A transgenic mice model of multiple sclerosis (ND4 mice) shows progressive visual loss as determined by pattern electroretinogram (PERG). Noninvasive evaluation (PERG, MRI, and OCT) of brain and optic nerve of ND4 mice has been described.
Abstract
Purpose.
To evaluate the ND4 transgenic mouse model of multiple sclerosis using noninvasive methods.
Methods.
Assessment of neurologic/behavioral abnormalities was made using pattern electroretinogram (PERG), magnetic resonance imaging (MRI), optic coherence tomography (OCT), and end point histologic analysis.
Results.
Electrophysiologic (PERG) recordings demonstrated functional deficits in vision commensurate with neurologic/behavioral abnormalities. In ND4 mice, the authors found PERG abnormalities preceded neurologic/gait abnormalities. MRI demonstrated subtle structural changes that progressed over time in correlation with behavioral abnormalities.
Conclusions.
The ND4 mouse model has been evaluated using well-defined parameters of noninvasive methods (PERG, MRI, and OCT), enabling objective identification of functional and structural deficits and their correlation with neurologic/gait abnormality.
Multiple sclerosis (MS) is a disease associated with the demyelination of neurons.1 MS features an underlying multifactorial etiology2 and presents a spectrum of clinical symptoms.2 Spasm and gait abnormalities are clinical symptoms of demyelinating disease.1,3 MS is frequently associated with visual impairment and vision loss.4 Modification of environmental circumstances, which are often thought to be contributory to the disease process, is severely restricted in human studies compared with studies in animal models. For these reasons, animal models are needed both to understand the underlying pathologic mechanisms and to evaluate novel therapeutic and reparative approaches. It is expected, given the complex nature of the disease and its multifactorial etiology, that a single animal model will not provide insight into all clinical, radiologic, pathologic, and genetic features of MS. In addition to frequently used rodent models,5 nonhuman primates6 and avian models7,8 are used in MS research, which has expanded our understanding of different pathologic aspects of MS. The most commonly studied major categories of animal/rodent models of MS include experimental autoimmune encephalomyelitis (EAE), virally induced (e.g., Theiler's murine encephalomyelitis virus [TMEV] infection) chronic demyelinating models, and toxin-induced models of demyelination such as the cuprizone and focal demyelination induced by lysophosphatidyl choline models.5,9 In addition to the rodent models described, the importance of genetically modified mice cannot be underestimated.10 As stated, vision loss is frequently associated with MS and usually follows a late onset and progressive course.11–13 A number of noninvasive techniques have been used to characterize structural/functional changes in the nervous systems and MS pathology, including functional electrophysiologic measurements,14 optic coherence tomography (OCT),15,16 and magnetic resonance imaging (MRI).11 Electrophysiologic measurements and, in particular, pattern electroretinogram (PERG) help assess functional status and have provided insight about the health of the optic nerve in MS patients.17 A transgenic mouse model (ND4 mice) of MS harboring multiple copies of the DM-20 isoform of myelin proteolipid protein (PLP) is available.18,19 The ND4 mouse is clinically healthy at 3 months of age and has minimal inflammatory signs. Around 3 months of age or immediately thereafter, without the injection of a myelin-specific antigen such as myelin basic protein, ND4 mice spontaneously undergo demyelination. It is, therefore, one of the genetically modified animal models for the study of demyelinating disease and offers intrinsic manifestations of MS symptoms without any external injections. At the same time, littermate controls can be generated to rule out the effects of confounding factors such as aging. MS has been known to be associated with vision loss, but most animal models, including the transgenic ND4 model, remain uncharacterized with respect to the visual system. Here we present a noninvasive characterization of the visual system in this model and extend some of the methods for characterization of the central nervous system. Noninvasive characterization provides the advantage of observing individual animals at different stages of the disease process, not only enabling determination of disease features at the population level but also capturing individual differences and the outcome measures of intervention strategies at individual levels.
Materials and Methods
Tissue Procurement and Animal Housing
We established an ND4 transgenic mouse colony at the Animal Facility of the University of Miami, Miller School of Medicine (Miami, FL) after receiving Institutional Animal Care and Use Committee approval and adhering to ARVO Statement for the Use of Animal in Ophthalmic and Vision Research. Breeding pairs were obtained from Mario Moscarello (Hospital for Sick Children, Toronto, Ontario, Canada), and a pathogen-free line was derived by embryo transfer performed at the Charles River Laboratory (Wilmington, MA). Our colony was maintained in a controlled and environmentally enriched environment. All mice (ND4 and their normal littermates in CD1 background) were weighed and scored for clinical signs each week by three independent observers. The animals were maintained in rooms with 12-hour light/12-hourdark cycles and fed ad libitum. For all noninvasive evaluation, animals were anesthetized by intraperitoneal administration of ketamine (90 mg/kg) and xylazine (10 mg/kg).
