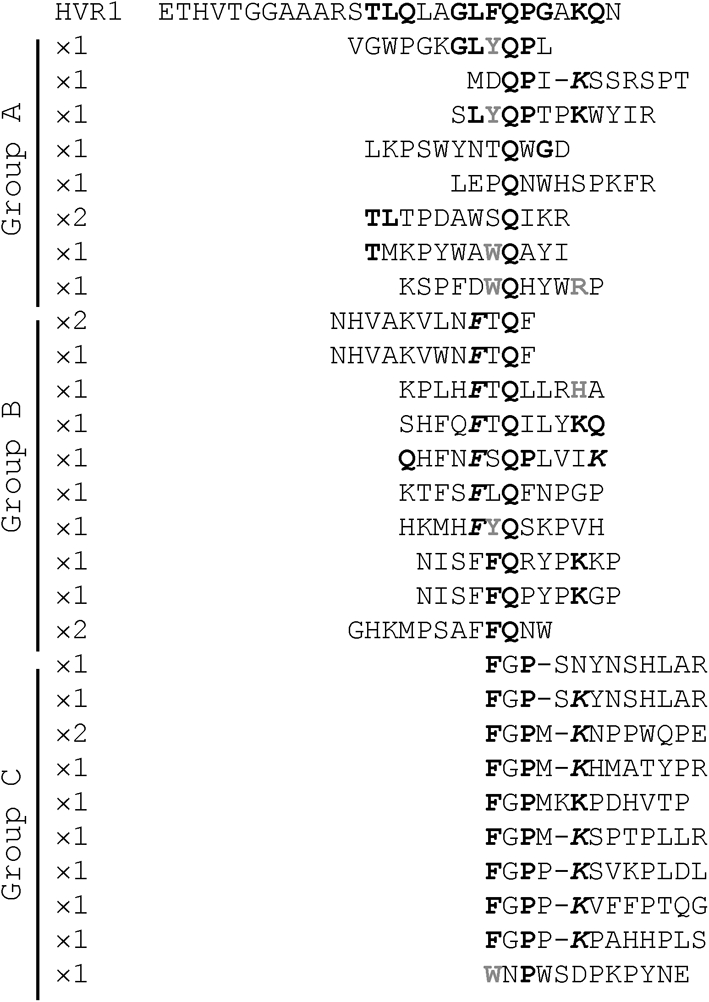
Epitope mapping of mAb AP213 by peptide phage display. A total of 32 peptides were selected after three rounds of panning of a random peptide phage display library against mAb AP213. Their amino acid sequence was aligned manually with the Gla HVR1 sequence. Residues in bold black represent residues present in the HVR1 sequence, aligned with or close to (italics) the HVR1 residue. Residues in bold grey are residues aligned with HVR1 residues having similar properties.
The C-terminal moiety of HVR1 is the main determinant for anti-HVR1 antibody binding
To delineate the epitope region of all three anti-HVR1 antibodies, a panel of E1E2 mutants was generated in a phCMV vector. They encoded, in an H77c background, a chimeric HVR1 consisting of the H77c HVR1 N terminus and the Gla HVR1 C terminus (HG chimera) or the Gla HVR1 N terminus and the H77c HVR1 C terminus (GH chimera) (Fig. 6a). Reactivity with mAb AP33 and the conformation-sensitive mAb H53 suggested that the protein expression levels and global conformation were similar to those for the parental (H77c) constructs (Fig. 6b, c). The chimeras were also functional in the HCVpp system, with at least 50 % of the infectivity of the parental strain preserved (data not shown). Nevertheless, differences in affinity for anti-HVR1 antibodies were observed. R1020 serum, specific for the H77c HVR1, did not recognize the HG chimera but reacted with the GH chimera (Fig. 6d). Importantly, residue N8, which was H77c specific (see Fig. 4 and above), was therefore not necessary for R1020 IgG binding. The Gla HVR1-specific mAb AP213 only recognized the HG chimera (Fig. 6e). Lastly, the R140 serum bound both constructs, with an increased affinity for the HG chimera (Fig. 6f). This indicated that the HVR1 C terminus was involved in antibody binding and strain specificity of all three antibodies, whilst the HVR1 N terminus was not sufficient for binding. Importantly, antibody affinity for the HVR1 chimeras was always lower than for the parental homologous complete HVR1. In addition, the spectrum of antibody neutralization towards HCVpp harbouring chimeric HVR1 (HG or GH chimeras; see Supplementary Table S1) correlated with the antibody affinity as assayed by ELISA (Fig. 6). Therefore, our anti-HVR1 antibodies reacted principally with the C-terminal moiety of HVR1, although the N-terminal portion may be important for HVR1 global conformation and optimal antibody binding affinity.
Fig. 6.
Reactivity of anti-HVR1 antibodies for a panel of E1E2 mutants. (a) Amino acid sequences of the H77c, Gla and chimeric HG or GH HVR1 (see Methods). Residues in bold differ between the H77c and Gla HVR1 sequence. All mutants were generated in the H77c-GlaHVR1 background. Clones A8N to Q21T consisted of H77c-GlaHVR1 E1E2 with single mutations in HVR1 replacing a residue from the Glasgow strain by its H77c counterpart. (b–f) Serially diluted lysates of HEK cells transfected with different E1E2 constructs were tested for recognition by different antibodies by GNA capture ELISA. For each antibody, a lysate dilution was chosen that gave absorbance readings in a linear range, and absolute absorbance values (at 450 nm) were plotted.
