Abstract
Bruxism is a movement disorder characterized by grinding and clenching of teeth. Awake bruxism is found more in females as compared to males while sleep bruxism shows no such gender prevalence. Etiology of bruxism can be divided into three groups psychosocial factors, peripheral factors and pathophysiological factors. Treatment modalities involve occlusal correction, behavioural changes and pharmacological approach. A literature search was performed using National Library of Medicine’s (NLM) Medical Subject Headings (MeSH) Database, Pubmed and Google search engines. The search term ‘Bruxism’ yielded 2,358 papers out of which 230 were review papers. Most of the papers selected were recently published during the period of 1996–2010 and very few of them were published before 1996.
Introduction
Tooth grinding is an activity particularly importantant to the dentist because of breakage of dental restorations, tooth damage, induction of temporal headache and temporomandibular disorders [1].
The term parafunction was introduced by Drum [2] to suggest distinction between occlusal stress exerted during mastication and swallowing and occlusal stress which are brought into action outside of the normal function. Parafunctional activities are non functional oromandibular or lingual activities that includes jaw clenching, bruxism, tooth grinding, tooth tapping, cheek biting, lip biting, object biting etc. that can occur alone or in combination and are different from functional activities like chewing, speaking and swallowing.
The term ‘la bruxomanie’ was first introduced by Marie Pietkiewicz in 1907 [3]. It was latter adopted as ‘bruxism’ to describe gnashing and grinding of the teeth occurring without a functional purpose. Glossary of Prosthodontic Terms (GPT-8) [4] defines bruxism as parafunctional grinding of teeth or an oral habit consisting of involuntary rhythmic or spasmodic non functional gnashing, grinding or clenching of teeth in other than chewing movements of the mandible which may lead to occlusal trauma. Bruxism can occur during wakefulness or during sleep. Bruxism during daytime is commonly a semivoluntary ‘clenching’ activity and is also known as ‘Awake Bruxism’ (AB) or Diurnal Bruxism (DB). AB can be associated with life stress caused by familial responsibility or work pressure. Bruxism during sleep either during daytime or during night is termed as ‘Sleep Bruxism’ (SB). SB is an oromandibular behavior that is defined as a stereotyped movement disorder occurring during sleep and characterized by tooth grinding and/or clenching [5]. Sleep bruxism was recently classified as sleep related movement disorder according to recent classification of Sleep Disorders [4].
Prevalence rate of AB and SB is about 20 and 8–16% respectively in adult population [6]. AB is found to occur predominantly among females while no such gender difference is seen for sleep bruxism [7]. Onset of SB is about 1 year of age soon after the eruption of deciduous incisors [8]. The disorder is appearing more frequently in the younger population [8]. The prevalence in children is between 14 to 20%. In adults aged above 60 years and over only 3% are being aware of frequent grinding [9].
Etiology
Bruxism is considered to have multifactorial etiology. SB and grinding have been associated with peripheral factors such as tooth interference in dental occlusion, psychosocial influences such as stress or anxiety and central or pathophysiological causes involving brain neurotransmitters or basal ganglia [3].
Central or Pathophysiological Factors
More and more pathophysiological factors are suggested to be involved in the precipitation of bruxism. As the bruxism often occurs during sleep, the physiology of sleep has been studied extensively especially the ‘arousal response’ in search of possible causes of disorder. Arousal response is a sudden change in the depth of the sleep during which the individual either arrives in the lighter sleep stage or actually wakes up. Such a response is accompanied by gross body movements, increased heart rate, respiratory changes and increased muscle activity. Macaluso et al. [10] in their study showed 86% of bruxism episodes were associated with arousal response along with involuntary leg movements. This shows that bruxism is a part of arousal response indeed.
Recently it is derived that disturbances in central neurotransmitter system may be involved in the etiology of the bruxism [11, 12]. It is hypothesized that the direct and indirect pathways of the basal ganglion, a group of five subcortical nuclei that are involved in the coordination of movements is disturbed in bruxer. The direct output pathway goes directly from the stratum to the thalamus from where afferent signals project to the cerebral cortex. The indirect pathway on the other hand passes by several other nuclei before reaching it to the thalamus [13]. If there is imbalance between both the pathways, movement disorder results like Parkinson’s disease. The imbalance occurs with the disturbances in the dopamine mediated transmission of action potential. In case of bruxism there may be an imbalance in both the pathways. Acute use of dopamine precursors like L-dopa inhibits bruxism activity [11] and chronic long term use of l-dopa results in increased bruxism activity [11]. SSRTs (serotonin reuptake inhibitors) which exert an indirect influence on the dopaminergic system [14] may cause bruxism after long term use. Amphetamine [11] which increases the dopamine concentration by facilitating its release has been observed to increase bruxism. Nicotine stimulates central dopaminergic activities which might explain the finding that cigarette smokers report bruxism two times more than the non smokers [15].
Psychosocial Factors
Number of studies is published in the literature regarding the role of psychosocial factors in the etiology of bruxism but none of these describe the conclusive nature because of the absence of large scale longitudinal trials. Bruxers differs from healthy individuals in the presence of depression, increased levels of hostility [16] and stress sensitivity [17]. Bruxing children are more anxious than non bruxers [18]. A multifactorial large scale population study to sleep bruxism revealed highly stressful life as a significant risk factor [19]. A study by Van Selms et al. [20] demonstrated that daytime time clenching could significantly be explained by experienced stress, although experienced stress and anticipated stress were unrelated to sleep bruxism as recorded with ambulatory devices [20]. All these studies show possible relationship between bruxism and various psychosocial factors is growing but not conclusive.
