Abstract
Background
The utility of defibrillation threshold testing in patients undergoing implantable cardioverter–defibrillator (ICD) implantation is controversial. Higher defibrillation thresholds have been noted in patients undergoing implantation of cardiac resynchronization therapy defibrillators (CRT-D). Since the risks and potential benefits of testing may be higher in this population, we sought to assess the impact of defibrillation safety margin or vulnerability safety margin testing in CRT-D recipients.
Methods and results
A total of 256 consecutive subjects who underwent CRT-D implantation between January 2003 and December 2007 were retrospectively reviewed. Subjects were divided into two groups based on whether (n= 204) or not (n= 52) safety margin testing was performed. Patient characteristics, tachyarrhythmia therapies, procedural results, and clinical outcomes were recorded. Baseline characteristics, including heart failure (HF) severity, were comparable between the groups. Four cases of HF exacerbation (2%), including one leading to one death, were recorded in the tested group immediately post-implantation. No complications were observed in the untested group. After a mean follow-up of 32 ± 20 months, the proportion of appropriate shocks in the two groups was similar (31 vs. 25%, P = 0.49). There were three cases of failed appropriate shocks in the tested group, despite adequate safety margins at implantation, whereas no failed shocks were noted in the untested group. Survival was similar in the two groups.
Conclusion
Defibrillation efficacy testing during implant of CRT-D was associated with increased morbidity and did not predict the success of future device therapy or improve survival during long-term follow-up.
Keywords: Biventricular implantable cardioverter–defibrillator, Defibrillation threshold, Defibrillation efficacy
Introduction
Cardiac resynchronization with defibrillation therapy (CRT-D) is an established treatment modality for patients with a prolonged QRS duration and advanced drug-refractory congestive heart failure.1 Implant testing can be performed by determining defibrillation threshold (DFT) or by scanning the T wave with shocks to assess the upper limit of vulnerability (ULV).2,3
With improved device and lead technology, the utility of DFT testing during implantable cardioverter–defibrillator (ICD) implantation has been questioned.4,5 Several studies conducted in the ICD patient population have failed to show a relationship between first-shock efficacy or survival and the results of DFT testing.4,6,7
However, in a CRT-D population, an increased incidence of high DFTs was found, nearly 12%.8,9 Patient characteristics correlated with high DFT are more common in this population, including prolonged QRS duration, low ejection fraction, high left ventricular (LV) mass, high end-diastolic diameter, high New York Heart Association (NYHA) functional class, and prolonged procedural time.8–10
Although rare, major complications from testing, including death and stroke, have been reported.8,11 Measures taken to correct a finding of high DFT may also increase the complication risk in a patient who may never need the defibrillation capabilities of the device.4 Considering the longer procedural times in a more severely ill patient population, it is suspected that defibrillation testing may pose a higher risk to patients undergoing CRT-D implantation.11
In this study we compared the outcomes of patients undergoing CRT-D implantation with and without defibrillation efficacy testing over a 5-year period at a single centre.
Methods
We retrospectively studied consecutive patients with heart failure (HF) who were referred to our institution for CRT-D implantation or upgrade between January 2003 and December 2007. All patients with a minimum follow-up of 6 months and those who died or received orthotopic heart transplant (OHT) during this time were included. Patients with a right-sided device and those in whom it was unclear whether testing was performed were excluded. The Institutional Review Board approved review of these data. Both vulnerability safety margin (VSM) and defibrillation safety margin (DSM) were tested in this group; these terms will be used throughout this manuscript.12,13
Device implantation and testing
All patients underwent CRT-D placement or upgrade from another device via a standard transvenous approach under conscious sedation or general anaesthesia. After adequate sensing and pacing thresholds were assured for both ventricular and atrial leads, some patients underwent DSM/VSM testing (tested group) and some did not (untested group). Several methods were used to test defibrillation and confirm adequate safety margins.