Single point mutants in HVR1 highlight residues important for antibody binding
Each of the eight residues differing between the H77c and Gla HVR1 sequence (Fig. 6a) were individually mutated, in the H77c–Gla HVR1 sequence, to their H77c counterpart. Antibody binding to these mutants was then analysed (Fig. 6b–e). None of these mutations significantly affected the protein expression levels (mAb AP33 binding) or the affinity for the conformation-sensitive mAb H53. However, mutation of F20 and Q21 into the H77c residue resulted in a complete loss of affinity for AP213 mAb (Fig. 6e). Moreover, the A17V mutation significantly decreased the Gla HVR1 affinity for both the AP213 and R140 antibodies (Fig. 6e, f). Conversely, a single point mutation in the Gla HVR1 sequence (Q21T) could restore some affinity for R1020 antibody, which is H77c specific, indicating that residue T21 plays an important part in the R1020 epitope.
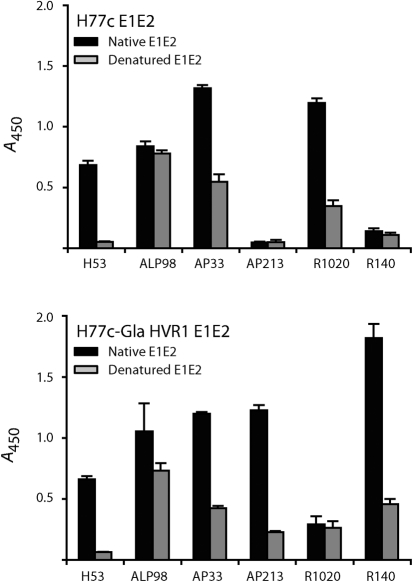
Anti-HVR1 antibodies are partially conformation sensitive
To test whether anti-HVR1 antibodies were conformation sensitive, their affinity for native or denatured E1E2 captured on GNA-coated plates was determined (Fig. 7). As expected (Cocquerel et al., 1998), the conformation-sensitive mAb H53 specifically recognized native H77c E1E2. The affinity of mAb ALP98 for both H77c and H77c-GlaHVR1 E1E2 was only mildly affected by antigen denaturation. As shown previously (Tarr et al., 2006), mAb AP33 exhibited partial conformation sensitivity for both antigens, although its epitope is linear. Interestingly, although two of them were raised against peptides, all three anti-HVR1 antibodies were also partially conformation sensitive. Of note, the peptides used to raise the R1020 and R140 antisera were relatively long (27 and 21 residues, respectively) and might fold in solution.
Fig. 7.
Conformation sensitivity of anti-HVR1 antibodies. Native or denatured HEK cell lysates expressing the H77c (top panel) or H77c-GlaHVR1 (bottom panel) E1E2 were captured on GNA-coated plates and tested for recognition by different antibodies. For each antibody, a lysate dilution was chosen that gave absorbance readings in a linear range and absolute absorbance values (at 450 nm) were plotted.
Anti-HVR1 antibodies are capable of post-attachment neutralization
To determine whether anti-HVR1 antibodies act by inhibiting HCVcc attachment or at a post-binding entry step, three protocols were used (Fig. 8, and see Methods) to distinguish between these events. Protocol C, in particular, measured post-attachment neutralization. mAb AP33 was efficient at inhibiting entry in protocol C, showing that this mAb was capable of post-attachment neutralization, as reported previously (Haberstroh et al., 2008). Nevertheless, AP33-mediated neutralization was more efficient in protocol B, suggesting an additional effect of this antibody in attachment inhibition or that its epitope was more readily available before virus attachment. Interestingly, anti-HVR1 antibodies behaved similarly to mAb AP33 in that they were capable of post-attachment neutralization but were more efficient at inhibiting HCVcc entry when present during the virus-binding stage. It should be noted that the antibodies were used at suboptimal concentrations so that the differences in neutralization between protocols B and C could be observed more easily, but up to 80 % post-attachment neutralization could be obtained with anti-HVR1 antibodies used at higher concentrations (data not shown). Also, because antibodies were used at suboptimal concentrations, more efficient neutralization was observed in protocol A where antibody was added twice in the experiment. Consistent with their role in binding inhibition (Chang et al., 2007; Koutsoudakis et al., 2006), heparin and the anti-ApoE serum were more efficient in protocol C. Note that we did observe some post-attachment neutralization with heparin that could correspond to the elution of a fraction of the cell-bound viruses. This effect was weaker or undetected in previous reports (Haberstroh et al., 2008; Koutsoudakis et al., 2006; Zeisel et al., 2007), possibly due to the use of different virus strains (Luc-Jc1 versus JFH-1 virus). In contrast, anti-CD81 mAb had similar effects in protocols B and C, consistent with the established role of CD81 in a post-attachment step of entry. Similar results were obtained with the HCVpp system (data not shown).
Fig. 8.
