11-cis retinal retards cone function and cell loss in mouse models for LCA2 if kept in the dark. Pigment formation appears critical for healthy cones, but with a limited supply of retinal, light negates any improvements to cones of treated mice.
Abstract
Purpose.
To determine the effect of light/dark cycles on the cones of 11-cis retinal–treated RPE65/rhodopsin double knockout (Rpe65−/−Rho−/−) mice. Studies have shown that cones degenerate in chromophore-deficient mouse models for Leber Congenital Amaurosis (LCA), but exogenous supplementation of the native 11-cis retinal chromophore can inhibit this degeneration, suggesting that 11-cis retinal could be used as a therapeutic agent for preserving functional cones in patients with LCA. However, these treated mice were maintained in the dark.
Methods.
11-cis Retinal was introduced into Rpe65−/−Rho−/− mice at postnatal day 10 as a single subcutaneous injection mixed with a basement membrane matrix. The mice were maintained in either normal light/dark cycles or constant dark conditions. Fluorescence microscopy was used to assess retinal morphology. Cone cell survival was determined by counting cone opsin–containing cells on flat-mounted P30 retinas. Cross-sections of P21 mouse retina were used to assess cone cell integrity by visualizing opsin localization. Cone function was determined by electroretinography (ERG).
Results.
Previous studies have shown that 11-cis retinal–treated mice lacking RPE65 and raised in constant dark have higher cone photoreceptor cell number, improved cone opsin localization, and enhanced cone ERG signals when compared with untreated mice. However, in this study the authors show that 11-cis retinal–treated Rpe65−/−Rho−/− mice raised in cyclic light did not show the improvements seen with the dark-reared mice.
Conclusions.
Thus, 11-cis retinal by itself, as well as other agents that form photosensitive pigments, will not be good therapeutic candidates for preserving cones in LCA.
Leber Congenital Amaurosis (LCA) is an early-onset childhood blinding disease.1–3 Mutations to proteins involved in the visual cycle, a process wherein the chromophore (11-cis retinal) for visual pigments is regenerated, have been implicated in several forms of LCA. LCA2 is a predominant form and associated with defective RPE65,4 a retinal pigment epithelium protein that is critical in the conversion of the visual chromophore from an all-trans to an 11-cis form.5–7 Recent studies on patients with LCA2 have noted early loss of visual acuity, in particular no observed blue color vision, and thinning of the fovea, consistent with early loss of cones.8–11 A mouse model for LCA2 in which RPE65 has been knocked out (Rpe65−/−) has been generated by Redmond et al.12 In this mouse, 11-cis retinal is not synthesized, visual pigments are not formed,12 and cones degenerate rapidly.13
Rohrer et al.14 further observed abnormal localization of both the middle- and short-wavelength sensitive (M- and S-, respectively) cone opsins within the first three weeks after birth before cone degeneration. Usually, cone opsins are predominantly localized in the cone outer segments; however, cone opsins in Rpe65−/− mice are distributed throughout the cone cell from the outer segments to the synapse pedicles. Interestingly, cone opsin localization and cone cell survival can be improved with early administration of 11-cis retinal when maintained in the dark.14–16 Another retinal analog, 9-cis retinyl acetate which is converted to 9-cis retinal,17 also improves cone morphology and function in similar mouse models.18 These results suggest that receptor-ligand (cone opsin-11-cis retinal) interactions can help prevent cone photoreceptor cell death. They also suggest the potential of such a ligand as a useful therapeutic agent in preserving cone cell integrity and function for patients with LCA2. However, the mice were maintained in the dark after treatment; this treatment protocol may have optimized the effectiveness of 11-cis retinal because 11-cis retinal and the pigments generated with 11-cis retinal and cone opsins are both highly light-sensitive.19 If the 11-cis form of the molecule is important, then photoisomerization might be destructive and would limit the efficacy of 11-cis retinal as a potential therapeutic agent for LCA2. In this study, we assessed the effectiveness of 11-cis retinal treatment to a mouse model for LCA, Rpe65−/−Rho−/− mice, under cyclic light conditions. We show that there was no improvement of cone cell health and function after treatment under cyclic light conditions.
Materials and Methods
Animal Use
Rpe65−/−Rho−/− mice were the generous gift from Mathias Seeliger (University of Tübingen, Tübingen, Germany). All experiments were designed and performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Medical University of South Carolina Animal Care and Use Committee. Unless stated otherwise, mice were maintained in the MUSC core animal facilities under 12 h light/12 h dark cyclic light conditions. Light intensity at cage level varied depending on the cage location in the rack and averaged 150 ± 20 lux. Mice were killed at the ages indicated in the text by carbon dioxide exposure and cervical dislocation.