Defibrillation safety margin testing
Ventricular fibrillation (VF) was induced once (DSM) or twice (DSM+) with the defibrillation shock output programmed at the discretion of the implanting physician. The difference between lowest energy of successful shock and maximal device output was considered the DSM.
Vulnerability safety margin testing
Vulnerability safety margin testing was conducted by pacing the ventricle for eight beats and then delivering a shock at the peak of the T wave, 20 and 40 ms before and 20 ms after the peak of the T wave. The shock output was programmed at the operator's discretion. If VF was not induced, the difference between the maximal device output and the energy applied was considered the VSM, approximately equivalent to the DSM.3
Combined testing
Some patients underwent both VSM and DSM testing. Defibrillation safety margin testing was conducted after VSM testing, which did or did not induce VF.
Deferred testing
Testing was deferred until a later date in several patients at the discretion of the implanting physician. These patients were included in the tested cohort.
All patients in sinus rhythm received an atrio-biventricular pulse generator programmed to DDDR mode. Data regarding device and shocking lead properties, system modification to increase the safety margins, and programmed anti-arrhythmic treatment were collected. The length of hospitalization after the implantation was also recorded.
Patient follow-up
Patients were seen at an outpatient clinic every 3–6 months for device interrogation and clinical follow-up. All arrhythmic events were assessed by an experienced electrophysiologist. Data regarding arrhythmic events, antitachycardia pacing (ATP), ICD shocks including energy delivered, shock appropriateness, and treatment success were collected. The occurrence of OHT, death, and cause of death were documented using patient records and the National Death Index database.
Statistical analysis
Continuous variables were expressed as mean ± SD. Statistical significance was assessed using the unpaired Student's t-test or the non-parametric Wilcoxon test when applicable. Categorical variables, expressed as numbers or percentages, were analysed using Fisher's exact test. The Kaplan–Meier method was used to compare arrhythmia-free survival and the combined event-free survival of death or need for OHT. All tests were two-tailed and P< 0.05 was considered significant.
Results
Clinical characteristics
The current study included 256 patients with CRT-D implantation or upgrade between January 2003 and December 2007 and either (i) ≥6 months follow-up or (ii) death or OHT within the first 6 months. Mean follow-up was 32 ± 20 months and interquartile range was 15–46 months. Nineteen patients (13 underwent DSM/VSM testing and 6 did not) had a follow-up of <6 months and were therefore excluded. Two patients with right-sided implant and two with inadequate documentation of testing were also excluded. Defibrillation safety margin/VSM was tested in 204 patients and not tested in 52 patients. Reasons for not testing included unstable haemodynamic status (n= 10), prolonged procedure (n= 5), intracardiac thrombus (n= 12), atrial fibrillation without TEE (n= 9), recent stroke (n= 3), and unavailability of anaesthesia coverage or physician discretion (n= 13).
Baseline clinical characteristics are presented in Table 1. Patients in the untested group were older (P= 0.049), with a trend towards lower left ventricular ejection fraction (LVEF) (20.9 vs. 23.0%, P= 0.056). The index procedure occurred during hospitalization for HF exacerbation in 38 (19%) and 14 (27%) cases from the tested and untested groups, respectively (P= 0.18). Clinical severity of HF as measured by NYHA functional class and B-type natriuretic peptide was comparable between the groups.
Table 1.