Anti-HVR1 antibodies are capable of post-attachment neutralization. (a) Schematic representation of the protocols used to distinguish between binding and entry stages of HCVcc entry (see Methods). Arrows correspond to the addition of inhibitors. The # symbol indicates that the inhibitor was pre-incubated with the appropriate HCVcc (heparin, 500 μg ml−1; anti-ApoE serum, 1 : 150; mAb AP33, 20 μg ml−1; mAb AP213, 20 μg ml−1; R1020 IgGs, 30 μg ml−1; R140 IgGs, 30 μg ml−1) or with the cells (anti-CD81 antibody at 5 μg ml−1) for 30 min at 37 °C before virus inoculation. Ab, Antibody. (b) HCVcc infectivity was assessed after adding the inhibitors at different stages of the entry process, according to protocols A, B and C. Infectivity was assessed by f.f.u. assay at 48 h p.i. and plotted as the percentage of infectivity observed in the absence of inhibitor.
DISCUSSION
Anti-HVR1 antibodies neutralize chimeric HCVcc
Anti-HVR1 antibodies are generally isolate specific, and, to our knowledge, no animal-raised anti-HVR1 antibody has been obtained against the genotype 2a HCVcc JFH1 isolate. HVR1 plays a crucial role in virus entry and is a major target of the neutralizing antibody response in vivo. Therefore, the lack of information on anti-HVR1 antibody-mediated neutralization represents a significant gap in our understanding of the mechanics of virus entry.
In this study, we investigated the role of HVR1 in HCVcc entry and the mechanisms of antibody-mediated neutralization against this region. First, functional HCVcc was generated with heterologous genotype 1a HVR1. Whilst HVR1 deletion drastically impairs HCV infectivity (Bankwitz et al., 2010; Bartosch et al., 2003c; Forns et al., 2000b; Wakita et al., 2005), this inter-genotypic swap in HVR1 had little effect on virus infectivity, consistent with the flexibility of this segment, its relative independence from the rest of E2 (Forns et al., 2000a; McCaffrey et al., 2007) and its tolerance to certain mutations. These chimeras were therefore suitable tools to study the effect of a panel of anti-HVR1 antibodies on HCVcc infectivity as they were raised against genotype 1a HVR1.
The anti-HVR1 antibodies inhibited HCV infection in the HCVpp and HCVcc systems in an isolate-specific manner. Interestingly, their epitopes mapped mostly to the C-terminal part of HVR1, consistent with a HVR1 model where the neutralization determinants are located in this portion and not in the less variable N-terminal part. Indeed, for example, the rat anti-HVR1 mAbs 6/16, 7/59, 6/82 (reactive against HVR1 N-terminal part) and 9/86 (recognizing the whole HVR1) do not neutralize HCVpp infectivity (Hsu et al., 2003). However, mAb 9/27, whose epitope includes the C-terminal part of HVR1 (Flint et al., 2000), neutralizes HCVpp infection (Bartosch et al., 2003a; Hsu et al., 2003). This antibody also blocks binding of soluble E2 to SR-BI. The mAbs 2P24 and 15H4 recognize an epitope within the HVR1 C terminus (23GXXQ26 motif) and inhibit HCV serum particles binding to MOLT-4 cells (Li & Allain, 2005; Li et al., 2001). In keeping with the latter, our phage display mapping data indicated that the 20FQPXXK25 motif is a key element of the mAb AP213 epitope. Lastly, statistical analyses have suggested the presence of two immunogenic domains on HVR1 (Penin et al., 2001): the first region encompassing the 14 first residues of HVR1 and the second region starting from residue 17. The compiled data obtained with various anti-HVR1 antibodies suggested that the second region is the main neutralization determinant in HVR1.
Further fine mapping identified the residues A17, F20 and Q21 in the Gla HVR1 sequence and T21 (and possibly L20) in the corresponding H77c sequence as essential for the AP213 and R1020 epitopes, respectively. Residue A17 was also crucial for the reactivity of R140 to Gla HVR1. Whether these residues are involved directly in antibody–antigen interaction or whether they are essential to confer an appropriate configuration to the antigen is unknown. However, this fits with the interaction interface model whereby a small number of hydrophobic residues, which are rare in HVR1, would constitute the contact points between two proteins, this hotspot for binding energy being surrounded by a more hydrophilic region exposed to the aqueous solution (Clackson & Wells, 1995).
Mechanisms of neutralization by anti-HVR1 antibodies
Anti-HVR1 antibodies had very little effect in vitro on CD81 binding and no effect on heparin binding (data not shown). This region has never been implicated in direct CD81 binding, although it was shown to modulate it (Bankwitz et al., 2010; Roccasecca et al., 2003). Consistently, we observed that mAb AP33 neutralization (which inhibits the E2–CD81 interaction) and also inhibition with a soluble form of CD81 (data not shown) were significantly attenuated with JFH1 HVR1 chimeras, although we could not detect any difference in mAb AP33 affinity for E1E2 extracted from infected cells (data not shown). Swapping the HVR1 loop might therefore increase the steric hindrance around the CD81-binding site, a phenomenon possibly accentuated at the surface of virus particles where glycoproteins might be more tightly packed together.
