Abstract
Objectives
A minority of HIV-infected patients taking an antiretroviral (ARV) regimen containing dideoxynucleosides (d-drugs) such as stavudine (d4T) and didanosine (DDI) experiences dose-limiting neuropathic pain and paraesthesias, usually within weeks of starting these drugs. Because d-drugs are among the few affordable options available in developing countries, continuing d-drug therapy would be a desirable strategy for many HIV-infected individuals. Therefore, we evaluated the safety of continuing d-drug therapy.
Methods
In a US cohort, we compared the rates of worsening neuropathic symptoms and signs in HIV-infected individuals on stable ARV regimens that did (n = 252) or did not (n = 250) include d-drugs. Rates of worsening were compared using proportional hazards model and the log-rank test.
Results
The risk ratios (RR) were not significantly larger for worsening neuropathy signs [0.94; 95% confidence interval (CI) 0.84–1.07] or symptoms (0.99; 95% CI 0.88–1.14) in patients taking d-drugs continuously compared to those not taking d-drugs.
Conclusions
Continued d-drug exposure among patients tolerating an initial trial did not increase the risk of worsening neuropathy compared to non-d-drug-containing regimens. If applicable in developing countries, these findings suggest that in most patients d-drugs can be continued safely in the long term without increasing the risk of worsening neuropathy.
Keywords: AIDS, dideoxynucleoside, HIV, neuropathy
Introduction
HIV-associated distal sensory-predominant polyneuropathy (DSPN) is an axon-length-dependent, painful, sensory neuropathy that often presents as paraesthesias and dysaesthesias in the feet [1–4]. Symptomatic DSPN is a frequent reason for patients to seek medical care, including analgesic therapy. Of all the neurological complications of HIV infection, DSPN is recognized most frequently by HIV care providers as having an impact on disability and antiretroviral (ARV) treatment choices.
HIV is thought to cause peripheral neuropathy by increasing macrophage activation in the peripheral nervous system. The use of dideoxynucleoside analogues [d-drugs: didanosine (ddI), stavudine (d4T), and zalcitabine (ddC)] in the context of ARV treatment frequently contributes to or exacerbates DSPN 1,5–8. The best available evidence suggests that the pathophysiological basis for polyneuropathy from dideoxynucleosides is mitochondrial toxicity [9]. Dideoxynucleoside analogues contain azido groups that compete with natural thymidine triphosphate as substrates of DNA polymerase γ. This competitive inhibition of DNA polymerase γ may disrupt mitochondrial DNA synthesis [6], resulting in abnormal mitochondria as well as a significantly decreased quantity of mtDNA.
Since highly active antiretroviral therapy (HAART) became widely available in 1996, HIV-related sensory neuropathy has become a highly prevalent neurological complication, affecting approximately half of patients with HIV [10,11]. While d-drugs are used infrequently in the USA because of the risk of various toxicities (including neuropathy) and because other options are readily available, in many resource-limited settings cost and manufacturing considerations lead to d-drugs being an essential component of regimens. Thus, in a 2006 survey of 23 developing countries representing 851000 patients receiving ARV treatment, d-drugs – particularly stavudine (d4T) – were included in 70% of first-line ARV regimens and 60% of second-line regimens [12]. If continued d-drug therapy produced cumulative peripheral nerve toxicity in such patients, the result would be increasing numbers of individuals with neurological disability and requirement for ongoing pain management.
To evaluate whether continuing d-drug therapy was associated with accumulating neuropathy risk, we compared the rates of worsening neuropathy signs and symptoms in patients on stable d-drug-containing regimens to those of patients on non-d-drug containing regimens.