Baseline clinical characteristics of the study population
| DSM/VSM tested (n= 204) | Untested (n= 52) | P value | |
|---|---|---|---|
| IHD/non-ischaemic CM | 94/110 | 27/25 | 0.53 |
| Age (mean ± SD), years | 60 ± 14.1 | 64.3 ± 14 | 0.049 |
| Males (%) | 160 (78) | 34 (65) | 0.07 |
| NYHA (mean ± SD) | 3 ± 0.48 | 3.1 ± 0.67 | 0.28 |
| Primary/secondary | 155/49 | 37/15 | 0.47 |
| BIV ICD upgrade (%) | 48 (24) | 17 (32) | 0.21 |
| LVEF (mean ± SD) | 23 ± 6.9 | 20.9 ± 7.5 | 0.056 |
| LVEDD (mm) | 65.4 ± 10.8 | 64.7 ± 9.9 | 0.66 |
| Labs | |||
| Creatinine (mg/dL) | 1.55 ± 1 | 1.55 ± 0.68 | 0.99 |
| BNP (pg/mL) | 1005 ± 4000 | 873 ± 817 | 0.83 |
| QRS duration (ms) | 154.4 ± 36.5 | 156.3 ± 37.3 | 0.75 |
| CV risks (%) | |||
| Hyperlipidaemia | 100 (49) | 29 (56) | 0.43 |
| Smoking/past smoker | 39 (19) | 8 (15) | 0.68 |
| Hypertension | 81 (40) | 25 (48) | 0.34 |
| Diabetes mellitus | 54 (26) | 17 (33) | 0.38 |
| Medications (%) | |||
| Amiodarone | 48 (24) | 15 (29) | 0.47 |
| ACE inhibitors/ARBs | 176 (86) | 40 (77) | 0.13 |
| Beta-blockers | 167 (82) | 40 (77) | 0.43 |
| Diuretics | 168 (82) | 43 (83) | 0.99 |
| Statins | 113 (55) | 28 (54) | 0.87 |
| Anticoagulants | 69 (34) | 24 (46) | 0.1 |
VSM, vulnerability safety margin; DSM, defibrillation safety margin; IHD, ischaemic heart disease; CM, cardiomyopathy; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; NYHA, New York Heart Association; BIV ICD, biventricular implantable cardioverter–defibrillator; CV, cardiovascular; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; BNP, B-type natriuretic peptide.
Results of defibrillation efficacy testing
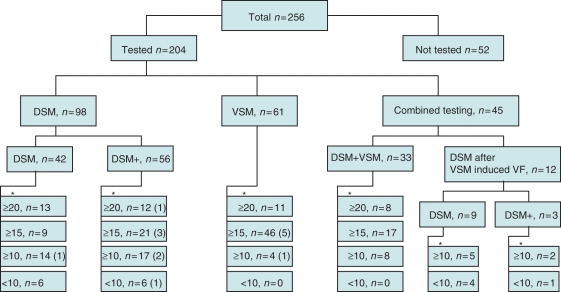
Figure 1 summarizes the results and methods of DSM/VSM testing. A safety margin of <10 J was found in 17 (8.3%) patients. In 10 (4.9%) of these 17 patients, no further invasive procedures were performed to improve the safety margin. Of these 10 patients, 9 had successful DSM testing with a <10 J safety margin (mean 5 ± 3 J) and 1 had both successful and unsuccessful DSM testing at maximal output. In three patients, repeat testing on another day revealed safety margin of at least 10 J. In one patient an adequate safety margin was achieved after programming tilt change of the biphasic shock waveform. Three patients underwent an invasive procedure to achieve adequate safety margin: in one by removing the superior vena cava coil and reversing defibrillation polarity and in two by adding a subcutaneous array (along with an azygos lead in one patient). Overall, 14 (7%) patients had testing deferred. Of these, 6 were tested 1 day post-procedure, 1 during the first week and 7 after 1–5 months.
Figure 1.
Summary of patient division into tested and untested groups and mode of testing. The numbers below the asterisks represent the achieved safety margins; in brackets are the number of patients with deferred testing.