11-cis Retinal Treatment
11-cis Retinal was introduced into postnatal day (P) 10 Rpe65−/−Rho−/− pups (4.5–6.0 g) by injecting a 200 μL mixture of 0.9 μmol 11-cis retinal (44 mM stock in ethanol) and basement membrane matrix (Matrigel; BD Biosciences, Bedford, MA) subcutaneously between the shoulder blades under dim red light conditions.20 Litter size was limited to 6 pups during the experiment. One set of 11-cis retinal–treated mice was maintained in constant dark as a positive control for the 11-cis retinal treatment; the experimental group of mice was treated with 11-cis retinal and returned to the core animal facilities on the same day and exposed to normal cyclic light conditions described above. As a negative control, one more group of mice was injected with ethanol (instead of an ethanolic solution containing 11-cis retinal) mixed with basement membrane matrix and then returned to the core animal facilities on the same day and exposed to normal cyclic light conditions.
Immunohistochemistry
Retinal Flat-mount.
Animals were killed at P30. The dorsal pole of mouse eyes was first marked using a cautery pen. After enucleation and dissection, the retina-lens complexes were fixed in freshly made 4% paraformaldehyde in phosphate-buffered saline (PBS), consisting of 10 mM Na2HPO4, 140 mM NaCl, 2.8 mM KCl, 1.8 mM KH2PO4, pH 7.4, for 2 hours on ice. Tissues were washed three times with PBS and then blocked with 5% normal donkey serum and 0.1% Triton X-100 in PBS for 1 hour at room temperature, followed by overnight incubation with primary antibody at 4°C. Tissues were rinsed with PBS and incubated with Texas Red–conjugated donkey anti-rabbit antibody (1:500, Jackson Immuno Research) for 2 hours at room temperature. After three PBS rinses, the lens was removed and the retina was mounted and flattened on a slide. The dorsal and ventral areas of the retinas were recorded by fluorescence microscopy (Axioplan II; Carl Zeiss Inc., Germany) using the 20× objective lens.
Retinal Cross-sections.
Animals were killed at P21. The dorsal pole of mouse eyes was first marked using a cautery pen. Eyes were enucleated, a 1- to 2-mm hole in the cornea was made, and the eyes were fixed in freshly made 4% paraformaldehyde in PBS for 2 hours on ice. The eyes were then transferred into 15% sucrose in PBS and equilibrated for 1 hour on ice, followed by overnight incubation at 4°C in 30% sucrose in PBS. Tissues were embedded in optimal cutting temperature (OCT) compound (Tissue Tek; Sakura Finetech, Torrance, CA) and sectioned at −26°C. The 14 μm sections were washed with PBS to remove OCT and blocked with the PBS containing 5% normal donkey serum and 0.1% Triton X-100 for 1 hour at room temperature. S-opsin was probed with a polyclonal antibody, kindly provided by Jeannie Chen (Department of Cell & Neurobiology, Department of Ophthalmology, Zilkha Neurogenetic Institute, University of Southern California) also diluted 1:200. The sections were washed three times with PBS for 15 minutes and incubated with Texas Red–conjugated donkey anti-rabbit antibody (1:500, Jackson Immuno Research) for 2 hours at room temperature. Nuclei were stained with the DNA dye DRAQ5 (1:500, Biostatus Limited) for 10 minutes at room temperature. Images were acquired on a confocal microscope (Leica, Bannockburn, IL).
Electroretinography
Mice at P19 were dark adapted for 24 hours and anesthetized with xylazine (20 mg/kg) and ketamine (80 mg/kg). Pupils were dilated with phenylephrine hydrochloride (2.5%) and atropine sulfate (1%). Contact lens electrodes were placed on cornea with one drop of methylcellulose. Full-field electroretinograms (ERGs) were recorded on P20 mice using the universal testing and electrophysiology LKC system (UTAS, LKC Technologies, Gaithersburg, MD). ERGs were recorded in response to 10 ms single white flashes with a fixed light intensity (24.8 cd s m−2) under scotopic conditions, which will not result in rod signal contributions because of the absence of rhodopsin in the mice studied. A single light intensity, rather than a family of light intensities, was chosen in order not to significantly bleach the limited number of newly formed pigment. b-Wave amplitudes are reported as a mean ± SE and analyzed by a two-tailed Student's t-test, accepting a significance value of P < 0.05.