Peripheral Factors
Several occlusal factors were suggested to be related to self reported bruxism in a study with children. Giffin [21] in his article has mentioned that for an effective management of bruxism, establishment of harmony between maximum intercuspation and centric relation is required. But most of studies published in the literature on this subject now agrees that there is no or hardly any relationship between clinically established bruxism and occlusal factors in adults [17, 22]. Manfredini et al. [23] in their review of literature have stated that there is still a lack of methodological sound studies to definitely refute the importance of occlusal factors in the etiology of bruxism.
Assessment/Diagnosis of Bruxism in the Clinic [24]
Although bruxism is not a life-threatening disorder, it can influence the quality of human life, especially through dental problems, such as tooth wear, frequent fractures of dental restorations and pain in the oro-facial region. Hence its early assessment is very essential. Some of the methods to assess bruxism in the clinic are mentioned below:
Questionnaires
Questionnaires are generally used in both research and clinical situations. This method can be applied to large population but the disadvantage with this method is that information obtained is sujective in nature.
Self reports to assess bruxism presence and absence of bruxism is convenient for both clinicians and researchers but it has been found that about 80% of bruxism episodes are not accompanied by noise [25]. So a large percentage of adults and children are unaware of their bruxism activity and thus fails to identify themselves as the bruxers (Table 1).
Table 1.
Questionnaire for detecting bruxer [26]
| Has anyone heard you grinding your teeth at night? |
| Is your jaw ever fatigue or sore on awakening in the morning? |
| Are you teeth or gums ever sore on awakening in the morning? |
| Do you ever experience temporal headache on awakening in the morning? |
| Are you ever aware of grinding your teeth during the day? |
| Are you ever aware of clenching your teeth during the day? |
Clinical Findings/Evaluation
Clinical Examination
The diagnosis of bruxism is based particularly on history, tooth mobility, tooth wear and other clinical findings listed in the Table 2.
Table 2.
| Report of tooth grinding or tapping sounds (usually reported by bed partner) |
| Presence of tooth wear seen within normal range of jaw movements or at eccentric position |
| Presence of masseter muscle hypertrophy on voluntary contraction |
| Complain of masticatory muscles discomfort, fatigue or stiffness in the morning (occasionally, headache in temporal muscle region) |
| Tooth or teeth hypersensitive to cold air or liquid |
| Clicking or locking of temporomandibular joint |
| Tongue on cheek indentation |
Tooth Wear
Tooth wear is considered to be analogous to bruxism. Several studies have demonstrated a positive relationship between tooth wear and bruxism [29] but others have not [30]. A number of systems for the classification and measurement of incisal and occlusal tooth wear have been introduced. One such system of classifications is an Individual (personal) Tooth-Wear Index which was given to rank persons with regard to incisal and occlusal wear and was developed to investigate the prevalence and severity of tooth wear [31].
First, the extent of incisal or occlusal wear for a single tooth was evaluated by the following four-point scale:
0: no wear or negligible wear of enamel;
1: obvious wear of enamel or wear through the enamel to the dentine in single spots;
2: wear of the dentine up to one-third of the crown height;
3: wear of the dentine up to more than one-third of the crown height; excessive wear of tooth restorative material or dental material in the crown and bridgework, more than one-third of the crown height.
Then, the individual (personal) tooth-wear index (IA) was calculated from the scores of incisal or occlusal wear for each tooth of that individual.
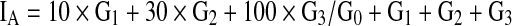 |
where G0, G1, G2 and G3 are the number of teeth with scores of 0, 1, 2 and 3 respectively. This method makes it possible to calculate the degree of individual (personal) tooth wear without being influenced by the number of missing teeth. Major disadvantage with tooth wear is that it neither proves ongoing bruxism nor static tooth clenching. Tooth wear is a cumulative record of both functional and parafunctional activities and various factors such as age, gender, diet and bruxism are associated with tooth wear. Erosion by acidic drink is considered to be major contributing factor to tooth wear [32]. All mechanisms of tooth wear rarely act alone and usually interacts with each other to cause wear. So the evaluation of tooth wear to for predicting actual bruxism is controvercial and is difficult to estimate the degree of contribution of bruxism to tooth wear alone.
Intraoral Appliance
Bruxism activity can be evaluated using the intra-oral appliance and is classified into two groups:
observation of wear facets of the intra-oral appliance [33, 34].
measurement of bite force loaded on the intra-oral appliance [35].
Wear Facets of Intra-oral Appliance
Wear Facets on Intraoral Appliance
Holmgren et al. [33] reported a repetitive wear pattern on the occlusal splint. They observed wear facets on full-arch acrylic resin splints, which reappeared in the same location with a similar pattern and direction, even after adjustment of the splints. Also, Korioth et al. [34] reported that parafunctional nocturnal dental activity on full-arch occlusal stabilization splints resulted in wear, which was both asymmetric and uneven. Unfortunately, no confirmation of the reliability of these methods has been reported.