Assessment of Transgene Status
Transgenic status was assessed by genotyping with DNA derived from tail clips using the polymerase chain reaction (PCR) assay. ND4 mice are heterozygous; as such, their normal littermates (the CD1 background) served as controls. For purposes of routine genotyping, the transgenic mice were determined to carry the transgene by PCR analysis of isolated DNA. Briefly, DNA was isolated from mouse tail clips (∼5 mm). The tail clips were immersed in 300 μL lysis reagent (DirectPCR, catalog no. 101-T; Viagen Biotech Inc., Los Angeles, CA), after which 9 μL proteinase K (10 mg/mL concentration; Sigma, St. Louis, MO) was added, and the mixture was incubated at 55°C overnight. After incubation, the mixture was centrifuged at 10,000g for 1 minute and incubated again at 85°C for 45 minutes. Approximately 1 μL (200 ng DNA) of lysate was used for each 50-μL PCR reaction. The presence of the transgene (proteolipid protein isoform DM-20) was confirmed using the following set of primers: forward, 5′-GTGGATGTGGACATGAAGCTCTC-3′; reverse, 5′-CAGGAGCCATACAACAGTC-3′. To determine whether the isolated DNA was a good substrate for PCR, the endogenous mouse β-globin gene was amplified with the primer pairs 5′-CCAATCTGCTCACACAGGATAGAGAGGGCAGG-3′ and 5′-CCTTGAGGCTGTCCAAGTGATTCAGGCCATCG-3′, with the expected amplification product of 494 base pairs.
Neurologic Abnormalities/Expanded Disability Status Scale
A clinical score was assigned for several signs for each animal during weekly examinations. Scoring was based on a scale of 0 (best) to 5 (worst) by three independent observers in the following manner: 0, no disease; 0.5, distal limp tail; 1, limp tail; 2, mild paraparesis, ataxia; 3, moderate paraparesis, occasional tripping; 3.5, one hind limb paralyzed; 4, complete hind limb paralysis; 4.5, complete hind limb paralysis, incontinence; 5, moribund, difficulty breathing, does not eat or drink. If the animals were part of this last group, they were euthanatized immediately on recognition of their condition. The cohort of control and transgenic mice was observed for approximately 12 months.
Electrophysiologic Assessments
Cohorts of three ND4 mice at different ages (3 months, 5 months, and 8 months) were used in each single batch of electrophysiologic experiments. Briefly, the mice were anesthetized using ketamine and xylazine and were gently restrained with the use of a bite bar and a nose holder that allowed unobstructed vision. They were kept at a constant body temperature of 37°C with a feedback-controlled heating pad. In anesthetized mice, eyes were typically wide open and steady, and pupils were undilated and pointing laterally and upward. The active electrode (0.25-mm diameter silver wire configured to a semicircular loop of 2-mm radius) was placed on the corneal surface by means of a micromanipulator and was positioned in such a way as to encircle the pupil without limiting the field of view. Reference and ground electrodes were stainless steel needles inserted under the skin of the scalp and tail, respectively.20 A small drop of balanced salt solution (Alcon, Fort Worth, TX) was topically applied on the cornea to prevent dehydration for the duration of the recording. A visual stimulus of contrast-reversing horizontal bars (field area, 50° × 58°; mean luminance, 50 cd/m2; spatial frequency, 0.05 cyc/deg; contrast, 98%; temporal frequency, 1 Hz) was aligned with the projection of the pupil at the viewing distance of 15 cm. Eyes were not refracted for the viewing distance given that the mouse eye has a large depth of focus because of the pinhole pupil. Retinal signals were amplified (10,000-fold) and bandpass filtered (1–30 Hz). Three consecutive responses to each of 600 contrast reversals were recorded. The responses were superimposed to check for consistency and then averaged (1800 sweeps). PERG is a light-adapted response. For a corresponding index of outer retinal function, a light-adapted flash ERG (FERG) was also recorded with undilated pupils in response to strobe flashes of 20 cd · m2/s superimposed on a steady background light of 12 cd/m2 and presented within a Ganzfeld bowl. Averaged PERG and FERG consisting of a major positive wave followed by a slower negative wave were automatically analyzed to evaluate the response amplitude. It was defined as the sum of the absolute values of maximum and minimum voltages (peak-to-trough amplitude). Statistical analysis was performed by Student's t-test for unpaired data. P < 0.05 was considered statistically significant.
Optical Coherence Tomography
Before the experiments, animals were anesthetized and then restrained on a mounting tube fixed on a six-axis movable platform. Ultra-high resolution (∼3 μm) OCT was used to image each mouse. This device was custom built and has been described in a previous report.21 Raster scans with an approximately 32° scan filed were used to image the retina of each mouse eye. Cross-sectional images were obtained, and en face fundus images were reconstructed from a data set of 128 OCT images.
Histology
As end point experiments, 8-month-old animals were subjected to euthanatization using carbon dioxide and further subjected to cervical dislocation. Eyes were enucleated, immediately immersed in 4% paraformaldehyde, and incubated for 24 hours at 4°C in the dark. After alcohol dehydration, the eyes were embedded in paraffin, and the whole globe was mounted. Hematoxylin and eosin staining was performed to assess for any differences in optic nerve integrity and morphology. A microscope (BX51; Olympus America Inc., Center Valley, PA) equipped with imaging software (QImaging Image-Pro Plus; Olympus America Inc.) was used for imaging the hematoxylin and eosin–stained globes.