Arrhythmic outcomes
During follow-ups of 33 ± 20 and 27 ± 18 months (P= 0.04), 63 (30%) and 13 (25%) patients of the tested and untested groups, respectively, had ventricular arrhythmic events resulting in device therapy (P= 0.49). In order to assess for implanting physician bias (not testing patients with a lower likelihood of arrhythmic events), the time to first ventricular arrhythmic event was examined. The Kaplan–Meier curves are presented in Figure 2 and demonstrate comparable time to the first event in both groups. The total number of ventricular tachycardia (VT)/VF episodes recorded in the tested and untested groups was 378 (mean = 1.85 per patient, median = 0 per patient) and 54 (mean = 1.04 per patient, median = 0 per patient) (P= 0.43), respectively. Of ventricular arrhythmic events, 104 (28%) and 18 (33%) were treated with shock therapy in the tested and untested groups, respectively (P= 0.82). Table 2 summarizes device programming and therapy. Despite programming to a lower mean first-shock output in the tested group, the mean energy output during arrhythmia treatment did not differ significantly between the groups (26.7 vs. 23.3 J, P= 0.31). Failed shocks were seen in three patients, all from the tested group, culminating in death in two patients. Both these patients had undergone VSM testing with a safety margins of 15 J (with device maximal outputs of 30 and 35 J). The third patient was successfully cardioverted by the third 30 J shock (the device maximal output); this patient had undergone DSM testing with failed 15 J and successful 20 J shock.
Figure 2.
Kaplan–Meier curves for arrhythmia-free survival. The time for first ventricular arrhythmic event was comparable between the groups.
Table 2.
Device settings and arrhythmia therapy
| DSM/VSM tested (n= 204) | Untested (n= 52) | P value | |
|---|---|---|---|
| Device settings | |||
| First-shock energy (J) | 23 ± 6.9 | 26.3 ± 7.7 | 0.007 |
| Maximal device output (J) | 33.3 ± 3 | 33.7 ± 2.6 | 0.46 |
| bpm threshold for VT zone | 162 ± 11 | 162 ± 12 | 0.91 |
| bpm threshold for VF zone | 206 ± 19 | 204 ± 23 | 0.56 |
| Arrhythmia therapy | |||
| Appropriate (%) | 63 (31) | 13 (25) | 0.49 |
| Energy delivered (J) | 26.7 ± 7.2 | 23.33 ± 7.5 | 0.31 |
| Failed DC therapy (%) | 3 (1.5) | 0 (0) | 0.61 |
| Inappropriate (%) | 31 (15) | 8 (15) | 0.97 |
VSM, vulnerability safety margin; DSM, defibrillation safety margin; bpm, beats per minute; VT, ventricular tachycardia; VF, ventricular fibrillation.
Complications
Among ambulatory patients, seven in the tested group (3.4%) and two in the untested group (3.8%) stayed in hospital for more than 1 day after implantation. In the tested group this was due to the need for repeat DSM testing (n= 2), pneumothorax (n= 1), and HF exacerbation (n= 4), including one patient requiring intubation and one that developed cardiogenic shock and later died in hospital. In the untested group one patient had pneumothorax and another had hypertensive urgency.
Primary endpoint
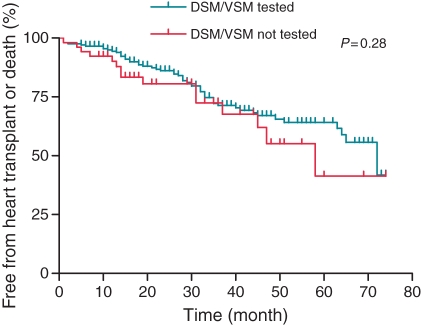
During follow-up, 36 (17.6%) and 10 (19.2%) patients died and 19 (9.3%) and 5 (9.6%) required OHT, from the tested and untested groups, respectively. The Kaplan–Meier curve for combined event-free survival of death or need for OHT is presented in Figure 3 and demonstrates comparable outcomes.
Figure 3.
Kaplan–Meier curves for the combined event-free survival of death or need for heart transplantation (OHT). Comparable outcome is demonstrated.