Bruxcore Plate
The Bruxcore Bruxism-Monitoring Device (BBMD) is an intra-oral appliance that was introduced as a device for measuring sleep bruxism activity objectively [36] and the Bruxcore plate evaluates bruxism activity by counting the number of abraded microdots on its surface and by scoring the volumetric magnitude of abrasion. The BBMD is a 0.51-mm-thick polyvinyl chloride plate that consists of four layers with two alternating colors and a halftone dot screen on the topmost surface. The number of missing microdots is counted to assess the abraded area and the number of layers uncovered represents the depth parameter. Both parameters are combined to obtain an index for the amount of bruxism activity. The major disadvantage with this method is that it is difficult to count the number of missing dots with good precision. Pieree and Gale [37] in their study did not find any significant co relation between the duration of bruxism analyzed with the EMG data and that with the bruxcore plate scores. In this respect, the bruxism activity assessed by Bruxcore plates may not be the same as that measured with a portable EMG device. However, the relationship between wear and bruxism activity is still questionable [38].
Detection of Bite Force
Takeuchi et al. [39] developed a recording device for sleep bruxism, an intra-splint force detector (ISFD), which uses an intra-oral appliance to measure the force being produced by tooth contact onto the appliance. The force is detected using a thin, deformation-sensitive piezoelectric film, which is embedded 1–2 mm below the occlusal surface of the appliance. It was confirmed that the duration of bruxism events during simulated bruxism, i.e. clenching, grinding, tapping and rhythmic clenching, evaluated with the ISFD was correlated with that of the masseter EMG. Even though the ISFD did not correctly capture force magnitudes during sustained clenching because of the characteristic of the piezoelectric film, i.e. this transducer is best at detecting rapid changes in force, not static forces. ISFD was not suitable for detecting the magnitude of force during steady-state clenching behaviour.
It is obvious, however, that the major problem of these methods is that subjects have to wear the intra-oral device and this may change the original bruxism activity. Well-designed comparative studies with polysomnographic recordings, are required to evaluate the possible influences of the intra-oral device on the original bruxism activity.
Masticatory Muscle Electromyographic Recording
Among the various methods for the assessment of bruxism, the EMG recording has been commonly used to measure actual sleep bruxism activity directly. The principal advantage is, that the occurrence of bruxism can be assessed without intra-oral devices, which may change natural bruxism activity.
Portable EMG Recording Device
Starting in the 1970s, sleep bruxism episodes were measured over an extended period in patients homes with the use of battery-operated EMG recording devices [40]. The portable EMG recording system has become easy for subjects to operate and can measure masticatory muscle activity more minutely, i.e. the number, duration and magnitude of bruxism events can be evaluated with fair accuracy. Criteria for the detection of sleep bruxism with the portable EMG recording system have been suggested [41] but their validity in a large population has not yet been confirmed.
The detection power of sleep bruxism is generally considered inferior to that in a sleep laboratory because other confounding oro-facial activities (e.g. sight, coughing and talking) cannot be discriminated from sleep bruxism. Also, other sleep disorders cannot be ruled out or other physiological changes related to sleep bruxism (e.g. microarousal, tachycardia and sleep-stage shift) cannot be monitored [42–44]. The implement for recording the heart rate was recommended as one of the compensatory measures for improving the accuracy of sleep bruxism recognition. Also, a surface EMG electrode with a built-in buffer-amplifier and a cordless type of EMG measurement system was developed to improve the reliability of recordings [45].
Miniature Self-Contained EMG Detector–Analyser
A miniature self-contained EMG detector–analyser (BiteStrip) was developed as a screening test for moderate to high level bruxers [46]. The device, which is comprised of EMG electrodes, an amplifier, a central processing unit (CPU) with software, a display which presents the outcome in the morning, a light emitting diode and a lithium battery records the number of masseter muscle activities above a preset threshold. The special feature of this device is that the number of bruxism events can be objectively estimated by simply attaching it to the skin over the masseter muscle. Minakuchi and Clark [46] examined the sensitivity and specificity of the BiteStrip recording versus masseter EMG recordings during a polysomnogram in five suspected bruxers. Overall, there was good specificity for all subjects but fair sensitivity for subjects that exhibit moderate to high levels of EMG determined bruxism. The device might be a cost-effective tool for screening moderate- to high level bruxism subjects.
More recently, a miniature self-contained EMG detector–analyser with a biofeedback function (Grindcare) was developed as a detector and biofeedback device for sleep bruxism [47]. It is comprised of EMG and stimulation electrodes, a microprocessor, a memory for data storage, a display for user interface, light-emitting diodes, a rechargeable battery, a plug-in USB connector for data connection to computer to a battery charger, and a strap for carrying the apparatus around the forehead. It enables the online recording of EMG activity of the anterior temporalis muscle, online processing of EMG signals to detect tooth grinding and clenching and also biofeedback stimulation for reducing sleep bruxism activities. Although scientific confirmation is needed for a large population, it is considered as one of the potent devices for detecting and also for managing sleep bruxism. The portable EMG recording system enables multiple-night recording in a natural environment for the subject with minimal expense.
Finally, a miniature self-contained EMG detector–analyser seems to be a potentially useful device for detecting sleep bruxism.