Determination of Marker Positive Retinal Ganglion Cell Frequency
Mouse eye–embedded cryosections (8 μm) were subjected to immunohistochemical analysis and imaging with a laser scanning confocal microscope (TCS-SP5; Leica, Exton, PA). Serial sections of retina from control and ND4 mice were stained with the retinal ganglion cell (RGC) marker Brn3b (catalog no. SC-6026, goat; Santa Cruz Biotechnology, Santa Cruz, CA) or NeuN (catalog no. MAB377, mouse; Chemicon International, Temecula, CA; secondary antibody coupled with Alexa 594) and DAPI. Area measurements in comparable regions in the retina and determinations of relative intensity were performed (Application Suite, Advanced Fluorescence, 1.7.0 Build 1240 software; Leica). Additional validation analyses were also performed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Cell counting was performed in equivalent regions of RGC layers within an equivalent area (i.e., 0.01 mm2 area was selected from equivalent regions of control and ND4 mouse retinas each time for 10 consecutive sections for Brn3b and NeuN). At least 10 sections were measured.
Magnetic Resonance Image Acquisition
In vivo MRI data sets were collected on a 4.7-Tesla (200 MHz) 40-cm bore magnet interfaced with a console (Avance; Bruker, Billerica, MA) using a gradient set with an inner diameter of 120 mm and a maximum gradient strength of 400 mT/m. After anesthesia induction, the subject was placed in an MRI-compatible cradle equipped with a nose cone to deliver gaseous anesthesia, head restraints to reduce motion, a respiratory sensor, and a body temperature regulating system. Approximately 0.8% to 1.5% isoflurane with 1 L/min oxygen was used to anesthetize the subject. The isoflurane percentage was adjusted to maintain a respiratory rate of approximately 90 breaths per minute. After subject placement in the animal cradle, the radio-frequency (RF) coils were attached to the cradle and subject. A two RF coil setup was used; a separate volume linear birdcage transmit coil and quadrature surface coil with active decoupling provided excellent excitation with maximal signal-to-noise ratio. The surface coil was placed on the subject's cranium, with dimensions matching the size of the mouse head for reception of the nuclear magnetic resonance signal. Pilot images were acquired in sagittal, coronal, and axial imaging planes to ensure proper slice positioning. MRI acquisitions used several two-dimensional T2_RARE (rapid acquisition relaxation enhanced) sequences acquired in the sagittal, coronal, and axial slice orientations. The coronal T2_RARE acquisition had the following settings: time of repetition, 4000 ms; echo time, 18 ms; effective echo time, 74 ms; field of view, 2.8 cm × 1.6 cm; matrix size, 232 × 136; in-plane resolution, 120 μm × 118 μm; slice thickness, 0.55 mm; acquisition time, 9 minutes. The sagittal T2_RARE acquisition had settings similar to those of the coronal acquisition: time of repetition, 4500 ms; echo time, 16 ms; effective echo time, 67 ms; field of view, 2.8 cm × 1.6 cm; matrix size, 224 × 136; in-plane resolution, 125 μm; slice thickness, 0.50 mm; acquisition time, 9.5 minutes. In addition, a 3D gradient echo (GE) sequence was acquired to visualize the optic nerve and to evaluate any atrophy or structural changes between controls and ND4 mice. Typical settings for the fat-suppressed 3D GE acquisitions consisted of a repetition time of 30 ms, an echo time of 3.3 ms, a flip angle of 25°, four averages, an acquisition time of 28 minutes, a field of view of 20 × 15 × 13 mm, and a matrix size of 170 × 128 × 110, resulting in a 118-μm isotropic resolution. These 3D GE acquisitions were analyzed in 3D visualization software (ParaVision; Bruker) to obtain the oblique cuts needed to properly visualize the optic nerve.
Results
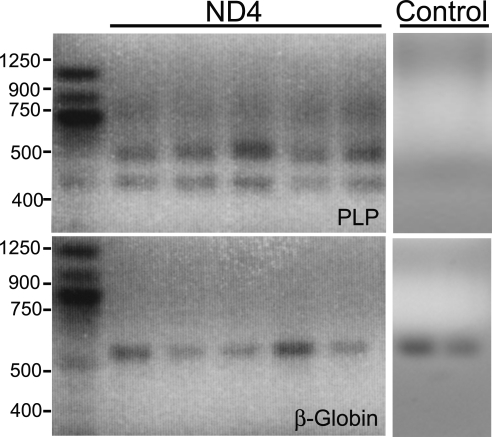
The ND4 mice were genotyped by amplification of the DM-20 variant of the PLP transgene and the β-globin gene as control using the primer pairs and protocol described in Materials and Methods (Fig. 1). The PLP transgene under these conditions showed a prominent (∼400-bp) DNA band, whereas the β-globin gene showed a 494-bp DNA band (Fig. 1). ND4 mice showed spastic tremor and gait abnormalities at approximately 3 months of age. From there the symptoms became increasingly severe, and the mice die approximately from 8 to 13 months of age.