Results
Light Reduces the Effectiveness of Improved Cone Survival with 11-cis Retinal
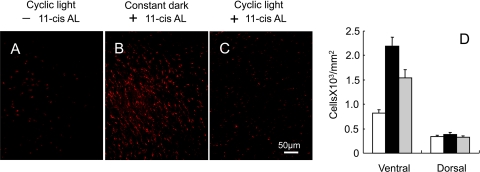
Cone cell survival was assessed by counting the number of cells containing cone opsins in flat-mounted P30 retinas from Rpe65−/−Rho−/− mice treated with and without 11-cis retinal (Fig. 1). The S-cone density was very low (823 ± 63 per mm2) in the Rpe65−/−Rho−/− ventral region of the mouse retina at P30 (Fig. 1A), while treatment with 11-cis retinal at P10 followed by dark rearing for 20 more days results in higher cone density in the same region of the retina (2187 ± 188 per mm2) (Fig. 1B). However, 11-cis retinal treatment to Rpe65−/−Rho−/− mice was not as effective in improving S-cone photoreceptor density when mice were subjected to 12 hours room light each day (Fig. 1C). M-cones were also counted and found to follow a similar pattern (not shown). A limitation to these measurements is that probing the whole-mounted retina with an opsin antibody indicates the number of cells that contain the opsin and is not necessarily indicative of the viability of the cell. Thus, the treated mice exposed to light appear to have not only fewer cells but also much less S-cone opsin based on the brightness and density of the fluorescence image when compared to mice treated in the same manner but maintained in the dark. However, the absolute number of S-cone opsin containing cells is higher in the ventral area for the treated animals than in the untreated mice regardless of light conditions (Fig. 1D).
Figure 1.
S-cone photoreceptor survival in Rpe65−/−Rho−/− retinas at P30. Flat-mounted retinas were probed for S-cone opsins from mice (A) not treated with 11-cis retinal (11-cis AL), followed by cyclic light–rearing, (B) treated with 11-cis retinal, followed by dark-rearing, and (C) treated with 11-cis retinal, followed by cyclic light–rearing. Images were taken from ventral region of the retinas. Scale bar, 50 μm. (D) Average density of photoreceptor cells containing S-opsin in ventral and dorsal regions of Rpe65−/−Rho−/− retinas for untreated (white), 11-cis retinal-treated mice maintained in the dark (black), and 11-cis retinal-treated mice maintained in cyclic light (gray). Data are presented as mean ± SE, n = 9.
11-cis Retinal Fails to Improve Cone Opsin Localization under Cyclic Light Conditions
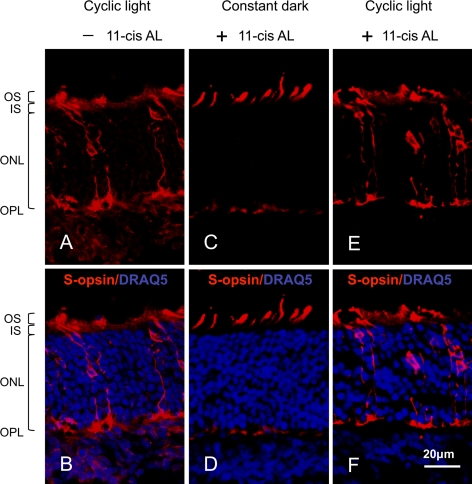
Cone opsin localization in cross-sections of three-week-old Rpe65−/−Rho−/− mouse retinas was used to assess the integrity of individual cone photoreceptor cells before their death. Consistent with previous studies,14,15 cross-sections of a retina from an untreated P21 Rpe65−/−Rho−/− mouse that were probed for S-cone opsin illustrate the delocalized pattern of opsin throughout the cell (Fig. 2A). Figure 2B adds nuclear staining with the DNA-binding dye DRAQ5 to further illustrate morphology and orientation of the retina. Consistent with earlier work,14,15 treating mice lacking RPE65 with 11-cis retinal followed by dark-rearing results in improved opsin localization in the outer segments, although there is still some visible fluorescence in the pedicles (Figs. 2C, 2D). A difference from the earlier studies, in which 11-cis retinal was introduced via multiple intraperitoneal injections over a 10- to 15-day period14,15 is that in this study, 11-cis retinal was introduced by a single subcutaneous injection within a basement membrane matrix at P10.20 However, the same 11-cis retinal treatment protocol followed by continued 12 h light/12 h dark conditions does not improve S-cone opsin; the delocalized opsin in Figures 2E and 2F appears essentially as if there had been no treatment with 11-cis retinal (Fig. 2A).