Polysomnography
Polysomnographic (sleep laboratory) recordings for sleep bruxism generally include electroencephalogram, EMG, electrocardiogram and thermally sensitive resistor (monitoring air flow) signals along with simultaneous audio–video recordings. Sleep bruxism activity is assessed based on EMG activity in the masticatory muscles (masseter and/or temporalis). Because the sleep laboratory setting offers a highly controlled recording environment, other sleep disorders (e.g. sleep apnoea and insomnia) can be ruled out and sleep bruxism can be discriminated from other orofacial activities (e.g. myclonus, swallowing and coughing) that occur during sleep [43, 44, 48]. Physiological changes related to sleep bruxism (e.g. microarousal, tachycardia and sleep-stage shift) can also be monitored. Hence, a polysomnographic study allows for multidimensional analyses of sleep-related physiological behaviours and studies on sleep laboratory EMG-based assessments are reported to be very reliable. One major limitation is that a change in the environment for sleep may influence the actual behaviour of bruxism. Another is the expense as multiple night recording is to be taken for the occurrence of sleep bruxism varies over a number of nights.
Management of Bruxism [49]
Occlusal Therapy
Occlusal Interventions
This category aims at achieving harmonious relationship between occluding surfaces but there exist controversies among dental clinicians and researchers. Butler [50] described an occlusal adjustment procedure for the treatment of bruxism without a proper theoretical basis. Similarly, Frumker [51] formulated a set of principles for a successful occlusal treatment on the basis of an unfounded idea that the better the occlusal anatomy and function, the easier the bruxers relieve tension in the masticatory and associated musculature. In a study, Holmgren and Sheikholeslam [52] tried to substantiate the effects of occlusal adjustment on the myoelectrical activity of the jaw-closing muscles. However, their brief daytime EMG recordings of postural activity and maximal voluntary clenching cannot be interpreted in terms of bruxism. Greene et al. [53]. stated that occlusal rehabilitation further mutilates the dentition beyond what bruxism has created. In short, there is no support in the literature for the use of ‘true’ occlusal interventions like equilibration, rehabilitation and orthodontic alignment in the management of bruxism. In view of the current insights into the etiology of bruxism that the disorder is mainly regulated centrally and not peripherally future research on this category of management strategies for bruxism seems impractical.
Occlusal Appliances
The second category of occlusal management strategies for bruxism contains the frequency used occlusal appliances. Most papers in the literature describes the clinical and laboratory procedures for the various types of splints. These splints have different names (e.g. occlusal bite guard, bruxism appliance, bite plate, night guard, occlusal device) and slightly different appearances and properties, but in essence most of them are hard acrylic-resin stabilization appliances, mostly worn in the upper jaw [54, 55]. Hard splints are generally preferred over soft splints for practical reasons (e.g. soft splints are more difficult to adjust than hard ones) to prevent inadvertent tooth movements and also because hard splints are suggested to be more effective in reducing bruxism activity than soft splints [56].
Four simple methods are described in the literature for the fabrication of occlusal splints. First two methods describe the chair side procedure for the fabrication of occlusal splints using hard acrylic resin and composite resins [57]. The third prescription describes the chair-side adjustment of the so-called ‘Nociceptive Trigeminal Inhibition (NTI) Clenching Suppression System’—a small anterior splint that is supposed to be effective amongst others in the management of bruxism [58]. No evidence for the NTI splint’s long-term efficacy or safety is available so far. Finally, the fourth prescription describes the scientifically unsupported concept of the pre-fabricated and chair-side adjustable “Bruxism ‘S’ Splint” that can be used in combination with active orthodontic treatment [59]. More research is needed to assess the efficacy and safety of such unconventional, chair-side solutions before their application in dental practice can be recommended. In an study by Clark et al. [60] it was shown that occlusal splint treatment resulted in a decrease in nocturnal EMG activities in about half of the patients, while in another half of the patients, no change or even increase in EMG activity was observed. Landry et al. [61]. performed a short-term RCT (Randomized Control Trials) to the efficacy of mandibular advancement devices (MAD; a bimaxillary appliance that is indicated for the management of snoring and sleep apnea) when compared with that of ‘regular’ maxillary occlusal splints. They observed only a moderate reduction in polysomnographically established sleep bruxism with the occlusal splint in situ, but a large decrease in bruxism activity when the MAD was worn regardless of the amount of protrusion of the appliance. The authors could not readily explain this result but they hypothesized, amongst others, that the fact that approximately two-thirds of their study sample reported localized pain with the MAD in situ may be responsible for the observed decrease, after all it has been reported that in the presence of pain, bruxism activity may reduce considerably [62]. Given the contradictory results of the above described studies and the scarcity of RCT on the efficacy of occlusal splints in the management of bruxism, it is prudent to limit the use of oral splints in the management of bruxism to the prevention or limitation of dental damage that is possibly caused by the disorder.
Biofeedback
Biofeedback is based on the principle that bruxers can ‘unlearn’ their behaviour when a stimulus makes them aware of their adverse jaw muscle activities (‘aversive conditioning’). This technique has been applied for bruxism during wakefulness as well as for sleep bruxism. While awake, patients can be trained to control their jaw muscle activities through auditory or visual feedback from a surface EMG. For sleep bruxism, auditory, electrical, vibratory and even taste stimuli can be used for feedback.