Patients and methods
Selection of patients
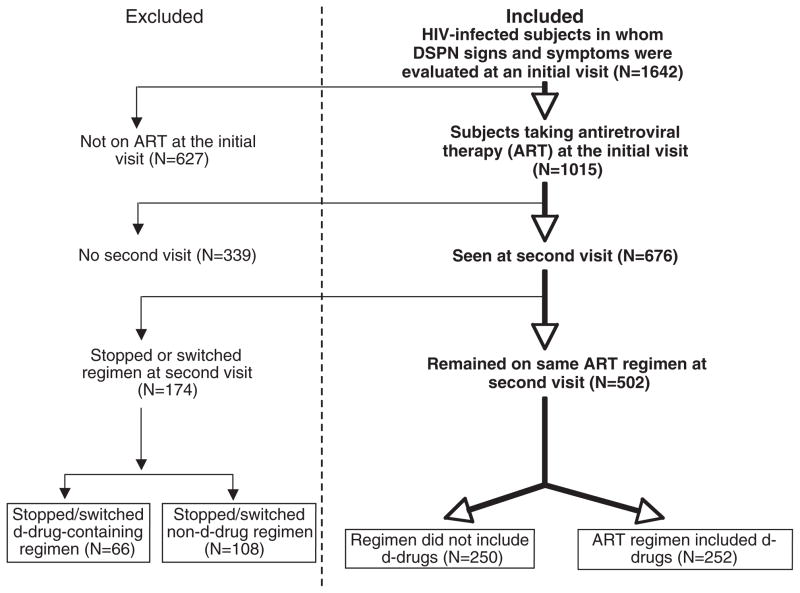
Patients were enrolled prospectively in clinical studies at the HIV Neurobehavioral Research Center (HNRC) at the University of California, San Diego between 1999 and 2005. Information on past and current ARV therapy was obtained by research nurses and physicians, trained and experienced in the evaluation of HIV-infected individuals. Figure 1 depicts the criteria used to select patients for this analysis: a stable ARV regimen for at least two consecutive visits at which trained research nurses obtained complete history and physical examinations, including assessment of neuropathy signs and symptoms. Patients were divided into two groups: those whose regimens included d-drugs (ddI, d4T, ddC) at the time of clinical assessment and those whose regimens did not include d-drugs.
Fig. 1.
Selection of patients for this analysis.
Immunological and virological measurements
In pre-enrolment questionnaires, patients were asked to report their lowest CD4 cell count. If their CD4 cell count was lower at their first visit or they could not recall a CD4 nadir, their first visit count was also used as their nadir count. Plasma viral loads were determined by standard viral load Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) (lower detection limit >400 HIV-1 RNA -copies/mL) in most cases (92%), or by ultrasensitive viral load RT-PCR (lower detection limit >50 copies/mL) in a minority (8%). All viral load levels below 400 copies/mL (2.60 log10 copies/mL) were considered undetectable on either assay. Viral load measurements were log10-transformed for analysis.
HIV-associated DSPN: signs and symptoms
Each of three signs was graded in severity on a three-point scale: 0 = normal; 1 = abnormal, mild severity; 2 = moderate to severe. For example, vibration sensation was considered normal if greater than or equal to 10 s, mildly abnormal if less than 10 s and severely impaired if completely absent. Patients were classified as having DSPN (symptomatic or asymptomatic) if they demonstrated two or more of the following three physical exam signs, regardless of symptoms: (1) vibration sensation at the great toes less than 10 s; (2) diminished or absent sensation to pinprick in the distal legs and feet; and (3) ankle reflexes diminished in comparison to knee reflexes. In all cases, signs were considered to be present only if findings were bilateral. Patients with symptomatic DSPN had at least one symptom of reported pain, paraesthesias or loss of sensation in a distal pattern consistent with neuropathy, whereas patients without these symptoms were labelled asymptomatic. Symptoms of pain, paraesthesias and loss of sensation were each graded on a four-point scale: 0 = absent; 1 = mild, non-disabling, not requiring treatment; 2 = resulting in some disability or requiring some treatment; 3 = disabling despite daily treatment. Thus the symptom scores ranged from 0 to 9 and the sign scores from 0 to 6.
Worsening neuropathy signs and symptoms
Clinical neuropathy outcomes were assessed in three ways. Firstly, we compared the number and severity of signs in each individual patient at the initial and subsequent visits. Signs were considered to have worsened if there were new neuropathic findings or if signs worsened from mild to moderate/severe. Secondly, we assessed the number and severity of symptoms at each visit. Symptoms were considered to have worsened if new neuropathic complaints were reported or if symptoms reported previously increased in severity. Finally, we considered changes in DSPN classification at follow-up visits.