Figure 2.
S-opsin localization in cone photoreceptors of cross-sectioned P21 Rpe65−/−Rho−/− retinas. (A, B) Untreated mice; (C, D) 11-cis retinal-treated mice maintained in the dark; and (E, F) 11-cis retinal-treated mice maintained in 12 h light/12 h dark conditions. The sections were probed for S-opsin (red) in both the top and bottom panels. Nuclei were stained with DRAQ5 (blue) in the bottom panels to help orient the cross-sections and indicate outer retina integrity. The locations of the photoreceptor outer segment (OS), photoreceptor inner segment (IS), outer nuclear layer (ONL), and outer plexiform layer (OPL) are labeled beside the first image. The scale bar represents 20 μm.
S-cone images shown were taken from ventral regions as S-opsin-containing cones are more dominant in the ventral than in the dorsal region of mouse retinas while M/L opsin are dominant in the dorsal region.21 At P21, M/L opsin appears only in dorsal regions of the Rpe65−/−Rho−/− mouse retinas. Although the improvement of M/L opsin localization is not as dramatic as S-opsin, the density of M/L opsin in outer segments is higher in dark-reared 11-cis retinal–treated mouse groups (data not shown). These results are slightly different from those of Rohrer et al.14 but consistent with those from Zhang et al.15 There is no apparent difference in the localization of either opsin between the untreated and cyclic light–reared, 11-cis retinal–treated mouse groups.
11-cis Retinal Fails to Improve Cone Function under Cyclic Light Conditions
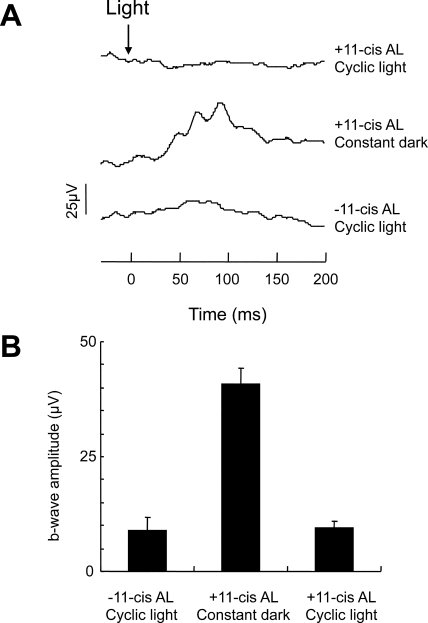
Previous studies showed marked improvement in light-induced ERG signals in mice lacking RPE65 treated with 11-cis or 9-cis retinal and maintained in the dark.20,22 We repeated these measurements with Rpe65−/−Rho−/− mice under our treatment protocol, followed by either constant dark or cyclic light–rearing conditions. Because the rod opsin is absent in Rpe65−/−Rho−/− mice, any photoresponse arises from cones. The b-wave amplitudes from dark-reared 11-cis retinal–treated Rpe65−/−Rho−/− mice were significantly enhanced (40 ± 3 μV) over those from untreated Rpe65−/−Rho−/− mice (9 ± 3 μV) (Fig. 3). This is generally consistent with previously published results14,20 although with different treatment and ERG protocols, the absolute amplitudes do differ. However, such increases in b-waves are not detected in the 11-cis retinal–treated cyclic light–reared group (9 ± 2 μV).
Figure 3.
ERG recordings from Rpe65−/−Rho−/− mice at P20. (A) Representative single-flash ERG in response to 24.8 cd s m−2 white light from mice treated in the following manner (bottom to top traces): untreated and maintained in cyclic light, treated with 11-cis retinal and maintained thereafter in the dark, and treated with 11-cis retinal and maintained in cyclic light. (B) Averages of the ERG b-wave amplitudes from the untreated and treated Rpe65−/−Rho−/− mice. Data are presented as mean ± SE, n = 10.