Discussion
Major findings
The major finding of this study is that in a group of patients undergoing CRT-D implantation or upgrade, DSM/VSM testing had no impact on patient survival and did not predict or improve success of shock therapy. In our cohort all three cases of failed shocks occurred in patients with adequate safety margins during device testing. Significant complications occurred among tested patients, including four cases of HF exacerbation and one death among ambulatory patients during the index admission for device implantation. These outcomes from a longitudinal cohort of patients with a mean follow-up of 32 months represent real-life experience with implant testing and device settings.
Comparison to previous studies
Several studies of patients undergoing ICD implantation have found worse outcomes in patients whose DFT was not tested.5,14,15 However, patients were often not tested due to more significant comorbidities, and this selection bias may explain the difference in outcomes. Indeed, a study that compared outcomes in hospitals that either tested or did not test DFT routinely, which minimized selection bias, found no mortality difference between the two strategies.7 In our study the tested and untested groups were comparable in terms of comorbidities and HF severity. In these relatively similar groups, there was no survival difference during the study period.
Other studies in patients with CRT-D found a higher incidence of elevated DFT in this population.8,9 In one of these studies elevated DFT was not associated with higher mortality.9 One possible explanation is that modifications were made to the device system to ensure adequate safety margins.9 However, another possibility is that DFT is not well correlated with therapy failure or mortality. No large studies have compared strategies of testing vs. not testing at CRT-D implantation. Here we studied long-term outcomes in untested patients, who had no modifications to increase safety margin, and found no survival difference.
Several explanations for the lack of benefit from DFT testing in this study can be considered.4,10,16 First, the incidence of inadequate safety margin, although higher than in previous studies of ICD patients, was still low in absolute terms. In addition, a relatively low incidence of significant arrhythmia during follow-up (25–30%) was seen in our cohort. Another factor may be that some appropriate shocks are not necessary shocks, as shown by the higher incidence of appropriate shocks compared to control-group sudden death in ICD trials.17 Implantation of CRT-D may improve patients' HF status and decrease LV volumes, or patients may fail to respond or even deteriorate rapidly due to advancing HF.18 In this dynamic group of patients, clinical characteristics may change more rapidly as compared with ICD patients, rendering DFT testing at the time of implantation less useful.8,9
Patients with HF and CRT-D may have clusters of ventricular arrhythmia as an epiphenomenon of preterminal HF deterioration (cardiogenic shock).19 In the SCD-HeFT cohort,6 10% of failed shocks occurred in patients with clinical HF deterioration culminating in death. Similarly, in our study, two of the three patients with failed shocks had intractable HF deterioration culminating in death. The DFT during this clinical scenario may be higher than at the time of implant. Also, sudden death in ICD patients with severe HF may result from electromechanical dissociation after successful defibrillation.20 Both these factors may make DFT testing less relevant to patient outcome in patients with severe HF.
One potential advantage of DFT/ULV testing is the possibility of using lower energy for the first shock,10 potentially lowering post-shock myocardial damage and stunning. Also, with shorter charging time, loss of consciousness may be prevented.10 However, delay for higher energy output is short in the latest generation of devices. Several trials have emphasized the importance of avoiding shock therapy,21 which can be achieved by delaying therapy to avoid treating non-sustained arrhythmias21 or ATP therapy, even at high rates.22 These measures may be more important than lowering device output. Interestingly, in this study even though the tested group had lower energy first shock programmed in the VT zone, delivered energy was not different between the groups. This was because many of the observed arrhythmias were in the fast VT or VF zones.
A potential disadvantage of testing is the occurrence of complications. Birnie et al.11 presented data on over 19 000 ICD implantations in Canada. There were three deaths and five strokes related to DFT testing. In a cohort of 501 patients undergoing CRT-D implantation, one patient died from induced VF that failed to convert and five had adverse hypotensive responses.8 We noted the death of one patient admitted electively in our cohort and another who experienced HF decompensation with intubation. Although not statistically significant, these major complications in the absence of proven benefit further call into question the rationale for testing.