Bruxism During Wakefulness/Daytime (AB)
One of the early publications on the use of biofeedback in the management of bruxism during wakefulness was a prescription by Mittelman [63]. He described an EMG technique that provides the daytime clencher with auditory feedback from his/her muscle activity letting him know the degree of muscle activity or relaxation that is taking place. A similar suggestion was given in the review articles by Cannistraci [64] in which he used a flat occlusal splint for biofeedback. The splint was inserted in the explicit understanding that the appliance serves to remind the daytime bruxer of adverse tooth contacts (i.e. contacts other than those involved in chewing and swallowing) and according to them 50% success was obtained by using this technique.
Sleep Bruxism
For the use of biofeedback in the management of sleep bruxism, Cherasia and Parks [65] published a prescription. Their technique used contingent arousal from sleep with actual awakenings. Although the authors were aware of the lack of validation of their technique, they stated that its potential effectiveness, ease of use and lack of risk warrant its consideration. Nissani [66] used a taste stimulus to awaken the patient. This stimulus was caused by the bruxism-related rupture of capsules, filled with an aversive substance (agreed upon with the patient) in the dental appliance. On the basis of a single case, the author claimed long-term success. In most of the case reports, a sound blast was applied as the aversive stimulus. The sound stimulus wakes up the patient, who is then supposed to switch off the sound and resume his/her sleep. The awakenings are a major disadvantage of such approaches because sleep disruption may lead to serious side effects like excessive daytime sleepiness [67].
No evidence is available for the long time use of biofeedback in the management of bruxism. Further, the possible consequences of the frequent arousals, like excessive daytime sleepiness, need further attention before this technique can be applied for the safe treatment of patients with bruxism.
Pharmacological Approach
The pharmacological management of bruxism has been studied increasingly over the past decades. Drugs that have paralytic effect on the muscles through an inhibition of acetylcholine release at the neuromuscular junction (botulinum toxin) decreases bruxism activity especially in severe cases with comorbidities like coma, brain injury, amphetamine abuse, Huntington’s disease [68] and autism [69].
Several studies have been performed to access the effects of serotonergic and dopaminergic medicines in the treatment of sleep bruxism [70]. In a placebo-controlled RCT, bruxism-related nocturnal EMG activity was not influenced by the serotonin precursor l-tryptophan. In contrast to that negative finding, a placebo-controlled sleep laboratory RCT showed that the catecholamine precursor l-dopa exerted a modest, attenuating effect on sleep bruxism. Likewise, sleep bruxism activity was reduced by the administration of low doses of the dopamine D1/D2 receptor agonist pergolide in a severe bruxism case. Huynh et al. [71] found no effects of the non-selective adrenergic beta-blocker propranolol on sleep bruxism, despite the positive response to this drug in two cases of antipsychotic induced bruxism. The selective alpha-2 agonist clonidine, on the other hand, does seem a promising medicine for the management of sleep bruxism, although further safety assessments are still required because severe morning hypotension was noted in approximately 20% of the participants [72].
Antidepressant drugs may exert deviating effects on bruxism: either they exacerbate the condition (selective serotonin reuptake inhibitors, SSRI) or they are inert in their effects (amitriptyline) [73]. Taking the above-described evidence together, it can be concluded that although some pharmacological approaches for bruxism seem promising, they all need further efficacy and safety assessments before clinical recommendations could be made.
Summary
Bruxism is a sleep related disorder. Studies show that bruxism is centrally mediated disorder and psychosocial factors like stress have little role in the etiology of bruxism. There are no reliable methods for assessing bruxism in the clinic which have reasonable diagnostic validity. Tooth wear is considered analogous to bruxism but it can also be the result of attrition, abrasion and erosion. Devices such as miniature self contained EMG detector analyzer have potential if they are scientifically verified in large population and proven to be useful in clinics in terms of easy use. In the absence of definitive evidence, bruxism can be best managed by occlusal appliances, counseling, change in lifestyle and pharmacological interventions.
Contributor Information
Shilpa Shetty, Email: drshilpashetty@gmail.com.
Varun Pitti, Email: varun_20012000@yahoo.com, Email: varunpitti@gmail.com.