Statistical methods
The proportion of patients excluded because of switching or stopping of regimens was compared between regimens including d-drugs or not, using Fisher’s exact test. The demographics and patient characteristics (year of enrolment, age, CD4 nadirs, current CD4 and proportion with DSPN at baseline) were compared between arms by Wilcoxon rank test; meanwhile, ethnicity and viral load were compared using Pearson’s χ2 test. A Kaplan–Meier analysis was performed on the time to the development of worsening of symptom score, sign score and DSPN classification, as described earlier. For ease of visual inspection, time-to-event plots were generated illustrating the accumulation of signs and symptoms over time. Events were defined as the first visit at which incidence or worsening occurred. The two treatment groups were compared using the log-rank test. In addition, the risk ratios of an event [and 95% confidence intervals (CIs)] were computed using a Cox proportional hazards model, with and without adjustment for viral load (<400 copies/mL vs. ≥ 400 copies/mL) and CD4 cell count. The incidence of DSPN cases was computed and compared between the d-drug and non-d-drug treatment groups using an exponential survival model.
Results
Among 676 patients on antiretroviral therapy (ART) at the first visit (Fig. 1), 174 (26%) were excluded from the analysis because they stopped or switched their first visit regimen prior to their second visit. These included 66/318 (21%) who stopped or switched d-drug-containing regimens and 108/358 (30%) who stopped or switched non-d-drug containing regimens (P = 0.006, Fisher’s exact test).
Among the remaining 502 patients on stable ART during the period of the study, 252 were on regimens that included at least one d-drug, and 250 had no d-drugs in their regimens. Table 1 summarizes the baseline characteristics of the two groups. Patients in the two groups did not differ significantly according to year of enrolment (d-drug group, median = 2000; non-d-drug group, median = 2001). The d-drug and non-d-drug groups were comparable in distribution of sex (86% and 85% male, respectively), ethnicity (51% and 57% White, respectively) and age (medians 42 and 41.8 years, respectively). They were also similar immunologically, with comparable CD4 nadirs [medians 131.5 and 154.5 cells/μL (P = 0.17), respectively] and current CD4s (medians 280 and 296 cells/μL, respectively). The proportion of patients with undetectable plasma viral loads (defined as <400 copies/mL) differed significantly (59% vs. 72% in the d-drug and non-d-drug groups, respectively; P = 0.003).
Table 1.
Patient demographics at first visit. All differences were non-significant, except plasma viral load (P = 0.003)
| Baseline sample characteristics | Active d-drug use (n = 252) | Non-d-drug use (n = 250) |
|---|---|---|
| Demographics | ||
| Male: n (%) | 216 (86%) | 212 (85%) |
| White: n (%) | 129 (51%) | 142 (57%) |
| Age: years, median (range) | 42 (18.5–64.8) | 41.8 (22.4–69.6) |
| Previous d-drug exposure: % | NA | 54% (n = 237) |
| Clinical data | ||
| CD4 nadir: median (IQR) [cells/μL] | 131.5 (29–278.5) | 154.5 (30.3–323) |
| CD4 count: median (IQR) [cells/μL] | 280 (131.5–465.5) | 296 (155–508) |
| Plasma viral load undetectable | 59.3% (n = 243) | 72.2% (n = 241) |
| Blood glucose: median (IQR) [mg/dL] | 90 (73–103) | 91 (80–103) |
| (n = 245) | (n = 244) | |
| DSPN | ||
| Symptom score: median (IQR) | 1 (0–3) | 2 (0–4) |
| Sign score: median (IQR) | 2 (0–3) | 2 (0–4) |
DSPN, distal sensory-predominant polyneuropathy; IQR, interquartile range.