Limitations
The present study is retrospective and mean follow-up time is longer in the tested group. However, survival analysis as presented in Kaplan–Meier curves adjusts for the differential follow-up. Another limitation is the unexpectedly low incidence of failed shock therapy in this cohort; however, this is reflective of real-world experience in an unbiased population.
In our study 17 patients in the tested group had a safety margin of <10 J, including 10 in whom no corrective measures were taken. We included these patients in the study population to avoid selection bias. Nevertheless, the results would not be different if these patients had been excluded.
Conclusion
Defibrillation safety margins/VSM testing in recipients of CRT-D did not correlate with successful device therapy or OHT-free survival. Several cases of HF exacerbation and one death appeared to be related to testing. Larger prospective studies are needed to validate our findings and further clarify the role of defibrillation testing in CRT-D patients.
Funding
This work was supported by the NHLBI (R01HL084261 and R01 HL067647 to K.S.).
Acknowledgements
We thank Ms Daniela Markovic, MS, and Dr. Jeffrey Gornbein, UCLA SBCC, for statistical analysis support.
Conflict of interest: none declared.
References
- 1.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. doi:10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 2.Marchlinski FE, Flores B, Miller JM, Gottlieb CD, Hargrove WC., III Relation of the intraoperative defibrillation threshold to successful postoperative defibrillation with an automatic implantable cardioverter defibrillator. Am J Cardiol. 1988;62:393–8. doi: 10.1016/0002-9149(88)90965-4. doi:10.1016/0002-9149(88)90965-4. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow CD, Shehata M, Chen PS. Using the upper limit of vulnerability to assess defibrillation efficacy at implantation of ICDs. Pacing Clin Electrophysiol. 2007;30:258–70. doi: 10.1111/j.1540-8159.2007.00659.x. doi:10.1111/j.1540-8159.2007.00659.x. [DOI] [PubMed] [Google Scholar]
- 4.Viskin S, Rosso R. The top 10 reasons to avoid defibrillation threshold testing during ICD implantation. Heart Rhythm. 2008;5:391–3. doi: 10.1016/j.hrthm.2008.01.006. doi:10.1016/j.hrthm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Russo AM, Sauer W, Gerstenfeld EP, Hsia HH, Lin D, Cooper JM, et al. Defibrillation threshold testing: is it really necessary at the time of implantable cardioverter–defibrillator insertion? Heart Rhythm. 2005;2:456–61. doi: 10.1016/j.hrthm.2005.01.015. doi:10.1016/j.hrthm.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Blatt JA, Poole JE, Johnson GW, Callans DJ, Raitt MH, Reddy RK, et al. No benefit from defibrillation threshold testing in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) J Am Coll Cardiol. 2008;52:551–6. doi: 10.1016/j.jacc.2008.04.051. doi:10.1016/j.jacc.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi S, Ricci RP, Biscione F, Sgreccia F, Di Belardino N, Rossi P, et al. Primary prevention implantation of cardioverter defibrillator without defibrillation threshold testing: 2-year follow-up. Pacing Clin Electrophysiol. 2009;32:573–8. doi: 10.1111/j.1540-8159.2009.02329.x. doi:10.1111/j.1540-8159.2009.02329.x. [DOI] [PubMed] [Google Scholar]
- 8.Schuger C, Ellenbogen KA, Faddis M, Knight BP, Yong P, Sample R. Defibrillation energy requirements in an ICD population receiving cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2006;17:247–50. doi: 10.1111/j.1540-8167.2006.00345.x. doi:10.1111/j.1540-8167.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 9.Mainigi SK, Cooper JM, Russo AM, Nayak HM, Lin D, Dixit S, et al. Elevated defibrillation thresholds in patients undergoing biventricular defibrillator implantation: incidence and predictors. Heart Rhythm. 2006;3:1010–6. doi: 10.1016/j.hrthm.2006.05.028. doi:10.1016/j.hrthm.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow CD, Russo AM, Degroot PJ. The dilemma of ICD implant testing. Pacing Clin Electrophysiol. 2007;30:675–700. doi: 10.1111/j.1540-8159.2007.00730.x. doi:10.1111/j.1540-8159.2007.00730.x. [DOI] [PubMed] [Google Scholar]