C. L. Satish Babu, Email: nithyasatish@hotmail.com
G. P. Surendra Kumar, Email: anuskumar@hotmail.com
References
- 1.Lavigne GJ, et al. Bruxism physiology and pathology: an overview of clinicians. J Oral Rehab. 2008;35:476–494. doi: 10.1111/j.1365-2842.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 2.Burton C (1983) Bruxism. Thesis, University of Sydney
- 3.Bader G, Lavigne G. Sleep bruxism; an overview of an oromandibular sleep movement disorder. Sleep Med Rev. 2000;4:27–43. doi: 10.1053/smrv.1999.0070. [DOI] [PubMed] [Google Scholar]
- 4.AASM (2005) International classification of sleep disorders, 2nd edn. American Academy of Sleep Medicine, Westchester
- 5.Laat A, Macaluso GM. Sleep bruxism as a motor disorder. Mov Disord. 2002;17:S67–S69. doi: 10.1002/mds.10064. [DOI] [PubMed] [Google Scholar]
- 6.Glaros AG. Incidence of diurnal and nocturnal bruxism. J Prosthet Dent. 1981;45:545–549. doi: 10.1016/0022-3913(81)90044-5. [DOI] [PubMed] [Google Scholar]
- 7.ICSD—International Classification of Sleep Disorders: Diagnostic and Coding manual. Diagnostic Classification Steering Committee, Thorpy, Chairman, Rochester, MN: American Sleep Disorders Association (1990)
- 8.Sari S, Sonmez H. The relationship between occlusal factors and bruxism in permanent and mixed dentition in Turkish children. J Clin Pediatr Dent. 2001;25:191–194. doi: 10.17796/jcpd.25.3.84m695q650622568. [DOI] [PubMed] [Google Scholar]
- 9.Lavigne G, Montplaisir JV. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–743. [PubMed] [Google Scholar]
- 10.Macaluso GM, et al. Sleep bruxism is an disorder related to periodic arousals of sleep. J Dent Res. 1998;77:565. doi: 10.1177/00220345980770040901. [DOI] [PubMed] [Google Scholar]
- 11.Lobbezoo F, Lavinge GJ, Tanguay R, Montplaier JY. The effect of the catecholamine precursor l-dopa on sleep bruxism: a controlled clinical trial. Mov Disord. 1997;12:73. doi: 10.1002/mds.870120113. [DOI] [PubMed] [Google Scholar]
- 12.Lobbezoo F, Soucy JP, Montplaster JY, Lavinge GJ. Striatal D2 receptor binding in sleep bruxism: a controlled study with iodine-123-iodobenzamide, single photon emission computed tomography. J Dent Res. 1996;75:1804. doi: 10.1177/00220345960750101401. [DOI] [PubMed] [Google Scholar]
- 13.Lobbezoo F, Naeije M. Bruxism is mainly regulated centrally and not peripherally. J Oral Rehab. 2001;35:1085–1091. doi: 10.1046/j.1365-2842.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 14.Lobbezoo F, Van Denderan RJ, et al. Reports of SSRI-associated bruxism in the family physician office. J Orofac Pain. 2001;15:340–346. [PubMed] [Google Scholar]
- 15.Ashroftt GW, Eccleston D, Waddell JL. Recognition of amphetamine addicts. Br Med J. 1965;1:57. doi: 10.1136/bmj.1.5426.57-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manfredini D, Landi N, Romagnoli M, Bosco M. Psychic and occlusal factors in bruxers. Aust Dent J. 2004;49:84–89. doi: 10.1111/j.1834-7819.2004.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 17.Molina OF, dos Santos J., Jr Hostility in TMD/bruxism patients and controls: a clinical comparison study and preliminary results. Cranio. 2002;20:282–288. doi: 10.1080/08869634.2002.11746220. [DOI] [PubMed] [Google Scholar]
- 18.Monaco A, Ciammella NM, Marci MC, Pirro R, Giannoni M. The anxiety in bruxer child. A case–control study. Minerva Stomatol. 2002;51:247–250. [PubMed] [Google Scholar]
- 19.Ohayon MM, Li KK, Guilleminault C. Risk factors for sleep bruxism in the general population. Chest. 2001;119:53–61. doi: 10.1378/chest.119.1.53. [DOI] [PubMed] [Google Scholar]
- 20.Selms MKA, Lobbezoo F, Wicks DJ, Hamburger HL, Naeije M. Craniomandibular pain, oral parafunctions, and psychological stress in a longitudinal case study. J Oral Rehabil. 2004;31:738–745. doi: 10.1111/j.1365-2842.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- 21.Giffin KM. Mandibular adaptive reposturing: the aetiology of a common and multifaceted autodestructive syndrome. Gen Dent. 2003;51:62–67. [PubMed] [Google Scholar]
- 22.Manfredini D, Landi N, Tognini F, Montagnani G, Bosco M. Occlusal features are not a reliable predictor of bruxism. Minerva Stomatol. 2004;53:231–239. [PubMed] [Google Scholar]
- 23.Manfredini D, Cantini E, Romagnoli M, Bosco M. Prevalence of bruxism in patients with different research diagnostic criteria for temporomandibular disorders (RDC/TMD) diagnoses. Cranio. 2003;21:279–285. doi: 10.1080/08869634.2003.11746263. [DOI] [PubMed] [Google Scholar]
- 24.Koyano K, et al. Assessment of Bruxism in the clinic. J Oral Rehab. 2008;35:495–508. doi: 10.1111/j.1365-2842.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 25.Lavigne GJ, Montplaisir JV. Bruxism: epidemiology, diagnosis, pathophysiology, and pharmacology. In: Fricton JR, Dubner R, editors. Orofacial pain and temporomandibular disorders: advances in pain research and therapy. New York: Raven Press; 1995. pp. 387–404. [Google Scholar]