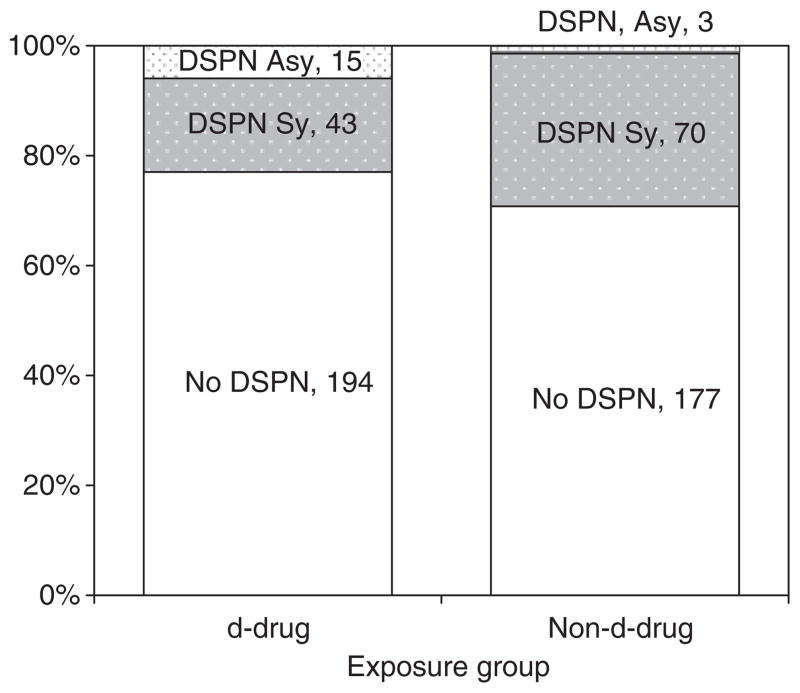
Figure 2 depicts DSPN classification at the first study visit. Patients taking d-drugs were significantly less likely to have symptomatic DSPN (P<0.001; see Discussion). Of 252 patients on d-drugs, 194 (77%) had no DSPN, 15 (6%) had asymptomatic DSPN and 43 (17.1%) had symptomatic DSPN. Of 250 not taking d-drugs, 177 (70.8%) did not meet criteria for DSPN, three (1.2%) had asymptomatic DSPN and 70 (28%) had symptomatic DSPN.
Fig. 2.
Distal sensory-predominant polyneuropathy (DSPN) classification according to antiretroviral exposure group at the first study visit. Patients taking d-drugs were significantly less likely to have symptomatic DSPN than those not taking d-drugs (P<0.001). Asy, asymptomatic; Sy, symptomatic.
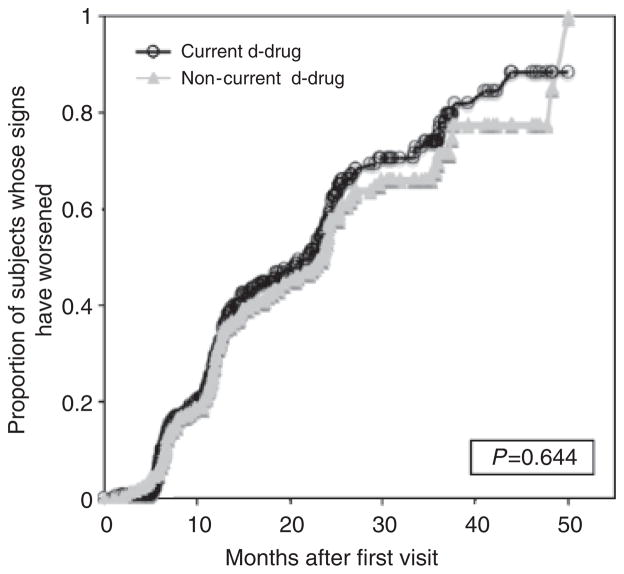
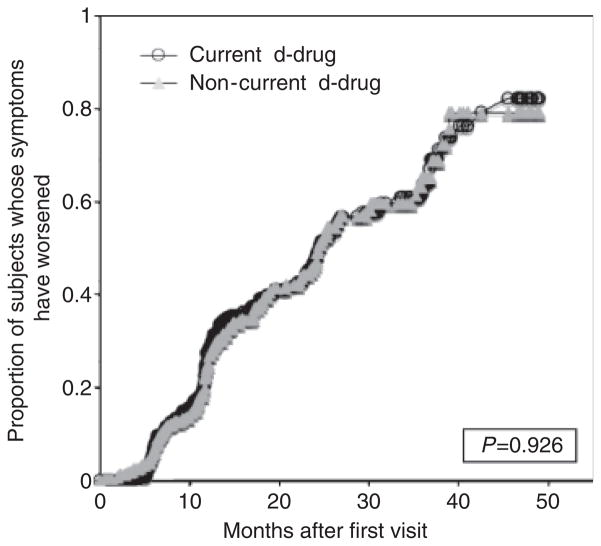
Figures 3 and 4 compare DSPN sign and symptom worsening in patients who did and did not take d-drugs during the study period. The median duration of follow-up was 19 months [interquartile range (IQR) 12–31] and did not differ significantly between the two groups in a non-parametric analysis [d-drug, 18 months (12–29); non-d-drug, 22 months (11–34)]. The hazard ratio (HR) for worsening neuropathy signs was not significantly different for patients taking non-d-drugs compared to those taking d-drugs (HR = 0.94; 95% CI 0.84–1.07; P = 0.64). Similarly, there was no increase in risk for worsening neuropathy symptoms (HR = 0.99; 95% CI 0.88–1.14; P = 0.93). There was no significant difference in risk between the two treatment groups after adjusting for baseline viral load (<400 copies/mL vs. ≥ 400 copies/mL).