Figure 1.
Representative PCR confirmation of the myelin PLP DM-20 variant transgene. The blood obtained by tail clipping of mice was subjected to PCR amplification. The DM-20 variant of the PLP transgene was confirmed by the presence of a band measuring approximately 400 bp. This band was absent in the control animals. The endogenous mouse β-globin gene was amplified and used as a control, giving an expected amplification product of 494 bp in both transgene and control animals.
Assessment of Disability (Expanded Disability Status Scale)
ND4 mice were assessed for measurement of disability. An average clinical score was calculated from all observations at each age, as detailed in Materials and Methods, ranging from 0 to 5. As shown in Table 1, the average clinical score for mice at different ages was calculated by three independent observers. Mice used in these studies showed disability scales of 0 or 1 at 3 months of age, 1.5 to 2.0 at 5 months of age, and 3.5 to 5.0 at 8 months of age.
Table 1.
Disability Rating of Mice
| Mouse Type | Mouse Age |
||
|---|---|---|---|
| 3 mo | 5 mo | 8 mo | |
| Control (CD1) | 0 | 0 | 0 |
| ND4 | 0–1.0 | 1.5–2.0 | 3.5–5.0 |
Measurement of Visual Dysfunction
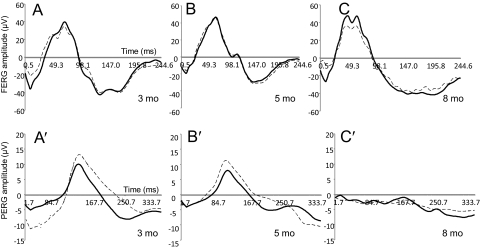
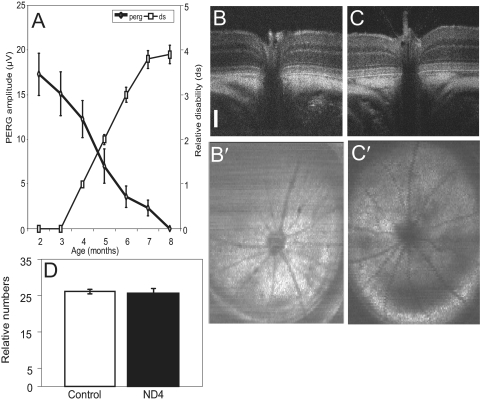
ND4 mice were subjected to assessment of visual dysfunction using FERG and PERG at all ages. At 3 months of age, the FERG (peak to trough) showed amplitudes of approximately 80 μV (Fig. 2A). Left and right eye ERG recordings showed similar responses (Fig. 2A, dashed and solid lines, respectively). FERG amplitude was similar at 5 and 8 months, respectively (Figs. 2B, 2C), with similar responses for left and right eyes. PERG measurements showed amplitudes of 10 to 15 μV at 3 months of age (Fig. 2A′). Left and right eye PERG amplitudes (Fig. 2A, dashed and solid lines, respectively) might have shown differences up to approximately 20%. PERG amplitude showed a marked decrease with respect to advancement of age and severity of disease (Figs. 2B′, 2C′). At 5 months, the PERG amplitude showed a discernible decrease, but at approximately 8 months, there was a marked decrease in PERG amplitude, and all amplitudes were virtually lost (Fig. 2C′). For mouse ND4, the PERG responses of right and left eyes showed variability (unpublished observations, 2010) that could have been as high as 25%, but the PERG amplitude for individual mice showed progressive decreases that correlated with the severity of the disease measured on a disability scale. However, the initial decrease in PERG amplitude preceded the onset of clinical symptoms and correlated well with disability score (Fig. 3A). The initial kinetics of PERG measurement showed visual dysfunction before the onset of clinical disability symptoms (Fig. 3A). The PERG decrease was also steeper than disability during rapid disease progression at a later stage. PERG kinetics showed a greater slope than that for disability (Fig. 3A).
Figure 2.
Representative FERG and PERG waveforms in ND4 mice of different ages. ND4 mice at ages 3 months (A, A′), 5 months (B, B′), and 8 months (C, C′) were analyzed with both left and right eyes, indicated as OS (solid lines) and OD (dashed lines), respectively. Waveforms for the ages 3, 5, and 8 months are shown for clinical symptoms before onset, at or around onset, and fully developed (tremors and gait abnormalities). Representative FERG waveforms (A–C) do not show any significant changes with aging in the ND4 mice. Representative PERG waveforms of the ND4 mouse (A′–C′) show reduced amplitude with aging (and disease progression), indicating the dysfunction of the RGCs in ND4 mice.
Figure 3.
Correlation between PERG and disability assessment and evaluation of optic nerve head using OCT. (A) Determination of correlation of PERG and disability in a representative ND4 mouse. Thick solid line with diamonds: PERG amplitude. Thin solid line with squares: disability measurement in Expanded Disability Status Scale. (B, C) Representative cross-sectional images of the retina and optic nerve of control (B, B′) and ND4 (C, C′) at 5 months of age using OCT. Scale bar, 100 μm. (D) Relative number of Brn3b-positive RGCs for an area of 0.01 mm2 and an equivalent region of retina from control and ND4 mice (at 5 months). Mean ± SEM using counts from 10 sections performed by two independent observers. Statistical t-test showed insignificant difference between the two groups.