- 26.Pintado MR, Anderson GC, Long R, Douglas WH. Variation in tooth wear in young adults over a two-year period. J Prosthet Dent. 1997;77:313–320. doi: 10.1016/S0022-3913(97)70189-6. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Thie NM, Montplaisir JY, Lavigne GJ. Bruxism and orofacial movements during sleep. Dent Clin N Am. 2001;45:657–684. [PubMed] [Google Scholar]
- 28.Rugh JD, Harlan J. Nocturnal bruxism and temporomandibular disorders. In: Jankovic J, Tolosa E, editors. Facial dyskinesias: advances in neurology. New York: Raven Press; 1988. pp. 329–341. [PubMed] [Google Scholar]
- 29.Knight DJ, Leroux BG, Zhu C, Almond J, Ramsay DS. A longitudinal study of tooth wear in orthodontically treated patients. Am J Orthod Dentofac Orthop. 1997;112:194–202. doi: 10.1016/S0889-5406(97)70246-4. [DOI] [PubMed] [Google Scholar]
- 30.Baba K, Haketa T, Clark GT, Ohyama T. Does tooth wear status predict ongoing sleep bruxism in 30-year-old Japanese subjects? Int J Prosthodont. 2004;17:39–44. [PubMed] [Google Scholar]
- 31.Ekfeldt A, Hugoson A, Bergendal T, Helkimo A. An individual tooth wear index and an analysis of factors correlated to incisal and occlusal wear in an adult Swedish population. Acta Odontol Scand. 1990;48:343–349. doi: 10.3109/00016359009033627. [DOI] [PubMed] [Google Scholar]
- 32.Johansson A, Johansson AK, Omer R, Carlsson GE. Rehabilitation of the worn dentition. J Oral Rehabil. 2008;35:548–566. doi: 10.1111/j.1365-2842.2008.01897.x. [DOI] [PubMed] [Google Scholar]
- 33.Holmgren K, Sheikholeslam A, Riise C. Effect of a full-arch maxillary occlusal splint on parafunctional activity during sleep in patients with nocturnal bruxism and signs and symptoms of craniomandibular disorders. J Prosthet Dent. 1993;69:293–297. doi: 10.1016/0022-3913(93)90109-2. [DOI] [PubMed] [Google Scholar]
- 34.Korioth TW, Bohlig KG, Anderson GC. Digital assessment of occlusal wear patterns on occlusal stabilization splints: a pilot study. J Prosthet Dent. 1998;80:209–213. doi: 10.1016/S0022-3913(98)70112-X. [DOI] [PubMed] [Google Scholar]
- 35.Baba K, Clark GT, Watanabe T, Ohyama T. Bruxism force detection by a piezoelectric film-based recording device in sleeping humans. J Orofac Pain. 2003;17:58–64. [PubMed] [Google Scholar]
- 36.Forgione A (1974) Simple but effective method quantifying bruxing behavior. J Dent Res 53(special issue)
- 37.Pierce CJ, Gale EN. Methodological considerations concerning the use of bruxcore plates to evaluate nocturnal bruxism. J Dent Res. 1989;68:1110–1114. doi: 10.1177/00220345890680061101. [DOI] [PubMed] [Google Scholar]
- 38.Lobbezoo F, Zaag J, Selms MKA, Hamburger HL, Naeije M. Principles for the management of bruxism. J Oral Rehabil. 2008;35:509–523. doi: 10.1111/j.1365-2842.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi H, Ikeda T, Clark GT. A piezoelectric film-based intrasplint detection method for bruxism. J Prosthet Dent. 2001;86:195–202. doi: 10.1067/mpr.2001.115487. [DOI] [PubMed] [Google Scholar]
- 40.Rugh JD, Solberg WK. Electromyographic studies of bruxist behavior before and during treatment. J Calif Dent Assoc. 1975;3:56–59. [PubMed] [Google Scholar]
- 41.Ikeda T, Nishigawa K. Criteria for the detection of sleep associated bruxism in humans. J Orofac Pain. 1996;10:270–282. [PubMed] [Google Scholar]
- 42.Kato T, Thie NM, Huynh N, Miyawaki S, Lavigne GJ. Topical review: sleep bruxism and the role of peripheral sensory influences. J Orofac Pain. 2003;17:191–213. [PubMed] [Google Scholar]
- 43.Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil. 2008;35:476–494. doi: 10.1111/j.1365-2842.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 44.Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Drugs and bruxism: a critical review. J Orofac Pain. 2003;17:99–111. [PubMed] [Google Scholar]
- 45.Yamaguchi T, Mikami S, Okada K. Validity of a newly developed ultraminiature cordless EMG measurement system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e22–e27. doi: 10.1016/j.tripleo.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Minakuchi H, Clark GT (2004) The sensitivity and specificity of miniature bruxism detection device. J Dent Res 83(special issue A)
- 47.Jadidi F, Castrillon E, Svensson P. Effect of conditioning electrical stimuli on temporalis electromyographic activity during sleep. J Oral Rehabil. 2007;34:152–159. doi: 10.1111/j.1365-2842.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 48.Velly-Miguel AM, Montplaisir J, Rompre PH, Lund JP, Lavigne GL. Bruxism and other orofacial movements during sleep. J Craniomandib Dis Fac Oral Pain. 1992;6:71–81. [Google Scholar]