- 11.Birnie D, Tung S, Simpson C, Crystal E, Exner D, Ayala Paredes FA, et al. Complications associated with defibrillation threshold testing: the Canadian experience. Heart Rhythm. 2008;5:387–90. doi: 10.1016/j.hrthm.2007.11.018. doi:10.1016/j.hrthm.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Day JD, Doshi RN, Belott P, Birgersdotter-Green U, Behboodikhah M, Ott P, et al. Inductionless or limited shock testing is possible in most patients with implantable cardioverter–defibrillators/cardiac resynchronization therapy defibrillators: results of the multicenter ASSURE Study (arrhythmia single shock defibrillation threshold testing versus upper limit of vulnerability: risk reduction evaluation with implantable cardioverter–defibrillator implantations) Circulation. 2007;115:2382–9. doi: 10.1161/CIRCULATIONAHA.106.663112. doi:10.1161/CIRCULATIONAHA.106.663112. [DOI] [PubMed] [Google Scholar]
- 13.Curtis AB. Defibrillator implantation without induction of ventricular fibrillation: good enough? Circulation. 2007;115:2370–2. doi: 10.1161/CIRCULATIONAHA.107.698548. doi:10.1161/CIRCULATIONAHA.107.698548. [DOI] [PubMed] [Google Scholar]
- 14.Pires LA, Johnson KM. Intraoperative testing of the implantable cardioverter–defibrillator: how much is enough? J Cardiovasc Electrophysiol. 2006;17:140–5. doi: 10.1111/j.1540-8167.2005.00294.x. doi:10.1111/j.1540-8167.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 15.Hall B, Jeevanantham V, Levine E, Daubert J, McNitt S, Hall F, et al. Comparison of outcomes in patients undergoing defibrillation threshold testing at the time of implantable cardioverter–defibrillator implantation versus no defibrillation threshold testing. Cardiol J. 2007;14:463–9. doi: [PubMed] [Google Scholar]
- 16.Curtis AB. Defibrillation threshold testing in implantable cardioverter–defibrillators: might less be more than enough? J Am Coll Cardiol. 2008;52:557–8. doi: 10.1016/j.jacc.2008.05.016. doi:10.1016/j.jacc.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter–defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1111–21. doi: 10.1016/j.jacc.2008.05.058. doi:10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. doi:10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 19.Lunati M, Gasparini M, Bocchiardo M, Curnis A, Landolina M, Carboni A, et al. Clustering of ventricular tachyarrhythmias in heart failure patients implanted with a biventricular cardioverter defibrillator. J Cardiovasc Electrophysiol. 2006;17:1299–306. doi: 10.1111/j.1540-8167.2006.00618.x. doi:10.1111/j.1540-8167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell LB, Pineda EA, Titus JL, Bartosch PM, Benditt DG. Sudden death in patients with implantable cardioverter defibrillators: the importance of post-shock electromechanical dissociation. J Am Coll Cardiol. 2002;39:1323–8. doi: 10.1016/s0735-1097(02)01784-9. doi:10.1016/S0735-1097(02)01784-9. [DOI] [PubMed] [Google Scholar]
- 21.Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, et al. Strategic programming of detection and therapy parameters in implantable cardioverter–defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541–50. doi: 10.1016/j.jacc.2008.05.011. doi:10.1016/j.jacc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, et al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter–defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–6. doi: 10.1161/01.CIR.0000145610.64014.E4. doi:10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]