Assessment of Structures of Optic Nerve and Brain
To determine whether the optic nerve experienced a structural change in ND4 mice compared with the functional impairment demonstrated by PERG amplitude, we used high-resolution OCT measurements of the retina and the optic nerve. In contrast to the control CD1 mouse (5 months of age; Figs. 3B, 3B′), no differences in optic nerve head or retinal structures were found in the 5-month-old control ND4 mice (Figs. 3C, 3C′), which showed significant differences in PERG amplitude compared with control. Mice that were 8 months older (control CD1 and ND4 mice) showed no significant differences in the optic nerve head or retina in OCT images (data not shown) or in histochemical assessment on optic nerve sections (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6425/-/DCSupplemental). Furthermore, counting of the RGC marker Brn3b (Fig. 3D) or NeuN (not shown) from an equivalent region and area of the retina showed no significant difference between control and ND4 mice at 5 months of age (Fig. 3D).
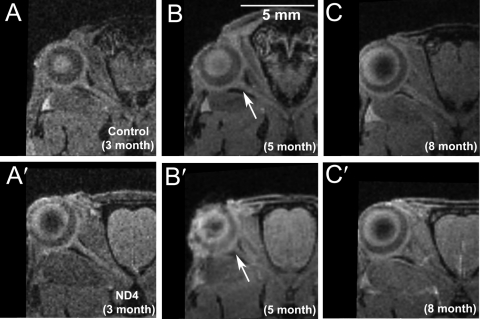
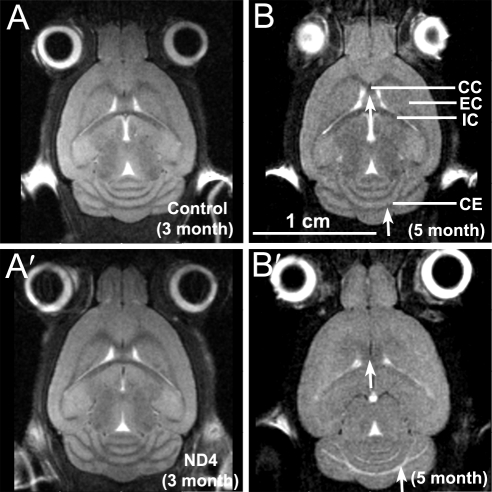
The 3D GE or the T2 RARE MRI data sets did not show differences in optic nerve structures between control (Figs. 4A–C) and ND4 (Figs. 4 A′–C′) mice when compared at ages 3, 5, or 8 months, respectively (Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6425/-/DCSupplemental). The T2 RARE coronal brain images of control mice showed normal brain structures at 3 and 5 months (Figs. 5A, 5B). In comparison, ND4 mice (Figs. 5A′, 5B′) showed an onset of demyelination in the brain structures at 5 months that indicated progressive increase at 8 months of age (data not shown), consistent with previous histochemical findings.22,23 The degree of demyelination varied among the ND4 mice and generally correlated with the extent of subjective assessment of disability score. The demyelination was also observed in sagittal images at 5 months in ND4 mice compared with its absence in controls (Supplementary Fig. S3, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6425/-/DCSupplemental). We found that the brain volumes of the ND4 mice underwent significant shrinkage; the 8-month-old animal showed only 85% of brain volume (539.97 mm3) compared with control animals (634.88 mm3). PERG amplitude (Figs. 2, 3A) showed early decreases compared with disability scores (Fig. 3A) before brain demyelination was evident by MRI measurement (Fig. 5, Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6425/-/DCSupplemental).
Figure 4.
3D GE MRI taken of 3-, 5-, and 8-month-old (A–C) control and ND4 (A′–C′) mice encompassing the ON. Arrows: intersection/convergence point of the ON at the globe.
Figure 5.
Coronal T2 RARE MRI brain images of control (A, B) and ND4 mice (A′, B′) at the ages indicated. Arrows: areas undergoing structural changes and demyelination. CC, corpus callosum; EC, external capsule; IC, internal capsule; CE, cerebellum.