- 49.Lobbezoo F, et al. Principles of Maganement of Bruxism. J Oral Rehab. 2008;35:509–523. doi: 10.1111/j.1365-2842.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- 50.Butler JH. Occlusal adjustment. Dent Dig. 1970;76:422–426. [PubMed] [Google Scholar]
- 51.Frumker SC. Occlusion and muscle tension. Basal facts. 1981;4:85–87. [PubMed] [Google Scholar]
- 52.Holmgren K, Sheikholeslam A. Occlusal adjustment and myoelectric activity of the jaw elevator muscles in patients with nocturnal bruxism and craniomandibular disorders. Scand J Dent Res. 1994;102:238–243. doi: 10.1111/j.1600-0722.1994.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 53.Greene CS, Klasser GD, Epstein JB. ‘Observations’ questioned. J Am Dent Assoc. 2005;136:852–853. doi: 10.14219/jada.archive.2005.0276. [DOI] [PubMed] [Google Scholar]
- 54.Allen DL. Accurate occlusal bite guards. Periodontics. 1967;5:93–95. [PubMed] [Google Scholar]
- 55.Nassif NJ, Al-Ghamdi KS (1999) Managing bruxism and temporomandibular disorders using a centric relation occlusal device. Compend Contin Educ Dent 20:1071–1074, 1076, 1078, 1086 [PubMed]
- 56.Okeson JP. The effects of hard and soft occlusal splints on nocturnal bruxism. J Am Dent Assoc. 1987;114:788–791. doi: 10.14219/jada.archive.1987.0165. [DOI] [PubMed] [Google Scholar]
- 57.Leib AM (1996) The occlusal bite splint—a noninvasive therapy for occlusal habits and temporomandibular disorders. Compend Contin Educ Dent 17:1081–1084, 1086, 1088 [PubMed]
- 58.Boyd JP. Improving TMD treatment and protecting restorative dentistry. Dent Today. 1998;17:144. [PubMed] [Google Scholar]
- 59.Sullivan TC. A new occlusal splint for treating bruxism and TMD during orthodontic therapy. J Clin Orthod. 2001;35:142–144. [PubMed] [Google Scholar]
- 60.Clark GT, Beemsterboer PL, Solberg WK, Rugh JD. Nocturnal electromyographic evaluation of myofascial pain dysfunction in patients undergoing occlusal splint therapy. J Am Dent Assoc. 1979;99:607–611. doi: 10.14219/jada.archive.1979.0348. [DOI] [PubMed] [Google Scholar]
- 61.Landary ML, et al. Reduction of sleep bruxism using a mandibular advancement device: an experimental controlled study. Int J Prosthodont. 2006;19:549–556. [PubMed] [Google Scholar]
- 62.Lavigne GJ, Rompre PH, Montplaisir JY, Lobbezoo F. Motor activity in sleep bruxism with concomitant jaw muscle pain: a retrospective pilot study. Eur J Oral Sci. 1997;105:92–95. doi: 10.1111/j.1600-0722.1997.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 63.Mittelman J. Biofeedback: new answer to dental pain. It can be administered easily and inexpensively in any dental office. Dent Manag. 1976;16(21–22):26–27. [PubMed] [Google Scholar]
- 64.Cannistraci AJ. A method to control bruxism: biofeedback assisted relaxation therapy. J Am Soc Prev Dent. 1976;6:12–15. [PubMed] [Google Scholar]
- 65.Cherasia M, Parks L. Suggestions for use of behavioral measures in treating bruxism. Psychol Rep. 1986;58:719–722. doi: 10.2466/pr0.1986.58.3.719. [DOI] [PubMed] [Google Scholar]
- 66.Nissani M. Can taste aversion prevent bruxism? Appl Psychophysiol Biofeedback. 2000;25:43–54. doi: 10.1023/A:1009585422533. [DOI] [PubMed] [Google Scholar]
- 67.Roehrs T, Carskadon MA, Dement WC, Roth T. Daytime sleepiness and alertness. In: Kryger M, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders; 2005. pp. 39–50. [Google Scholar]
- 68.Nash MC, Ferrell RB, Lombardo MA, Williams RB. Treatment of bruxism in Huntington’s disease with botulinum toxin. J Neuropsychiatry Clin Neurosci. 2004;16:381–382. doi: 10.1176/jnp.16.3.381-a. [DOI] [PubMed] [Google Scholar]
- 69.Monroy PG, Da Fonseca MA. The use of botulinum toxin-a in the treatment of severe bruxism in a patient with autism: a case report. Spec Care Dent. 2006;26:37–39. doi: 10.1111/j.1754-4505.2006.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 70.Tan EK, Jankovic J. Treating severe bruxism with botulinum toxin. J Am Dent Assoc. 2000;131:211–216. doi: 10.14219/jada.archive.2000.0149. [DOI] [PubMed] [Google Scholar]
- 71.Huynh N, Lavigne GJ, Lanfranchi PA, Montplaisir JY, Champlain J. The effect of 2 sympatholytic medications—propranolol and clonidine on sleep bruxism: experimental randomized controlled studies. Sleep. 2006;29:307–316. doi: 10.1093/sleep/29.3.307. [DOI] [PubMed] [Google Scholar]
- 72.Etzel KR, Stockstill JW, Rugh JD, Fisher JG. Tryptophan supplementation for nocturnal bruxism: report of negative results. J Craniomandib Disord. 1991;5:115–120. [PubMed] [Google Scholar]
- 73.Amir I, Hermesh H, Gavish A. Bruxism secondary to antipsychotic drug exposure: a positive response to propranolol. Clin Neuropharmacol. 1997;20:86–89. doi: 10.1097/00002826-199702000-00011. [DOI] [PubMed] [Google Scholar]