Similarly to mAb AP33, anti-HVR1 antibodies were capable of post-attachment neutralization, but were more efficient when present during the virus-binding stage. This could suggest that anti-HVR1 antibodies also inhibit virus binding or that their epitope is more available before virus attachment. Interestingly, we quantified viral RNA bound to the cell surface at 4 °C and found that attachment was not significantly affected by virus pre-incubation with anti-HVR1 antibodies (data not shown) but was strongly inhibited by heparin treatment (Vieyres et al., 2009). Although one might expect an attenuated binding to SR-BI in presence of anti-HVR1 antibodies, it is likely that binding occurs mainly via virus-associated lipoproteins and is therefore not blocked by anti-HVR1 antibodies. Thus, the role of HVR1 in HCV infection is not limited to cell-surface attachment, through glycosaminoglycans binding for instance (Barth et al., 2006; Basu et al., 2004); on the contrary, this region seems to play an active role in entry.
In conclusion, the chimeric HCVcc constructs and anti-HVR1 antibodies described here constitute new tools to investigate further the role of HVR1 in the HCV life cycle. Antibodies targeting the HVR1 C terminus were able to neutralize HCVcc infectivity and notably inhibited a post-attachment step of entry, unravelling new roles for HVR1 in HCVcc infection.
METHODS
Cell culture and antibodies.
Human hepatoma Huh7 cells (Nakabayashi et al., 1982) and human epithelial kidney (HEK) 293T cells (ATCC CRL-1573) were propagated as described elsewhere (Vieyres et al., 2009). The mouse mAbs AP213, AP33, ALP98 and H53 and the rabbit polyclonal serum R1020 have been described previously (Clayton et al., 2002; Cocquerel et al., 1998; Owsianka et al., 2005; Patel et al., 2000). The rabbit polyclonal antiserum R140 was raised against a synthetic peptide (1084.B; see below) corresponding to the HCV genotype 1a strain Gla HVR1, as described previously (Patel et al., 2000). The rabbit serum R1353 directed against an irrelevant protein was used as a control. The mouse monoclonal and sheep polyclonal anti-HCV NS5A antibodies were kind gifts from C. M. Rice and M. Harris, respectively (Lindenbach et al., 2005; Macdonald et al., 2003). Anti-CD81 mAb (clone JS-81) and anti-ApoE serum were obtained from BD Biosciences and Millipore, respectively. Alexa Fluor 488-conjugated donkey anti-mouse and anti-rabbit antibodies were purchased from Invitrogen. HRP-conjugated protein A or antibodies were from Sigma. Total IgGs from R1020, R140 and R1353 sera were purified using protein G affinity chromatography for use in neutralization assays.
Plasmid constructs.
Plasmid phCMVcE1E2(H77c) encoding the full-length E1E2 of the genotype 1a strain H77c (Yanagi et al., 1997) has been described previously (Owsianka et al., 2005). The HVR1 (aa 384 – 410 of the viral polyprotein) in the E2 gene of this plasmid was replaced with the corresponding sequence of the genotype 1a Gla strain to form plasmid phCMVcE1E2(H77c-GlaHVR1). This plasmid also carried an A373V amino acid substitution in the H77c E1 gene. Chimeric HVR1 sequences were generated by fusion PCR and inserted into phCMVcE1E2(H77c). Point mutants in HVR1 were generated in the phCMVcE1E2(H77c-GlaHVR1) plasmid using a QuikChange II Site-directed Mutagenesis kit (Stratagene). E1E2-encoding plasmids were expressed by transfection of HEK cells and cell lysates were harvested 3 days later.
The plasmid pUC-JFH1 carries the full-length cDNA of the genotype 2a HCVcc strain JFH1 (Wakita et al., 2005). The plasmid pUC-JFH1/GND is identical except that it carries the replication knockout GND mutation in the NS5B-encoding sequence (Wakita et al., 2005). Chimeric JFH1 genomic cDNAs were constructed that carried the Gla or H77c HVR1 in the plasmid pUC-JFH1. All constructs were checked by restriction analysis and nucleotide sequencing of the E1E2-encoding region.
Generation of HCVcc and infectivity assays.
HCVpp and HCVcc were generated essentially as described previously (Bartosch et al., 2003b; Kato et al., 2006). Briefly, linearized in vitro-transcribed HCV genomic RNA was electroporated into Huh7 cells. Cells were split at 2 days post-electroporation. The filtered supernatant harvested at 5 days post-electroporation was titrated and used to infect naïve cells at an m.o.i. of 0.005. These infected cells were serially passaged and the supernatant evaluated for virus infectivity at each passage. High-titre stocks were generated from infected cells at passages 5–7, titrated, stored in aliquots at −70 °C and used in neutralization experiments. In addition, 105 naïve Huh7 cells were infected in six-well dishes with these virus stocks and total RNA was extracted after 3 days. HCV RNA corresponding to the E1E2-encoding region was reverse transcribed and amplified by PCR. The cDNA fragments obtained were purified on gels and sequenced to check for the appearance of adaptive mutations.
HCVcc infectivity was assessed as described previously by TCID50 or f.f.u. methods (Lindenbach et al., 2005; Zhong et al., 2005). In both cases, cells were fixed in methanol 2 days p.i. and immunostained for NS5A using mAb 9E10 or sheep anti-NS5A antiserum.
Binding/entry assay.