Discussion
Multiple sclerosis is a group of heterogeneous diseases in its clinical presentation and has a multifactorial etiology. It is frequently associated with visual impairment and progressive vision loss.11–13 Although the late onset and progressive nature of vision loss are frequently associated with MS, vision loss has also been observed in late-onset MS.13 Acute demyelination of the optic nerve (optic neuritis—the anterior afferent pathway) is common in MS. However, clinically silent lesions are also frequently found in the posterior afferent visual pathway.11 Usually myelination stops at the optic nerve head, but aberrant myelination extending to the retina also exists. Injury-associated remyelination often results in abnormal myelination and extends to the retina.24 Mitochondrial mutations also have been shown to result in MS-like symptoms.25 Deterioration of visual acuity and problems with eye movement are frequently associated with episodes of MS, followed by recovery of visual acuity and of the visual field. Uhthoff's symptom of visual loss after exercise or a hot bath and anomalous perception of motion in depth (Pulfrich's phenomenon) may also occur. Imaging, neurophysiologic studies, and pathologic studies indicate that despite persistent tissue loss, recovery continues long after the acute attack. Human MS optic nerve imaging by various methods has shown atrophy that has been attributed to demyelination and axonal loss. The extent of atrophy often has been reported to correlate with disease duration. Atrophy of smaller neurons (parvocellular layer of lateral geniculate nucleus) occurs in MS, which is consistent with transsynaptic degeneration and suggests an increased susceptibility of smaller axons to MS-related injury.11 Several noninvasive methods, such as OCT, scanning laser polarimetry (SLP), and confocal scanning laser ophthalmoscopy (CSLO), have been used to image the retina and to determine retinal layer thickness in MS patients. OCT was found to be superior to SLO and CSLO in terms of strength of association between structure and function. We have adopted OCT and made measurements on ND4 and control mice.11 MRI, another noninvasive imaging method, has also been extensively used for diagnosis of optic nerve atrophy in MS-related optic neuropathies. One of the advantages of MRI is the ability to acquire images sensitive to different pathologic changes, such as inflammation, axonal degeneration, blood-brain barrier leakage, water molecule displacement, and macromolecular changes.11 Analysis of gray matter by different MRI methods has shown significant reduction in visual cortex bilaterally in MS patients compared with controls.11 Changes in visual acuity and specific magnetization transfer ratio reduction in visual cortex may be attributed to trans-synaptic morphologic changes occurring in corresponding gray matter specifically caused by a remote white matter tract lesion. In human patients and in mice, it has been difficult to distinguish optic nerve structures and fat signals on conventional T2-weighted images. All mouse optic nerve images acquired here have used fat suppression (Fig. 4). MR spectroscopy of the brain has been helpful in providing cell-specific measures of neuronal damage and glial activation or proliferation. The optic nerve is a difficult structure for MRI methods because it is a small, inherently mobile structure surrounded by fat and residing in bony orbital cavity. Further advances are required to apply it to localized areas in the CNS, particularly the optic nerve.11
A multicenter North American Optic Neuritis Treatment Trial has linked optic neuritis (ON) with MS.11 A large percentage of MS patients experience transient loss of vision as assessed by visual acuity measurements. Visual acuity determination frequently shows a recovery of vision. However, multiple studies have shown decreased retinal nerve layer thickness (RNFL) and aberrant P50 or N95 PERG parameters in MS patients, irrespective of whether the MS patient has ON.11,14,26 Such decreased RNFL and PERG abnormalities continue to exist even after the recovery of vision is shown by visual acuity measurements. Thus, visual acuity measurements alone do not capture the entire spectrum of visual disability in MS patients. In other words, the visual abnormalities detected by RNFL and PERG are not transient and do not recover even though visual acuity shows transient loss and recovery. People who have had ON are at increased risk for MS. We would like to emphasize that the PERG amplitude (Figs. 2, 3A) showed early decreases compared with neurologic/gait abnormality or disability score (Fig. 3A) and before brain demyelination was evident by MRI measurement (Fig. 5; Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6425/-/DCSupplemental). Functional visual deficits may be apparent before detectable structural abnormalities, and they continue to worsen progressively. Future studies in subjects with MS or thought to have MS may help determine whether functional deficit measurements with PERG may be of predictive value for rate of disease progression.
It is conceivable that a single animal model is unlikely to provide insight into all the genetic, pathologic, clinical, and radiologic features of MS. Different types of rodents,5 nonhuman primates,6 and avian models7,8 have been also used in MS research, which has enriched our insight into different pathologic aspects of MS. The commonly studied major categories of rodent models are the EAE model, the virally induced (e.g., TMEV) infection chronic demyelinating models, toxin (e.g., cuprizone and lysophosphatidyl choline-induced demyelination) models,5,9 and the genetically altered mouse.10
Rodent models of EAE have greatly enhanced our understanding of pathogenetic, diagnostic, and therapeutic aspects of MS. In the EAE models of disease, the course varies according to the genetic background of the animal, the source of antigen, and the mode of application. Active EAE induced by subcutaneous injection of myelin oligodendrocyte glycoprotein peptide 33–55 or PLP peptide 139–151 and adoptive transfer of EAE by intravenous injection of myelin-reactive CD4+ Th1 lymphocytes into naive animals are examples of EAE models. EAE models have helped in the development of a number of MS therapies such as glatiramer acetate (GA or copolymer 1), which suppresses EAE by the induction of the Th2/Th3-mediated anti-MBP immune response and immunosuppressive mitoxantrone.9
Genetic models, despite inherent heterogeneity at the age of disease onset and differences in disease severity, confer the advantage of spontaneous development of the disease process contributed by changes within rather than external injections and provide relative homogeneity at age of onset and degree of disability over external immunization models.