Fig. 3.
Distal sensory-predominant polyneuropathy (DSPN) bilateral sign worsening of patients currently on/off d-drugs.
Fig. 4.
Distal sensory-predominant polyneuropathy (DSPN) symptomatic worsening of patients currently on/off d-drugs.
A multivariate analysis, which used initial CD4 cell count as a covariate, showed a significant increase in the hazard for worsening DSPN signs and symptoms in more immunocompromised patients. Using the median of the entire cohort (291 cells/μL) as the cut-off demonstrated that those with a count less than the median were 23% more likely to develop worsening signs (HR = 0.77; 95% CI 0.68–0.87; P<0.001). Similarly, those with a count less than the median were 17% more likely to develop worsening symptoms compared to their less immunocompromised counterparts (HR = 0.83; 95% CI 0.73–0.95; P = 0.007).
Adjusting for both baseline viral load and CD4 cell count in a multivariate analysis did not change the outcome between the two arms. The worsening of signs remained insignificantly different, with an HR of 0.97 (95% CI 0.86–1.10; P = 0.69) for those not taking d-drugs. Similarly, the worsening of symptoms also remained insignificant (HR = 1.01; 95% CI 0.88–1.15; P = 0.88).
To determine the risk of a new clinical diagnosis of DSPN with continued d-drug use, we analysed data separately from those who were originally DSPN-free. During 359 person-years (P-Y) of follow-up in 194 patients taking d-drugs continuously, 53 incident cases of DSPN developed (0.148/P-Y). In comparison, during 311 P-Y of follow-up among 177 non-d-drug-taking patients, 49 incident cases of DSPN developed (0.158/P-Y; P = 0.76).
To ascertain whether d-drug use prior to study entry influenced accumulation of neuropathic symptoms and signs during the period of observation, a Cox proportional hazards analysis was performed. The cohort not taking a d-drug-containing regimen was divided into two subgroups: those with past d-drug exposure (n = 130) and those never exposed to d-drugs (n = 107). There were no significant differences between the arms, including in baseline plasma viral load (P = 0.15, Wilcoxon), baseline CD4 cell count (P = 0.45, Wilcoxon) or DSPN prevalence (25% of those never exposed; 33% of those previously exposed; P = 0.19). In univariate analyses, the HRs did not differ significantly between patients never exposed to d-drugs and those only exposed previously for worsening neuropathy symptoms (HR = 0.89; 95% CI 0.73–1.08; P = 0.24) and signs (HR = 1.17; 95% CI 0.98–1.41; P = 0.08). To ascertain if past d-drug exposure might be masking an effect, those with current d-drug use (n = 252) were compared to those never exposed to d-drugs. In the Cox proportional hazards analysis, the HRs did not differ significantly between those never exposed and those currently taking d-drugs, in both the rate of worsening symptoms (HR = 1.06; 95% CI 0.89–1.27; P = 0.51) and worsening signs (HR = 0.97; 95% CI 0.83–1.13; P = 0.65). Therefore, those with past exposure and no exposure to d-drugs were grouped into one ‘not currently taking d-drugs’ group.
Discussion
Despite highly successful viral suppression with combination ARV therapies, peripheral neuropathy remains a frequent and disabling condition. In fact, most HIV care providers would agree that neuropathy is the most common neurological complication that impacts patient management by limiting the choice of ARVs. Furthermore, neuropathy impacts quality of life negatively, and may require additional medications for symptomatic relief.