Three protocols were used to determine whether anti-E2 antibodies inhibited virus attachment to the cells or acted post-binding. In protocol A, HCVcc was pre-incubated for 20 min at 37 °C with antibody, chilled briefly and then adsorbed onto pre-cooled target cells for 1 h at 4 °C. Unbound virus was removed by washing the cells twice with cold medium. Antibody was added again to the culture for 10 min at 4 °C, after which the cells were shifted to 37 °C to allow HCV entry to occur. In protocols B and C, antibody was only added reciprocally before or after HCVcc binding, respectively. Anti-CD81 mAb was pre-incubated with the cells rather than with HCVcc. The inoculum was replaced with fresh medium after 3 h at 37 °C. At 2 days p.i., cells were fixed and stained for counting of f.f.u.
GNA capture ELISA.
Detection of E2 glycoprotein by ELISA was performed essentially as described previously (Patel et al., 2000). Briefly, E1E2 glycoproteins from clarified lysates of transfected HEK cells were captured onto GNA lectin-coated ELISA plates (Immulon 2HB; Thermo Electro Corp.). The bound glycoproteins were detected with specific anti-E2 antibodies, followed by HRP-conjugated anti-species IgG and 3,3′,5,5′-tetramethylbenzidine substrate (Invitrogen). Reactions were stopped with 0.5 M H2SO4 and absorbance values were determined at 450 nm. To test for conformation sensitivity of antibody epitopes, HEK cells expressing E1E2 were lysed in PBS by freeze-thawing and sonication. Clarified cell lysates were either left untreated (native E1E2) or boiled for 10 min in the presence of SDS (0.1 % final) and DTT (20 mM final) (denatured E1E2). NP-40 was added to both native and denatured samples at a final concentration of 1 %. The glycoproteins were then captured on GNA-coated plates and tested for antibody recognition as described above.
Peptide competition assay.
Branched peptides 1084.B [residues 390–410 of HCV strain Gla HVR1: (GAAARSTLQLAGLFQPGAKQN)4K3A] and 2060.10 [residues 604–621 of HCV strain H77c E2: (TPRCMVDYPYRLWHYPCT)4K3A] were incubated in a range of concentrations with mAbs AP33 and AP213 or R140 antiserum at room temperature for 30 min. The peptide/antibody mix was then tested for reactivity against GNA-captured H77c-GlaHVR1 E1E2 in an ELISA as described above.
AP213 epitope mapping by random peptide phage display.
A PhD-12 Phage Display Peptide Library kit (New England Biolabs) was used according to the manufacturer's instructions. Briefly, the peptide library was enriched for mAb AP213-specific peptides by three rounds of panning. At each round, phages were incubated in a well of an Immulon 2HB plate (Thermo Electro Corp.) coated with 10 μg mAb AP213 ml−1 and bound phages were eluted in low-pH buffer. After the third round of panning, 52 eluted phages were isolated, amplified and tested by ELISA for their reactivity with mAb AP213 or an isotype control (mAb AP33). The DNA sequence of 32 AP213-specific phages was determined following the manufacturer's instructions. The deduced peptide sequences were aligned with that of Gla E1E2 using clustal w and the alignment with Gla HVR1 was further adjusted manually.
Supplementary Material
Acknowledgments
We are grateful to T. Wakita, C. M. Rice, F.-L. Cosset, J. A. McKeating, J. K. Ball and M. Harris for providing us with reagents. We thank A. G. N. Angus and S. Dhillon for their help. This work was funded by the Medical Research Council, UK. G. V. was supported by a fellowship from the French Ministry of Research and the Région Nord-Pas-de-Calais.
Footnotes
Antibody neutralization data is available as supplementary data in JGV Online.
References
- Agnello, V., Abel, G., Elfahal, M., Knight, G. B. & Zhang, Q. X. (1999). Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A 96, 12766–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankwitz, D., Steinmann, E., Bitzegeio, J., Ciesek, S., Friesland, M., Herrmann, E., Zeisel, M. B., Baumert, T. F., Keck, Z. Y. & other authors (2010). Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol 84, 5751–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth, H., Schafer, C., Adah, M. I., Zhang, F., Linhardt, R. J., Toyoda, H., Kinoshita-Toyoda, A., Toida, T., Van Kuppevelt, T. H. & other authors (2003). Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem 278, 41003–41012. [DOI] [PubMed] [Google Scholar]
- Barth, H., Schnober, E. K., Zhang, F., Linhardt, R. J., Depla, E., Boson, B., Cosset, F. L., Patel, A. H., Blum, H. E. & Baumert, T. F. (2006). Viral and cellular determinants of the hepatitis C virus envelope–heparan sulfate interaction. J Virol 80, 10579–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosch, B., Bukh, J., Meunier, J. C., Granier, C., Engle, R. E., Blackwelder, W. C., Emerson, S. U., Cosset, F. L. & Purcell, R. H. (2003a). In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci U S A 100, 14199–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosch, B., Dubuisson, J. & Cosset, F. L. (2003b). Infectious hepatitis C virus pseudo-particles containing functional E1–E2 envelope protein complexes. J Exp Med 197, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosch, B., Vitelli, A., Granier, C., Goujon, C., Dubuisson, J., Pascale, S., Scarselli, E., Cortese, R., Nicosia, A. & Cosset, F. L. (2003c). Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem 278, 41624–41630. [DOI] [PubMed] [Google Scholar]