ND4 mice were generated by microinjection of the DM-20 variant of PLP transgene into the pronuclei of fertilized eggs that were subsequently transferred into pseudopregnant females. Four stable transgenic lines—ND2, ND3A, ND4, ND5—were thus established bearing 2 to 70 copies of the transgene. Line ND1 was able to produce offspring, but the offspring of ND3B did not breed. ND4 mice bore approximately 70 copies of the transgene integrated into the genome and did differ from jimpy mice in that they had less severe manifestation and normal myelin production at the initial stages.18 They manifested wobbling gait and seizures between 3 to 6 months and died by 8 to 13 months. ND4 mice manifest genetically transmitted demyelinating disease and thus are a useful tool for studying demyelination and associated disease.18,19,27
The PLP DM-20 variant has a unique and not very well understood role in myelination. The DM-20 variant is a product of alternate splicing of PLP with the deletion of 35 highly positive amino acids of PLP. Molecularly, DM-20 and PLP may form adhesive pores composed of complexes within the plane of the lipid bilayer, thus forming conduits that span the whole multilayered myelin sheath.28 Insight into the role of PLP in demyelination emerged from X-linked spastic paraplegia (SPG). In SPG, a point mutation in PLP exon 3B was found that affects PLP though DM-20 remains normal.29 In Pelizaeus-Merzbacher disease (PMD), which is a similar though much more severe neurologic disorder than SPG, mutations in the PLP gene affecting both PLP and DM-20 have been identified.29 The mildness and late onset of disabilities in SPG relative to PMD support the possibility of a role for DM-20 in the CNS different from that of PLP.
The DM-20 isoform is responsible for myelination in younger rodents (up to 3 months of age). The level of the DM-20 variant decreases in rodents older than 3 months of age. Failure of the DM-20 variant of PLP to decrease causes the demyelination of neurons. The PLP isoform plays a role in later months of life. Experimental results indicate that it is the overexpression of DM-20, not just a reduction in the total amount of PLP and its DM-20 variant, that causes CNS demyelination in ND4 mice.18,19
In summary, noninvasive measurements are good for direct application on humans. They reduced animal numbers and allow for more humane and painless treatment of animal study subjects. Development of such methods may facilitate noninvasive detection of select target molecules. For example, specific proteins (such as isoforms of ETX130) can be assessed using magnetomotive OCT,31 and molecules may be investigated at different stages of disease resulting in demonstration of their correlation with disease stage and/or alteration of organ/tissue structures. We have attempted to use noninvasive methods for characterizing functional and structural changes in the visual pathway and found functional changes to be early predictors of disease before the onset of clinical symptoms and before discernible structural changes in the brain. Various improvements in MRI and OCT for use in ND4 mice are in development. As noted earlier, the optic nerve is a difficult structure for MRI because it is a small, inherently mobile structure surrounded by fat and residing in bony orbital cavity. This is especially true in rodents because the rodent optic nerve structure is much smaller than the primate optic nerve structure. Attempts are under way to adopt additional MRI sequences, such as short tau inversion recovery, selective partial inversion recovery, and magnetization transfer methods, to obtain qualitative and quantitative data on the mouse optic nerve. The development of such methods is expected to provide better insight into the disease process and to help reduce the number of animals necessary for evaluation.
Supplementary Material
Acknowledgments
The authors thank Mario Moscarello and Fabrizio Mastronardi for their gift of ND4 mice and for generous consultation; Gabriel Gaidosh, Eleut Hernandez, and Archana Gupta for assistance; Ming Li and Hong Jiang for assistance on OCT imaging and for comments on the manuscript; and Ashley Crane for critical reading of the manuscript.
Footnotes
Supported by a career award (SKB) and unrestricted funds from Research to Prevent Blindness and by National Institutes of Health Grants EY019077 and P30-EY14801.
Disclosure: M. Enriquez-Algeciras, None; D. Ding, None; T.-H. Chou, None; J. Wang, None; K.R. Padgett, None; V. Porciatti, None; S.K. Bhattacharya, None
References
- 1. Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269 [DOI] [PubMed] [Google Scholar]
- 2. Ziemssen T. Modulating processes within the central nervous system is central to therapeutic control of multiple sclerosis. J Neurol. 2005;252(suppl 5):v38–v45 [DOI] [PubMed] [Google Scholar]