Discussion
In healthy wild-type cone photoreceptor cells, opsins are primarily localized in the outer segments. However, in mice lacking the ability to produce 11-cis retinal, cone cells are lost approximately 1 month after birth.13 Earlier studies demonstrated that multiple injections of 11-cis retinal into mouse models for LCA2 could impede these events, suggesting a potentially useful therapeutic agent for LCA2 patients, but the mice were maintained in the dark once treatment with 11-cis retinal commenced.13,14,16 Two other mouse models for LCA2 (Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/−) also showed improved cone morphology and function when treated with 9-cis retinyl acetate and maintained in the dark.18 Maeda et al.17 had previously reported that 9-cis retinyl acetate treatment results in increased levels of 9-cis retinal in the retina, which can form visual pigments, but that the cis retinal disappeared with light. If 11-cis or 9-cis retinal were to be useful for preserving cone photoreceptor cell integrity and function in LCA patients, we reasoned that the improved effects of exogenously added 11-cis retinal needs to occur under cyclic light living conditions. However, we find that the presence of light eliminates the benefits of treating RPE65-deficient mice with 11-cis retinal.
In this study, we have used the Rpe65−/−Rho−/− mouse because 11-cis retinal is not produced and rod opsins would not compete for exogenously added 11-cis retinal nor obscure light-driven ERG signals as a mixture of rod and cone signals.23 Although the lack of rhodopsin in mice results in degeneration of the retina,24–26 its absence in the double knockout for this study does not appear to interfere with our results. Cones have been reported to appear quite normal within the first month in Rho−/− mice26; morphologic properties of treated and untreated double knockout mice are similar to the single Rpe65−/− mice.15
Delocalized cone opsins followed by cone cell death has been observed in the retinas of other knockout mouse strains.15,16,27 Furthermore, other membrane proteins normally associated with the outer segment were also found to be mislocalized in the different mouse models.15,27,28 Similar to the targeting of rhodopsin to rod outer segments as proposed by Deretic et al.,29 Karan et al.30 have proposed cone opsins as part of a post-Golgi transport vesicle targeting the cilium, but unlike in rods, trafficking in cones involves vesicles comprised of a complex of multiple signaling proteins such that their transport to the outer segment is tightly coupled. Thus, one could argue that the role 11-cis retinal plays is to induce a conformation of the opsin that favors it being packaged into this transport vesicle complex; whereas, the apoprotein is in an unfavorable conformation. As 11-cis retinal is an inverse agonist of cone opsins,31,32 it maintains the protein in a conformation that can differ from the apoprotein. Furthermore, cone pigment formation within the inner segment appears possible, especially with recent studies implicating Müller cells as a source of 11-cis retinoids33–36 and a previous study indicating that cones can take up 11-cis retinoids from the inner segment.37 As to why bleaching is not a problem for wild-type retinas, we can only speculate it might be due to the constant availability of 11-cis retinal in wild-type mice compared with limited supply in the 11-cis retinal–treated knockout mice. Perhaps there is a threshold of number of pigments necessary to form a transport vesicle complex.
Finally, we would like to address why a therapeutic agent would be useful for LCA2. Gene therapy replacing missing or mutant RPE65 gene has shown great promise.38–41 In human trials, the time at which gene therapy was administered has likely limited the degree of restoration42–44 as a significant amount of cones could have already irreversibly degenerated. Jacobson et al.10 reported early thinning of the fovea as well as diminished visual acuity and no observed blue color vision—all consistent with loss of cones. Results from an age-dependent study of RPE65 gene therapy on humans are consistent with this notion.45 Thus cone photoreceptor preservation and overall integrity of the retina are important components in the eventual overall rescue of vision in LCA. Our results indicate that there might be great success in accomplishing this with an agent that is a ligand for cone opsins but does not form a photosensitive pigment.
Footnotes
Supported by NIH Grants R01-EY019515 (MK) and R01-EY004939 (RKC); an unrestricted grant to the Department of Ophthalmology at MUSC from the Research to Prevent Blindness; and Shanghai Laboratory of Fundus Diseases 07Z22911 (JF). RKC is a Research to Prevent Blindness Senior Scientist.