Surveys in developing countries have indicated a high rate of neuropathy, both symptomatic and asymptomatic [13–16]. Those with asymptomatic neuropathy may be at increased risk of conversion to symptomatic neuropathy and may show greater vulnerability to peripheral nerve insults related to neurotoxic medications including d-drugs. As a result, there is concern that d-drug exposure, in the context of low-cost ARV regimens being introduced to thousands of such individuals [12], might lead to a large increase in the number of individuals experiencing disabling neuropathic symptoms.
In this US cohort, 72% of HIV-infected patients on a d-drug-containing ARV regimen at their first visit remained on that regimen for a median of 18 months during follow-up. Despite the known peripheral nerve toxicity of d-drugs, individuals tolerating their initial trial of a d-drug-containing regimen demonstrated no subsequent increase in risk of worsening neuropathy signs or symptoms compared to patients taking non-d-drug-containing ARV regimens. These findings suggest that an important majority of patients with HIV is able to tolerate continued d-drug therapy without increased risk of worsening neuropathy.
The failure to detect an increased risk of d-drug exposure in this study was not attributable to differences in immune status, age or other demographic or medical factors, because these were similar between groups at the initial visit. However, patients on d-drug-containing regimens had higher initial plasma viral loads, which in previous studies have been shown to confer an increased risk of DSPN worsening [17]. This difference between the groups might bias our study to find a greater risk of neuropathy worsening in the d-drug-treated patients. Despite this bias, we found no difference in worsening between the two groups. Additionally, we evaluated a multivariate Cox proportional hazards model in which initial viral load was included as a covariate along with d-drug group. This analysis also showed no increase in risk with d-drugs.
At the first study visit, symptomatic DSPN was significantly less frequent in those taking d-drugs compared to those not taking them. We believe that this difference in part reflects intentional stopping or switching of d-drugs in symptomatic patients prior to the first study visit: it is well known among providers that d-drug therapy leads to neuropathic symptoms in some patients. Because of the observational nature of this study, we were unable to capture detailed information on reasons for switching therapy reliably. However, because d-drugs are associated with additional dose-limiting toxicities beyond just DSPN, it is likely that these other toxicities contributed. Dose-limiting toxicities are known to occur with non-d-drug containing regimens as well. In fact, the rate at which d-drug and non-d-drug-containing regimens were stopped or switched was not significantly different.
The design of this study limits in several ways the conclusions that can be drawn from its findings. Because patients were not randomized to continue d-drug- vs. non-d-drug-containing therapy, it is possible that unobserved differences between the groups at baseline contributed to a failure to find differences in neuropathy worsening. Such differences might include hepatitis C virus (HCV) seropositivity, diabetes mellitus and exposure to other peripheral nerve toxins such as alcohol. While data were available only for sub-groups of these patients, we did not find differences in random serum glucose levels, haemoglobin A1c or HCV serostatus (data not shown). We did not assess genetic factors related to susceptibility to d-drug toxicity, which may differ in non-Western racial groups.
Our analyses might have missed a small increase in risk of continuing d-drug therapy (i.e. type II error), but the confidence limits we report are consistent with HRs no larger than 0.88 (or 0.86 if adjusted for viral load and CD4 cell count) – a relatively small increase in risk that is of questionable clinical significance. By way of comparison, our study found that CD4 cell counts less than 291 copies/μL were associated with an HR of 1.30 for worsening neuropathy signs, similar to that found in previous studies [10,18,19].
Our study supports the continued use of d-drugs in developing countries, where large numbers of individuals are treated with d-drug-containing regimens because of their relatively low cost. The prevalence of neuropathy in resource-limited settings appears similar to that found in the USA, ranging from 20% to 75% of HIV/AIDS patients, with similar increases in risk caused by increasing age and decreasing CD4 cell counts [13,16,20–23].
Our findings are similar to those of Cherry et al. (2006), who found that despite three- and seven-fold increases in the risk of acquiring symptomatic neuropathy among those with exposure to ddI and d4T, there was no significant difference in risk if participants remained on the d-drug(s) for durations longer than 12 months. Two international studies observed neuropathy to account for approximately 67% of ARV clinical toxicities, including 13–27% of regimen switches [21,24]. In combination, these studies support careful monitoring of early presenting neuropathy, and suggest that if neuropathy does not present within the first year of d-drug therapy patients continuing on d-drugs will have no greater risk of developing DSPN than those taking non-d-drug-containing regimens.