- 3. Gallo V, Armstrong RC. Myelin repair strategies: a cellular view. Curr Opin Neurol. 2008;21:278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Libbey JE, McCoy LL, Fujinami RS. Molecular mimicry in multiple sclerosis. Int Rev Neurobiol. 2007;79:127–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denic A, Johnson AJ, Bieber AJ, Warrington AE, Rodriguez M, Pirko I. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hart BA, Laman JD, Bauer J, Blezer E, van Kooyk Y, Hintzen RQ. Modelling of multiple sclerosis: lessons learned in a non-human primate. Lancet Neurol. 2004;3:588–597 [DOI] [PubMed] [Google Scholar]
- 7. Rose NR. Avian models of autoimmune disease: lessons from the birds. Poult Sci. 1994;73:984–990 [DOI] [PubMed] [Google Scholar]
- 8. Kinutani M, Coltey M, Le Douarin NM. Postnatal development of a demyelinating disease in avian spinal cord chimeras. Cell. 1986;45:307–314 [DOI] [PubMed] [Google Scholar]
- 9. Mix E, Meyer-Rienecker H, Zettl UK. Animal models of multiple sclerosis for the development and validation of novel therapies—potential and limitations. J Neurol. 2008;255(suppl 6):7–14 [DOI] [PubMed] [Google Scholar]
- 10. Schreiner B, Heppner FL, Becher B. Modeling multiple sclerosis in laboratory animals. Semin Immunopathol. 2009;31:479–495 [DOI] [PubMed] [Google Scholar]
- 11. Kolappan M, Henderson AP, Jenkins TM, et al. Assessing structure and function of the afferent visual pathway in multiple sclerosis and associated optic neuritis. J Neurol. 2009;256:305–319 [DOI] [PubMed] [Google Scholar]
- 12. Beeton C, Garcia A, Chandy KG. Induction and clinical scoring of chronic-relapsing experimental autoimmune encephalomyelitis. J Vis Exp. 2007:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Safran AB. Late onset multiple sclerosis: clinical pattern of optic nerve involvement. Metab Pediatr Syst Ophthalmol. 1989;12:58–60 [PubMed] [Google Scholar]
- 14. Holder GE, Gale RP, Acheson JF, Robson AG. Electrodiagnostic assessment in optic nerve disease. Curr Opin Neurol. 2009;22:3–10 [DOI] [PubMed] [Google Scholar]
- 15. Pula JH, Reder AT. Multiple sclerosis, I: neuro-ophthalmic manifestations. Curr Opin Ophthalmol. 2009;20:467–475 [DOI] [PubMed] [Google Scholar]
- 16. Kallenbach K, Frederiksen J. Optical coherence tomography in optic neuritis and multiple sclerosis: a review. Eur J Neurol. 2007;14:841–849 [DOI] [PubMed] [Google Scholar]
- 17. Bodis-Wollner I. Sensory evoked potentials: PERG, VEP, and SEP. Curr Opin Neurol Neurosurg. 1992;5:716–726 [PubMed] [Google Scholar]
- 18. Johnson RS, Roder JC, Riordan JR. Over-expression of the DM-20 myelin proteolipid causes central nervous system demyelination in transgenic mice. J Neurochem. 1995;64:967–976 [DOI] [PubMed] [Google Scholar]
- 19. Mastronardi FG, Ackerley CA, Arsenault L, Roots BI, Moscarello MA. Demyelination in a transgenic mouse: a model for multiple sclerosis. J Neurosci Res. 1993;36:315–324 [DOI] [PubMed] [Google Scholar]
- 20. Porciatti V. The mouse pattern electroretinogram. Doc Ophthalmol. 2007;115:145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruggeri M, Wehbe H, Jiao S, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–1814 [DOI] [PubMed] [Google Scholar]
- 22. Mastronardi FG, Min W, Wang H, et al. Attenuation of experimental autoimmune encephalomyelitis and nonimmune demyelination by IFN-beta plus vitamin B12: treatment to modify notch-1/sonic hedgehog balance. J Immunol. 2004;172:6418–6426 [DOI] [PubMed] [Google Scholar]
- 23. Mastronardi FG, Tsui H, Winer S, et al. Synergy between paclitaxel plus an exogenous methyl donor in the suppression of murine demyelinating diseases. Mult Scler. 2007;13:596–609 [DOI] [PubMed] [Google Scholar]
- 24. Blakemore WF, Franklin RJ. Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 2008;318:193–212 [DOI] [PubMed] [Google Scholar]
- 25. Bhatti MT, Newman NJ. A multiple sclerosis-like illness in a man harboring the mtDNA 14484 mutation. J Neuroophthalmol. 1999;19:28–33 [PubMed] [Google Scholar]
- 26. Holder GE. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res. 2001;20:531–561 [DOI] [PubMed] [Google Scholar]
- 27. Mastronardi FG, Ackerley CA, Roots BI, Moscarello MA. Loss of myelin basic protein cationicity in DM-20 transgenic mice is dosage dependent. J Neurosci Res. 1996;44:301–307 [DOI] [PubMed] [Google Scholar]
- 28. Kitagawa K, Sinoway MP, Yang C, Gould RM, Colman DR. A proteolipid protein gene family: expression in sharks and rays and possible evolution from an ancestral gene encoding a pore-forming polypeptide. Neuron. 1993;11:433–448 [DOI] [PubMed] [Google Scholar]
- 29. Saugier-Veber P, Munnich A, Bonneau D, et al. X-linked spastic paraplegia and Pelizaeus-Merzbacher disease are allelic disorders at the proteolipid protein locus. Nat Genet. 1994;6:257–262 [DOI] [PubMed] [Google Scholar]
- 30. Iragavarapu S, Algeciras ME, Lee RK, Bhattacharya SK. ETX1 is over-expressed in the glaucomatous trabecular meshwork. Mol Vis. 2009;15:2061–2067 [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J, Wang MR, Jiang H, Shen M, Cui L, Bhattacharya SK. Detection of magnetic particles in live DBA/2J mouse eyes using magnetomotive optical coherence tomography. Eye Contact Lens. 2010;36:346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.