Disclosure: J. Fan, None; R.K. Crouch, None; M. Kono, None
References
- 1. Schappert-Kimmijser J, Henkes HE, Van Den Bosch J. Amaurosis congenita (Leber). AMA Arch Ophthalmol. 1959;61:211–218 [DOI] [PubMed] [Google Scholar]
- 2. Kaplan J, Bonneau D, Frezal J, Munnich A, Dufier JL. Clinical and genetic heterogeneity in retinitis pigmentosa. Hum Genet. 1990;85:635–642 [DOI] [PubMed] [Google Scholar]
- 3. Perrault I, Rozet JM, Gerber S, et al. Leber congenital amaurosis. Mol Genet Metab. 1999;68:200–208 [DOI] [PubMed] [Google Scholar]
- 4. Marlhens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber's congenital amaurosis. Nat Genet. 1997;17:139–141 [DOI] [PubMed] [Google Scholar]
- 5. Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lambert SR, Kriss A, Taylor D, Coffey R, Pembrey M. Follow-up and diagnostic reappraisal of 75 patients with Leber's congenital amaurosis. Am J Ophthalmol. 1989;107:624–631 [DOI] [PubMed] [Google Scholar]
- 9. Koenekoop RK. An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol. 2004;49:379–398 [DOI] [PubMed] [Google Scholar]
- 10. Jacobson SG, Aleman TS, Cideciyan AV, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci U S A. 2007;104:15123–15128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walia S, Fishman GA, Jacobson SG, et al. Visual acuity in patients with Leber's congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010;117:1190–1198 [DOI] [PubMed] [Google Scholar]
- 12. Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351 [DOI] [PubMed] [Google Scholar]
- 13. Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46:1473–1479 [DOI] [PubMed] [Google Scholar]
- 14. Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–3882 [DOI] [PubMed] [Google Scholar]
- 15. Zhang H, Fan J, Li S, et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28:4008–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. Rpe65−/− and Lrat−/− mice: comparable models of leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2008;49:2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maeda T, Maeda A, Casadesus G, Palczewski K, Margaron P. Evaluation of 9-cis-retinyl acetate therapy in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2009;50:4368–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maeda T, Cideciyan AV, Maeda A, et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet. 2009;18:2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rando RR. Polyenes and vision. Chem Biol. 1996;3:255–262 [DOI] [PubMed] [Google Scholar]
- 20. Tang PH, Fan J, Goletz PW, Wheless L, Crouch RK. Effective and sustained delivery of hydrophobic retinoids to photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:5958–5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Applebury ML, Antoch MP, Baxter LC, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523 [DOI] [PubMed] [Google Scholar]
- 22. Rohrer B, Goletz P, Znoiko S, et al. Correlation of regenerable opsin with rod ERG signal in Rpe65−/− mice during development and aging. Invest Ophthalmol Vis Sci. 2003;44:310–315 [DOI] [PubMed] [Google Scholar]
- 23. Seeliger MW, Grimm C, Stahlberg F, et al. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet. 2001;29:70–74 [DOI] [PubMed] [Google Scholar]
- 24. Humphries MM, Rancourt D, Farrar GJ, et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219 [DOI] [PubMed] [Google Scholar]
- 25. Lem J, Krasnoperova NV, Calvert PD, et al. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci U S A. 1999;96:736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaissle GB, May CA, Reinhard J, et al. Evaluation of the rhodopsin knockout mouse as a model of pure cone function. Invest Ophthalmol Vis Sci. 2001;42:506–513 [PubMed] [Google Scholar]
- 27. Baehr W, Karan S, Maeda T, et al. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang H, Li S, Doan T, et al. Deletion of PrBP/δ impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci U S A. 2007;104:8857–8862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci U S A. 2005;102:3301–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karan S, Zhang H, Li S, Frederick JM, Baehr W. A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vision Res. 2008;48:442–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kono M. Constitutive activity of a UV cone opsin. FEBS Lett. 2006;580:229–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Isayama T, Chen Y, Kono M, et al. Differences in the pharmacological activation of visual opsins. Vis Neurosci. 2006;23:899–908 [DOI] [PubMed] [Google Scholar]
- 33. Das SR, Bhardwaj N, Kjeldbye H, Gouras P. Muller cells of chicken retina synthesize 11-cis-retinol. Biochem J. 1992;285:907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mata NL, Radu RA, Clemmons RS, Travis GH. Isomerization and oxidation of vitamin A in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci. 2009;12:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J-S, Kefalov VJ. An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol. 2009;19:1665–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jin J, Jones GJ, Cornwall MC. Movement of retinal along cone and rod photoreceptors. Vis Neurosci. 1994;11:389–399 [DOI] [PubMed] [Google Scholar]
- 38. Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95 [DOI] [PubMed] [Google Scholar]
- 39. Lai CM, Yu MJ, Brankov M, et al. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65−/− knockout mouse eye results in limited rescue. Genet Vaccines Ther. 2004;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2006;47:1177–1184 [DOI] [PubMed] [Google Scholar]
- 41. Bemelmans AP, Kostic C, Crippa SV, et al. Lentiviral gene transfer of Rpe65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3:e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bainbridge JWB, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 43. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]