- Bartosch, B., Verney, G., Dreux, M., Donot, P., Morice, Y., Penin, F., Pawlotsky, J. M., Lavillette, D. & Cosset, F. L. (2005). An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol 79, 8217–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, A., Beyene, A., Meyer, K. & Ray, R. (2004). The hypervariable region 1 of the E2 glycoprotein of hepatitis C virus binds to glycosaminoglycans, but this binding does not lead to infection in a pseudotype system. J Virol 78, 4478–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh, J., Miller, R. H. & Purcell, R. H. (1995). Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin Liver Dis 15, 41–63. [DOI] [PubMed] [Google Scholar]
- Callens, N., Ciczora, Y., Bartosch, B., Vu-Dac, N., Cosset, F. L., Pawlotsky, J. M., Penin, F. & Dubuisson, J. (2005). Basic residues in hypervariable region 1 of hepatitis C virus envelope glycoprotein E2 contribute to virus entry. J Virol 79, 15331–15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, K. S., Jiang, J., Cai, Z. & Luo, G. (2007). Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J Virol 81, 13783–13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson, T. & Wells, J. A. (1995). A hot spot of binding energy in a hormone–receptor interface. Science 267, 383–386. [DOI] [PubMed] [Google Scholar]
- Clayton, R. F., Owsianka, A., Aitken, J., Graham, S., Bhella, D. & Patel, A. H. (2002). Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles. J Virol 76, 7672–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquerel, L., Meunier, J. C., Pillez, A., Wychowski, C. & Dubuisson, J. (1998). A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J Virol 72, 2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon, S., Witteveldt, J., Gatherer, D., Owsianka, A. M., Zeisel, M. B., Zahid, M. N., Rychlowska, M., Foung, S. K., Baumert, T. F. & other authors (2010). Mutations within a conserved region of the hepatitis C virus E2 glycoprotein that influence virus–receptor interactions and sensitivity to neutralizing antibodies. J Virol 84, 5494–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux, M., Pietschmann, T., Granier, C., Voisset, C., Ricard-Blum, S., Mangeot, P. E., Keck, Z., Foung, S., Vu-Dac, N. & other authors (2006). High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J Biol Chem 281, 18285–18295. [DOI] [PubMed] [Google Scholar]
- Evans, M. J., von Hahn, T., Tscherne, D. M., Syder, A. J., Panis, M., Wolk, B., Hatziioannou, T., McKeating, J. A., Bieniasz, P. D. & Rice, C. M. (2007). Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446, 801–805. [DOI] [PubMed] [Google Scholar]
- Farci, P., Shimoda, A., Wong, D., Cabezon, T., De Gioannis, D., Strazzera, A., Shimizu, Y., Shapiro, M., Alter, H. J. & Purcell, R. H. (1996). Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci U S A 93, 15394–15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farci, P., Shimoda, A., Coiana, A., Diaz, G., Peddis, G., Melpolder, J. C., Strazzera, A., Chien, D. Y., Munoz, S. J. & other authors (2000). The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288, 339–344. [DOI] [PubMed] [Google Scholar]
- Flint, M., Dubuisson, J., Maidens, C., Harrop, R., Guile, G. R., Borrow, P. & McKeating, J. A. (2000). Functional characterization of intracellular and secreted forms of a truncated hepatitis C virus E2 glycoprotein. J Virol 74, 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns, X., Allander, T., Rohwer-Nutter, P. & Bukh, J. (2000a). Characterization of modified hepatitis C virus E2 proteins expressed on the cell surface. Virology 274, 75–85. [DOI] [PubMed] [Google Scholar]
- Forns, X., Thimme, R., Govindarajan, S., Emerson, S. U., Purcell, R. H., Chisari, F. V. & Bukh, J. (2000b). Hepatitis C virus lacking the hypervariable region 1 of the second envelope protein is infectious and causes acute resolving or persistent infection in chimpanzees. Proc Natl Acad Sci U S A 97, 13318–13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstroh, A., Schnober, E. K., Zeisel, M. B., Carolla, P., Barth, H., Blum, H. E., Cosset, F. L., Koutsoudakis, G., Bartenschlager, R. & other authors (2008). Neutralizing host responses in hepatitis C virus infection target viral entry at postbinding steps and membrane fusion. Gastroenterology 135, 1719–1728. [DOI] [PubMed] [Google Scholar]
- Hijikata, M., Kato, N., Ootsuyama, Y., Nakagawa, M., Ohkoshi, S. & Shimotohno, K. (1991). Hypervariable regions in the putative glycoprotein of hepatitis C virus. Biochem Biophys Res Commun 175, 220–228. [DOI] [PubMed] [Google Scholar]
- Hsu, M., Zhang, J., Flint, M., Logvinoff, C., Cheng-Mayer, C., Rice, C. M. & McKeating, J. A. (2003). Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci U S A 100, 7271–7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iro, M., Witteveldt, J., Angus, A. G., Woerz, I., Kaul, A., Bartenschlager, R. & Patel, A. H. (2009). A reporter cell line for rapid and sensitive evaluation of hepatitis C virus infectivity and replication. Antiviral Res 83, 148–155. [DOI] [PubMed] [Google Scholar]