We believe that susceptibility to neuropathy with d-drugs differs markedly between individuals and that most can tolerate these effective, widely available ARV agents long-term. An option for many patients with intermediate susceptibility is to use lower doses of stavudine, which has been shown to have a lower incidence of neuropathy compared to current standard doses [25].
Acknowledgments
The HNRC is supported by Center award MH 62512 from National Institute of Mental Health (NIMH). The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Appendix
The San Diego HNRC group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego and the Veterans Affairs San Diego Healthcare System. It includes the following individuals. Director: Igor Grant MD; Co-Directors: J. Hampton Atkinson MD, Ronald J. Ellis MD PhD, J. Allen McCutchan MD; Centre Manager: Thomas D. Marcotte PhD; Naval Hospital San Diego: Braden R. Hale MD MPH (PI). Neuromedical component: Ronald J. Ellis MD PhD (PI), J. Allen McCutchan MD, Scott Letendre MD, Edmund Capparelli PharmD, Rachel Schrier PhD. Neurobehavioural component: Robert K. Heaton PhD (PI), Mariana Cherner PhD, Steven Paul Woods PsyD. Neuroimaging component: Terry Jernigan PhD (PI), Christine Fennema-Notestine PhD, Sarah L. Archibald MA, John Hesselink MD, Jacopo Annese PhD, Michael J. Taylor PhD, Brian Schweinsburg PhD. Neurobiology component: Eliezer Masliah MD (PI), Ian Everall FRCPsych FRCPath PhD, T. Dianne Langford PhD. Neurovirology component: Douglas Richman MD (PI), David M. Smith MD. International component: J. Allen McCutchan MD (PI). Developmental component: Ian Everall FRCPsych FRCPath PhD (PI), Stuart Lipton MD PhD. Clinical trials component: J. Allen McCutchan MD, J. Hampton Atkinson MD, Ronald J. Ellis MD PhD, Scott Letendre MD. Participant accrual and retention unit: J. Hampton Atkinson MD (PI), Rodney von Jaeger MPH. Data management unit: Anthony C. Gamst PhD (PI), Clint Cushman BA (Data Systems Manager), Daniel R. Masys MD (Senior Consultant). Statistics unit: Ian Abramson PhD (PI), Florin Vaida PhD, Christopher Ake PhD.
References
- 1.Blum AS, Dal Pan GJ, Feinberg J, et al. Low-dose zalcitabine-related toxic neuropathy: frequency, natural history, and risk factors. Neurology. 1996;46:999–1003. doi: 10.1212/wnl.46.4.999. [DOI] [PubMed] [Google Scholar]
- 2.Marra CM, Boutin P, Collier AC. Screening for distal sensory peripheral neuropathy in HIV-infected persons in research and clinical settings. Neurology. 1998;51:1678–1681. doi: 10.1212/wnl.51.6.1678. [DOI] [PubMed] [Google Scholar]
- 3.Moyle GJ, Sadler M. Peripheral neuropathy with nucleoside antiretrovirals: risk factors, incidence and management. Drug Saf. 1998;19:481–494. doi: 10.2165/00002018-199819060-00005. [DOI] [PubMed] [Google Scholar]
- 4.Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:153–161. [PubMed] [Google Scholar]
- 5.Cherry CL, McArthur JC, Hoy JF, Wesselingh SL. Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol. 2003;26:195–207. doi: 10.1016/s1386-6532(02)00118-x. [DOI] [PubMed] [Google Scholar]
- 6.Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2′3′-dideoxycytidine (ddC) Lab Invest. 2001;81:1537–1544. doi: 10.1038/labinvest.3780367. [DOI] [PubMed] [Google Scholar]
- 7.Moore RD, Wong WM, Keruly JC, McArthur JC. Incidence of neuropathy in HIV-infected patients on monotherapy vs. those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS. 2000;14:273–278. doi: 10.1097/00002030-200002180-00009. [DOI] [PubMed] [Google Scholar]
- 8.Simpson DM, Haidich AB, Schifitto G, et al. Severity of HIV-associated neuropathy is associated with plasma HIV-1 RNA levels. AIDS. 2002;16:407–412. doi: 10.1097/00002030-200202150-00012. [DOI] [PubMed] [Google Scholar]