- Johansson, D. X., Voisset, C., Tarr, A. W., Aung, M., Ball, J. K., Dubuisson, J. & Persson, M. A. (2007). Human combinatorial libraries yield rare antibodies that broadly neutralize hepatitis C virus. Proc Natl Acad Sci U S A 104, 16269–16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, T., Date, T., Murayama, A., Morikawa, K., Akazawa, D. & Wakita, T. (2006). Cell culture and infection system for hepatitis C virus. Nat Protoc 1, 2334–2339. [DOI] [PubMed] [Google Scholar]
- Keck, Z. Y., Xia, J., Cai, Z., Li, T. K., Owsianka, A. M., Patel, A. H., Luo, G. & Foung, S. K. (2007). Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J Virol 81, 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoudakis, G., Kaul, A., Steinmann, E., Kallis, S., Lohmann, V., Pietschmann, T. & Bartenschlager, R. (2006). Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J Virol 80, 5308–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki, M., Enomoto, N., Marumo, F. & Sato, C. (1993). Rapid sequence variation of the hypervariable region of hepatitis C virus during the course of chronic infection. Hepatology 18, 1293–1299. [PubMed] [Google Scholar]
- Lavillette, D., Tarr, A. W., Voisset, C., Donot, P., Bartosch, B., Bain, C., Patel, A. H., Dubuisson, J., Ball, J. K. & Cosset, F. L. (2005). Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology 41, 265–274. [DOI] [PubMed] [Google Scholar]
- Law, M., Maruyama, T., Lewis, J., Giang, E., Tarr, A. W., Stamataki, Z., Gastaminza, P., Chisari, F. V., Jones, I. M. & other authors (2008). Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med 14, 25–27. [DOI] [PubMed] [Google Scholar]
- Li, C. & Allain, J. P. (2005). Chimeric monoclonal antibodies to hypervariable region 1 of hepatitis C virus. J Gen Virol 86, 1709–1716. [DOI] [PubMed] [Google Scholar]
- Li, C., Candotti, D. & Allain, J. P. (2001). Production and characterization of monoclonal antibodies specific for a conserved epitope within hepatitis C virus hypervariable region 1. J Virol 75, 12412–12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach, B. D., Evans, M. J., Syder, A. J., Wolk, B., Tellinghuisen, T. L., Liu, C. C., Maruyama, T., Hynes, R. O., Burton, D. R. & other authors (2005). Complete replication of hepatitis C virus in cell culture. Science 309, 623–626. [DOI] [PubMed] [Google Scholar]
- Liu, S., Yang, W., Shen, L., Turner, J. R., Coyne, C. B. & Wang, T. (2009). Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent superinfection. J Virol 83, 2011–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, A., Crowder, K., Street, A., McCormick, C., Saksela, K. & Harris, M. (2003). The hepatitis C virus non-structural NS5A protein inhibits activating protein-1 function by perturbing ras–ERK pathway signaling. J Biol Chem 278, 17775–17784. [DOI] [PubMed] [Google Scholar]
- Mancini, N., Diotti, R. A., Perotti, M., Sautto, G., Clementi, N., Nitti, G., Patel, A. H., Ball, J. K., Clementi, M. & Burioni, R. (2009). Hepatitis C virus (HCV) infection may elicit neutralizing antibodies targeting epitopes conserved in all viral genotypes. PLoS ONE 4, e8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey, K., Boo, I., Poumbourios, P. & Drummer, H. E. (2007). Expression and characterization of a minimal hepatitis C virus glycoprotein E2 core domain that retains CD81 binding. J Virol 81, 9584–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens, L., Bertaux, C., Cukierman, L., Cormier, E., Lavillette, D., Cosset, F. L. & Dragic, T. (2008). The tight junction proteins claudin-1, -6, and -9 are entry cofactors for hepatitis C virus. J Virol 82, 3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour, D., Penin, F. & Rice, C. M. (2007). Replication of hepatitis C virus. Nat Rev Microbiol 5, 453–463. [DOI] [PubMed] [Google Scholar]
- Nakabayashi, H., Taketa, K., Miyano, K., Yamane, T. & Sato, J. (1982). Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res 42, 3858–3863. [PubMed] [Google Scholar]
- Owsianka, A., Clayton, R. F., Loomis-Price, L. D., McKeating, J. A. & Patel, A. H. (2001). Functional analysis of hepatitis C virus E2 glycoproteins and virus-like particles reveals structural dissimilarities between different forms of E2. J Gen Virol 82, 1877–1883. [DOI] [PubMed] [Google Scholar]
- Owsianka, A., Tarr, A. W., Juttla, V. S., Lavillette, D., Bartosch, B., Cosset, F. L., Ball, J. K. & Patel, A. H. (2005). Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol 79, 11095–11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianka, A. M., Tarr, A. W., Keck, Z. Y., Li, T. K., Witteveldt, J., Adair, R., Foung, S. K., Ball, J. K. & Patel, A. H. (2008). Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J Gen Virol 89, 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A. H., Wood, J., Penin, F., Dubuisson, J. & McKeating, J. A. (2000). Construction and characterization of chimeric hepatitis C virus E2 glycoproteins: analysis of regions critical for glycoprotein aggregation and CD81 binding. J Gen Virol 81, 2873–2883. [DOI] [PubMed] [Google Scholar]
- Pawlotsky, J. M. (2003). Hepatitis C virus genetic variability: pathogenic and clinical implications. Clin Liver Dis 7, 45–66. [DOI] [PubMed] [Google Scholar]