- 9.Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther. 2000;22:685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- 10.Cherry CL, Skolasky RL, Lal L, et al. Antiretroviral use and other risks for HIV-associated neuropathies in an international cohort. Neurology. 2006;66:867–873. doi: 10.1212/01.wnl.0000203336.12114.09. [DOI] [PubMed] [Google Scholar]
- 11.Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61:546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 12.Renaud-Thery F, Nguimfack BD, Vitoria M, et al. Use of antiretroviral therapy in resource-limited countries in 2006: distribution and uptake of first- and second-line regimens. AIDS. 2007;21 (Suppl 4):89–95. doi: 10.1097/01.aids.0000279711.54922.f0. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa M, Maruyama Y, Sugita H, Osame M. Nationwide survey of neurologic manifestations of acquired immunodeficiency syndrome in Japan. Intern Med. 1997;36:175–178. doi: 10.2169/internalmedicine.36.175. [DOI] [PubMed] [Google Scholar]
- 14.Robertson K, Kopnisky K, Mielke J, et al. Assessment of neuroAIDS in Africa. J Neurovirol. 2005;11 (Suppl 1):7–16. [PubMed] [Google Scholar]
- 15.Wong MH, Robertson K, Nakasujja N, et al. Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology. 2007;68:350–355. doi: 10.1212/01.wnl.0000252811.48891.6d. [DOI] [PubMed] [Google Scholar]
- 16.Zanetti C, Manzano GM, Gabbai AA. The frequency of peripheral neuropathy in a group of HIV-positive patients in Brazil. Arq Neuropsiquiatr. 2004;62:253–256. doi: 10.1590/s0004-282x2004000200012. [DOI] [PubMed] [Google Scholar]
- 17.Schifitto G, McDermott MP, McArthur JC, et al. Markers of immune activation and viral load in HIV-associated sensory neuropathy. Neurology. 2005;64:842–848. doi: 10.1212/01.WNL.0000152981.32057.BB. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein KA, Armon C, Baron A, Moorman AC, Wood KC, Holmberg SD. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV Outpatient Study Cohort. Clin Infect Dis. 2005;40:148–157. doi: 10.1086/426076. [DOI] [PubMed] [Google Scholar]
- 19.Simpson DM, Wolfe DE. Neuromuscular complications of HIV infection and its treatment. AIDS. 1991;5:917–926. doi: 10.1097/00002030-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology. 2005;65:1778–1781. doi: 10.1212/01.wnl.0000187119.33075.41. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins C, Achenbach C, Fryda W, Ngare D, Murphy R. Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr. 2007;45:304–310. doi: 10.1097/QAI.0b013e318050d66c. [DOI] [PubMed] [Google Scholar]
- 22.Nicholas PK, Kemppainen JK, Canaval GE, et al. Symptom management and self-care for peripheral neuropathy in HIV/AIDS. AIDS Care. 2007;19:179–189. doi: 10.1080/09540120600971083. [DOI] [PubMed] [Google Scholar]
- 23.Parry O, Mielke J, Latif AS, Ray S, Levy LF, Siziya S. Peripheral neuropathy in individuals with HIV infection in Zimbabwe. Acta Neurol Scand. 1997;96:218–222. doi: 10.1111/j.1600-0404.1997.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumarasamy N, Vallabhaneni S, Cecelia AJ, et al. Reasons for modification of generic highly active antiretroviral therapeutic regimens among patients in southern India. J Acquir Immune Defic Syndr. 2006;41:53–58. doi: 10.1097/01.qai.0000188123.15493.43. [DOI] [PubMed] [Google Scholar]
- 25.Hill A, Ruxrungtham K, Hanvanich M, et al. Systematic review of clinical trials evaluating low doses of stavudine as part of antiretroviral treatment. Expert Opin Pharmacother. 2007;8:679–688. doi: 10.1517/14656566.8.5.679. [DOI] [PubMed] [Google Scholar]