- Penin, F., Combet, C., Germanidis, G., Frainais, P. O., Deleage, G. & Pawlotsky, J. M. (2001). Conservation of the conformation and positive charges of hepatitis C virus E2 envelope glycoprotein hypervariable region 1 points to a role in cell attachment. J Virol 75, 5703–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perotti, M., Mancini, N., Diotti, R. A., Tarr, A. W., Ball, J. K., Owsianka, A., Adair, R., Patel, A. H., Clementi, M. & Burioni, R. (2008). Identification of a broadly cross-reacting and neutralizing human monoclonal antibody directed against the hepatitis C virus E2 protein. J Virol 82, 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileri, P., Uematsu, Y., Campagnoli, S., Galli, G., Falugi, F., Petracca, R., Weiner, A. J., Houghton, M., Rosa, D. & other authors (1998). Binding of hepatitis C virus to CD81. Science 282, 938–941. [DOI] [PubMed] [Google Scholar]
- Ploss, A., Evans, M. J., Gaysinskaya, V. A., Panis, M., You, H., de Jong, Y. P. & Rice, C. M. (2009). Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457, 882–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccasecca, R., Ansuini, H., Vitelli, A., Meola, A., Scarselli, E., Acali, S., Pezzanera, M., Ercole, B. B., McKeating, J. & other authors (2003). Binding of the hepatitis C virus E2 glycoprotein to CD81 is strain specific and is modulated by a complex interplay between hypervariable regions 1 and 2. J Virol 77, 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, D., Campagnoli, S., Moretto, C., Guenzi, E., Cousens, L., Chin, M., Dong, C., Weiner, A. J., Lau, J. Y. & other authors (1996). A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci U S A 93, 1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarselli, E., Ansuini, H., Cerino, R., Roccasecca, R. M., Acali, S., Filocamo, G., Traboni, C., Nicosia, A., Cortese, R. & Vitelli, A. (2002). The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J 21, 5017–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield, D. J., Bartosch, B., Shimizu, Y. K., Allander, T., Alter, H. J., Emerson, S. U., Cosset, F. L. & Purcell, R. H. (2005). Human monoclonal antibodies that react with the E2 glycoprotein of hepatitis C virus and possess neutralizing activity. Hepatology 42, 1055–1062. [DOI] [PubMed] [Google Scholar]
- Shimizu, Y. K., Hijikata, M., Iwamoto, A., Alter, H. J., Purcell, R. H. & Yoshikura, H. (1994). Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol 68, 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds, P. (1995). Variability of hepatitis C virus. Hepatology 21, 570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr, A. W., Owsianka, A. M., Timms, J. M., McClure, C. P., Brown, R. J., Hickling, T. P., Pietschmann, T., Bartenschlager, R., Patel, A. H. & Ball, J. K. (2006). Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology 43, 592–601. [DOI] [PubMed] [Google Scholar]
- Troesch, M., Meunier, I., Lapierre, P., Lapointe, N., Alvarez, F., Boucher, M. & Soudeyns, H. (2006). Study of a novel hypervariable region in hepatitis C virus (HCV) E2 envelope glycoprotein. Virology 352, 357–367. [DOI] [PubMed] [Google Scholar]
- Vieyres, G., Angus, A. G., Haberstroh, A., Baumert, T. F., Dubuisson, J. & Patel, A. H. (2009). Rapid synchronization of hepatitis C virus infection by magnetic adsorption. J Virol Methods 157, 69–79. [DOI] [PubMed] [Google Scholar]
- Voisset, C., Callens, N., Blanchard, E., Op De Beeck, A., Dubuisson, J. & Vu-Dac, N. (2005). High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J Biol Chem 280, 7793–7799. [DOI] [PubMed] [Google Scholar]
- Wakita, T., Pietschmann, T., Kato, T., Date, T., Miyamoto, M., Zhao, Z., Murthy, K., Habermann, A., Krausslich, H. G. & other authors (2005). Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11, 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, A. J., Brauer, M. J., Rosenblatt, J., Richman, K. H., Tung, J., Crawford, K., Bonino, F., Saracco, G., Choo, Q. L. & other authors (1991). Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology 180, 842–848. [DOI] [PubMed] [Google Scholar]
- Weiner, A. J., Geysen, H. M., Christopherson, C., Hall, J. E., Mason, T. J., Saracco, G., Bonino, F., Crawford, K., Marion, C. D. & other authors (1992). Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci U S A 89, 3468–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi, M., Purcell, R. H., Emerson, S. U. & Bukh, J. (1997). Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci U S A 94, 8738–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel, M. B., Koutsoudakis, G., Schnober, E. K., Haberstroh, A., Blum, H. E., Cosset, F. L., Wakita, T., Jaeck, D., Doffoel, M. & other authors (2007). Scavenger receptor class B type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology 46, 1722–1731. [DOI] [PubMed] [Google Scholar]
- Zheng, A., Yuan, F., Li, Y., Zhu, F., Hou, P., Li, J., Song, X., Ding, M. & Deng, H. (2007). Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J Virol 81, 12465–12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, J., Gastaminza, P., Cheng, G., Kapadia, S., Kato, T., Burton, D. R., Wieland, S. F., Uprichard, S. L., Wakita, T. & Chisari, F. V. (2005). Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A 102, 9294–9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibert, A., Schreier, E. & Roggendorf, M. (1995). Antibodies in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology 208, 653–661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.